Abstract
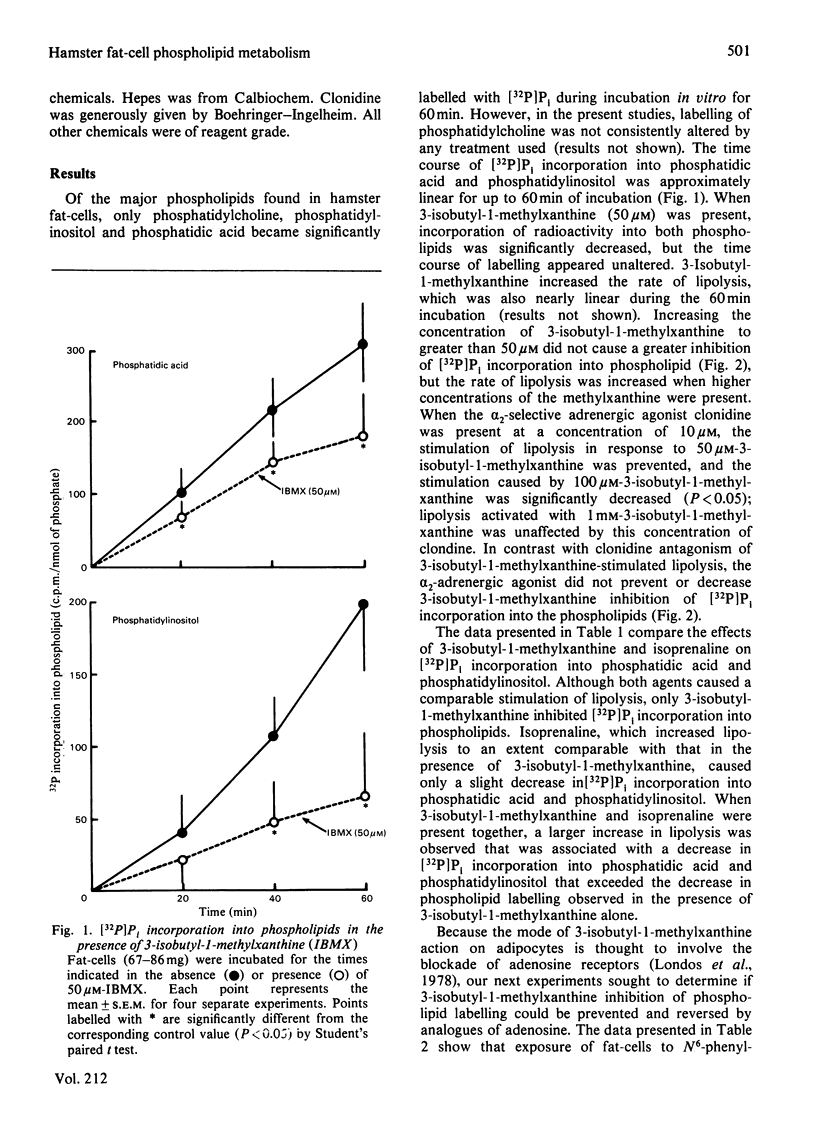
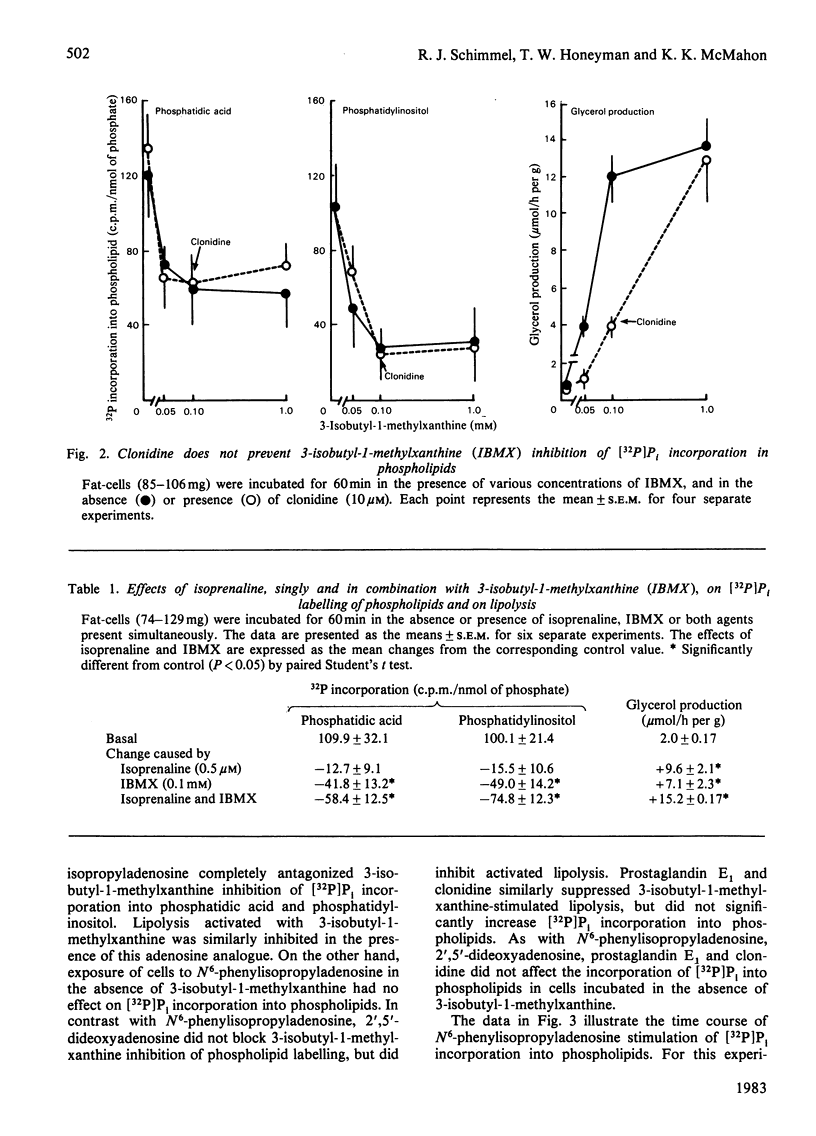
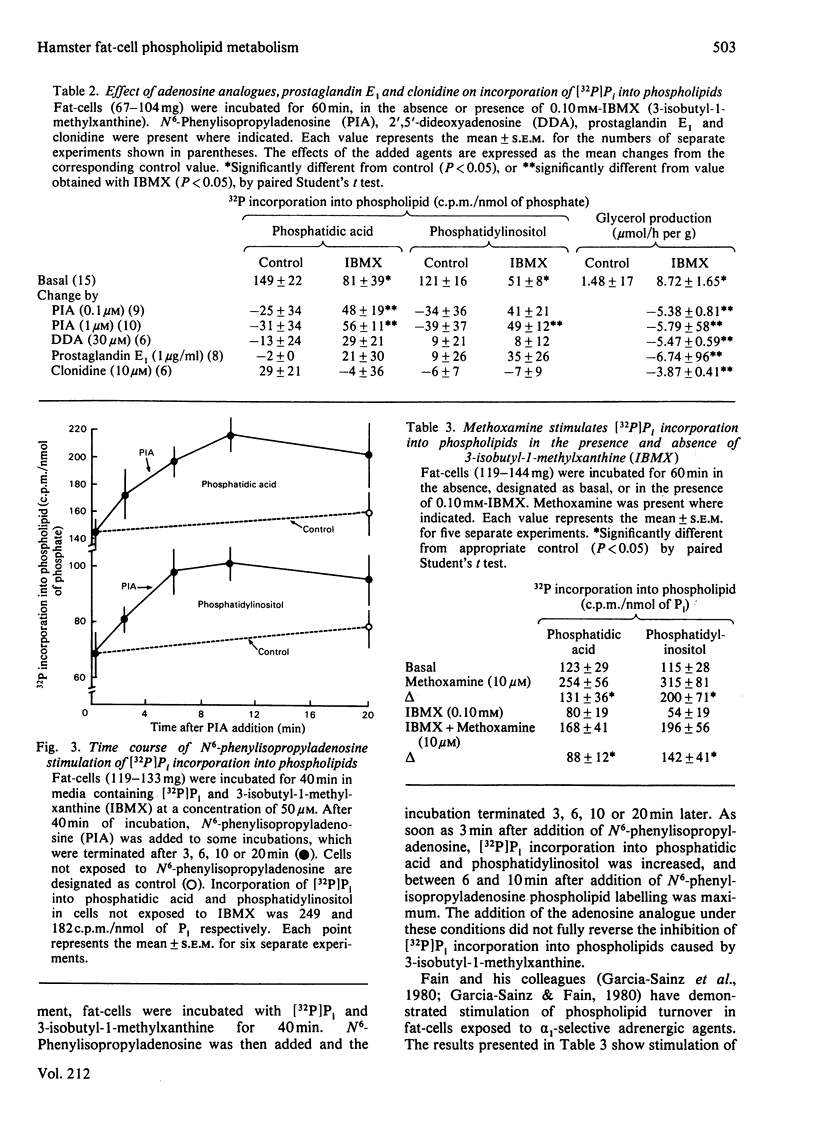
Incorporation of [32P]Pi into phosphatidic acid and phosphatidylinositol of hamster epididymal adipocytes was partially inhibited by 3-isobutyl-1-methylxanthine. This effect of 3-isobutyl-1-methylxanthine was antagonized by isopropyl-N6-phenyladenosine but not by 2',5'-dideoxyadenosine, prostaglandin E1 or clonidine. N6-Phenylisopropyladenosine did not affect incorporation of [32P]Pi into phosphatidic acid or phosphatidylinositol when 3-isobutyl-1-methylxanthine was not present. In contrast with 3-isobutyl-1-methylxanthine inhibition of [32P]Pi incorporation into phospholipids, which was blocked only by N6-phenylisopropyladenosine, accelerated lipolysis was blocked by prostaglandin E1, clonidine and 2',5'-dideoxyadenosine as well as by N6-phenylisopropyladenosine. Phospholipid labelling was also decreased in the presence of adenosine deaminase, but not in the presence of isoprenaline (isoproterenol). The stimulatory effect of N6-phenylisopropyladenosine on [32P]Pi incorporation into phospholipids in cells exposed to 3-isobutyl-1-methylxanthine was evident as soon as 3 min after addition of the adenosine analogue and maximum 10 min after its addition. As observed by others, [32P]Pi incorporation into phospholipids was increased by the alpha 1-selective agonist methoxamine. The stimulatory effect of methoxamine occurred with a time course similar to that of N6-phenylisopropyladenosine and was present at nearly equal magnitude in the absence or presence of 3-isobutyl-1-methylxanthine. The inhibitory effects of 3-isobutyl-1-methylxanthine and adenosine deaminase on phospholipid labelling are attributed to blockade of the action, or to the enzymic removal, of adenosine formed in and released from the fat-cells during their incubation. Supporting this view is the selective reversal of the actions of 3-isobutyl-1-methylxanthine and of adenosine deaminase by N6-phenylisopropyladenosine. These findings suggest an important role for endogenous adenosine in regulation of phospholipid turnover in adipocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowyer D. E., King J. P. Methods for the rapid separation and estimation of the major lipids of arteries and other tissues by thin-layer chromatography on small plates followed by microchemical assays. J Chromatogr. 1977 Sep 1;143(5):473–490. doi: 10.1016/s0378-4347(00)81794-6. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Salmon D. M., Honeyman T. W. Phosphatidic acid inhibition of PGE1-stimulated cAMP accumulation in WI-38 fibroblasts: similarities with carbachol inhibition. J Cyclic Nucleotide Res. 1980;6(1):37–49. [PubMed] [Google Scholar]
- Daly J. W., Bruns R. F., Snyder S. H. Adenosine receptors in the central nervous system: relationship to the central actions of methylxanthines. Life Sci. 1981 May 11;28(19):2083–2097. doi: 10.1016/0024-3205(81)90614-7. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Molecular mechanisms involved in alpha-adrenergic responses. Mol Cell Endocrinol. 1981 Sep;23(3):233–264. doi: 10.1016/0303-7207(81)90123-4. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Larson R. E., Sabir M. A. Insulin acutely increases phospholipids in the phosphatidate-inositide cycle in rat adipose tissue. J Biol Chem. 1982 Apr 25;257(8):4042–4045. [PubMed] [Google Scholar]
- Freholm B. B., Möllby R., Malmquist T., Smyth C. J. Inhibition of noradrenaline-stimulated lipolysis and cyclic AMP accumulation in isolated rat adipocytes by purified phospholipase C and theta-toxin from Clostridium perfringens. Acta Pharmacol Toxicol (Copenh) 1978 Jan;42(1):23–34. doi: 10.1111/j.1600-0773.1978.tb02167.x. [DOI] [PubMed] [Google Scholar]
- García-Sáinz J. A., Fain J. N. Effect of insulin, catecholamines and calcium ions on phospholipid metabolism in isolated white fat-cells. Biochem J. 1980 Mar 15;186(3):781–789. doi: 10.1042/bj1860781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sínz J. A., Hoffman B. B., Li S. Y., Lefkowitz R. J., Fain J. N. Role of alpha 1 adrenoceptors in the turnover of phosphatidylinositol and of alpha 2 adrenoceptors in the regulation of cyclic AMP accumulation in hamster adipocytes. Life Sci. 1980 Sep 15;27(11):953–961. doi: 10.1016/0024-3205(80)90105-8. [DOI] [PubMed] [Google Scholar]
- Green D. E., Fry M., Blondin G. A. Phospholipids as the molecular instruments of ion and solute transport in biological membranes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):257–261. doi: 10.1073/pnas.77.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel J. J., Cushman S. W., Jeanrenaud B. Cell-associated fatty acid levels and energy-requiring processes in mouse adipocytes. Am J Physiol. 1974 Jan;226(1):16–24. doi: 10.1152/ajplegacy.1974.226.1.16. [DOI] [PubMed] [Google Scholar]
- Honeyman T. W., Strohsnitter W., Scheid C. R., Schimmel R. J. Phosphatidic acid and phosphatidylinositol labelling in adipose tissue. Relationship to the metabolic effects of insulin and insulin-like agents. Biochem J. 1983 May 15;212(2):489–498. doi: 10.1042/bj2120489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya K., Ui M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin Chim Acta. 1966 Sep;14(3):361–366. doi: 10.1016/0009-8981(66)90114-8. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K. The hamster adipocyte adenylate cyclase system. I. Regulation of enzyme stimulation and inhibition by manganese and magnesium ions. Biochim Biophys Acta. 1981 Aug 5;676(1):51–58. doi: 10.1016/0304-4165(81)90008-8. [DOI] [PubMed] [Google Scholar]
- Kanfer J. N. Base exchange reactions of the phospholipids in rat brain particles. J Lipid Res. 1972 Jul;13(4):468–476. [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Schlegel W., Rodbell M. Adenosine analogs inhibit adipocyte adenylate cyclase by a GTP-dependent process: basis for actions of adenosine and methylxanthines on cyclic AMP production and lipolysis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5362–5366. doi: 10.1073/pnas.75.11.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Van De Walle C. M., Haddas R. A. Is phosphatidic acid a calcium ionophore under neurohumoral control? Nature. 1980 Mar 27;284(5754):345–347. doi: 10.1038/284345a0. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Jones A. B. Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J Biol Chem. 1966 Jan 10;241(1):140–142. [PubMed] [Google Scholar]
- Rosenthal J. W., Fain J. N. Insulin-like effect of clostridial phospholipase C, neuraminidase, and other bacterial factors on brown fat cells. J Biol Chem. 1971 Oct 10;246(19):5888–5895. [PubMed] [Google Scholar]
- Salmon D. M., Honeyman T. W. Proposed mechanism of cholinergic action in smooth muscle. Nature. 1980 Mar 27;284(5754):344–345. doi: 10.1038/284344a0. [DOI] [PubMed] [Google Scholar]
- Schimmel R. J., Honeyman T. W., McMahon K. K., Serio R., Clark R. B. Inhibition of cyclic AMP accumulation in hamster adipocytes with phosphatidic acid: differences and similarities with alpha adrenergic effects. J Cyclic Nucleotide Res. 1980;6(6):437–449. [PubMed] [Google Scholar]
- Schimmel R. J., McMahon K. K., Serio R. Interactions between alpha-adrenergic agents, prostaglandin E1, nicotinic acid, and adenosine in regulation of lipolysis in hamsters epididymal adipocytes. Mol Pharmacol. 1981 Mar;19(2):248–255. [PubMed] [Google Scholar]
- Schwabe U., Ebert R., Erbler H. C. Adenosine release from isolated fat cells and its significance for the effects of hormones on cyclic 3',5'-AMP levels and lipolysis. Naunyn Schmiedebergs Arch Pharmacol. 1973;276(2):133–148. doi: 10.1007/BF00501186. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Schönhöfer P. S., Ebert R. Facilitation by adenosine of the action of insulin on the accumulation of adenosine 3':5'-monophosphate, lipolysis, and glucose oxidation in isolated fat cells. Eur J Biochem. 1974 Aug 1;46(3):537–545. doi: 10.1111/j.1432-1033.1974.tb03647.x. [DOI] [PubMed] [Google Scholar]
- Teo T. S., Ooi S. O., Wong E. H. Stimulation of rat fat cell phosphodiesterase by adenosine. FEBS Lett. 1981 Jun 1;128(1):75–78. doi: 10.1016/0014-5793(81)81083-6. [DOI] [PubMed] [Google Scholar]
- Trost T., Schwabe U. Adenosine receptors in fat cells. Identification by (-)-N6-[3H]phenylisopropyladenosine binding. Mol Pharmacol. 1981 Mar;19(2):228–235. [PubMed] [Google Scholar]
- Trost T., Stock K. Effects of adenosine derivatives on cAMP accumulation and lipolysis in rat adipocytes and on adenylate cyclase in adipocyte plasma membranes. Naunyn Schmiedebergs Arch Pharmacol. 1977 Aug;299(1):33–40. doi: 10.1007/BF00508634. [DOI] [PubMed] [Google Scholar]


