Abstract
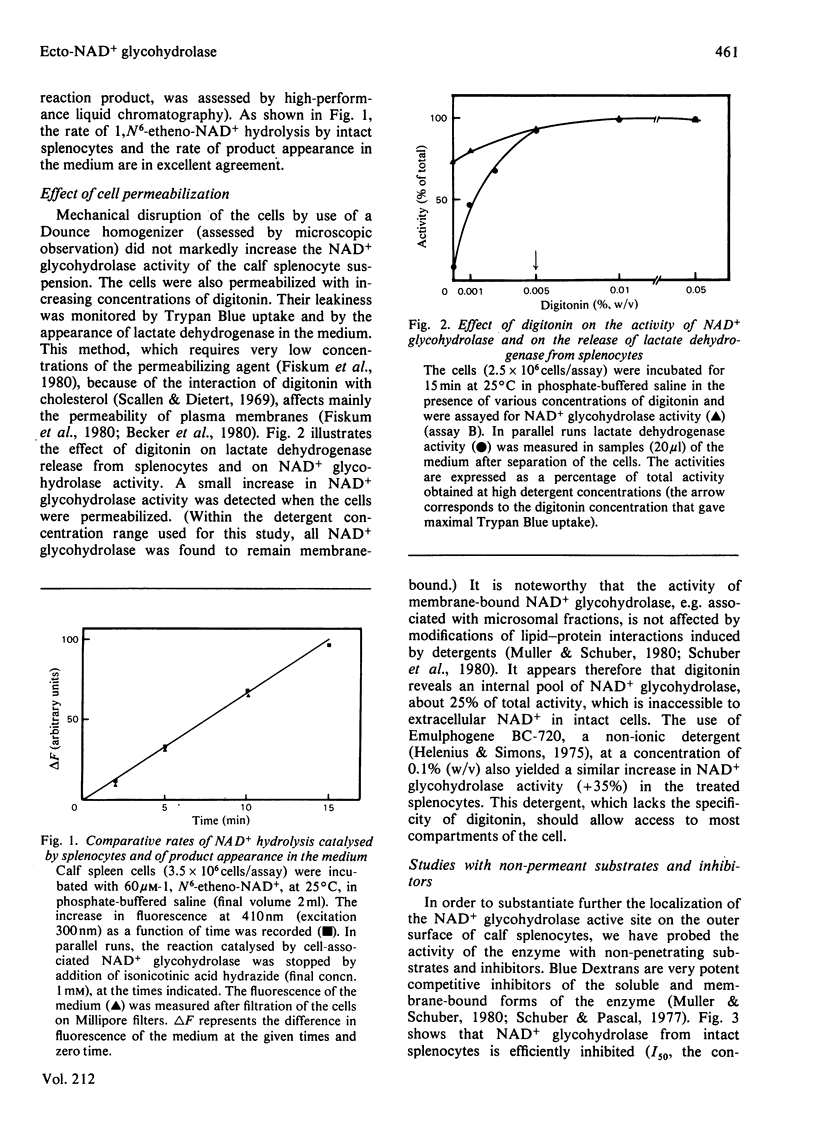
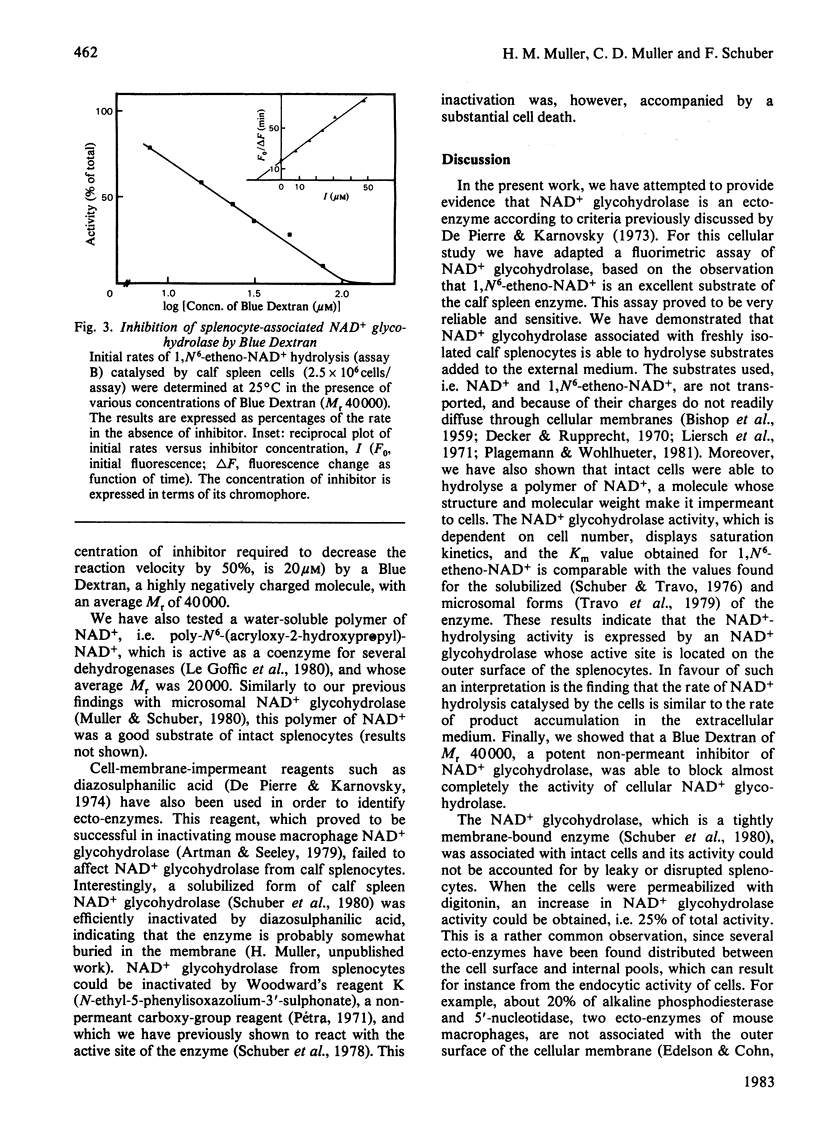
By using a sensitive fluorimetric assay of NAD+ glycohydrolase (EC 3.2.2.6), we showed that calf spleen cells are able to hydrolyse 1,N6-etheno-NAD+ given in the medium. The observed rates of substrate hydrolysis and product accumulation in the medium are equivalent. Moreover, the splenocytes are able to cleave the nicotinamide-ribose bond of a water-soluble polymer of NAD+, and their NAD+ glycohydrolase activity is fully inhibited by a high-molecular-weight Blue Dextran. Selective permeation of the cellular membrane digitonin revealed an intracellular pool of NAD+ glycohydrolase, which accounts for 25% of the total activity. We conclude that NAD+ glycohydrolase associated with the splenocytes has the characteristics of an ecto-enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARTMAN M., BEKIERKUNST A., BARKAI E. SUBMICROSOMAL LOCALIZATION OF MOUSE-LIVER NICOTINAMIDE-ADENINE DINUCLEOTIDE GLYCOHYDROLASE. Biochim Biophys Acta. 1964 Mar 9;81:614–617. doi: 10.1016/0926-6569(64)90152-x. [DOI] [PubMed] [Google Scholar]
- Alivisatos S. G., Ungar F., Gerber M., Arora R., Levitt L. P., Tabakoff B. Cellular distribution of nicotinamide adenine dinucleotide glycohydrolase in the central nervous system. Biochem Pharmacol. 1974 Jul 15;23(14):2060–2062. doi: 10.1016/0006-2952(74)90265-2. [DOI] [PubMed] [Google Scholar]
- Artman M., Seeley R. J. Nicotinamide adenine dinucleotide splitting enzyme: a characteristic of the mouse macrophage. Science. 1978 Dec 22;202(4374):1293–1295. doi: 10.1126/science.214853. [DOI] [PubMed] [Google Scholar]
- Artman M., Seeley R. J. Nicotinamide adenine dinucleotide splitting enzyme: a plasma membrane protein of murine macrophages. Arch Biochem Biophys. 1979 Jun;195(1):121–127. doi: 10.1016/0003-9861(79)90333-3. [DOI] [PubMed] [Google Scholar]
- BISHOP C., RANKINE D. M., TALBOTT J. H. The nucleotides in normal human blood. J Biol Chem. 1959 May;234(5):1233–1237. [PubMed] [Google Scholar]
- Becker G. L., Fiskum G., Lehninger A. L. Regulation of free Ca2+ by liver mitochondria and endoplasmic reticulum. J Biol Chem. 1980 Oct 10;255(19):9009–9012. [PubMed] [Google Scholar]
- Bekierkunst A. Effect of isoniazid on preservation of nicotinamide dinucleotide in animal cells. Arch Biochem Biophys. 1967 Mar 20;118(3):549–555. doi: 10.1016/0003-9861(67)90389-x. [DOI] [PubMed] [Google Scholar]
- Bernofsky C., Pankow M. Protein binding of nicotinamide adenine dinucleotide and regulation of nicotinamide adenine dinucleotide glycohydrolase activity in homogenates of rabbit skeletal muscle. Arch Biochem Biophys. 1973 May;156(1):143–153. doi: 10.1016/0003-9861(73)90351-2. [DOI] [PubMed] [Google Scholar]
- Bock K. W., Gäng V., Beer H. P., Kronau R., Grunicke H. Localization and regulation of two NAD nucleosidases in Ehrlich ascites cells. Eur J Biochem. 1968 Apr;4(3):357–363. doi: 10.1111/j.1432-1033.1968.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- CIOTTI M. M., KAPLAN N. O. Chemistry and properties of the 3-acetylpyridine analogue of diphosphopyridine nucleotide. J Biol Chem. 1956 Aug;221(2):823–832. [PubMed] [Google Scholar]
- Clark J. B., Pinder S. Control of the steady-state concentrations of the nicotinamide nucleotides in rat liver. Biochem J. 1969 Sep;114(2):321–330. doi: 10.1042/bj1140321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. I. Evidence for an ecto-adenosine monophosphatase, adenosine triphosphatase, and -p-nitrophenyl phosphates. J Biol Chem. 1974 Nov 25;249(22):7111–7120. [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K., Rupprecht E. Permeation von NAD aus der Blutbahn in die Leberzellen. Hoppe Seylers Z Physiol Chem. 1970 Jan;351(1):15–24. doi: 10.1515/bchm2.1970.351.1.15. [DOI] [PubMed] [Google Scholar]
- DiAugustine R. P., Abe T., Voytek P. Purification and comparative properties of the glycoprotein nicotinamide adenine dinucleotide glycohydrolase from rat liver microsomal and plasma membranes. Biochim Biophys Acta. 1978 Oct 12;526(2):518–530. doi: 10.1016/0005-2744(78)90142-0. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. 5'-Nucleotidase activity of mouse peritoneal macrophages. II. Cellular distribution and effects of endocytosis. J Exp Med. 1976 Dec 1;144(6):1596–1608. doi: 10.1084/jem.144.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson P. J., Erbs C. Plasma membrane localization and metabolism of alkaline phosphodiesterase I in mouse peritoneal macrophages. J Exp Med. 1978 Jan 1;147(1):77–86. doi: 10.1084/jem.147.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G., Craig S. W., Decker G. L., Lehninger A. L. The cytoskeleton of digitonin-treated rat hepatocytes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3430–3434. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukishima M., Okayama H., Takahashi Y., Hayaishi O. Characterization of the NAD+ glycohydrolase associated with the rat liver nuclear envelope. J Biochem. 1976 Jul;80(1):167–176. doi: 10.1093/oxfordjournals.jbchem.a131248. [DOI] [PubMed] [Google Scholar]
- Gholson R. K. The pyridine nucleotide cycle. Nature. 1966 Nov 26;212(5065):933–935. doi: 10.1038/212933a0. [DOI] [PubMed] [Google Scholar]
- Green S., Dobrjansky A. Nicotinamide adenine dinucleotide glycohydrolases from Ehrlich ascites tumor cell nuclei: isolation, partial purification, and properties. Biochemistry. 1972 Oct 24;11(22):4108–4113. doi: 10.1021/bi00772a013. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- JACOBSON K. B., KAPLAN N. O. Distribution of enzymes cleaving pyridine nucleotides in animal tissues. J Biophys Biochem Cytol. 1957 Jan 25;3(1):31–43. doi: 10.1083/jcb.3.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S. Metabolism of NAD and N1-methylnicotinamide in growing and growth-arrested cells. Eur J Biochem. 1980 Dec;112(3):635–641. doi: 10.1111/j.1432-1033.1980.tb06128.x. [DOI] [PubMed] [Google Scholar]
- Le Goffic F., Sicsic S., Vincent C. A two-step synthesis of new water-soluble polymers of NAD+ and ADP. The biological properties of these polymers. Eur J Biochem. 1980;108(1):143–148. doi: 10.1111/j.1432-1033.1980.tb04705.x. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Everse J. Studies on the properties of 1,N 6 -ethenoadenine derivatives of various coenzymes. Arch Biochem Biophys. 1973 Jul;157(1):83–90. doi: 10.1016/0003-9861(73)90392-5. [DOI] [PubMed] [Google Scholar]
- Liersch M., Grotelüschen H., Decker K. Zur Frage der Permeation von NAD in die Leberzelle. Hoppe Seylers Z Physiol Chem. 1971 Feb;352(2):267–274. [PubMed] [Google Scholar]
- Mellors A., Lun A. K., Peled O. N. Evidence for NAD nucleosidase in rabbit-liver lysosomes. Can J Biochem. 1975 Feb;53(2):143–148. doi: 10.1139/o75-022. [DOI] [PubMed] [Google Scholar]
- Muller H., Schuber F. Studies on the association of NAD glycohydrolase with membranes in calf spleen. Eur J Biochem. 1980 Mar;104(2):489–500. doi: 10.1111/j.1432-1033.1980.tb04451.x. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Steinman R. M., Cohn Z. A. The membrane proteins of the vacuolar system. II. Bidirectional flow between secondary lysosomes and plasma membrane. J Cell Biol. 1980 Jul;86(1):304–314. doi: 10.1083/jcb.86.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala P. H., Anderson B. M. Studies of bovine erythrocyte NAD glycohydrolase. J Biol Chem. 1978 Oct 25;253(20):7453–7459. [PubMed] [Google Scholar]
- Scallen T. J., Dietert S. E. The quantitative retention of cholesterol in mouse liver prepared for electron microscopy by fixation in a digitonin-containing aldehyde solution. J Cell Biol. 1969 Mar;40(3):802–813. doi: 10.1083/jcb.40.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Levitzki A. Molecular basis of negative co-operativity in rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1974 Feb 5;82(4):547–561. doi: 10.1016/0022-2836(74)90248-4. [DOI] [PubMed] [Google Scholar]
- Schneider Y. J., Tulkens P., de Duve C., Trouet A. Fate of plasma membrane during endocytosis. II. Evidence for recycling (shuttle) of plasma membrane constituents. J Cell Biol. 1979 Aug;82(2):466–474. doi: 10.1083/jcb.82.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuber F., Muller H., Schenherr I. Amphipathic properties of calf spleen NAD glycohydrolase. FEBS Lett. 1980 Jan 14;109(2):247–251. doi: 10.1016/0014-5793(80)81097-0. [DOI] [PubMed] [Google Scholar]
- Schuber F., Pascal M. Interaction of Blue Dextran and and Cibacron Blue F3GA with calf spleen NAD+-glycohydrolase. Biochimie. 1977;59(8-9):735–737. doi: 10.1016/s0300-9084(77)80254-x. [DOI] [PubMed] [Google Scholar]
- Schuber F., Pascal M., Travo P. Calf-spleen nicotinamide-adenine dinucleotide glycohydrolase. Properties of the active site. Eur J Biochem. 1978 Feb 1;83(1):205–214. doi: 10.1111/j.1432-1033.1978.tb12085.x. [DOI] [PubMed] [Google Scholar]
- Schuber F., Travo P. Calf-spleen nicotinamide--adenine dinucleotide glycohydrolase. Solubilization purification and properties of the enzyme. Eur J Biochem. 1976 May 17;65(1):247–255. doi: 10.1111/j.1432-1033.1976.tb10411.x. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Edwards M. R., Luzio J. P. Subcellular distribution and movement of 5'-nucleotidase in rat cells. Biochem J. 1980 Jan 15;186(1):59–69. doi: 10.1042/bj1860059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamulevicius P., Streffer C., Roscic O., Hubert E. Localization of oxidized nocotinamide--adenine dinucleotide glycohydrolase in the mouse liver nuclear envelope. Biochem J. 1979 Feb 15;178(2):467–473. doi: 10.1042/bj1780467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982 Feb;92(2):417–424. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travo P., Muller H., Schuber F. Calf spleen NAD glycohydrolase. Comparison of the catalytic properties of the membrane-bound and the hydrosoluble forms of the enzyme. Eur J Biochem. 1979 May 2;96(1):141–149. doi: 10.1111/j.1432-1033.1979.tb13023.x. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Schneider Y. J., Pierre B., Baudhuin P., Trouet A. Evidence for a continual exchange of 5'-nucleotidase between the cell surface and cytoplasmic membranes in cultured rat fibroblasts. Cell. 1982 Jan;28(1):61–70. doi: 10.1016/0092-8674(82)90375-0. [DOI] [PubMed] [Google Scholar]
- ZATMAN L. J., KAPLAN N. O., COLOWICK S. P., CIOTTI M. M. The isolation and properties of the isonicotinic acid hydrazide analogue of diphosphopyridine nucleotide. J Biol Chem. 1954 Aug;209(2):467–484. [PubMed] [Google Scholar]


