Abstract
Purpose
Colorectal cancer (CRC) surgery in elderly patients with hypertension poses challenges due to potential complications and prolonged recovery. This study aimed to assess the impact of multimodal opioid-sparing anesthesia on intestinal function and prognosis of elderly hypertension patients undergoing CRC surgery.
Methods
A total of 80 elderly hypertension patients who underwent open surgery for CRC in the People’s Hospital of Xinjiang Uygur Autonomous Region from October 2020 to October 2022 were selected and randomly divided into two group (A and B, n = 40) through the random number table method. Group A received multimodal opioid-sparing anesthesia, defined as low-dose opioid general anesthesia combined with a transversus abdominis plane block, incision infiltration with local anesthetics, and postoperative analgesia via a patient-controlled analgesia (PCA) pump, with the remifentanil dose set at one-third (± 10%) of the conventional group’s dose. Group B received conventional opioid anesthesia, involving standard general anesthesia maintained with remifentanil at 0.4–0.5 µg/(kg·min), incision infiltration with local anesthetics, and postoperative PCA. Primary outcomes included mean arterial pressure (MAP) and heart rate (HR), changes in albumin, C-reactive protein (CRP) and white blood cell (WBC), indicators of intestinal function recovery (the recovery time of bowel sounds, the first exhaust time, the first defecation time and the feeding recovery time), and visual analogue scale (VAS) pain scores. Second outcomes included postoperative complications and total hospital stays.
Results
After excluding 8 patients, 72 were included in the final analysis. Compared with patients in the B group, patients in the A group exhibited shorter recovery time of bowel sounds, first exhaust time and feeding recovery time (P < 0.05), higher levels of postoperative albumin, and lower levels of CRP and WBC (P < 0.05). Moreover, the incidence of nausea and vomiting was lower and the total hospital stays were fewer in the A group than in the B group (P < 0.05).
Conclusion
Multimodal opioid-sparing anesthesia contributes to rapid recovery of postoperative intestinal function and reduction of postoperative adverse reactions. Therefore, it is safe and feasible to apply multimodal opioid-sparing anesthesia to elderly hypertension patients receiving open surgery for CRC.
Keywords: Opioid-sparing, Multimodal anesthesia, Hypertension, Open surgery for colorectal cancer, Intestinal function
Introduction
Colorectal cancer (CRC), one of the common digestive system malignant tumors worldwide [1]. Estimates from GLOBOCAN suggest that in 2020 there are estimated to be more than 1.9 million new cases of CRC and 930,000 deaths, while by 2040, CRC is projected to increase to 3.2 million new cases and 1.6 million deaths [2]. At present, surgery, as the first choice for radical treatment of CRC, greatly improves the survival rate of patients with CRC [3]. However, the trauma and stress caused by surgery usually induce some postoperative complications and affect intestinal function to some content. Moreover, patients with CRC complicated with hypertension have more obvious circulation fluctuation during surgery, not only inducing higher surgery risks but also directly affecting the quality of postoperative rehabilitation [4]. CRC surgery causes a strong traumatic stress response in elderly patients, even affects the recovery of bowel function and prolongs hospital stays [5]. The intestinal regulatory function of elderly patients is decreased, so early recovery of postoperative intestinal function is one of the key points for the prognosis of patients.
Currently, opioids are commonly used postoperative analgesics. However, elderly patients are prone to respiratory depression, gastrointestinal dysfunction and urinary retention, accompanied with adverse effects such as dry mouth, abdominal distention and constipation, if they take too many opioids [6]. Elderly patients, especially CRC with comorbid hypertension, experience more pronounced circulatory fluctuations during surgery, heightening the risk of adverse surgical outcomes and impacting postoperative recovery [7]. The use of opioids for postoperative pain management, while effective, can exacerbate complications such as respiratory depression and gastrointestinal dysfunction, which are particularly detrimental in the elderly hypertensive demographic [8]. These complications can impede early postoperative recovery, essential for the prognosis of elderly patients.
Recently, perioperative multi-mode analgesia has become an important part of promoting rapid recovery of patients [9]. Multimodal analgesia refers to the combined application of analgesic drugs or analgesic methods acting on different pain conduction pathway targets during the whole perioperative period. Briefly, multimodal analgesia can achieve additional or synergistic analgesic effects, reduce the dosage of opioids and related adverse reactions, and promote postoperative function recovery of patients [10]. Nowadays, multimodal low-opioid anesthesia has been applied in the management of anaesthesia for various surgeries [11]. This approach is hypothesized to preserve hemodynamic stability, enhance postoperative intestinal function, and shorten hospital stays, and can help to maintain more stable blood pressure and reduce cardiovascular complications by reducing the amount of opioids used [12, 13]. Although the benefits of multimodal opioid-sparing anaesthesia have been tentatively recognised, the actual outcomes and best practices for older hypertensive patients require further research. In this study, multimodal opioid-sparing anesthesia was defined as the intraoperative administration of 1/3 dosage of conventional opioids. The objective of this study was to observe the impact of multimodal opioid-sparing anesthesia on intestinal function and prognosis of elderly hypertension patients after CRC surgery. The outcomes could guide anesthesia practices and pain management strategies, potentially setting new standards for the care of elderly hypertensive patients undergoing major surgeries.
Materials and methods
Research objects
This study was registered with the Clinical Trials Registry (Registration number: ISRCTN18251692). Research objects (n = 80) were selected from elderly hypertension patients who underwent open surgery for CRC in the People’s Hospital of Xinjiang Uygur Autonomous Region from October 2020 to October 2022. This study was single-blind and participants did not know the treatment assignment group, and they were randomly divided into an A group and a B group (40 cases per group) through the random number table method. In the A group, patients received multimodal opioid-sparing anesthesia. In the B group, patients were given opioids at a conventional dosage.
Inclusion and exclusion criteria were shown as follows. Inclusion criteria included patients (1) aged 60–80 years; (2) receiving CRC surgery at ASA II-III (the American Society of Anesthesiology classification); (3) with body mass index (BMI) ranging from 18.5 to 30 kg/m2; (4) with a history of hypertension, a systolic blood pressure ≥ 140 mmHg, and a pulse pressure ≥ 60 mmHg; (5) with drug-controlled hypertension; (6) without contraindications to anesthesia; (7) without systemic infection before surgery. Exclusion criteria consisted of patients with (1) tumors at other sites; (2) a history of long-term use of sedative and analgesic drugs; (3) previous history of gastrointestinal surgery; (4) uncontrollable hypertension, hyperglycemia and severe liver and kidney dysfunction; Unmanageable hypertension is defined as unsatisfactory blood pressure control after adequate treatment with at least five different types of antihypertensive drugs, including one long-acting thiazide diuretic and one salocorticoid receptor antagonist; (5) long-term use of adrenergic neuroleptics (rifampicin); (6) other surgical contraindications. All patients were informed consent to the content and significance of this study, and signed an informed consent form. Besides, this study was approved by the Ethics Committee of People’s Hospital of Xinjiang Uygur Autonomous Region (KY2021031901).
Sample size calculation and random grouping method
The sample size calculation was performed based on pretest results and using PASS15.0 Software (NCSS Statistical Software, Kaysville, Utah). Pretest data indicated a population standard deviation (σ) of 2.6, which was based on the formula
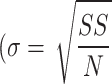 |
, SS, sample variance; N, total number of samples).
An effect size (δ) of 2 was used, based on clinically relevant differences observed in previous research. To ensure adequate power for detecting a difference with a two-tailed significance level (α) of 0.05 and a power (1-β) of 0.90, the sample size estimate suggested a minimum of 29 patients per group. For intermediate exclusion cases and lost follow-up cases, the actual sample size of each group was 40 cases, and a total of 80 patients were included in the two groups.
The random number table method was used for grouping. 1) Generate random numbers: The “Random Number Generator” of SPSS software was used to generate 80 random numbers between 0 and 1, and then in the “Convert” - “Calculate Variable”, it was defined as “Random Number” and the numerical expression was set to “RV.UNIFORM (0,1)”. “Calculation variables” will be defined as “random numbers” and set the numerical expression to “RV.UNIFORM (0,1)”, giving each patient a random number for subsequent grouping. UNIFORM (0,1)”, giving each patient a random number for subsequent grouping. 2) Randomization grouping: use the “conversion” in the “visual sub-box” on the random number of grouping; Set the amount of change in the split box to “Group” and divide it into two groups; Set the number of split points to 1 to get the variable “group”; Patients in group 1 were included in the low opioid multimodal analgesia group (group A) and patients in group 2 were included in the conventional opioid analgesia group (group B). Randomization was completed with 40 patients in each group.
Administration methods
Patients are prohibited from meat and solid foods 8 h prior to the surgery and from drinking (no liquids of any kind) 4 h before surgery. Diuretics were discontinued in all patients 2–3 days before surgery; angiotensin receptor blockers (ARBs) were discontinued on the day of surgery; β-blockers, calcium channel blockers, and sympathomimetic depressants (colistin) did not have to be discontinued preoperatively; and ACEIs were analysed individually according to the patient’s preoperative potassium status. Patients were sent to an operating room for conventional electrocardiography monitoring, and the blood pressure and heart rate (HR) of patients were recorded when they entered the room. Additionally, the peripheral venous access was opened, and the radial artery puncture and catheterization were performed under local anesthesia for invasive arterial pressure monitoring.
Before anesthesia induction: Firstly, bispectral index (BIS) monitoring was established. Besides, 30 min before induction, patients in the A and B groups were given 30 mg of intravenous nonsteroidal anti-inflammatory agents ketorolac tromethamine (1 mL: 30 mg, the batch number: 035210425-2, Shandong New Time Pharmaceutical Co., Ltd.) for preemptive analgesia. Next, 10 mg of dexamethasone (1 mL: 5 mg, the batch number: H42020019, Hubei TIANYAO Pharmaceutical Co., Ltd.) was given slowly.
Anesthesia induction: After sufficient oxygen inhalation, patients in the two groups were given 0.05 mg/kg of midazolam (2 mL: 10 mg, the batch number: 11G07011A3, Yichang Humanwell Pharmaceutical Co., Ltd.), 1–2 mg/kg of propofol (20 mL: 200 mg, the batch number: 22102021-5, Xi’an Libang Pharmaceutical Co., Ltd.), 0.15 mg/kg of cisatracurium (5 mL: 10 mg, the batch number: 22111311, Hangzhou Ausia Biological Tech Co., Ltd.), 0.4 µg/kg of sufentanil (1 mL: 50 µg, the batch number: 11A05091A3, Yichang Humanwell Pharmaceutical Co., Ltd.), and low-dose dexmedetomidine (2 mL: 0.2 mg, the batch number: 22011831, Yangtze River Pharmaceutical Group). Then, tracheal intubation was placed to assist breathing. The mechanical ventilation parameters included respiratory rate (12–16 breaths per minute), tidal volume (6–8 mL/kg), FiO2 (65%) and partial pressure of end-tidal CO2 (PETCO2) (35–45 mmHg). Blood pressure and HR at the time of intubation were recorded. Subsequently, under the guidance of ultrasound, right internal jugular vein catheterization was performed. During surgery, active warming therapy was performed to ensure that the body temperature of patients was above 36.7 ℃.
Anesthesia maintenance: In the A group, bilateral transversus abdominis plane block (TAPB) was performed under the guidance of ultrasound, and 15 mL of 0.33% ropivacaine (10 mL: 100 mg, the batch number: 21122401, Shandong Ruiyang Pharmaceutical Technology Co., Ltd.) was injected into the bilateral transversus abdominis plane of patients. In the B group, patients did not receive TAPB treatment. During surgery, patients in the B and A groups were given propofol [4–6 mg/(kg·h)] + remifentanil [0.4–0.5 µg/(kg·min)] (1 mg, the batch number: 10A11101, Yichang Humanwell Pharmaceutical Co., Ltd.) and propofol [4–6 mg/(kg·h)] + remifentanil [one-third (± 10%) of group B] by a continuous infusion pain pump, respectively. Moreover, both groups of patients received dexmedetomidine [0.4 µg/(kg·h)]. Next, the patient’s vital signs were closely monitored and the depth of anaesthesia was maintained in the range of 40–60. During surgery, hemodynamic stability was maintained, and the mean arterial pressure (MAP) was kept above 60 mmHg. If the patient’s blood pressure dropped by more than 20% of the preoperative level, fluid replacement therapy was performed with Ringer’s lactate solution immediately. If the blood pressure did not increase obviously, patients were injected with 6 mg of ephedrine intravenously or given norepinephrine. If hypertension occurred, 15–25 mg of urapidil was given to patients. If the HR was less than 50 beats/min, 0.5 mg of atropine was administrated. Intravenous anesthetics were discontinued 3 min before the end of the surgery, and both groups of patients were given 10 mL of 0.5% ropivacaine hydrochloride injection for incision infiltration anesthesia.
Postoperative analgesia: After completion of surgery, patients in the A group were administrated with low-opioid patient controlled intravenous analgesia (PCIA). The medicine formula was shown as follows: 8 mg of butorphanol tartrate injection (1 mL: 1 mg, the batch number: 230130BP, Jiangsu Hengrui Pharmaceuticals Co., Ltd.) + 200 mg of flurbiprofen axetil injection (5 mL: 50 mg, the batch number: 3E013A, Beijing Tide Pharmaceutical Co., Ltd.) + 20 mg of metoclopramide dihydrochloride injection (1 mL: 10 mg, the batch number: 62112181, Shanghai Harvest Pharmaceutical Co., Ltd.). Patients in the B group were given opioids PCIA after surgery, and the medicine formula was 1.5 µg/kg of sufentanil + 200 mg of flurbiprofen axetil injection + 20 mg of metoclopramide dihydrochloride injection. PCIA lasted for two days postoperatively in both groups. Medicines received by both groups of patients were diluted to 100 mL with normal saline, with a continuous administration of 2 mL/h, 2 mL of PCA (patient controlled analgesia) for a single dosage, and a locking time of 10 min. The patient can eat after the occurrence of exhaust gas.
We assigned the mean duration of surgery for both groups as a; the mean weight of both groups as b. The following formula was used to calculate the intraoperative and postoperative opioid dosage of the patients.
Intraoperative opioid dosage for patients in group A: 0.4*b + 1/3 (0.4–0.5)*ab;
Intraoperative opioid dosage for patients in group B: 0.4*b + (0.4–0.5)*ab;
Postoperative opioid dosage for patients in group A: (analgesic with opioid receptor activation) Butorphanol tartrate injection 8 mg;
Postoperative opioid dosage for patients in group B: 1.5*b.
Outcome measures
Primary outcome: Intestinal function recovery indicators, such as the recovery time of bowel sounds (the time when normal bowel sounds are first auscultated), the first exhaust time (the time when the patient first expels gas from the anus after surgery), the first defecation time and the feeding recovery time (when the patient first tolerates a soft liquid diet after surgery), were record. The vital signs, including MAP and heart rate (HR), were recorded at four time points: upon entry (T0), at intubation (T1), at extubation (T2) and 1 h after extubation (T3). Prognostic indicators, including the changes in albumin, C-reactive protein (CRP) and white blood cell (WBC) on the first day before and after surgery, as well as the visual analogue scale (VAS) pain scores 3 days after surgery (1–3 points indicated mild pain; 4–10 points indicated moderate and severe pain), were monitored.
Secondary outcomes: General information and intraoperative factors were collected, including gender, age, BMI, ASA classification, surgery type (right hemicolectomy only/left hemicolectomy only/total colectomy /Hartmann procedure/others), surgery time, anesthesia time and hemorrhage volume. Postoperative complications (such as fever, nausea and vomiting, deep vein thrombosis, intestinal obstruction) and total hospital stays were record.
Statistical analysis
SPSS 25.0 and GraphPad Prism 8.0 software were employed for statistical analysis. Firstly, the Shapiro-Wilk test was applied to check the normal distribution of measurement data. The measurement data conforming to the normal distribution were expressed by the mean ± standard deviation (SD), and the T test was used for comparison between groups. The measurement data conforming to the skewed distribution were expressed by median (the 1st quartile, the 3rd quartile) [M (Q1, Q3)], and Mann-Whitney U test was adopted for comparison between groups. The enumeration data were expressed by n (%), and χ2 was used for comparison between groups. P < 0.05 indicated a significant difference.
Results
Comparison of general information and intraoperative indexes between the two groups
In this study, 3 patients gave up surgery, 2 patients withdrew halfway, and 3 patients lost follow-up. A total of 8 patients were excluded, and 72 patients were included in the statistical analysis. As shown in Table 1, there was no significant difference in gender, age, BMI, ASA classification, surgery type (right hemicolectomy only/left hemicolectomy only/total colectomy /Hartmann procedure/others), surgery time, anesthesia time and hemorrhage volume between the two groups (P > 0.05).
Table 1.
Comparison between two groups of patients in general information and intraoperative indexes [n (%), mean ± SD, median (Q1, Q3)]
| Indexes | A group (n = 36) | B group (n = 36) | t/z/χ2 | P values |
|---|---|---|---|---|
| Gender (case, male/ female) | 25/11 | 23/13 | 0.250 | 0.617 |
| Age (year, mean ± SD) | 69.86 ± 4.02 | 70.42 ± 4.87 | -0.528 | 0.599 |
| BMI (kg/m2, mean ± SD) | 22.77 ± 2.48 | 23.39 ± 2.89 | -0.984 | 0.328 |
| ASA classification (II/III) | 22/14 | 25/11 | 0.551 | 0.458 |
| Surgery type (right hemicolectomy only/left hemicolectomy only/total colectomy /Hartmann procedure/others) | 12/6/6/8/4 | 11/7/6/9/3 | 0.322 | 0.988 |
| Surgery time (h, mean ± SD) | 3.68 ± 1.06 | 3.59 ± 1.02 | 0.351 | 0.726 |
| Anesthesia time (h, mean ± SD) | 4.35 ± 1.03 | 4.36 ± 0.97 | -0.035 | 0.972 |
| Hemorrhage volume [mL, M(Q1, Q3)] | 100 (50,150) | 100 (50,138) | -0.302 | 0.763 |
A group: multimodal opioid-sparing anesthesia; B group: anesthesia with conventional dosage of opioids; BMI: body mass index; ASA: American Society of Anesthesiology
Comparison of mean arterial pressure (MAP) and heart rate (HR) between the two groups at different time points
As maintaining haemodynamic stability during surgery in elderly hypertensive patients is critical, fluctuations in MAP and HR can indicate cardiovascular stress or instability. Therefore, we first measured MAP and HR. As displayed in Table 2, the difference was not significant in MAP and HR at the time of entry (T0), intubation (T1), extubation (T2) and 1 h after extubation (T3) between the groups (P > 0.05) (Table 2).
Table 2.
Comparison between two groups of patients in mean arterial pressure (MAP) and heart rate (HR) at different time points (mean ± SD)
| A group (n = 36) | B group (n = 36) | t | P | ||
|---|---|---|---|---|---|
| MAP | T0 | 98.07 ± 8.81 | 97.34 ± 8.33 | 0.359 | 0.720 |
| T1 | 100.13 ± 8.68 | 101.68 ± 8.45 | -0.771 | 0.444 | |
| T2 | 94.80 ± 7.77 | 95.78 ± 7.92 | -0.530 | 0.598 | |
| T3 | 93.94 ± 7.57 | 94.27 ± 7.74 | -0.186 | 0.853 | |
| HR | T0 | 79.25 ± 9.30 | 78.56 ± 7.48 | 0.349 | 0.728 |
| T1 | 82.58 ± 9.10 | 82.50 ± 7.70 | 0.042 | 0.967 | |
| T2 | 76.33 ± 9.18 | 75.72 ± 6.99 | 0.318 | 0.752 | |
| T3 | 75.44 ± 8.49 | 74.92 ± 7.21 | 0.284 | 0.777 |
A group: multimodal opioid-sparing anesthesia; B group: anesthesia with conventional dosage of opioids; MAP: mean arterial pressure; HR: heart rate. T0: the time at entry; T1: the time at intubation; T2: the time at extubation; T3: 1 h after extubation
Comparison of postoperative recovery of intestinal function between two groups of patients
Subsequently, we reflected postoperative gastrointestinal function recovery by analysing intestinal function recovery indicators. Compared with the B group, the A group showed a shorter recovery time of bowel sounds (1.98 ± 0.49 vs. 2.43 ± 0.59 days), first exhaust time (2.60 ± 0.53 vs. 3.05 ± 0.57 days), and feeding recovery time (3.88 ± 0.50 vs. 4.25 ± 0.62 days), with a significant difference (P < 0.05). However, the first defecation time was not significantly different between the two groups (P > 0.05) (Table 3).
Table 3.
Postoperative recovery for intestinal function in two groups of patients (mean ± SD)
| Indexes | A group (n = 36) | B group (n = 36) | t | P values |
|---|---|---|---|---|
| Recovery time of bowel sounds (days) | 1.98 ± 0.49 | 2.43 ± 0.59 | -3.517 | < 0.001 |
| The first exhaust time (days) | 2.60 ± 0.53 | 3.05 ± 0.57 | -3.469 | < 0.001 |
| The first defecation time (days) | 3.44 ± 0.51 | 3.68 ± 0.60 | -1.825 | 0.072 |
| Feeding recovery time (days) | 3.88 ± 0.50 | 4.25 ± 0.62 | -2.769 | 0.007 |
A group: multimodal opioid-sparing anesthesia; B group: anesthesia with conventional dosage of opioids
Comparison of clinical prognosis between the two groups before and after surgery
Changes in albumin, CRP, and WBC counts on the first day before and after surgery, provide insights into the body’s inflammatory response and nutritional status, both of which influence recovery and overall prognosis. Before surgery, there was no significant difference in the changes of albumin, CRP and WBC between the A and B groups (P > 0.05). After surgery, relative to the B group, the A group displayed a higher level of albumin (34.61 ± 3.32 vs. 31.66 ± 4.47) while lower levels of CRP (30.56 ± 12.57 vs. 77.25 ± 24.51) and WBC (11.20 ± 1.86 vs. 13.19 ± 3.00), with a significant difference (P < 0.05) (Table 4).
Table 4.
Changes in albumin, C-reactive protein (CRP) and white blood cell (WBC) between two groups of patients before and after surgery (mean ± SD)
| A group (n = 36) | B group (n = 36) | t | P | ||
|---|---|---|---|---|---|
| Albumin (g/L) | Preoperative | 36.27 ± 3.35 | 36.62 ± 3.94 | -0.403 | 0.688 |
| Postoperative | 34.61 ± 3.32 | 31.66 ± 4.47 | 3.187 | 0.002 | |
| CRP (mg/L) | Preoperative | 28.58 ± 13.85 | 60.62 ± 16.76 | -0.633 | 0.529 |
| Postoperative | 30.56 ± 12.57 | 77.25 ± 24.51 | -3.361 | < 0.001 | |
| WBC (×109/L) | Preoperative | 7.26 ± 1.59 | 6.76 ± 1.48 | 1.388 | -3.377 |
| Postoperative | 11.20 ± 1.86 | 13.19 ± 3.00 | 0.169 | < 0.001 |
A group: multimodal opioid-sparing anesthesia; B group: anesthesia with conventional dosage of opioids; CRP: C-reactive protein; WBC: white blood cell
Comparison of postoperative complications between two groups of patients
There was no significant difference in VAS pain scores between the A and B groups on the 1st, 2nd and 3rd days after surgery (P > 0.05). Concerning postoperative complications, the incidence of fever between the A group and B group was not significantly different (P > 0.05); the incidence of nausea and vomiting in the A group was lower than that in the B group (5.6% vs. 27.8%), with significant differences (P < 0.05); neither deep venous thrombosis nor intestinal obstruction occurred in the two groups. Besides, the total hospital stays of patients in the A group were fewer than those in the B group (12.00 ± 3.05 vs. 14.61 ± 3.42), and the difference was significantly different (P < 0.05) (Table 5).
Table 5.
Comparison of clinical prognosis between two groups of patients (mean ± SD) [n (%)]
| Indexes | A group (n = 36) | B group (n = 36) | x2/t | P |
|---|---|---|---|---|
| VAS pain score on the 1st day | 0.158 | 0.691 | ||
| Mild pain | 33 (91.7) | 32 (88.9) | ||
| Moderate and severe pain | 3 (8.3) | 4 (11.1) | ||
| VAS pain score on the 2nd day | 0.107 | 0.743 | ||
| Mild pain | 30 (83.3) | 31 (86.1) | ||
| Moderate and severe pain | 6 (16.7) | 5 (13.9) | ||
| VAS pain score on the 3rd day | 0.348 | 0.555 | ||
| Mild pain | 35 (97.2) | 34 (94.4) | ||
| Moderate and severe pain | 1 (2.8) | 2 (5.6) | ||
| Postoperative complications | ||||
| Fever | 7 (19.4) | 9 (25.0) | 0.321 | 0.571 |
| Nausea and vomiting | 2 (5.6) | 10 (27.8) | 6.400 | 0.011 |
| Deep venous thrombosis | 0 | 0 | - | - |
| Intestinal obstruction | 0 | 0 | - | - |
| Total hospital stays (days) | 12.00 ± 3.05 | 14.61 ± 3.42 | -3.415 | < 0.001 |
A group: multimodal opioid-sparing anesthesia; B group: anesthesia with conventional dosage of opioids; VAS: visual analogue scale
Discussion
Early recovery of intestinal function after surgical treatment is very important for clinical prognosis of patients with CRC [14]. Due to the changes of immune function and intestinal flora, the elderly patients’ gastrointestinal motility is weakened, and their intestinal response is more fragile and sensitive. Therefore, elderly patients often suffer from functional gastrointestinal diseases and are prone to pain, constipation and nausea, which severely affect their quality of life [15]. Recently, perioperative multimodal opioid-sparing anesthesia has become the key to rapid postoperative rehabilitation of elderly patients [10]. However, opioids may cause a series of adverse effects in elderly patients, such as delayed recovery of intestinal function and intestinal dysfunction after surgery [16]. Hence, in this study, 80 elderly hypertension patients with CRC aged 65–80 years who underwent open surgery for CRC were included, aiming to research the effect of multimodal opioid-sparing anesthesia on postoperative intestinal function and prognosis of patients.
In this study, a total of 8 subjects were excluded due to surgery withdrawal or loss to follow-up, leaving 72 for statistical analysis. There were no significant differences between groups A and B regarding patient demographics, type of surgery, surgery duration, anesthesia time, or blood loss. Both groups exhibited comparable hemodynamic stability, with no significant differences between the multimodal opioid-sparing and traditional opioid analgesia approaches. This indicates that it can be safely and effectively used in colorectal cancer surgeries, fulfilling the sedation and analgesia needs of the patients without causing severe hemodynamic fluctuations. Moreover, patients in the multimodal opioid-sparing analgesia group experienced earlier recovery of intestinal function post-surgery compared to those in the conventional opioid analgesia group, with statistically significant differences. Generally, bowel sounds and anal exhaust serve as marks of clinical recovery from intestinal peristalsis [17], and are utilized to assess the early recovery of gastrointestinal function in patients. In this study, the A group showed shorter times for the return of bowel sounds, the first passage of flatus, and the feeding recovery time, significantly enhancing early patient recovery. Opioid receptors act on the submucosal plexus of the enteric nervous system, resulting in slow intestinal peristalsis, motor dysfunction, late presence of bowel sounds and anal exhaust [18]. The recovery of postoperative bowel function was relatively slower in group B in this study, which increased the number of days of hospitalisation to some extent. Fortunately, under the premise of ensuring the effect of analgesia, multimodal opioid-sparing anesthesia can reduce the adverse reactions of opioids, effectively control surgical trauma and accelerate postoperative rehabilitation process [16].
The most distinctive feature of this study is its focus on the application of multimodal opioid-sparing anesthesia in elderly patients with hypertension undergoing colorectal cancer surgery. This patient population is particularly vulnerable to perioperative complications due to the interplay between hypertension, age-related physiological decline, and the effects of anesthesia. Hypertension can exacerbate perioperative risks by making patients more prone to hemodynamic instability, increased cardiovascular complications, and slower recovery postoperatively [19, 20]. In this context, multimodal opioid-sparing anesthesia offers a targeted approach that minimizes opioid use, which is known to negatively affect both respiratory function and hemodynamic stability, especially in the elderly [21, 22]. By reducing opioid consumption and using alternative analgesic techniques, such as the TAPB and non-opioid analgesics, we aimed to maintain more stable blood pressure and reduce cardiovascular stress during surgery. This is particularly relevant in hypertensive patients, where large fluctuations in blood pressure could lead to adverse outcomes, such as myocardial infarction or stroke [23].
Interestingly, our findings did not show significant differences in perioperative blood pressure between the multimodal opioid-sparing group and the conventional opioid group. This could be explained by the fact that the anesthesia management protocols for both groups included measures to maintain stable hemodynamics, such as fluid management, vasopressors, and the use of dexmedetomidine, which has known antihypertensive properties. Thus, the lack of significant differences in blood pressure may reflect the overall effectiveness of modern anesthesia protocols in controlling hemodynamic variables, even when opioid-sparing techniques are used. This suggests that multimodal opioid-sparing anesthesia can safely be implemented in hypertensive elderly patients without increasing the risk of perioperative blood pressure instability.
The importance of this finding lies in the confirmation that multimodal opioid-sparing anesthesia does not compromise cardiovascular stability in elderly hypertensive patients. Previous studies on multimodal anesthesia have primarily focused on general populations or specific surgical outcomes but have not specifically addressed hypertensive elderly patients. Our study, therefore, contributes new insights by demonstrating that multimodal opioid-sparing anesthesia can be safely applied in this high-risk group, offering the benefits of enhanced intestinal recovery without increasing perioperative cardiovascular risks.
In addition, peripheral nerve blocks under visualisation techniques have become an important part of low opioid multimodal analgesic management, TAPB has been proved to be an effective auxiliary analgesia management technique [24]. TAPB can be performed before and after surgery and injected once or multiple times, characterized by safety, reliability and good analgesic effect. In this research, there was no significant difference in VAS scores between the A and B groups 3 days after surgery, which indicated that TAPB performed in the A group could achieve the same analgesic effect as that in the B group. Furthermore, the incidence of nausea and vomiting in the A group was lower than that in the B group, which suggested that the combination of low-opioid analgesia and TAPB was effective and could reduce the related adverse reactions of opioids. Moreover, the level of postoperative inflammatory response in the A group was lower, and the total hospital stays were fewer. Such outcomes once again verified that multimodal anesthesia can not only achieve satisfactory analgesia, but also reduce postoperative complications, relieve stress-related inflammatory response and maintain the relative stability of hemodynamics [25].
This study’s strengths include its randomized design, which reduces selection bias, and its focus on a vulnerable patient population (elderly patients with hypertension), making the results more applicable to high-risk surgical groups. Additionally, by examining both physiological parameters (e.g., MAP, HR, albumin, CRP) and clinical outcomes (e.g., intestinal recovery, pain management), we provide a comprehensive evaluation of the impact of opioid-sparing anesthesia.
In discussing the findings of this study, it is crucial to acknowledge certain limitations that should inform future research directions. Firstly, the measures used to assess the recovery of intestinal function, such as bowel sounds and the timing of the first passage of flatus, are somewhat subjective and can introduce observer bias, especially since the assessors were not blinded to the treatment groups. Although efforts were made to standardize the assessment procedures to minimize variability, future studies would benefit from incorporating more objective measures or a blinded assessment to enhance the reliability of the findings. Additionally, the sample size, although adequate to detect statistically significant differences, was relatively small, which might limit the power to detect smaller yet clinically relevant differences. Future research with larger sample sizes and diverse demographics is recommended to confirm and extend these findings. Lastly, while the study focused on elderly patients with hypertension undergoing colorectal cancer surgery, the specific impact of varying degrees of hypertension and other comorbid conditions was not extensively analyzed, which could influence the postoperative recovery and analgesic needs. Therefore, a large sample size study is needed to further explore the best analgesic and rapid rehabilitation program in the future.
Conclusion
This study demonstrates that multimodal opioid-sparing anesthesia significantly improves the postoperative recovery of intestinal function and reduces complications in elderly hypertensive patients undergoing colorectal cancer surgery. Specifically, it shortens the recovery time for bowel sounds, first exhaust, and feeding, and decreases the incidence of nausea, vomiting, and overall hospital stay duration, compared to conventional opioid anesthesia. Clinically, these findings suggest that multimodal opioid-sparing anesthesia could be a safer and more effective alternative in managing anesthesia for elderly patients, minimizing opioid-related adverse effects while promoting faster recovery.
Author contributions
Yan-kai Ma conceptualized and designed the study, drafted the initial manuscript. Li Qu, Nan Chen, Zhe Chen, Yin Li, A Li Mu Jiang·Si Ma Yi and Xiao-liang Zhao collected the data and carried out the initial analyses. Gui-ping Xu critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This study is supported by Autonomous Region Key R&D Program Project “Research on the Prevention and Treatment System and Key Technologies of Elderly Related Diseases” (2022B03009-4).
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval
This study was approved by the Ethics Committee of People’s Hospital of Xinjiang Uygur Autonomous Region (KY2021031901). All patients provided written informed consent prior to enrollment in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baidoun F, et al. Colorectal Cancer epidemiology: recent trends and Impact on outcomes. Curr Drug Targets. 2021;22(9):998–1009. [DOI] [PubMed] [Google Scholar]
- 2.Morgan E, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–44. [DOI] [PubMed] [Google Scholar]
- 3.Shinji S, et al. Recent advances in the treatment of Colorectal Cancer: a review. J Nippon Med Sch. 2022;89(3):246–54. [DOI] [PubMed] [Google Scholar]
- 4.Cai P, Chen Y. Application of rapid rehabilitation nursing in colorectal cancer patients complicated with hypertension after laparoscopic radical resection during perioperative period. Xinxueguanbing Fangzhi Zhishi. 2022;12(14):3. [Google Scholar]
- 5.Shi J. Contrastive analysis about the postoperative clinical characteristics of elderly patients with colorectal cancer in different age groups. Zhonghua Yi Xue Za Zhi. 2022;102(8):6. [DOI] [PubMed] [Google Scholar]
- 6.Association. TeagapmgoAboCM At. National Clinical Research Center for Geriatrics NAoGA, Expert consensus on multimodal low opioid anesthesia for elderly patients during perioperative period (2021 edition). 2021. 101(3): p. 15.
- 7.Yu JH, et al. [Feasibility and safety of enhanced recovery after surgery for Elderly patients with Colorectal Cancer]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40(6):730–5. [DOI] [PubMed] [Google Scholar]
- 8.Gupta K, et al. Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses. BMJ Open. 2018;8(12):e024086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Association. Te.a., Association. CM. and N.A.o.G.A. National Clinical Research Center for Geriatrics, Expert consensus on the perioperative multimodal low-opioid anesthesia for elderly patients (2021 edition). 2021. 101(3): p. 15.
- 10.Falzone E, Hoffmann C, Keita H. Postoperative analgesia in elderly patients. Drugs Aging. 2013;30(2):81–90. [DOI] [PubMed] [Google Scholar]
- 11.Loskutov capital O C et al. Multimodal Low-Opioid Anesthesia - a New Approach to the issue of adequate intraoperative Analgesia. Georgian Med News. 2019(289): pp. 7–11. [PubMed]
- 12.Olmos AV et al. Increasing the use of multimodal analgesia during adult surgery in a tertiary academic anaesthesia department. BMJ Open Qual. 2021;10(3). [DOI] [PMC free article] [PubMed]
- 13.Stepan M, et al. Effects of multimodal low-opioid anesthesia protocol during on-pump coronary artery bypass grafting: a prospective cohort study. J Cardiothorac Surg. 2023;18(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafsson UO, et al. Guidelines for Perioperative Care in Elective colorectal surgery: enhanced recovery after surgery (ERAS((R))) Society recommendations: 2018. World J Surg. 2019;43(3):659–95. [DOI] [PubMed] [Google Scholar]
- 15.Karakan T et al. Gut-brain-microbiota Axis: Antibiotics and Functional Gastrointestinal disorders. Nutrients, 2021. 13(2). [DOI] [PMC free article] [PubMed]
- 16.Wang T, L. and, Mei W. The low-dose opioids therapy of perioperative multi-mode analgesia is pivotal to the enhanced recovery after surgery for elderly patients. 2021;101(3):3. [DOI] [PubMed]
- 17.Silinsky JD, et al. Preoperative intravenous meloxicam for moderate-to-severe pain in the immediate post-operative period: a phase IIIb randomized clinical trial in 55 patients undergoing primary open or laparoscopic colorectal surgery with bowel resection and/or anastomosis. Pain Manag. 2021;11(1):9–21. [DOI] [PubMed] [Google Scholar]
- 18.Lawrentschuk N, Hewitt PM, Pritchard MG. Elective laparoscopic cholecystectomy: implications of prolonged waiting times for surgery. ANZ J Surg. 2003;73(11):890–3. [DOI] [PubMed] [Google Scholar]
- 19.Lien SF, Bisognano JD. Perioperative hypertension: defining at-risk patients and their management. Curr Hypertens Rep. 2012;14(5):432–41. [DOI] [PubMed] [Google Scholar]
- 20.Lizano-Diez I, et al. The burden of perioperative hypertension/hypotension: a systematic review. PLoS ONE. 2022;17(2):e0263737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kianian S, et al. Perioperative multimodal analgesia: a review of efficacy and safety of the treatment options. Anesthesiology Perioperative Sci. 2024;2(1):9. [Google Scholar]
- 22.Huang L, et al. Postoperative Multimodal Analgesia Strategy for enhanced recovery after surgery in Elderly Colorectal Cancer patients. Pain Ther. 2024;13(4):745–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thune JJ, et al. Effect of antecedent hypertension and follow-up blood pressure on outcomes after high-risk myocardial infarction. Hypertension. 2008;51(1):48–54. [DOI] [PubMed] [Google Scholar]
- 24.Li Y. Postoperative analgesic effect of transversus abdominis plane block in the anesthesia of open intestinal surgeries. Int J Anesthesiology Resusc. 2019;40(1):4. [Google Scholar]
- 25.Dong J, Y. and, Shi Y. Multimodal analgesia application progress and development trend. Int J Anesthesiology Resusc. 2014;35(2):6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


