Abstract
1. Circulating and locally formed Angiotensin II regulates the cerebral circulation through stimulation of AT1 receptors located in cerebrovascular endothelial cells and in brain centers controlling cerebrovascular flow.
2. The cerebrovascular autoregulation is designed to maintain a constant blood flow to the brain, by vasodilatation when blood pressure decreases and vasoconstriction when blood pressure increases.
3. During hypertension, there is a shift in the cerebrovascular autoregulation to the right, in the direction of higher blood pressures, as a consequence of decreased cerebrovascular compliance resulting from vasoconstriction and pathological growth. In hypertension, when perfusion pressure decreases as a consequence of blockade of a cerebral artery, reduced cerebrovascular compliance results in more frequent and more severe strokes with a larger area of injured tissue.
4. There is a cerebrovascular angiotensinergic overdrive in genetically hypertensive rats, manifested as an increased expression of cerebrovascular AT1 receptors and increased activity of the brain Angiotensin II system. Excess AT1 receptor stimulation is a main factor in the cerebrovascular pathological growth and decreased compliance, the alteration of the cerebrovascular eNOS/iNOS ratio, and in the inflammatory reaction characteristic of cerebral blood vessels in genetic hypertension. All these factors increase vulnerability to brain ischemia and stroke.
5. Sustained blockade of AT1 receptors with peripheral and centrally active AT1 receptor antagonists (ARBs) reverses the cerebrovascular pathological growth and inflammation, increases cerebrovascular compliance, restores the eNOS/iNOS ratio and decreases cerebrovascular inflammation. These effects result in a reduction of the vulnerability to brain ischemia, revealed, when an experimental stroke is produced, in protection of the blood flow in the zone of penumbra and substantial reduction in neuronal injury.
6. The protection against ischemia resulting is related to inhibition of the Renin–Angiotensin System and not directly related to the decrease in blood pressure produced by these compounds. A similar decrease in blood pressure as a result of the administration of β-adrenergic receptor and calcium channel blockers does not protect from brain ischemia.
7. In addition, sustained AT1 receptor inhibition enhances AT2 receptor expression, associated with increased eNOS activity and NO formation followed by enhanced vasodilatation. Direct AT1 inhibition and indirect AT2 receptor stimulation are associated factors normalizing cerebrovascular compliance, reducing cerebrovascular inflammation and decreasing the vulnerability to brain ischemia.
8. These results strongly suggest that inhibition of AT1 receptors should be considered as a preventive therapeutic measure to protect the brain from ischemia, and as a possible novel therapy of inflammatory conditions of the brain.
KEY WORDS: renin–angiotensin system, Angiotensin II receptors, stroke, brain circulation, nitric oxide, brain inflammation
INTRODUCTION
Angiotensin II (Ang II), initially described as a peripheral circulating hormone regulating systemic blood pressure and fluid homeostasis, was later recognized as a brain neuromodulator inducing fluid and salt intake and blood pressure increase through stimulation of its physiological receptors, the AT1 receptor type (Saavedra, 2005). There are two closely integrated central Ang II systems, one responding to Ang II generated in the brain and stimulating receptors inside the blood brain barrier (Saavedra, 1992) and another with Ang II receptors in circumventricular organs and in cerebrovascular endothelial and smooth muscle cells (Fig. 1) (Zhou et al., 2005), responding to circulating Ang II of peripheral origin, and/or to locally generated Ang II (Saavedra, 2005). AT1 receptors located in the cerebrovascular endothelium and in specific brain areas participate in the regulation of the cerebrovascular circulation (Saavedra, 2005).
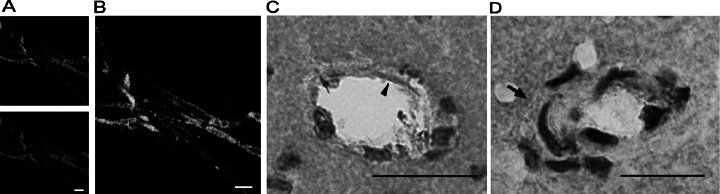
Fig. 1.
Localization of AT1 receptors in brain microvessels and small arterioles. (A) Immunofluorescence pictures of GLUT-1 (top), indicating brain endothelial cells, and AT1 receptor (bottom) in isolated brain microvessels from SHR. White bar is 10 μm. (B) Colocalization of AT1 receptors and endothelium cells in isolated brain microvessels detected by confocal microscopy in SHR. White bar is 10 μm. (C) Immunohistochemistry. The black arrowhead points to endothelial AT1 receptors in a microvessel from the cortex of SHR. Black bar is 40 μm. (D) Immunohistochemistry. The arrow points to AT1 receptor expression in smooth muscle cells of small arterioles less than 50 μm diameter in SHR. Black bar is 20 μm. Reproduced from Fig. 1 of Zhou et al. (2005).
CEREBROVASCULAR AUTOREGULATION
One important characteristic of the cerebral circulation is the capacity to adapt to variations in systemic or local blood pressure by regulating the degree of vasoconstriction and vasodilatation of the cerebral arteries, maintaining a constant blood flow to the brain. Under normal conditions, decreases in perfusion pressure result in vasodilatation, while increases in pressure produce vasoconstriction, to maintain a constant overall blood flow to the brain, a system of cerebrovascular autoregulation (Fig. 2).
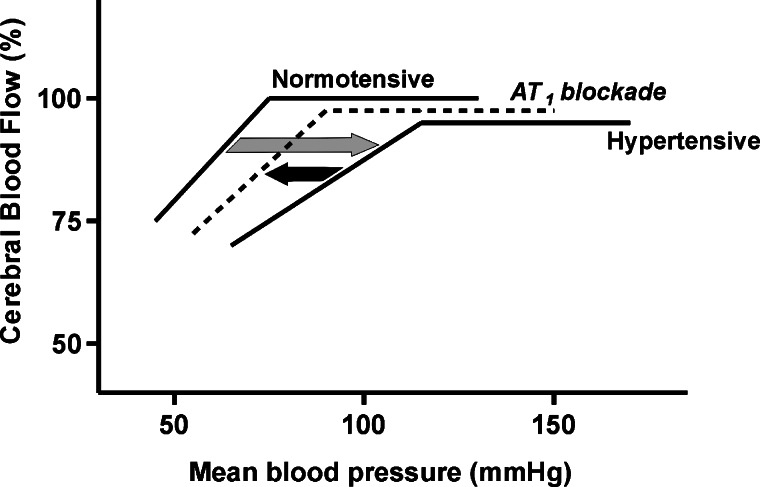
Fig. 2.
Cerebrovascular autoregulation. Cerebral blood flow is maintained constant over a range of systemic blood pressures by changes in the degree of vasodilatation and vasoconstriction of cerebral arterioles. During hypertension, decreased cerebrovascular compliance shifts the autoregulatory curve to the right, in the direction of higher blood pressures (grey arrow). This explains the vulnerability to reduction in perfusion pressure during hypertension. Brain arteries are less able to dilate and this results in reductions of blood flow to the brain, ischemia and neuronal injury. AT1 blockade improves cerebrovascular compliance reducing the shift to the right in the autoregulatory curve (black arrow) and the risk of ischemia.
CEREBROVASCULAR AUTOREGULATION IN HYPERTENSION
In established hypertension there is pathological growth and remodeling of the cerebral circulation, with increased medial thickness, collagen formation, cell growth and number and reduction of arterial lumen and vasoconstriction (Fig. 3). In spontaneously hypertensive rats (SHR) these alterations reduce cerebrovascular compliance, the capacity of the arteries to dilate when faced with a reduction of pressure and blood flow. There is a shift of the autoregulatory curve to the right, in the direction of higher blood pressures (Nishimura et al., 2000,b). The practical consequence is that, in hypertension, because the capacity for vasodilatation is reduced, the cerebral blood flow is more vulnerable to decreased perfusion pressure (Fig. 2). Alterations in endothelial function and fibrinolysis lead to arteriosclerosis and further reduction of arterial caliber. For these reasons ischemia and stroke are frequent in hypertension (Ross, 1993; Harrison, 1997).
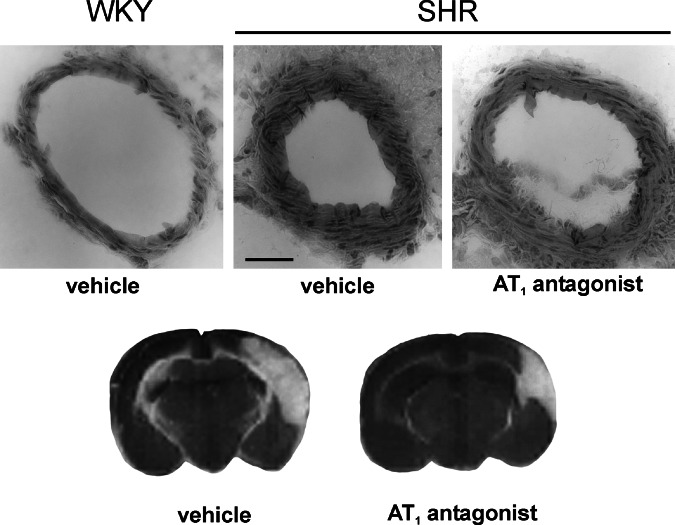
Fig. 3.
Reduction of cerebrovascular remodeling and protection from brain ischemia by pretreatment with an Angiotensin II receptor blocker. Upper figures: Coronal sections of the middle cerebral artery from a Wistar Kyoto (WKY) rat treated with vehicle, and SHR treated with vehicle or candesartan, 1 mg/kg per day for 4 weeks, sc. via osmotic minipumps. Note the increased growth of the medial layer and decreased lumen in the SHR treated with vehicle, when compared to the WKY rat, and the increase in lumen and decrease in medial layer growth after sustained treatment with candesartan in SHR. Bar is 20 μm. Lower figures: Coronal sections of a brain from an SHR treated with vehicle or candesartan as above, after permanent occlusion of the middle cerebral artery. Note the reduction in brain edema and size of the necrotic area in the rat pretreated with candesartan. Reproduced with modifications from Fig. 1 of Ando et al. (2004).
Hypertension increases the frequency of sudden reductions of blood flow in principal arteries such as the medial cerebral artery. Collateral arteries attempt to dilate and maintain blood flow to the region affected. The degree of preservation of blood flow by the collateral circulation determines the size of the ischemic area and neuronal injury, and therefore, the clinical consequences of the ischemic episode. Strokes following occlusion of cerebral arteries are characterized by a development of a necrotic core (Fig. 3), the area receiving most of its flow from the blocked artery, surrounded by a zone of “penumbra” where neuronal survival depends on the preservation of the collateral circulation. Below a certain threshold of blood flow there is permanent neuronal injury and death (Ito et al., 2002). The response of the collateral circulation to ischemia is compromised in hypertension because of the reduced arterial compliance as explained previously and for these reasons strokes are more frequent and more severe in hypertensivesubjects.
CEREBROVASCULAR ANGIOTENSINERGIC OVERDRIVE IN HYPERTENSION AND ITS CONSEQUENCES
The activity of the brain Ang II system is enhanced in SHR (Saavedra, 1992) and these animals express higher numbers of AT1 receptors in the cerebral vasculature, (Ando et al., 2004). The result of excess AT1 receptor stimulation is an important cause of cerebrovascular vasoconstriction, increased pathological growth and decreased cerebrovascular compliance in hypertension (Nishimura et al., 2000b).
In addition, the cerebral vasculature of hypertensive animals exhibits signs of inflammation and endothelial dysfunction, including macrophage attachment and infiltration (Ando et al., 2004) (Fig. 4), enhanced expression of inflammatory markers such as ICAM-1 and TNF-α and increased heat shock protein (HSP) expression in the microvessel endothelium (Zhou et al., 2005) (Fig. 5). Heat shock proteins induce proinflammatory responses including secretion of adhesion molecules and cytokines (Asea et al., 2000; Wallin et al., 2002), and the induction of HSPs previously primed by inflammation accelerates cell death by apoptosis (Buchman et al., 1993; Abello and Buchman, 1994). This is consistent with the observation that long-term administration of Ang II induces expression of HSPs by mechanisms unrelated to hypertension and dependent on AT1 activation (Ishizaka et al., 2002). Endothelial dysfunction and vascular inflammation enhance vulnerability to hypertensive brain damage and are major risk factors for brain ischemia and stroke (Amenta et al., 2003; Lawes et al., 2004). Vascular inflammation is at least partially due to excess Ang II formation (Pastore et al., 1999; Pueyo et al., 2000; Ruiz-Ortega et al., 2000; Touyz, 2003) and to increased AT1 receptor stimulation (Ando et al., 2004; Zhou et al., 2005).
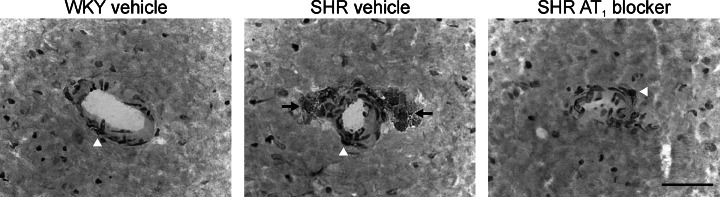
Fig. 4.
Macrophage infiltration in cerebral microvessels from genetically hypertensive rats. WKY and SHR were treated with vehicle or candesartan as in Fig. 3. Figures represent microvessels situated in the cerebral cortex. Middle figure: Black arrows indicate infiltrating macrophages in a microvessel from an SHR treated with vehicle. Right figure: White arrowhead indicates a dramatic reduction of macrophage infiltration after treatment with candesartan. Bar is 20 μm. Reproduced with modifications from Fig. 5 of Ando et al. (2004).
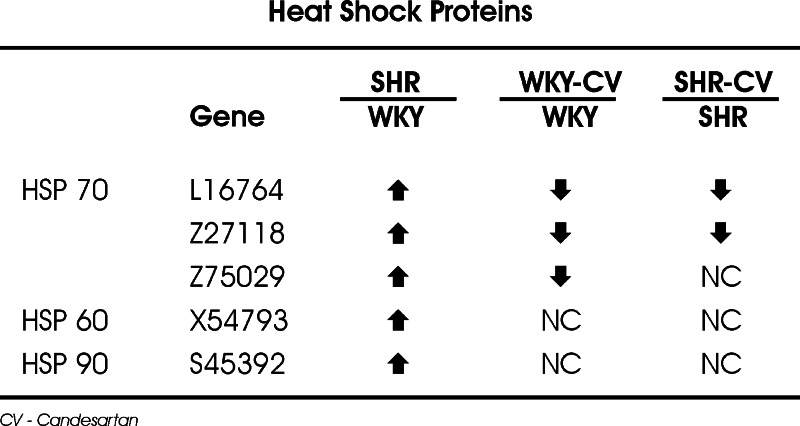
Fig. 5.
Regulation of heat shock protein transcripts in microvessels from SHR and WKY rats and effects of sustained AT1 receptor blockade. Expression of transcripts for heat shock proteins (HSP) 70, 60 and 90 was studied with the use of Affymetrix GeneChip U34A arrays (Zhou et al., 2005). Note increased expression of HSP 70, HSP 60 and HSP 90 in SHR when compared to WKY rats, and decreased expression of HSP 70 after candesartan treatment (modified from Zhou et al., 2005).
There are prominent alterations in nitric oxide (NO) production in the cerebral vasculature in hypertension. The complex role of nitric oxide (NO) production in cerebral arteries is linked to both vasodilatation and to inflammation, and there is a well-known association between the Ang II and NO systems (Briones et al., 2002). Nitric oxide synthase (NOS) isoenzymes are selectively localized in cerebral vessels, endothelial NOS (eNOS) located in the endothelium and inducible NOS (iNOS) in the adventitia. In hypertension, there is decreased eNOS and increased iNOS expression, and this alteration of the eNOS/iNOS ratio favors inflammation and reduces vasodilatation (Yamakawa et al., 2003). AT1 receptor stimulation decreases eNOS expression (Yamakawa et al., 2003). NOS inhibition promotes ICAM-1 expression (Luvara et al., 1998) and macrophage infiltration (Luvara et al., 1998; Usui et al., 2000) and adherence (Kiarash et al., 2001; Ando et al., 2004) and increases production of interleukin-1β, (Bauer et al., 1995) a proinflammatory cytokine that upregulates ICAM-1 expression (McCarron et al., 1994; Galea et al., 1998; Staykova et al., 2000; Schoning et al., 2002). These alterations explain the decreased vasodilatation and enhanced formation of reactive oxygen species correlated with arterial vasoconstriction and inflammation.
THERAPEUTIC MEASURES FOR THE PREVENTION OF STROKE
Inhibition of the mechanisms leading to decreased vascular compliance and inflammation is one of the principal strategies for the prevention of brain ischemia during hypertension. A sustained inhibition of peripheral and central Ang II AT1 receptors with peripheral administration of candesartan, an AT1 receptor blocker (ARB) with access to the brain (Nishimura et al., 2000) normalizes cerebrovascular compliance in SHR, correcting the shift to the right characteristic of cerebrovascular autoregulation during hypertension (Nishimura et al., 2000b) (Fig. 2). The practical consequence of the correction of the autoregulatory curve is that after blockade of a major cerebral artery, collateral arteries recover their capacity to dilate, maintaining the blood flow above the required threshold in the periphery of the lesion, the penumbra zone, and reducing the area of ischemia and neuronal injury (Fig. 3). There is a good correlation between the area of blood flow below the required protective threshold and the area of neuronal injury and death, both significantly reduced by AT1 receptor blockade (Ito et al., 2002). The underlying mechanisms of the ARB effect include a blockade of the vasoconstrictive and pro-growth effects of Ang II (Fig. 3).
The inflammatory response in cerebral microvessels of SHR depends on Ang II stimulation (Takemori et al., 2000; Suzuki et al., 2001; Ando et al., 2004) and can be suppressed by blockade of its AT1 receptors. Sustained treatment with the ARB reverses the alterations in eNOS expression, (Yamakawa et al., 2003) normalizing the eNOS/iNOS ratio. The upregulation of eNOS activity decreases ICAM-1 expression (Scalia et al., 2000; Buras et al., 2000), the transcription and expression of HSPs and inflammatory markers (Fig. 5), and prevents macrophage infiltration (Luvara et al., 1998; Usui et al., 2000; Yamakawa et al., 2003; Ando et al., 2004; Zhou et al., 2005). In this case, reversal of increased HSP and HSF-1 expression by AT1 receptor blockade might be considered an important mechanism to prevent inflammation, cell damage and apoptosis in brain microvessels, contributing to the end-organ protective effect of Ang II system blockade, as it is the case in peripheral organs (Hilgers et al., 2001; Dandona et al., 2003). These antiinflammatory effects can explain some of the underlying mechanisms of the decrease in cardiovascular morbidity and mortality, which follow a 7-day course of candesartan when the treatment is administered during the first week after acute stroke (Schrader et al., 2003).
THE ROLE OF BLOOD PRESSURE DECREASE AND OF THE SELECTIVE INHIBITION OF THE RENIN–ANGIOTENSIN SYSTEM
Treatment with candesartan normalizes blood pressure in SHR. It could be argued that protection against ischemia and reduced inflammation is the direct consequence of the blood pressure reduction. However, while both AT1 receptor blockade or ACE inhibition, which decreases Ang II synthesis, are effective (Nishimura et al., 2000b; Ito et al., 2002) no protection occurs after treatment with β-adrenergic or calcium-blocking agents (Nishimura et al., 2000a,b; Ito et al., 2002). In addition, treatment with a low dose of candesartan (0.1 mg/kg) reduced the stroke incidence and urinary protein excretion without affecting the blood pressure (Inada et al., 1997) and in peripheral organs, the antiinflammatory effect of AT1 antagonists is independent of their effects on blood pressure (Dohi et al., 2003).
THE ROLE OF AT RECEPTORS
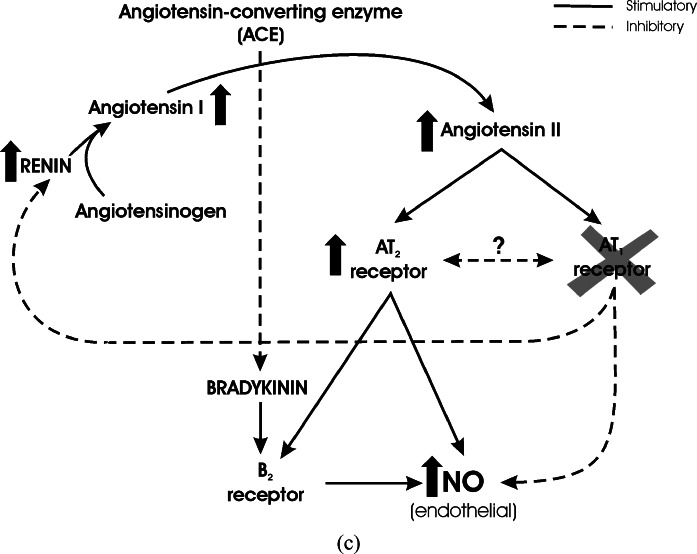
The protective effects of candesartan against brain ischemia may not be only related to direct inhibition of AT1 receptor stimulation. Treatment with candesartan increases the expression of Ang II AT2 receptors in brain microvessels (Zhou et al., submitted) (Fig. 6). AT2 receptor stimulation, the effects of which have not been completely clarified (Saavedra, 1999) was proposed to result in vasodilatation and inhibition of growth, balancing AT1 receptor effects (Carey, 2005). Stimulation of AT2 receptors enhances endothelial NO formation directly (Hiyoshi et al., 2005) and through stimulation of Bradykinin B2 receptors in the endothelium (Abadir et al., 2003; Ritter et al., 2003; Batenburg et al., 2004; Fukada et al., 2005) (Fig. 7a). Stimulation of NO formation is therefore influenced negatively by AT1 receptor action, and positively by AT2 receptor (Ritter et al., 2003) and B2 receptor activity (Fig. 7a). There are two pharmacological manipulations to decrease AT1 receptor stimulation, increasing AT2 and/or B2 effects on endothelial NO. Inhibition of Ang II formation by inhibition of the Angiotensin Converting Enzyme (ACE) enhances Bradykinin levels by inhibition of its degradation, decreases AT1 receptor stimulation by decreasing Ang II formation, and the net result is an increase in endothelial NO formation (Chen et al., 2003) (Fig. 7b). Direct inhibition of AT1 receptors with ARBs enhances NO formation by removing the inhibitory effect of AT1 receptor stimulation and increasing AT2 effects by stimulation of AT2 receptor expression and increased Ang II formation as a result of decreased inhibitory feed-back of renin production (Fig. 7c). This explains the protective effects of both ACE inhibitors and ARBs in brain ischemia (Nishimura et al., 2000b).
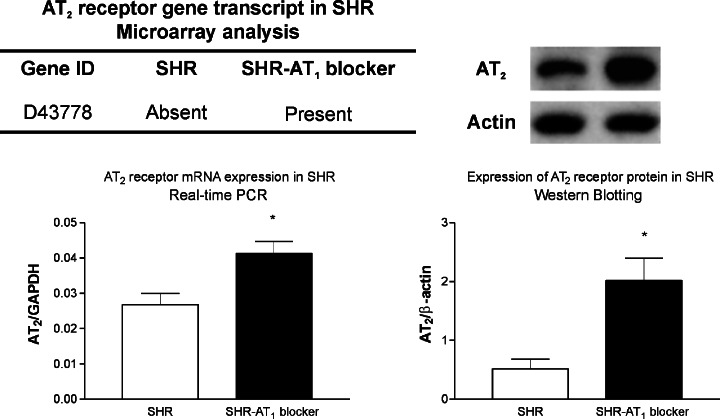
Fig. 6.
Angiotensin II AT2 receptor mRNA and protein in brain microvessels after sustained AT1 receptor blockade. SHR were treated with vehicle or with candesartan as in Fig. 3. The AT2 receptor transcript was absent in SHR treated with vehicle, and present in SHR treated with candesartan, as determined with the U34A array. Increased AT2 receptor mRNA and protein in SHR after sustained treatment with candesartan was confirmed with the use of RT-PCR and Western blotts. * p < 0.05, candesartan treated vs. vehicle-treated rats.
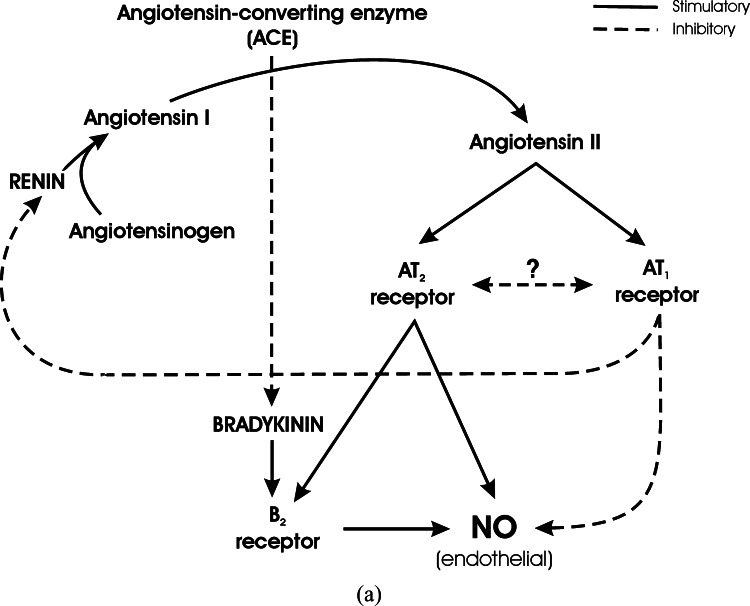
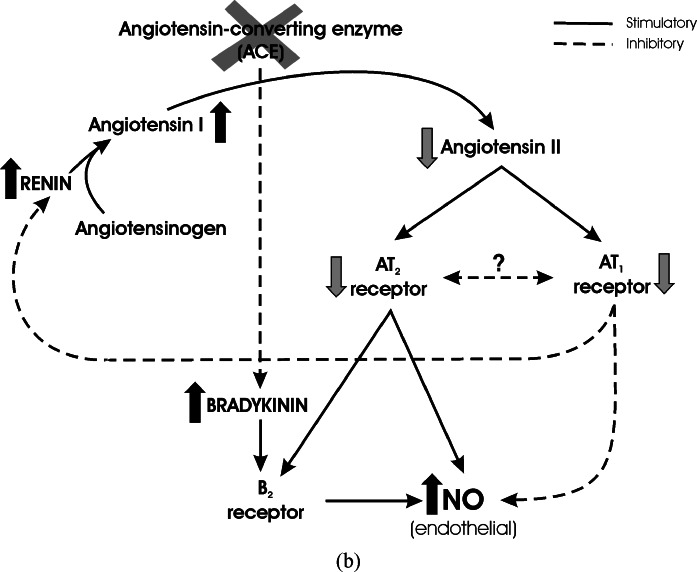
Fig. 7.
Regulation of NO formation by Angiotensin II receptor types, and influence of ACE or AT1 receptor blockade. (a) Interaction between Angiotensin II and Bradykinin in the regulation of NO formation. Renin converts Angiotensinogen to Angiotensin I, and Angiotensin Converting Enzyme (ACE) converts Angiotensin I to the active principle Angiotensin II. Angiotensin II stimulates two receptor types, AT1 and AT2 receptors. Stimulation of AT1 receptors decreases NO formation at endothelial sites, whereas AT2 receptor stimulation has an opposite effect. The action of AT2 receptors results from direct effect on endothelial NO formation, and from stimulation of Bradykinin B2 receptors. There is a still undetermined balance between AT1 and AT2 receptor effect and expression. AT1 stimulation is part of a feed-back inhibition of renin formation, to balance Angiotensin II production. Besides formation of Angiotensin II, ACE degrades Bradykinin and decreases Bradykinin B2 receptor stimulation. Full arrows represent stimulation. Broken arrows represent inhibition. (b) Inhibition of Angiotensin Converting Enzyme. Inhibition of ACE decreases Angiotensin II formation and AT1/AT2 receptor stimulation. Additionally, Bradykinin levels increase as a result of decreased degradation. This produces enhanced B2 receptor stimulation and increased endothelial NO, in spite of decreased stimulation by AT2 receptors. Full arrows represent stimulation. Broken arrows represent inhibition. (c) Inhibition of AT1 receptors. Inhibition of AT1 receptors increases endothelial NO formation by decreasing AT1 receptor inhibition, by increasing the expression of AT2 receptors, and by increasing Angiotensin II formation and AT2 receptor stimulation due to decreased inhibitory feed-back on renin formation. Full arrows represent stimulation. Broken arrows represent inhibition.
The question whether or not AT2 receptor stimulation plays a significant protective role after brain ischemia has not yet been resolved. There are reports of a possible inhibition of brain ischemia by AT2 receptor stimulation (Iwai et al., 2004). This report is based on the finding of enhanced neurological deficits in AT2 receptor knockout mice following middle cerebral artery occlusion. The authors did not consider that failure of AT2 receptor transmission results in enhanced brain AT1 receptor expression (Armando et al., 2002), a finding which can explain the increased sensitivity of AT2 receptor knockout mice to ischemia. In addition, blockade of Ang II synthesis with ACE inhibitors should result in a decrease in both AT1 and AT2 receptor stimulation. Since protection against brain ischemia occurs not only after AT1 receptor blockade but also after administration of ACE inhibitors, the logical conclusion is that inhibition of AT1 receptors is necessary and sufficient for the protection of brain ischemia.
CONCLUSIONS
The reversal of the cerebrovascular pathological growth and inflammation by AT1 receptor blockade indicates that AT1 receptor overstimulation is a mayor mechanism leading to increase vulnerability to brain ischemia during hypertension. In addition to their antihypertensive and antigrowth properties, the cerebrovascular antiinflammatory effects of Ang II AT1 receptor antagonists might be of major importance to protect the brain against neuronal damage due to ischemia. The suppression of inflammation in brain vessels suggests important therapeutic advantages of AT1 receptor antagonists not only in the prevention of brain ischemia but also in the treatment of inflammatory diseases of the brain.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institute of Mental Health, NIH, DHHS. The authors thank Astra-Zeneca for their supply of candesartan.
REFERENCES
- Abadir, P. M., Carey, R. M., and Siragy, H. M. (2003). Angiotensin AT2 receptors directly stimulate renal nitric oxide in Bradykinin B2-receptor-null mice. Hypertension42:600–604. [DOI] [PubMed] [Google Scholar]
- Abello, P. A., and Buchman, T. G. (1994). Heat shock-induced cell death in murine microvascular endothelial cells depends on priming with tumor necrosis factor-alpha or interferon-gamma. Shock2:320–323. [DOI] [PubMed] [Google Scholar]
- Amenta, F., Di Tullio, M. A., and Tomassoni, D. (2003). Arterial hypertension and brain damage—evidence from animal models. Clin. Exp. Hypertens.25:359–380. [DOI] [PubMed] [Google Scholar]
- Ando, H., Zhou, J., Macova, M., Imboden,H., and Saavedra, J. M. (2004). Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke35:1726–1731. [DOI] [PubMed] [Google Scholar]
- Armando, I., Terrón, J. A., Falcón-Neri, A., Ito, T., Häuser, W., Inagami, T., and Saavedra, J. M. (2002). Increased Angiotensin II AT1 receptor expression in paraventricular nucleus and hypothalamic–pituitary–adrenal axis stimulation in AT2 receptor gene disrupted mice. Neuroendocrinology76:137–147. [DOI] [PubMed] [Google Scholar]
- Asea, A., Kraeft, S. K., Kurt-Jones, E. A., Stevenson, M. A., Chen, L. B., Finberg, R. W., Koo, G. C., and Calderwood, S. K. (2000). HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med.6:435–442. [DOI] [PubMed] [Google Scholar]
- Batenburg, W. W., Garrelds, I. M., Bernasconi, C. C., Juillerat-Jeanneret, L., van Kats, J. P., Saxena, P. R., and Danser, A. H. (2004). Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation109:2296–2301. [DOI] [PubMed] [Google Scholar]
- Bauer, J., Huitinga, I., Zhao, W., Lassmann, H., Hickey, W. F., and Dijkstra, C. D. (1995). The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia15:437–446. [DOI] [PubMed] [Google Scholar]
- Briones, A. M., Alonso, M. J., Hernanz, R., Miguel, M., and Salaices M. (2002). Alterations of the nitric oxide pathway in cerebral arteries from spontaneously hypertensive rats. J. Cardiovasc. Pharmacol.39:378–388. [DOI] [PubMed] [Google Scholar]
- Buchman, T. G., Abello, P. A., Smith, E. H., and Bulkley, G. B. (1993). Induction of heat shock response leads to apoptosis in endothelial cells previously exposed to endotoxin. Am. J. Physiol.265:H165–H170. [DOI] [PubMed] [Google Scholar]
- Buras, J. A., Stahl, G. L., Svoboda, K. K., and Reenstra, W. R. (2000). Hyperbaric oxygen downregulates ICAM-1 expression induced by hypoxia and hypoglycemia: The role of NOS. Am. J. Physiol.278:C292–C302. [DOI] [PubMed] [Google Scholar]
- Carey, R. M. (2005). Update on the role of the AT2 receptor. Curr. Opin. Nephrol. Hypertens.14:67–71. [DOI] [PubMed] [Google Scholar]
- Chen, R., Iwai, M., Wu, L., Suzuki, J., Min, L. J., Shiuchi, T., Sugaya, T., Liu, H. W., Cui, T. X., and Horiuchi, M. (2003). Important role of nitric oxide in the effect of angiotensin-converting enzyme inhibitor imidapril on vascular injury. Hypertension42:542–547. [DOI] [PubMed] [Google Scholar]
- Dandona, P., Kumar, V., Aljada, A., Ghanim, H., Syed, T., Hofmayer, D., Mohanty, P., Tripathy, D., and Garg, R. (2003). Angiotensin II receptor blocker valsartan suppresses reactive oxygen species generation in leukocytes, nuclear factor-κB, in mononuclear cells of normal subjects: Evidence of an antiinflammatory action. J. Clin. Endocrinol. Metab.88:4496–4501. [DOI] [PubMed] [Google Scholar]
- Dohi, Y., Ohashi, M., Sugiyama, M., Takase, H., Sato, K., and Ueda, R. (2003). Candesartan reduces oxidative stress and inflammation in patients with essential hypertension. Hypertens. Res.26:691–697. [DOI] [PubMed] [Google Scholar]
- Fukada, S. Y., Tirapelli, C. R., de Godoy, M. A. F., and de Oliveira, A. M. (2005). Mechanisms underlying the endothelium-independent relaxation induced by Angiotensin II in rat aorta. J. Cardiovasc. Pharmacol.45:136–143. [DOI] [PubMed] [Google Scholar]
- Galea, E., Glickstein, S. B., Feinstein, D. L., Golanov, E. V., and Reis, D. J. (1998). Stimulation of cerebellar fastigial nucleus inhibits interleukin-1beta-induced cerebrovascular inflammation. Am. J. Physiol.275:H2053–H2063. [DOI] [PubMed] [Google Scholar]
- Harrison, D. G. (1997). Cellular and molecular mechanisms of endothelial cell dysfunction. J. Clin. Invest.100:2153–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers, K. F., Hartner, A., Porst, M., Veelken, R., and Mann, J. F. (2001). Angiotensin II type 1 receptor blockade prevents lethal malignant hypertension: Relation to kidney inflammation. Circulation104:1436–1440. [DOI] [PubMed] [Google Scholar]
- Hiyoshi, H., Yayama, K., Takano, M., and Okamoto, H. (2005). Angiotensin type 2 receptor-mediated phosphorylation of eNOS in the aortas of mice with 2-kidney, 1-clip hypertension. Hypertension45:967–973. [DOI] [PubMed] [Google Scholar]
- Inada, Y., Wada, T., Ojima, M., Sanada, T., Shibouta, Y., Kanagawa, R., Ishimura, Y., Fujisawa, Y., and Nishikawa, K. (1997). Protective effects of candesartan cilexetil (TCV-116) against stroke, kidney dysfunction and cardiac hypertrophy in stroke-prone spontaneously hypertensive rats. Clin. Exp. Hypertens.19:1079–1099. [DOI] [PubMed] [Google Scholar]
- Ishizaka, N., Aizawa, T., Ohno, M., Usui, S., Mori, I., Tang, S. S., Ingelfinger, J. R. Kimura, S., and Nagai, R. (2002). Regulation and localization of HSP70 and HSP25 in the kidney of rats undergoing long-term administration of Angiotensin II. Hypertension39:122–128. [DOI] [PubMed] [Google Scholar]
- Ito, T., Yamakawa, H., Bregonzio, C., Terrón, J. A., Falcón-Neri, A., and Saavedra, J. M. (2002). Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an Angiotensin II AT1 antagonist. Stroke33:2297–2303. [DOI] [PubMed] [Google Scholar]
- Iwai, M., Liu, H.-W, Chen, R., Ide, A., Okamoto, S., Hata, R., Sakanaka, M., Shiuchi, T., and Horiuchi, M. (2004). Possible inhibition of focal cerebral ischemia by Angiotensin II type 2 receptor stimulation. Circulation110:843–848. [DOI] [PubMed] [Google Scholar]
- Kiarash, A., Pagano, P. J., Tayeh, M., Rhaleb, N. E., and Carretero, O. A. (2001). Upregulated expression of rat heart intercellular adhesion molecule-1 in Angiotensin II—but not phenylephrine-induced hypertension. Hypertension37:58–65. [DOI] [PubMed] [Google Scholar]
- Lawes, C. M., Bennett, D. A., Feigin, V. L., and Rodgers, A. (2004). Blood pressure and stroke: An overview of published reviews. Stroke35:1024–1033. [PubMed] [Google Scholar]
- Luvara, G., Pueyo, M. E, Philippe, M., Mandet, C., Savoie, F., Henrion, D., and Michel, J. B. (1998). Chronic blockade of NO synthase activity induces a proinflammatory phenotype in the arterial wall: Prevention by Angiotensin II antagonism. Arterioscler. Thromb. Vasc. Biol.18:1408–1416. [DOI] [PubMed] [Google Scholar]
- McCarron, R. M., Wang, L., Siren, A. L., Spatz, M., and Hallenbeck, J. M. (1994). Monocyte adhesion to cerebromicrovascular endothelial cells derived from hypertensive and normotensive rats. Am. J. Physiol.267:H2491–H2497. [DOI] [PubMed] [Google Scholar]
- Nishimura, Y., Ito, T., Hoe, K.-L., and Saavedra, J. M. (2000a). Chronic peripheral administration of the Angiotensin II AT1 receptor antagonist candesartan blocks brain AT1 receptors. Brain Res.871:29–38. [DOI] [PubMed] [Google Scholar]
- Nishimura, Y., Ito, T., and Saavedra, J. M. (2000b). Angiotensin II AT1 blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke31:2478–2486. [DOI] [PubMed] [Google Scholar]
- Pastore, L., Tessitore, A., Martinotti, S., Toniato, E., Alesse, E., Bravi, M. C., Ferri, C., Desideri, G., Gulino, A., and Santucci, A. (1999). Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increases soluble ICAM-1 release in vivo. Circulation100:1646–1652. [DOI] [PubMed] [Google Scholar]
- Pueyo, M. E., Gonzalez, W., Nicoletti, A., Savoie, F., Arnal, J. F., and Michel, J. B. (2000). Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-κB activation induced by intracellular oxidative stress. Arterioscler. Thromb. Vasc. Biol.20:645–651. [DOI] [PubMed] [Google Scholar]
- Ritter, O., Schuh, K., Brede, M., Rothlein, N., Burkard, N., Hein, L., and Neyses, L. (2003). AT2 receptor activation regulates myocardial eNOS expression via the calcineurin-NF-AT pathway. FASEB J.17:283–285. [DOI] [PubMed] [Google Scholar]
- Ross, R. (1993). The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature362:801–809. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega, M., Lorenzo, O., Rupérez, M., König, S., Wittig, B., and Egido, J. (2000). Angiotensin II activates nuclear transcription factor κB through AT1 and AT2 in vascular smooth muscle cells: Molecular mechanisms. Circ. Res.86:1266–1272. [DOI] [PubMed] [Google Scholar]
- Saavedra, J. M. (1992). Brain and pituitary angiotensin II. Endoc. Revs.13:329–380. [DOI] [PubMed] [Google Scholar]
- Saavedra, J. M. (1999). Emerging features of brain angiotensin receptors. Reg. Pept.85:31–45. [DOI] [PubMed] [Google Scholar]
- Saavedra, J. M. (2005). Brain Angiotensin II: New developments, unanswered questions and therapeutic opportunities. Cell Mol. Neurobiol.25:485–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalia, R., Coyle, K. M., Levine, B. J., Booth, G., and Lefer, A. M. (2000). C-peptide inhibits leukocyte–endothelium interaction in the microcirculation during acute endothelial dysfunction. FASEB J.14:2357–2364. [DOI] [PubMed] [Google Scholar]
- Schoning, B., Elepfandt, P., Daberkow, N., Rupprecht, S., Stockhammer, F., Stoltenburg, G., Volk, H. D., and Woiciechowsky, C. (2002). Differences in immune cell invasion into the cerebrospinal fluid and brain parenchyma during cerebral infusion of interleukin-1beta. J. Neurol. Sci.23:211–218. [DOI] [PubMed] [Google Scholar]
- Schrader, J., Luders, S., Kulschewski, A., Berger, J., Zidek, W., Treib, J., Einhaupl, K., Diener, H. C., and Dominiak, P. (2003). The ACCESS Study: Evaluation of acute candesartan cilexetil therapy in stroke survivors. Stroke34:1699–1703. [DOI] [PubMed] [Google Scholar]
- Staykova, M., Maxwell, L., and Willenborg, D. (2000). Kinetics and polarization of the membrane expression of cytokine-induced ICAM-1 on rat brain endothelial cells. J. Neuropathol. Exp. Neurol.59:120–128. [DOI] [PubMed] [Google Scholar]
- Suzuki, K., Masawa, N., and Takatama, M. (2001). The pathogenesis of cerebrovascular lesions in hypertensive rats. Med. Electron Microsc.34:230–239. [DOI] [PubMed] [Google Scholar]
- Takemori, K., Ito, H., and Suzuki, T. (2000). Effects of the AT1 receptor antagonist on adhesion molecule expression in leukocytes and brain microvessels of stroke-prone spontaneously hypertensive rats. Am. J. Hypertens.13:1233–1241. [DOI] [PubMed] [Google Scholar]
- Touyz, R. M. (2003). The role of Angiotensin II in regulating vascular structural and functional changes in hypertension. Curr. Hypertens. Rep.5:155–164. [DOI] [PubMed] [Google Scholar]
- Usui, M., Egashira, K., Tomita, H., Koyanagi, M., Katoh, M., Shimokawa, H., Takeya, M., Yoshimura, T., Matsushima, K., and Takeshita, A. (2000). Important role of local Angiotensin II activity mediated via type 1 receptor in the pathogenesis of cardiovascular inflammatory changes induced by chronic blockade of nitric oxide synthesis in rats. Circulation101:305–310. [DOI] [PubMed] [Google Scholar]
- Yamakawa, H., Jezova, M., Ando, H., and Saavedra, J. M. (2003). Normalization of endothelial and inducible nitric oxide synthase expression in brain microvessels of spontaneously hypertensive rats by Angiotensin II AT1 receptor inhibition. J. Cereb. Blood Flow Metab.23:371–380. [DOI] [PubMed] [Google Scholar]
- Wallin, R. P. Lundqvist, A., Moré, S. H., von Bonin, A., Kiessling, R., and Ljunggren, H. G. (2002). Heat-shock proteins as activators of the innate immune system. Trends Immunol.23:130–135. [DOI] [PubMed] [Google Scholar]
- Zhou, J., Ando, H., Macova, M., Dou, J., and Saavedra, J. M. (2005). Angiotensin II AT1 receptor blockade abolishes brain microvascular inflammation and heat shock protein responses in hypertensive rats. J. Cereb. Blood Flow Metab.25:878–886. [DOI] [PubMed] [Google Scholar]