Abstract
1. Time with Julie in his laboratory at the NIH in the early 1970s is remembered. The experience led to a life-long interest in the regulation of catecholamine secretion. Here are summarized aspects of this work.
2. The relationship between ATP-dependent priming of exocytosis and the polyphosphoinositides is reviewed. In addition, studies are summarized in which total internal reflection fluorescent microscopy (TIRFM) was used to visualize secretory granule behavior before exocytosis and individual exocytotic events.
3. Quantitative optical analysis indicates that chromaffin granule motion is highly restricted but regulated. Granules can undergo significant motion in the 100 ms prior to fusion and interactions with the plasma membrane leading to fusion can occur within this time. The small motions may permit granules adjacent to the plasma membrane to repetitively sample microdomains of the plasma membrane, thereby increasing the probability of fruitful interactions that lead to fusion.
Key Words: Axelrod, exocytes, catecholamine, chromaffin cells, adrenal medulla, TIRFM, granula motion
REMEMBRANCES OF THE JULIE AXELROD LABORATORY
I came to the Axelrod laboratory in the early 1970s and soon became intrigued with the possibility of manipulating different aspects of the sympathetic nerve terminal. I was tutored by Julie in asking the right questions, while weighing chemicals on the only sensitive balance in the lab, which was immediately adjacent to his desk. I knew when he was interested in my ideas. He would turn from what he was doing at his desk and ask me about my experiments. When my discussion went afield of his interest, he would pick up the New York Times. I also knew when it was time to write a paper. Upon his return from his daily hour of reading in the library after lunch, he would tell me that he had read an interesting paper about a topic similar to what I was working on. Oh, he forgot the author, but by the way, he would ask, when are you going to write it up?
I was helped in my endeavors in the laboratory by incredible colleagues. They graciously introduced me to the world of biochemistry and pharmacology.
The thrust of my research continues to concern catecholamines, especially catecholamine secretion from adrenal medullary cells. With the development of techniques to maintain these primary, non-dividing cells in culture, chromaffin cells have become one of the most studied model systems for regulated exocytosis. Because large numbers of cells can be obtained from bovine adrenal medulla, the cultures are suitable for biochemical as well as physiological studies. We and others discovered that secretion was maintained in cells when the plasma membrane was permeabilized by electroporation (Baker and Knight, 1978) or low concentrations of digitonin (Dunn and Holz, 1983; Wilson and Kirshner, 1983; Brooks and Treml, 1983) or streptolysin O (Ahnert-Hilger et al., 1989). This opened the way for a large number of studies in which the cell interior could be manipulated and secretion stimulated directly by micromolar Ca2+.
THE ATP-DEPENDENCY OF SECRETION AND THE MAINTENANCE OF PTDINS(4,5)P2
Studies in permeabilized chromaffin cells first demonstrated that the secretory pathway could be separated into distinct kinetic steps with different biochemical characteristics (Holz et al., 1989; Bittner and Holz, 1992a,b). A slow ATP-dependent priming step precedes a rapid Ca2+-dependent triggering step.
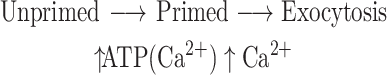 |
Ca2+, in addition to triggering exocytosis, also enhances ATP-dependent priming. Similar pathways exist in PC12 cells (Hay and Martin, 1992), melanotrophs (Parsons et al., 1995) and bipolar neurons (Heidelberger, 1998) and are likely to operate in most if not all differentiated secretory cells. It was subsequently discovered that a major component (as much as 70%) of the ATP-dependency of secretion in permeabilized chromaffin cells reflects the maintenance of the polyphosphoinositides (Eberhard et al., 1990). The enzymatic removal of PtdIns in permeabilized cells results in the subsequent decline in PtdIns(4,5)P2 and PtdIns(5)P and the specific inhibition of ATP-dependent secretion (Eberhard et al., 1990). The study demonstrated that the removal of either substrate of PtdIns-4 kinase, PtdIns or ATP, inhibited exocytosis. Studies in PC12 cells strongly advanced the concept of the role of the polyphosphoinositides in secretion. Two cytosolic factors that are necessary for ATP dependent priming of exocytosis were identified as a phosphatidylinositol transfer protein (Hay and Martin, 1993) and PtdIns(4)P-5 kinase (Hay et al., 1995). These studies implicated the following reactions in ATP- dependent priming of secretion:
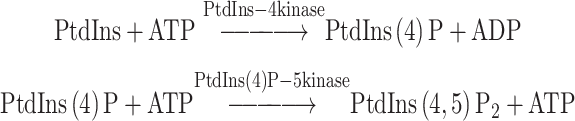 |
The experiments in chromaffin (Eberhard et al., 1990 ) and PC12 cells (Hay and Martin, 1993; Hay et al., 1995) were the first evidence that the polyphosphoinositides play important roles in vesicular trafficking reactions. Subsequently, polyphosphoinositides were implicated in numerous vesicular trafficking pathways (reviewed in (De Camilli et al., 1996) including synaptic vesicle exocytosis (Wiedemann et al., 1998; Khvotchev and Sudhof, 1998), ARF1-dependent trafficking, endocytic recycling of synaptic vesicle membrane, and endocytosis of G-protein coupled receptors (Sorensen et al., 1999). Polyphosphoinositides labeled in the three position of the inositol ring are necessary for vesicular trafficking between the Golgi and vacuole/lysosome in yeast and mammalian cells (Schu et al., 1993; Wurmser et al., 1999).
PtdIns-4,5-P2 is generally thought to be located on the plasma membrane (Whipps et al., 1987; Hokin and Hokin, 1964; Eichberg and Dawson, 1965). However, it has also long been known that PtdIns-4 kinase is an integral membrane protein of the chromaffin granule membrane (Buckley et al., 1971; Phillips, 1973; Muller and Kirshner, 1975; Husebye and Flatmark, 1988). PtdIns-4 kinase is also associated with synaptic vesicles (Wiedemann et al., 1998) and mast cell granules (Kurosawa and Parker, 1986). It is, therefore, possible that PtdIns-4,5-P2 could be synthesized on the secretory granule membrane through the sequential action of granule PtdIns-4 kinase and PtdIns-4-P-5 kinase. We investigated the localization of PtdIns-4,5-P2 that is involved in exocytosis by transiently expressing in chromaffin cells a pleckstrin homology (PH) domain that specifically binds PtdIns-4,5-P2 and is fused to green fluorescent protein (GFP). Transiently expressed PH-GFP almost exclusively labeled the plasma membrane of chromaffin cells, with no detectable labeling of chromaffin granules (Holz et al., 2000).
Expression of PH-GFP on the plasma membrane inhibited secretion measured biochemically in intact and permeabilized cells, and electrophysiologically by capacitance changes (Holz et al., 2000). Thus, it is the plasma membrane pool of PtdIns-4,5-P2 that is required for exocytosis.
This work has been expanded to neurons. The regulation by neuronal activity of presynaptic PtdIns-4,5-P2 was investigated in cultured hippocampal neurons transiently expressing PH-GFP (Micheva et al., 2001). As in chromaffin cells, PH-GFP selectively labeled the plasma membrane. Synaptic vesicles were not labeled. Upon electrical stimulation PH-GFP accumulated in the center of boutons where endocytic, clathrin-coated vesicles accumulate. Because PtdIns-4,5-P2 is metabolized by the lipid phosphatase synaptojanin to permit uncoating of clathrin from endocytic vesicles (Cremona et al., 1999), the dynamics of PH-GFP labeling may reflect a PtdIns-4,5-P2 cycle important in exocytosis and endocytosis. Newly formed endocytic vesicles contain PtdIns-4,5-P2 from the plasma membrane but must lose the lipid in order to mature into synaptic vesicles. The lack of PtdIns-4,5-P2 in the membranes of both synaptic vesicles and secretory granules from the protein biosynthetic pathway (e.g., chromaffin granules) may have important implications for the process of exocytosis itself. Surprising, PtdIns-4,5-P2 in the bouton is regulated by a retrograde signaling pathway involving post-synaptic, NMDA receptors and the formation of NO with effects on endocytosis and exocytosis (Micheva et al., 2001; Micheva et al., 2003).
How might plasma membrane PtdIns(4,5)P2 be involved in exocytosis? The initial experiments ruled out the involvement of PtdIns(4,5)P2 in secretion as a substrate for phospholipase C (Eberhard et al., 1990). Instead, the lipid is likely to act in the secretory pathway as an allosteric regulator of specific protein function. Three proteins associated with the chromaffin granule membrane bind in a specific manner PtdIns-4,5-P2–synaptotagmin (Schiavo et al., 1996; Bai et al., 2004), Rabphilin3 (Chung et al., 1998) and calcium-dependent activator protein for secretion (CAPS) (Loyet et al., 1998). The Ca2+-regulated interaction of one or more of the proteins with PtdIns-4,5-P2 in the plasma membrane may modulate protein function and could possibly be directly involved in the fusion reaction.
Additionally, the requirement of plasma membrane PtdIns-4,5-P2 in secretion may reflect a role for the lipid in regulating cytoskeletal dynamics immediately adjacent to the plasma membrane during exocytosis. Chromaffin cells have a 200 nm thick actin layer immediately adjacent to the plasma membrane (Nakata and Hirokawa, 1992). The polyphosphoinositides including PtdIns-4,5-P2 and 3- phosphorylated forms interact with and regulate numerous cytoskeletal proteins and proteins involved in vesicular trafficking (reviewed in (Martin, 1997; Wurmser et al., 1999)). The actin cytoskeleton in chromaffin cells is especially sensitive to reductions in PtdIns-4,5-P2 (Bittner and Holz, 2005). Cortical actin is decreased by transient expression of PH-GFP in intact cells and by removal of ATP in permeabilized cells. Surprisingly, the cortical actin network in chromaffin cells is resistant to latrunculin B, mycalide and cytochalasin D, drugs which by binding actin disrupt lamellapodia and stress fibers. These results suggest differences in the manner in which f-actin is controlled in different cellular locations.
SECRETORY GRANULE BEHAVIOR ADJACENT TO THE PLASMA MEMBRANE BEFORE AND DURING EXOCYTOSIS
Virtually all models of exocytosis invoke granule movement to the plasma membrane and a plasma membrane docking step. The precise nature of these events has been surmised but not directly investigated. “Morphological” docking has been defined by static electron microscopic images (Steyer et al., 1997). Inferences concerning the dynamics of events immediately before fusion are based upon the kinetics of secretion. In these experiments granule pools are defined by the rates at which granules fuse with the plasma membrane after a Ca2+stimulus and the manner in which the kinetics are altered by biochemical manipulations. Another view of granule behavior comes from quantitative studies of granule motion using wide field, confocal and especially total internal reflection fluorescence microscopy (TIRFM) of fluorescent-tagged secretory granules. The latter technique selectively illuminates the aqueous phase immediately adjacent to a glass interface with an exponentially decaying excitation (the evanescent field, decay constant 50–100 nm) (Axelrod, 1981, 2003). A distance into the cell extending less than a granule diameter is selectively visualized. Motions of granules labeled with GFP-tagged lumenal cargo or granule membrane protein can be determined with resolution of ∼10 nm. Motions perpendicular to the glass interface (z-motions) are calculated from changes in fluorescence intensity, whereas granule motions parallel to the glass interface (R-motions) are determined by extrapolated center of intensity measurements (Johns et al., 2001; Allersma et al., 2004). A common finding in TIRFM studies in chromaffin cells is that most (>98%) of the granules adjacent to the plasma membrane are highly restricted in their motion (Steyer et al., 1997; Oheim et al., 1998; Han et al., 1999; Johns et al., 2001; Ohara-Imaizumi et al., 2002), as if tethered or caged.
Granules undergo priming steps before secretion that are regulated by ATP and calcium (see above). Recent studies indicate that these co-factors also affect granule motion (Allersma et al., 2006). Removal of ATP in permeabilized cells caused granule motion to decrease. Nicotinic stimulation caused a calcium-dependent increase in average granule motion. This effect was more pronounced for granules that undergo exocytosis than for those that do not. Thus, there is a regulated component to these small motions that may be important in exocytosis.
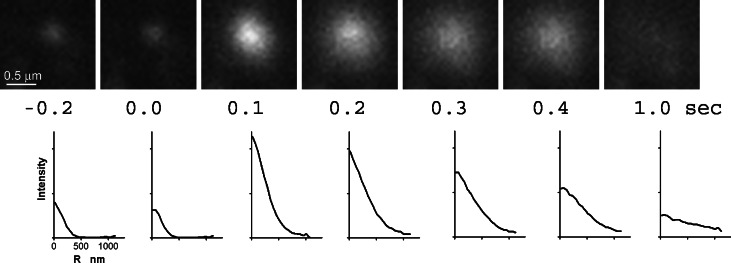
TIRFM was used to investigate fusion events from the point of view of a membrane protein that diffuses from the granule to the plasma membrane upon fusion (Allersma et al., 2004). Secretory granules labeled with the v-SNARE, VAMP-GFP, showed distinct signatures upon exocytosis when viewed by TIRFM. In approximately 90% of fusion events, we observed a large increase in fluorescence intensity coupled with a transition from a small punctate appearance to a larger, spreading cloud with free diffusion of the VAMP-GFP into the plasma membrane (Fig. 1). Quantitation indicates that these events reflect an initially fused and spherical granule, with subsequent free diffusion of VAMP-GFP through the fusion junction with possible flattening of the granule membrane into the plane of the plasma membrane (Allersma et al., 2004).
Fig. 1.
An intensity burst characterizes the fusion of an individual VAMP-GFP-labeled granule with the plasma membrane. Successive frames show the fusion of a VAMP-GFP-containing granule and the subsequent spread of the VAMP-GFP in the plasma membrane in TIRFM. The frame just prior to fusion is t=0.0 s. Below each image is the VAMP-GFP intensity (with background subtracted) averaged as a function of radial distance r away from the granule center. Peak intensity is in the center of the intensity profile. The fluorescence almost completely disappeared after one second. [from (Allersma et al., 2004)].
Most of the granules that undergo exocytosis are observed jittering in place adjacent to the plasma membrane, undergoing motions of 10–200 nm within 100–200 ms of fusion. Absence of correlation of motions of even immediately adjacent granules suggests that the motions of these granules are not directly coupled to hypothetical motions of extended areas of the plasma membrane. However, granules may by attached directly or indirectly by flexible tethers to the plasma membrane.
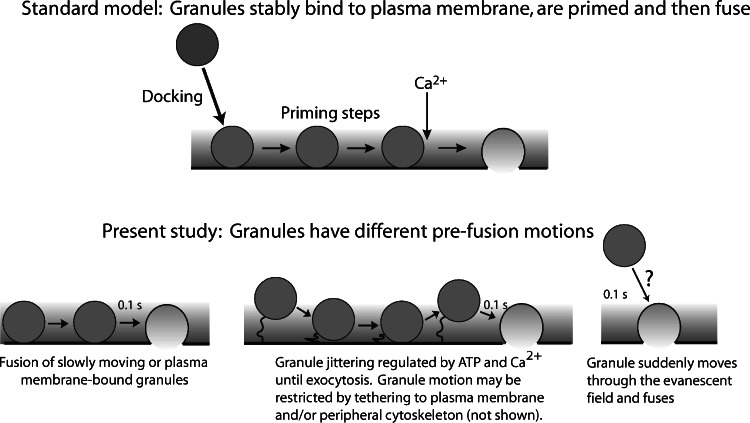
These observations of granule motion before exocytosis contrast with the common assumption that granules that are morphologically close to the plasma membrane (within a granule diameter) are stably interacting with the membrane and that this bound state is required for priming and subsequent exocytosis. Instead, they suggest that granule can undergo significant motion within 100 ms of fusion and that interactions with the plasma membrane leading to fusion can occur within this time (Fig. 2). These considerations may also apply to fast synaptic transmission. Indeed, rapid interactions between synaptic vesicles and the plasma membrane occur in the very fast events associated with synaptic transmission in inner hair cells (Griesinger et al., 2005).
Fig. 2.
Behavior of granules before exocytosis. A standard model for exocytosis is that granules translocate from the cell interior to the plasma membrane and stably bind or dock to the plasma membrane. After subsequent priming steps, the granule is able to fuse with the plasma membrane in respond to elevated Ca2+ a recent study indicates that granules that undergo exocytosis can have numerous behaviors immediately preceding fusion. Some allersma et al 2006 granules do not undergo detectable motion and may be bound to the plasma membrane. Others are jittering in place within 100 ms of fusion, as if on flexible tethers. A few granules are not present in the evanescent field before fusion and may move through it within 100 ms of fusion. Granule and plasma membrane priming events can occur before the final docking step leading to fusion.
What is the significance to exocytosis of the small, regulated motions of secretory granules? They may permit granules adjacent to the plasma membrane to repetitively sample microdomains of the plasma membrane, thereby increasing the probability of fruitful interactions that lead to fusion. The initial burst of exocytosis in chromaffin cells upon flash photolysis of caged Ca2+(a total of approximately 200 granules) (Voets, 2000) reflects the fraction of the ∼1000 granules within a granule diameter of the plasma membrane that are favorably interacting with the plasma membrane during the Ca2+stimulus. The duration of the initial burst, approximately 0.5 s, is sufficient time for the jittering motions to randomly sample the fusion environment on the plasma membrane, and, thereby, influence the rapid kinetics of secretion from the chromaffin cell.
ACKNOWLEDGMENTS
This work was funded by grants from National Institutes of Health grant RO1-DK50127 (to RWH), R01-NS38129 (to Daniel Axelrod, University of Michigan) and a Michigan Economic Development Corporation and the Michigan Life Sciences Corridor Grant (to RWH).
Abbreviation
- PtdIns
phosphatidyl inositol
- TIRFM
total internal reflection fluorescence microscopy.
REFERENCES
- Ahnert-Hilger, G., Bader, M. F., Bhakdi, S., and Gratzl, M. (1989). Introduction of macromolecules into bovine adrenal medullary chromaffin cells and rat pheochromocytoma cells (PC12) by permeabilization with streptolysin O: inhibitory effect of tetanus toxin on catecholamine secretion. J. Neurochem. 52:1751–1758. [DOI] [PubMed] [Google Scholar]
- Allersma, M. W., Bittner, M. A., Axelrod, D., and Holz, R. W. (2006). Motion Matters: Secretory Granule Motion Adjacent to the Plasma Membrane and Exocytosis. Mol. Biol. Cell E05–E10. [DOI] [PMC free article] [PubMed]
- Allersma, M. W., Wang, L., Axelrod, D., and Holz, R. W. (2004). Visualization of Regulated Exocytosis with a Granule-Membrane Probe using Total Internal Reflection Microscopy. Mol. Biol. Cell15:4658–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod, D. (1981). Cell-substrate contacts illuminated by total internal reflection fluorescence. J. Cell Biol. 89:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod, D. (2003). Total internal reflection fluorescence microscopy in cell biology. Methods Enzymol. 361:1–33. [DOI] [PubMed] [Google Scholar]
- Bai, J., Tucker, W. C., and Chapman, E. R. (2004). PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11:36–44. [DOI] [PubMed] [Google Scholar]
- Baker, P. F., and Knight, D. E. (1978). Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature276:620–622. [DOI] [PubMed] [Google Scholar]
- Bittner, M. A., and Holz, R. W. (1992a). A temperature-sensitive step in exocytosis. J. Biol. Chem. 267:16226–16229. [PubMed] [Google Scholar]
- Bittner, M. A., and Holz, R. W. (1992b). Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J. Biol. Chem. 267:16219–16225. [PubMed] [Google Scholar]
- Bittner, M. A., and Holz, R. W. (2005). Phosphatidylinositol-4,5-bisphosphate:actin dynamics and the regulation of ATP-dependent and independent secretion. Mol. Pharmacol. 67:1089–1098. [DOI] [PubMed] [Google Scholar]
- Brooks, J. C., and Treml, S. (1983). Catecholamine secretion by chemically skinned cultured chromaffin cells. J. Neurochem. 40:468–473. [DOI] [PubMed] [Google Scholar]
- Buckley, J. T., Lefebvre, Y. A., and Hawthorne, J. N. (1971). Identification of an actively phosphorylated component of adrenal medulla chromaffin granules. Biochim. Biophys. Acta239:517–519. [DOI] [PubMed] [Google Scholar]
- Chung, S.-H., Song, W.-J., Kim, K., Bednarski, J. J., Chen, J., Prestwich, G. D., and Holz, R. W. (1998). The C2 domains of Rabphilin3a specifically bind PtdIns(4,5)P2-containing vesicles in a Ca2+-dependent manner: characteristics and possible physiological significance. J. Biol. Chem. 273:10240–10248. [DOI] [PubMed] [Google Scholar]
- Cremona, O., Di Paolo, G., Wenk, M. R., Luthi, A., Kim, W. T., Takei, K., Daniell, L., Nemoto, Y., Shears, S. B., Flavell, R. A., McCormick, D. A., and De Camilli, P. (1999). Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell99:179–188. [DOI] [PubMed] [Google Scholar]
- De Camilli, P., Emr, S. D., McPherson, P. S., and Novick, P. (1996). Phosphoinositides as regulators of membrane traffic. Science271:1533–1539. [DOI] [PubMed] [Google Scholar]
- Dunn, L. A., and Holz, R. W. (1983). Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J. Biol. Chem. 258:4989–4993. [PubMed] [Google Scholar]
- Eberhard, D. A., Cooper, C. L., Low, M. G., and Holz, R. W. (1990). Evidence that the inositol phopholipids are necessary for exocytosis: loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem. J. 268:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichberg, J., and Dawson, R. M. C. (1965). Polyphosphoinositides in myelin. Biochem. J. 96:644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesinger, C. B., Richards, C. D., and Ashmore, J. F. (2005). Fast vesicle replenishment allows indefatigable signaling at the first auditory synapse. Nature 212–215. [DOI] [PubMed]
- Han, W., Ng, Y. K., Axelrod, D., and Levitan, E. S. (1999). Neuropeptide release by efficient recruitment of diffusing cytoplasmic secretory vesicles. Proc. Natl. Acad. Sci. USA96:14577–14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, J. C., Fisette, P. L., Jenkins, G. H., Fukami, K., Takenawa, T., Anderson, R. A., and Martin, T. F. J. (1995). ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature374:173–177. [DOI] [PubMed] [Google Scholar]
- Hay, J. C., and Martin, T. F. J. (1992). Resolution of regulated secretion into sequential MgATP-dependent and calcium-dependent stages mediated by distinct cytosolic proteins. J. Cell Biol. 119:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, J. C., and Martin, T. F. J. (1993). Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca2+-activated secretion. Nature366:572–575. [DOI] [PubMed] [Google Scholar]
- Heidelberger, R. (1998). Adenosine triphosphate and the late steps in calcium-dependent exocytosis at a ribbon synapse. J. Gen. Physiol. 111:225–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin, L., and Hokin, M. R. (1964). The incorporation of 32P from triphosphate into polyphosphoinositides [γ-32P]adenosine and phsophatidic acid in erythrocyte membranes. Biochim. Biophys. Acta84:563–575. [DOI] [PubMed] [Google Scholar]
- Holz, R. W., Bittner, M. A., Peppers, S. C., Senter, R. A., and Eberhard, D. A. (1989). MgATP-independent and MgATP-dependent exocytosis. Evidence that MgATP primes adrenal chromaffin cells to undergo exocytosis. J. Biol. Chem. 264:5412–5419. [PubMed] [Google Scholar]
- Holz, R. W., Hlubek, M. D., Sorensen, S. D., Fisher, S. K., Balla, T., Ozaki, S., Prestwich, G. D., Stuenkel, E. L., and Bittner, M. A. (2000). A pleckstrin homology domain specific for PtdIns-4-5-P2 and fused to green fluorescent protein identifies plasma membrane PtdIns-4-5-P2 as being important in exocytosis. J Biol Chem. 275:17878–17885. [DOI] [PubMed] [Google Scholar]
- Husebye, E. S., and Flatmark, T. (1988). Phosphatidylinositol kinase of bovine adrenal chromaffin granules: Kinetic properties and inhibition by low concentrations of Ca2+. Biochim. Biophys. Acta968:261–265. [DOI] [PubMed] [Google Scholar]
- Johns, L. M., Levitan, E. S., Shelden, E. S., Holz, R. W., and Axelrod, D. (2001). Restriction of secretory granule motion near the plasma membrane of chromaffin cells. J. Cell Biol. 153:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khvotchev, M., and Sudhof, T. C. (1998). Newly synthesized phosphatidylinositol phosphates are required for synaptic norepinephrine but not glutamate or gamma-aminobutyric acid (GABA) release. J. Biol. Chem. 273:21451–21454. [DOI] [PubMed] [Google Scholar]
- Kurosawa, M., and Parker, C. (1986). A phosphatidylinositol kinase in rat mast cell granules. J. Immunol. 136:616–622. [PubMed] [Google Scholar]
- Loyet, K. M., Kowalchyk, J. A., Chaudhary, A., Chen, J., Prestwich, G. D., and Martin, T. F. J. (1998). Specific binding of phosphatidylinositol 4,5-bisphosphate to calcium-dependent activator protein for secretion (CAPS), a potential phosphoinositide effector protein for regulated exocytosis. J. Biol. Chem. 273:8337–8343. [DOI] [PubMed] [Google Scholar]
- Martin, T. F. (1997). Phosphoinositides as spatial regulators of membrane traffic. Curr. Opin. Neurobiol. 7:331–338. [DOI] [PubMed] [Google Scholar]
- Micheva, K. D., Buchanan, J., Holz, R. W., and Smith, S. J. (2003). Retrograde regulation of synaptic vesicle endocytosis and recycling. Nat. Neurosci. 6:925–932. [DOI] [PubMed] [Google Scholar]
- Micheva, K. D., Holz, R. W., and Smith, S. J. (2001). Regulation of presynaptic phosphatidylinositol 4,5-bisphosphate by neuronal activity. J. Cell Biol. 154:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, T. W., and Kirshner, N. (1975). ATPase and phosphatidylinositol kinase activities of adrenal chromaffin vesicles. J. Neurochem. 24:1155–1161. [DOI] [PubMed] [Google Scholar]
- Nakata, T., and Hirokawa, N. (1992). Organization of cortical cytoskeleton of cultured chromaffin cells and involvement in secretion as revealed by quick-freeze, deep-etching, and double-label immunoelectron microscopy. J. Neurosci. 12:2186–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara-Imaizumi, M., Nakamichi, Y., Tanaka, T., Ishida, H., and Nagamatsu, S. (2002). Imaging exocytosis of single insulin secretory granules with evanescent wave microscopy. Distinct behavior of granule motion in biphasic insulin release. J. Biol. Chem. 277:3805–3808. [DOI] [PubMed] [Google Scholar]
- Oheim, M., Loerke, D., Stuhmer, W., and Chow, R. H. (1998). The last few milliseconds in the life of a secretory granule. Docking, dynamics and fusion visualized by total internal reflection fluorescence microscopy (TIRFM). Eur. J. Biophys. 27:83–98. [DOI] [PubMed] [Google Scholar]
- Parsons, T. D., Coorssen, J. R., Horstmann, H., and Almers, W. (1995). Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron15:1085–1096. [DOI] [PubMed] [Google Scholar]
- Phillips, J. H. (1973). Phosphatidylinositol kinase. Biochem. J. 136:579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo, G., Gu, Q.-M., Prestwich, G. D., Sollner, T., and Rothman, J. E. (1996). Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc. Natl. Acad. Sci. USA93:13327–13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu, P. V., Takegawa, K., Fry, M. J., Stack, J. H., Waterfield, M. D., and Emr, S. D. (1993). Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science260:88–91. [DOI] [PubMed] [Google Scholar]
- Sorensen, S. D., Linseman, D. A., McEwen, E. L., Heacock, A. M., and Fisher, S. K. (1999). A role for a wortmannin-sensitive phsophatidylinositol-4-kinase in the endocytosis of muscarinic cholinergic receptors. J. Pharmacol. Exp. Ther. 52:827–836. [PubMed] [Google Scholar]
- Steyer, J. A., Horstman, H., and Almers, W. (1997). Transport, docking and exocytosis of single secretory granules in live chromaffin cells. Nature388:474–478. [DOI] [PubMed] [Google Scholar]
- Voets, T. (2000). Dissection of three Ca2+-dependent steps leading to secretion in chromaffin cells from mouse adrenal slices. Neuron28:537–545. [DOI] [PubMed] [Google Scholar]
- Whipps, D. E., Armston, A. E., Pryor, H. J., and Halestrap, A. P. (1987). Effects of glucagon and Ca2+ on the metabolism of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5- bisphosphate in isolated rat hepatocytes and plasma membranes. Biochem. J. 241:835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann, C., Schafer, T., Burger, M. M., and Sihra, T. S. (1998). An essential role for a small synaptic vesicle-associated phosphatidylinositol 4-kinase in neurotransmitter release. J. Neurosci. 18:5594–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S. P., and Kirshner, N. (1983). Calcium-evoked secretion from digitonin-permeabilized adrenal medullary chromaffin cells. J. Biol. Chem. 258:4994–5000. [PubMed] [Google Scholar]
- Wurmser, A. E., Gary, J. D., and Emr, S. D. (1999). Phosphoinositide 3-kinases and their FYVE domain-containing effectors as regulators of vacuolar/lysosomal membrane trafficking pathways. J. Biol. Chem. 274:9129–9132. [DOI] [PubMed] [Google Scholar]