Abstract
Background
There is a need to better understand ulcerative colitis (UC) patient and healthcare provider (HCP) treatment satisfaction, acceptability, and preferences.
Methods
Two international, cross-sectional, web-based surveys were conducted among participants of a phase 3 mirikizumab study (NCT03519945). The questions captured moderate-to-severe UC patients’ experience, HCPs’ perception of patients’ experience, and HCPs’ own experience with mirikizumab administration through intravenous (IV) infusions and subcutaneous (SC) injections.
Results
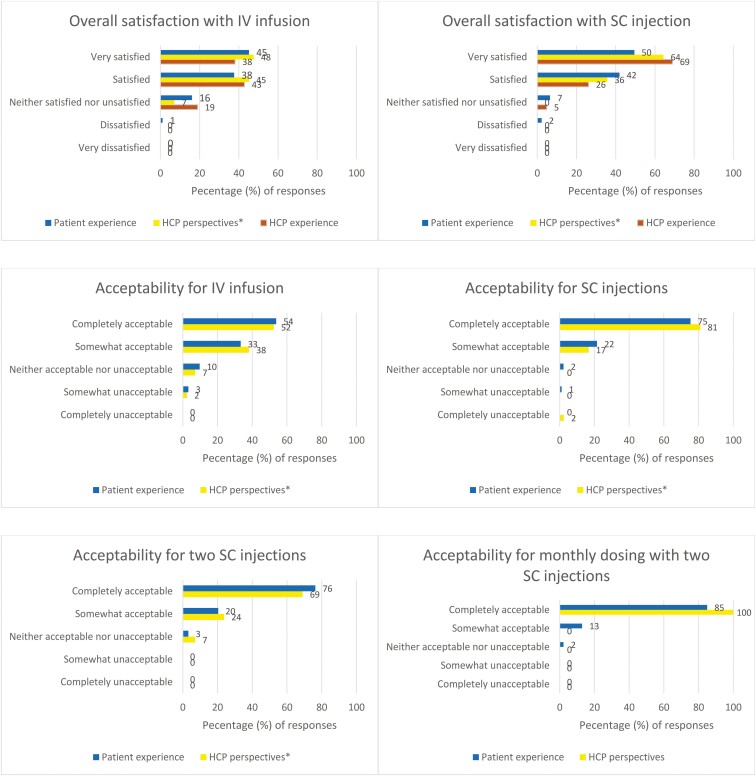
Respondents included 93 patients and 42 HCPs from 11 countries. The majority of patients had UC >4 years (74.2%), were bionaive (68%), in remission at the time of the survey (63%). HCPs were primarily from the United States (57%), generally nurses (41%) or gastroenterologists (26%) with ≥6 years of experience in treating UC (57%). Most patients were “very satisfied/satisfied” (IV, 83%; SC, 91%), “completely/somewhat” accepting of mirikizumab administration (IV, 87%; SC, 97%), and agreed that improvement to their UC outweighed any administration dissatisfaction (90%). HCPs’ perspectives of patients’ experiences were higher: “very satisfied/satisfied” (IV, 93%; SC, 100%); “completely/somewhat” accepting (IV, 90%; SC, 98%). HCPs themselves were “very satisfied/satisfied” (IV, 81%; SC, 95%); gastroenterologists were “very satisfied” (IV, 82%; SC, 82%) more than nurses (IV, 29%; SC, 65%) who were generally at least “satisfied” (IV, 53%; SC, 35%). Two SC and monthly SC injections were “completely acceptable” by the patients (76% and 85%) and per HCPs’ perceptions of patients’ preferences (69% and 100%).
Conclusions
Both patients and HCPs were satisfied with and accepted mirikizumab IV induction followed by monthly maintenance SC injections. UC improvement outweighed any administration dissatisfaction.
Keywords: administration, dosing, injection, infusion, mirikizumab
Introduction
Ulcerative colitis (UC) therapy options have significantly advanced during the past decades.1 A large variety of treatment alternatives are available in different formulations, with different routes and frequencies of administration.2–5 These include conventional therapy with aminosalicylates, glucocorticoids, immunomodulators, and TNF-α inhibitors as well as the most recent biologics (IL-12/23, IL-23, and integrin inhibitors) and small molecules (Janus kinase inhibitors and sphingosine-1-phosphate receptor agonists) that have improved the management of patients with UC because they addressed specific pathogenetic mechanisms.4–8
However, many patients do not respond to induction therapy or lose response during the maintenance treatment period.9–11 Moreover, some of these targeted therapies may not be suitable for a specific patient due to their medical history or medication risk profile.2,6,9,12,13 Consequently, finding more effective medications is still a therapeutic need that has not been satisfied.14
Mirikizumab is a humanized IgG4 monoclonal antibody directed against the p19 subunit of IL-23, a signaling cytokine involved in the inflammatory cascade associated with UC.15–17 Mirikizumab was recently approved for the treatment of moderately-to-severely active UC. It is administered intravenously (IV) during induction and subcutaneously (SC) during maintenance.18,19 Mirikizumab demonstrated significant clinical remission, compared to placebo, at week 12 of the induction trial (24.2% vs 13.3%, P < 0.001) and week 40 of the maintenance trial after 52 weeks of continuous treatment (49.9% vs 25.1%, P < 0.001), with an acceptable safety profile associated with a positive benefit-risk ratio.20
Despite the many years of rigorous clinical research on novel medications, there is little evidence of UC treatment administration preferences and associated satisfaction with treatment administration. Such information is important for making educated treatment decisions, particularly when there are multiple alternatives available.1 Moreover, perceptual differences between patients and their treating physicians may result in patients having suboptimal treatment.21,22
To understand mirikizumab treatment administration satisfaction, acceptability, and preferences, 2 surveys were conducted: (1) a patient survey among patients with UC receiving mirikizumab and (2) a healthcare provider (HCP) survey to capture their perspectives on their patients’ experiences as well as their own experiences. The study examined the differences in perceptions about treatment administration between patients and HCPs, explored differences between patients based on key clinical history and sociodemographic differences, and explored patient and HCP beliefs regarding benefit versus mirikizumab administration burden.
Methods
Study Design
This study involved 2 international, cross-sectional, non-interventional, web-based one-time surveys among patients with moderately-to-severely active UC and their treating HCPs. The optional surveys were included as a protocol addendum substudy to LUCENT-3 (AMAP), a phase 3, multicenter, open-label, 160-week, long-term extension study evaluating the efficacy and safety of mirikizumab (NCT03519945).20 All eligible patients and HCPs were offered the opportunity to complete the surveys, however, it was not a LUCENT-3 study protocol requirement.
The patient survey evaluated patients’ experiences with mirikizumab administration. The HCP survey assessed HCPs’ perceptions of patients’ experiences as well as the HCPs’ own experiences. Both surveys were administered from October 2022 to July 2023 and took approximately 30 minutes to complete.
Participants
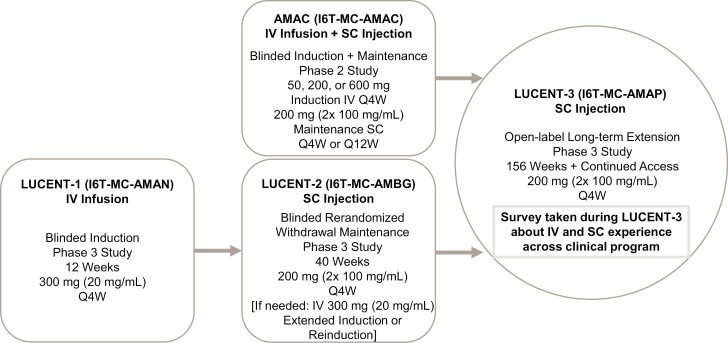
Participants in the LUCENT-3 study were recruited from among the completers of 2 previous multicenter, double-blind, placebo-controlled mirikizumab studies in patients with moderately-to-severely active UC who were allowed to have prior exposure to a biological agent: (1) the phase 2 induction and maintenance study (AMAC, NCT02589665),18,23 and (2) the phase 3 withdrawal maintenance study (LUCENT-2 [AMBG], NCT03524092) in which the enrolled patients had completed a previous induction phase 3 study (LUCENT-1 [AMAN], NCT03518086; Figure 1). Mirikizumab was administered during induction as a 300 mg (20 mg/mL) intravenous (IV) infusion once every 4 weeks for 3 total infusions.18 During maintenance, mirikizumab was administered as a 200 mg subcutaneous (SC) injection delivered in 2 consecutive 100 mg/mL injections every 4 weeks (Figures 2 and 3).
Figure 1.
Patient flow and mirikizumab administration experience across the LUCENT clinical program. Abbreviations: IV, intravenous; Q4W, every 4 weeks; Q12W, every 12 weeks; SC, subcutaneous; UC, ulcerative colitis.
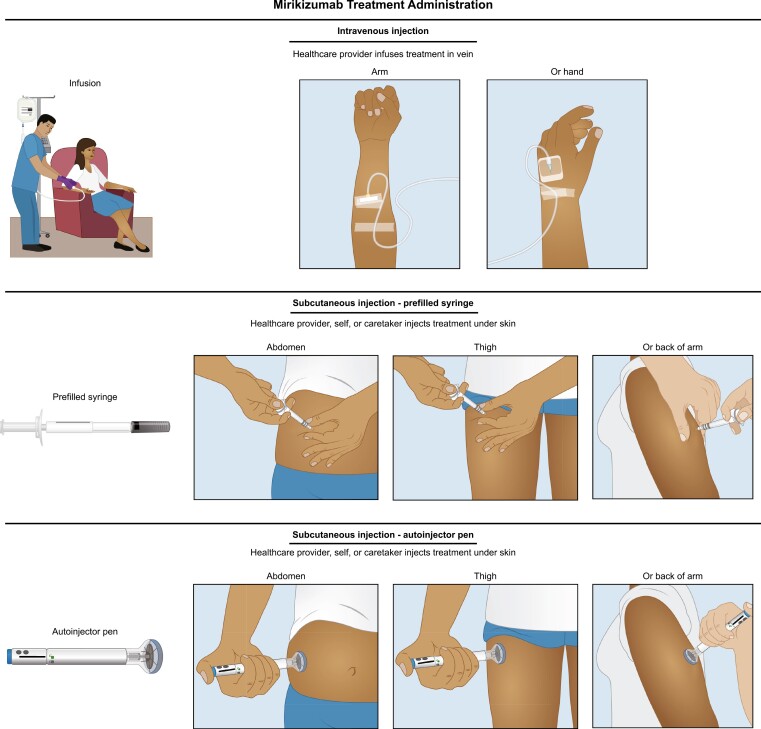
Figure 2.
Illustrations for mirikizumab administration as intravenous infusion or subcutaneous injection. Infusion: 300 mg (20 mg/mL). Prefilled syringe or autoinjector pen (200 mg [2× 100 mg/mL]).
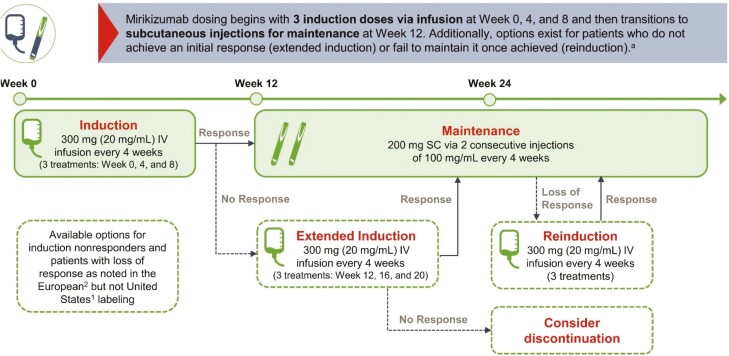
Figure 3.
Mirikizumab dosing and administration. Mirikizumab IV infusion occurs over at least 30 minutes. Patients should be monitored for at least 1 hour after dosing, according to the local standard of care. Abbreviations: IV, intravenous; SC, subcutaneous. aSource: Mirikizumab summary of product characteristics.19
Patients could participate in the survey if they gave written informed consent, were actively enrolled and participating in LUCENT-3, were willing and able to complete the web-based survey, were able to read and enter digital responses to complete the survey, and had access to the internet and a computer, tablet, or mobile device.
HCPs could participate in the survey if they were physicians, nurses, or other site support staff responsible for administering or overseeing administration of mirikizumab via IV infusion, SC injection, or both in AMAC, LUCENT-1, LUCENT-2, or LUCENT-3 studies for at least 6 months combined, and also met the criteria noted for patients.
Survey Structure and Content
The survey questions were designed to capture satisfaction with the mode of administration (IV and SC), acceptability of IV and SC, preferences for SC, and overall administration burden versus treatment satisfaction. Patients and HCPs were asked to answer the questions based on their holistic experiences across their participation in the clinical development program. Because IV infusions were not administered in LUCENT-3, patients and HCPs were asked to recall their experiences with IV infusions, administered every 4 weeks, in the induction periods of the LUCENT-3 parent studies. The SC injections’ location and device were determined by study protocols across the induction, maintenance, and extension studies. Patients were able to choose to self-inject only after they had completed 7 months in LUCENT-3; until that point, all SC injections were administered by the site staff (HCPs). Moreover, in the survey, the patients were asked to rank the SC injection options—HCP, self-injection, and caregiver—that they experienced during the study; if patients experienced only one method, this was ranked first, and if they experienced all 3 options, they were asked to rank them in order of preference.
Most survey questions were multiple-choice, with responses based on 5-point Likert-type scales. Some questions included ranked response options (e.g., patients’ most and least preferred location to receive an SC injection) or a list of relevant response options. The survey was originally developed in English and then translated for each country. The surveys were pretested with 3 patients and 2 HCPs and revised based on their feedback (Supplementary Material, Survey Instrument Development).
The patient survey (Supplementary Material, Patient survey) consisted of 28 closed-ended questions about participants satisfaction, acceptability, and preferences with different methods of study medication administration (SC or IV) as well as their satisfaction with overall treatment administration and injection device (prefilled syringe or autoinjector pen) usability. The survey did not contain questions on patients’ demographic information and their clinical characteristics, as these data were captured as part of the clinical program.
The HCP survey (Supplementary Material, HCP survey) consisted of 32 closed-ended questions about the HCP’s perception regarding their patients’ treatment satisfaction, acceptability, preferences (Q1-Q25; Supplementary Material, HCP survey), and their personal experience administering the study medication (Q26-Q32; Supplementary Material, HCP survey). At the beginning of the HCP survey, 8 questions captured the HCPs’ clinical background, including their specialty, their primary study role, the number of years they have provided care to patients with UC (excluding medical training), their primary practice setting, the percentage of working time spent actively interacting with patients (including patients with UC), their HCP experience with study medication administration methods, the number of infusions or injections they had administered or overseen during the mirikizumab clinical development program, and their country of origin.
Statistical Methods
Descriptive statistics were applied and presented for both patients’ and HCPs’ responses and were stratified by subgroups. The subgroup analyses were detailed and extended within the patient (Supplementary Table S1) and HCP (Supplementary Table S2) groups of survey participants. For all descriptive analyses, frequency distributions and cross-tabulations were constructed to evaluate and characterize the distribution properties of each variable assessed in the surveys. Chi-square tests were used to evaluate differences between the subgroups for categorical data when specified. Analysis of variance was used to evaluate the differences between subgroups for continuous data when specified. All statistical tests were 2-tailed and were conducted with a type I error probability fixed at 0.05. No formal hypotheses were being tested, and, therefore, no multiplicity adjustment was performed. All data entered by patients and HCPs on the web-based survey platform was provided as a clean, deidentified, fully documented dataset. For subgroup analyses, survey variables were merged with the variables from the clinical trial, including baseline demographics and patient characteristics. The analysis was performed using the SAS Enterprise Guide 7.15 HF6 (SAS Institute Inc., Cary, NC).
Ethical Considerations
Country-specific and, where applicable, site-specific institutional review board (IRB) approval was obtained prior to any data collection efforts. All participants provided written (patients) or electronic (HCPs) informed consent. Data collection complied with ISO 27001 and the European Union Data Protection Directive 95/46/EC.
Results
There were 316 patients who completed the LUCENT-2 maintenance study and entered the LUCENT-3 extension study and an estimated 183 HCPs (investigators and study coordinators) involved in treatment administration participating in the LUCENT-3 study addendum from the 11 of 35 total participating countries (Australia, Austria, Czech Republic, Germany, Hungary, Japan, Mexico, Poland, Spain, Switzerland, and United States) that were invited to voluntarily respond to the survey (Table 1). Of these, survey respondents included 93 patients and 42 HCPs.
Table 1.
Patient characteristics, n = 93.
| Age, mean years (SD) | 43 (11.8) |
|---|---|
| Male sex, n (%) | 51 (55) |
| Region, n (%) | |
| Europe | 41 (44) |
| North America | 25 (27) |
| Asia | 20 (22) |
| Central or South America | 5 (5) |
| Rest of the world | 2 (2) |
| Race, n (%) | |
| White | 65 (70) |
| Asian | 21 (23) |
| American Indian or Alaska Native | 5 (5) |
| Black or African American | 2 (2) |
| Disease duration ≥ 4 years, n (%) | 69 (74) |
| Prior bionaïve before entering mirikizumab program, n (%) | 63 (68) |
| Corticosteroid or immunomodulator use, n (%) | 57 (61) |
| Symptomatic remissiona achieved prior to entering LUCENT-3, n (%) | 59 (63) |
| MMS Score,bn (%) | |
| Moderate (MMS = 4-6) | 47 (51) |
| Severe (MMS > 6) | 43 (46) |
| Missing | 3 (3) |
| Mirikizumab SC injection experience through time of survey, n (%) | |
| >2 years to ≤3 years | 32 (34) |
| >3 years | 40 (43) |
| Missing | 21 (23) |
| Mirikizumab IV infusion experience through time of survey, n (%) | |
| 3 IVs (12 weeks) | 47 (51%) |
| 6 IVs (24 weeks) | 32 (34) |
| Missing | 14 (15) |
| Mirikizumab self-injection experience through time of survey, n (%) | 41 (44) |
| Fear of needles prior to taking study medication, n (%) | |
| Not at all afraid | 57 (61) |
| A little afraid | 23 (25) |
| Moderately afraid | 8 (9) |
| Very afraid | 2 (2) |
| Extremely afraid | 3 (3) |
aSymptomatic remission is based on Modified Mayo Score Stool Frequency (SF) and Rectal Bleeding (RB) components: SF = 0 or SF = 1 with a 1-point decrease in MMS from baseline; RB = 0.
bUlcerative colitis severity subgroups were defined by the Modified Mayo Score: Moderate (MMS = 4-6) and Severe (MMS > 6).
Abbreviations: IV, intravenous; MMS, Modified Mayo Score; SC, subcutaneous; SD, standard deviation.
The subinvestigators may perform the endoscopic evaluations or fill in occasionally but they are more like the other partners in the practice referring patients and not the target population for this substudy.
Based upon the induction study baseline, 3 in 4 patients (69; 74%) had UC for more than 4 years. For 68% of patients, mirikizumab was their first biologic therapy, and 61% were receiving corticosteroids or immunomodulators (Table 1). Nearly all (97%) had either moderate or severe UC as indicated with modified Mayo Scores ≥4. Based upon the last visit with symptomatic remission captured before entering LUCENT-3, the majority of patients (63%) had achieved symptomatic remission when they entered LUCENT-3. The survey was taken during LUCENT-3. At the time of the survey, patients were generally experienced in receiving injections, given that 85% had received at least 3 IV infusions and 77% had received mirikizumab SC injections for at least 2 years. As expected, based on the clinical study design, experience with self-injections was limited (44%). The majority of patients who took the survey did not report any fear of needles at the clinical study baseline (61%; Table 1).
The HCPs who responded to the survey were primarily from the United States (57%) and most frequently nurses (41%) or gastroenterologists (26%; Table 2). Most HCPs were experienced (≥6 years) in treating patients with UC (57%) and provided primarily office-based patient care (71%; Table 2). When asked about time spent actively seeing patients, HCPs most frequently reported 26%-50% (n = 15, 36%) or 76%-100% (n = 13, 31%).
Table 2.
HCP characteristics, n = 42.
| Type of HCP, n (%) | |
|---|---|
| Nursea | 17 (41) |
| Gastroenterologistb | 11 (26) |
| Internal medicine or other physician | 2 (5) |
| Other | 12 (29) |
| Years providing care to patients with UC (excluding medical training), n (%) | |
| 0-5 years | 18 (43) |
| 6-10 years | 8 (19) |
| 11-15 years | 6 (14) |
| 16-20 years | 7 (17) |
| ≥21 years | 3 (7) |
| Primary medical practice setting providing care to patients, n (%) | |
| Office-based | 30 (71) |
| Hospital-based (non-university) | 7 (17) |
| University-based | 5 (12) |
| Percentage of working time spent actively seeing patients, n (%) | |
| 0-25% | 4 (10) |
| 26%-50% | 15 (36) |
| 51%-75% | 10 (24) |
| 76%-100% | 13 (31) |
| Country, n (%) | |
| United States | 24 (57) |
| Australia | 4 (10) |
| Switzerland | 4 (10) |
| Hungary | 3 (7) |
| Japan | 2 (5) |
| Poland | 2 (5) |
| Austria | 1 (2) |
| Germany | 1 (2) |
| Spain | 1 (2) |
aIncludes nurses, nurse practitioners, or other nurse specialists.
bIncludes gastroenterologists or physicians with gastroenterology specialty.
Abbreviations: HCP, healthcare provider; UC, ulcerative colitis.
Patients Survey Responses
IV Infusions
Satisfaction overall and by subgroups
Patients were generally either “very satisfied” or “satisfied” with the administration of IV infusion (n = 77, 83%; Figure 4). Only one patient reported being “dissatisfied” with IV infusions, and none of the patients reported being “very dissatisfied.” Subgroup findings of interest are:
Figure 4.
Satisfaction and acceptability rates associated with mirikizumab IV infusions and SC injections by patients’ actual experience (n = 93), HCP perspectives of patients’ experiences, and HCP own experiences (n = 42). Abbreviations: HCP, healthcare professionals; IV, intravenous; SC, subcutaneous. *HCPs’ perspectives of patients’ experiences.
Women were more often “very satisfied” or “satisfied” (n = 39, 93%) than men (n = 38, 76%); only a few women (n = 3, 7%) were neither satisfied nor dissatisfied, as opposed to one in every 4 men (n = 12, 24%; p = 0.0159).
Most patients were “very satisfied” or “satisfied” with IV regardless of their previous experience with corticosteroids or immunomodulators (n = 51, 89% vs n = 26, 72%; p = 0.1104).
Similarly, most patients were “satisfied” or “very satisfied” with IV regardless of their experience with biologics (n = 48, 84% vs n = 21, 81%; p = 0.4216).
Satisfaction with IV infusion did not change over time for most patients (n=69, 74%). Of patients who reported satisfaction improvement over time (n=17, 18%), this change was more frequent among patients with <4 years’ experience with UC (n=9, 38%) than patients with >4 years’ experience (n = 8, 12%; p = 0.0374).
Asian patients were less likely to be “very satisfied” (n = 4, 19%), a trend in the data that should be interpreted with caution due to being underpowered.
With similar caution, patients were more likely to be “very satisfied” if they had Inflammatory Bowel Disease Questionnaire (IBDQ) response (≥16 improvement from baseline24–26; n = 37, 49%); had severe UC at induction baseline (n = 23, 54%); had self-injection experience (n = 21, 51%); had achieved symptomatic remission (n = 30, 51%); were not afraid of needles (n = 30, 53%). Younger (<40 years) patients (n = 20, 67%) chose “scheduling the IV infusions was easy” as a reason for satisfaction more than older (≥40 years) patients (n = 23, 45%).
Acceptability overall and by subgroups
To the question about the acceptability of receiving medication through IV infusion, the most frequent responses were “completely acceptable” or “somewhat acceptable” (n = 81, 87%; Figure 4). Subgroup findings of interest are:
Patients who found IV administration of study medication completely acceptable were older (≥40 years old; n = 33, 59%), men (n = 29, 57%), who had not experienced prior biologic failure (n = 35, 61%), were bionaïve (n = 35, 61%), had UC for <4 years (n = 18, 75%), had more severe UC (n = 26, 61%), had achieved IBDQ remission (IBDQ score ≥ 17024–26; n = 40, 56%), had achieved symptomatic remission in parent studies (n = 34, 58%), and had no fear of needles (n = 32, 56%). However, these subgroup comparisons were not statistically significant except for the comparison by disease duration (<4 years vs ≥4 years; p = 0.0467).
When examining reasoning for the responses of “completely” or “somewhat acceptable” by subgroups, there were a few response option trends that were clear. Nearly all patients with higher disease severity (ie, Modified Mayo Score [MMS] score > 6; n = 35, 92%) and most patients with moderate disease severity (MMS score 4-6; n = 35, 83%) at baseline of the parent induction studies selected “I feel it helped my UC.”
Women answered “it allowed me to interact with HCPs” more often than men (n = 17, 45% vs n = 10, 23%; p = 0.0407).
Patients with <4-year experience with UC more frequently selected the “length of time while receiving IV infusion was acceptable” than patients with ≥4-year experience with UC (n = 15, 65% vs n = 21, 36%; p = 0.0178).
SC Injections
Satisfaction overall and by subgroups
Patients were generally either “very satisfied” or “satisfied” with SC injections (n = 85, 91%; Figure 4). Two patients were dissatisfied with SC injections; no one was very dissatisfied. Subgroup findings of interest are:
Although subgroup comparison differences did not meet statistical significance, patients who were most frequently “very satisfied” were bionaïve (n = 32, 56%), had achieved IBDQ remission (n = 37, 51%), had moderate disease severity at baseline (n = 28, 60%), had achieved symptomatic remission at the end of the maintenance study LUCENT-3 (n = 31, 53%), and did not have a fear of needles (n = 31, 54%).
Some patients reported their satisfaction improved over time (n = 17, 18%). This change was statistically significant by disease duration (33% <4 years vs 13% ≥4 years; p = 0.0121), IBDQ remission (74% yes vs 53% no; p = 0.0035), symptomatic remission achieved at the end of the maintenance study LUCENT-3 (76% yes vs 57% no; p = 0.0195).
Acceptability overall and by subgroups
Nearly all patients (n = 90, 97%) found SC injections “completely” or “somewhat acceptable” (Figure 4). Two patients found SC injection “neither acceptable nor unacceptable” and one patient found it “somewhat unacceptable.” When asked about the acceptability of receiving 2 SC injections, most patients found it “completely acceptable” (n = 71, 76%) or “somewhat acceptable” (n = 19, 20%). Only 3 patients selected “neither acceptable nor unacceptable” (3%; Figure 4). The patients were then asked about acceptability of receiving monthly SC injections, and most selected “completely acceptable” (n = 79, 85%). Fewer patients selected “somewhat acceptable” (n = 12, 13%) or “neither acceptable nor unacceptable” (n = 2, 2%; Figure 4).
Subgroup findings of interest are:
Acceptability of monthly SC injections expressed as “completely acceptable” increased with years of injection experience from 75% (n = 24) among those with 2-3 years of experience to 93% (n = 37) among those with >3 years of experience (p = 0.0354).
Asian patients favored the “somewhat acceptable” (n = 11, 52%) response rather than “completely acceptable” (n = 9, 43%) that was favored by the patients from other geographic regions (p = 0.0097).
Although comparisons were not statistically significant, those who believed that SC injections were “completely acceptable” were older (≥40 years old; n = 46, 82%), men (n = 39, 77%), did not experience prior biologic failure (n = 43, 75%), were bionaïve (n = 43, 75%), had UC for <4 years (n = 22, 92%), had moderate disease severity (n = 37, 79%), had achieved IBDQ remission (n = 56, 78%), had achieved symptomatic remission before entering the LUCENT-3 study (n = 48, 81%), and had no fear of needles (n = 46, 81%).
Administration Options and Preferences
Patients most frequently preferred to receive their SC injections from HCPs (n=78, 84%), followed by self-injection (n=41, 44%), and caregivers (n=8, 9%); however, for patients with self-injection experience, self-injection was the preference. Patients’ injection-site preferences were abdomen (n = 72, 77%), followed by the back of the upper arm (n = 32, 34%), and the thigh (n = 8, 9%).
The patients were then asked to rank by preference the administration options that they experienced during their clinical trial participation. The ranking data should be interpreted with caution because some patients might not have experienced all options. Those patients who only experienced receiving the injection from an HCP ranked HCPs as either first (n = 56, 72%) or third (n = 22, 28%) preferred option. However, when patients also had self-injection experience, they ranked this option as their first (n = 31, 76%), third (n = 9, 22%), or second (n = 1, 2%) preference. Younger patients (n = 15, 83%) were more likely to rank self-injection as their first preference. Almost all of those who experienced receiving the injection from a caregiver, ranked caregiver as their first (n = 6, 75%) preferred option. For patients who found SC injections acceptable, the reason most patients selected was “I feel it helped my UC” (n = 87; 97%); “I liked the self-injection option” (n = 18; 51%), and “I liked having the option to receive the injections at home” (n = 19; 54%) were chosen more by younger UC patients.
Most patients reported they “strongly agree” when asked about the convenience of using the self-injection device (n = 24; 59%). Thirteen patients reported “agree” (n = 13; 32%), while only one patient reported “neither agree nor disagree” (n = 1; 2%) and 3 patients reported “disagree” (n = 3; 7%). Moreover, when asked whether they found the device “easy to use,” most patients selected “strongly agree” (n = 25; 61%) and “agree” (n = 14; 34%). The patients responded “strongly agree” (n = 34; 83%) and “agree” (n = 7; 17%) when the patients were asked whether they understood instructions to self-inject. Similarly, they responded “strongly agree” (n = 32; 78%) and “agree” (n = 7; 17%) when asked whether they understood instructions to store the medication at home.
Overall, the most reported strategy used by patients to help with SC injection administration was to “let study medication warm to room temperature before injecting” (n = 60; 65%), followed by “pinch skin and squeeze while injecting” (n = 47; 51%). Nineteen patients (20%) did not use any strategies to ease the administration burden.
Treatment Satisfaction and Administration Burden
Nearly all patients indicated that they were “very satisfied or satisfied” (98%) with the overall study medication administration (Table 3). Similarly, most patients (n = 88, 95%) expressed complete acceptability to the medication’s administration (Table 3). Subgroup findings of interest are as follows:
Table 3.
Overall experience with administration of study medication.
| Patients’ experiences (N = 93) | HCP perspectives of patients’ experiences (N = 42) | HCP own experiences (N = 42) | |
|---|---|---|---|
| Improvement in UC outweighed any dissatisfaction with the administration of mirikizumab, n (%) | |||
| Strongly agree | 67 (72) | 20 (48) | 26 (62) |
| Agree | 17 (18) | 17 (41) | 14 (33) |
| Neither agree nor disagree | 4 (4) | 5 (12) | 1 (2) |
| Disagree | 4 (4) | 0 (0) | 1 (2) |
| Strongly disagree | 1 (1) | 0 (0) | 0 (0) |
| Overall satisfaction with mirikizumab, n (%) | |||
| Very satisfied | 71 (76) | 27 (64) | 28 (67) |
| Satisfied | 20 (22) | 13 (31) | 13 (31) |
| Neither satisfied nor unsatisfied | 2 (2) | 1 (2) | 1 (2.4) |
| Unsatisfied | 0 (0) | 1 (2) | 0 (0) |
| Very unsatisfied | 0 (0) | 0 (0) | 0 (0) |
| Overall acceptability of administration of mirikizumab, n (%) | |||
| Completely acceptable | 88 (95) | 39 (93) | NA |
| Somewhat acceptable | 3 (3) | 2 (5) | NA |
| Neither acceptable nor unacceptable | 1 (1) | 1 (2) | NA |
| Somewhat unacceptable | 1 (1) | 0 (0) | NA |
| Completely unacceptable | 0 (0) | 0 (0) | NA |
| Recommend mirikizumab to someone with UC, n (%) | |||
| Strongly agree | 69 (74) | 28 (67) | NA |
| Agree | 21 (23) | 13 (31) | NA |
| Neither agree nor disagree | 2 (2) | 1 (2) | NA |
| Disagree | 1 (1) | 0 (0) | NA |
| Strongly disagree | 0 (0) | 0 (0) | NA |
| Found SC self-injection device easy to use, n (%) | |||
| Strongly agree | 25 (61) | 23 (55) | 32 (76) |
| Agree | 14 (34) | 9 (21) | 5 (12) |
| Neither agree nor disagree | 1 (2) | 8 (19) | 3 (7) |
| Disagree | 1 (2) | 2 (5) | 2 (5) |
Abbreviations: HCP, healthcare provider; NA, not applicable; SC, subcutaneous; UC, ulcerative colitis.
Overall satisfaction was statistically significantly higher among patients who had an IBDQ response (100% vs 86%; p = 0.0054).
Complete acceptability was expressed more frequently among older patients (aged ≥ 40 years) than among younger patients (98% vs 89%, p = 0.0369).
With regards to the potential administration burden, almost all patients (90%) “agreed” or “strongly agreed” that the improvement in their UC outweighed any dissatisfaction they may have had with the administration of the medication (Table 3). These responses were more frequent among patients who achieved IBDQ response (92% vs 79% without response; p = 0.0287) and achieved symptomatic remission (93% vs 83% without remission; p = 0.0207) at the end of the maintenance study before entering the LUCENT-3 study when the survey was administered.
Finally, almost all (97%) patients responded that they would recommend mirikizumab to someone with UC (Table 3). The willingness to recommend mirikizumab was stronger (ie, “strongly agree” answers) among non-Asian patients (n = 62, 85%) than among Asian patients (n = 7, 35%; p = 0.0009 vs other regions).
HCPs Survey Responses
IV Infusions
HCPs perspectives of patients’ experiences
HCPs believed that patients would be “very satisfied” or “satisfied” (n = 39, 93%) with IV infusions, and no one thought that there would be any dissatisfied patient (Figure 4). Subgroup findings of interest are as follows:
HCP perspective of patient overall satisfaction with administration of IV infusion was statistically significant by HCP type (p = 0.0048), years providing UC care (p = 0.0027), and average IV infusion experience (p = 0.0192).
When looking at HCP type, we found that all gastroenterologists (n=11, 100%) and nearly all nurses (n=16, 94%) believed that their patients were “very satisfied” or “satisfied”; however, the physicians’ answers were grouped within “very satisfied” (n=10), while nurses’ responses were more evenly divided between “very satisfied” (n = 7) and “satisfied” (n = 9).
Despite this, the proportion of HCPs thinking patients were “very satisfied” or “satisfied” was similar between HCP types. However, some HCPs were potentially overestimating their patients’ satisfaction from “satisfied” to “very satisfied” compared to the patients’ actual satisfaction.
HCPs with ≥16 years of providing UC care were more likely to believe that their patients were “very satisfied” (n = 9, 90%) with IV infusion compared with those who had <5 years of experience (n = 2, 11%; p = 0.0027).
Similarly, HCPs who had provided >30 IV infusions (n = 12, 75%) were more likely to believe patients were “very satisfied” with IV infusion than HCPs who had administered <10 infusions (n = 4, 36%: p = 0.0192).
Most HCPs said that patients’ satisfaction with the administration of IV infusion “did not change over time” (n=32, 76%). Seven HCPs said patients’ satisfaction “improved over time” mostly after the second or third infusion (n = 6) while one HCP believed that change occurred already after the first infusion.
Regarding acceptability, HCPs reported that they thought patients found it “completely” or “somewhat acceptable” (n = 38, 90%) to receive the IV infusion (Figure 4). Subgroup findings of interest are as follows:
Gastroenterologists were more likely to select “completely acceptable” than nurses (n = 11, 100% vs n = 7, 41%; p = 0.0008).
HCPs in Western Europe did not select “completely acceptable” at all and were the most likely to select “somewhat acceptable” (n = 6, 86%; p = 0.0296 vs among geographical regions).
HCPs believed that the main reason for acceptability was that the treatment helped patients with their UC (n = 33, 87%).
HCPs own experiences
HCPs themselves were mainly “very satisfied” (n = 16, 38%) or “satisfied” (n = 18, 43%) with IV infusions. Few HCPs (n = 8, 19%) were “neither satisfied or unsatisfied” and no one was unsatisfied or very unsatisfied (Figure 4). The gastroenterologists were mostly “very satisfied” (n = 9, 82%) while the nurses were mostly “satisfied” (n = 9, 53%) and to a lesser extent “very satisfied” (n = 5, 29%).
SC Injections
HCPs perspectives of patients’ experiences
When asked about their perspective on patients’ overall satisfaction with the administration of SC injections, the HCPs believed that their patients were “very satisfied” (n = 27, 64%) or “satisfied” (n = 15, 36%; Figure 4); gastroenterologists preferentially selected “very satisfied” (n = 9, 82%) while nurses distributed their responses between “very satisfied” (n = 10, 59%) and “satisfied” (n = 7, 41%). Most HCPs (n = 31, 74%) believed that patients’ satisfaction did not change over time. However, a few HCPs (n = 9, 21%) said their patients’ satisfaction improved over time.
Overall, most HCPs perceived their patients found it “completely acceptable” (n = 34, 81%) or “somewhat acceptable” (n = 7, 17%) to receive study medication via SC injection. Most HCPs also believed that patients accepted SC injections mainly because they helped their UC (n = 38, 93%) and that it was easy to schedule the injections (n = 29, 71%).
HCPs perspective on patient acceptability of receiving 2 SC injections (“completely acceptable”: n = 29, 69%; “somewhat acceptable”: n = 10, 24%) were lower than patients’ actual experience (Figure 4). Regarding the monthly dosing of 2 SC injections, all HCPs believed that their patients found it “completely acceptable” (n = 42, 100%; Figure 4).
HCPs own experiences
HCPs themselves were mainly “very satisfied” (n = 29, 69%) with SC injections. Some were “satisfied” (n = 11, 26%), only a few (n = 2, 5%) were “neither satisfied or unsatisfied” and no one was unsatisfied or very unsatisfied (Figure 4). The gastroenterologists were mostly “very satisfied” (n = 9, 82%) while the nurses were mostly “satisfied” (n = 11, 65%) and to a lesser extent “very satisfied” (n = 6, 35%).
Administration Options and Preferences
HCPs perspectives of patients’ experiences
HCPs were asked to provide a ranking for all administration options. They were asked to rank what they believed were patient preferences for who (self, caregiver, or HCP) administered the SC injections. When looking at the total sample, HCPs most frequently chose “HCP” (n = 29, 69%) followed by “caregiver” (n = 22, 52%), and “self-administration” (n = 20, 48%). Based on these results, HCPs believed that patients preferred the HCP to give the injection, while patients who had performed self-injections primarily preferred the “self-injection” option, suggesting an HCP-patient disconnect on the topic of preference for who should do the injections.
HCPs were also asked to rank what they believe were the patients’ preferences for the location (abdomen, thigh, and back of upper arm) of SC injections. As the first preferred option, they ranked “abdomen” (n = 26, 62%), which was aligned with the patients ranking. As the second preferred option, they reported “thigh” (n = 16, 38%) and “abdomen” (n = 14, 33%). As the third preferred option, they reported “thigh” (n = 24, 57%) and “back of upper arm” (n = 16, 38%).
HCPs own experiences
When HCPs were asked to rank their own preferences for the preferred administration method of SC injections, as the first preferred option, they most frequently ranked “SC injection, administered by the patient” (n = 18, 43%); as the second preferred option, they ranked “SC injection, administered by HCP” (n = 23, 55%); and, as the third preferred option, they ranked “SC injection, administered by caregiver” (n = 20, 48%).
Treatment Satisfaction and Administration Burden
HCPs perspectives of patients’ experiences
Most HCPs believed that patients were “very satisfied” or “satisfied” with the administration of the study medication (95%) and considered it “completely” or “somewhat acceptable” (98%; Table 3). In the opinion of the HCPs, patients believed that the therapeutic advantages of the medication outweighed any dissatisfaction they had with its administration (88% “agree” and “strongly agree”; Table 3). This opinion was expressed by all gastroenterologists (n = 11, 100%) but not by all nurses (n = 9, 75%; p=0.0746). In concordance with patients’ experiences, almost all HCPs (98%) agreed that their patients would recommend the study medication to someone with UC (Table 3).
Regarding HCP perspectives of patients’ beliefs of injector usability, patients responded that injector device was convenient (90% “strongly agree” and “agree”) and easy to use (95% “strongly agree” and “agree”) more than HCPs believed they would (79% and 76% respectively). Patients also responded that the injector device instructions were understood (100% “strongly agree” and “agree”) more than HCPs (91%) believed they would. Patients (54%) used injection instruction documents less than HCPs (95%) believed they did. Patients felt responses from their HCPs to their questions were more helpful (100%) than HCPs (94%) believed they were. Patients felt more confident they were using the injection device correctly (98%) and getting a full dose (100%) than HCPs (81% and 86%, respectively) believed they were.
HCPs own experiences
HCPs responses about their own opinions showed that most HCPs were “satisfied” or “very satisfied” with IV infusions (n = 34, 81%), SC injections (n = 40, 95%), and with overall mirikizumab treatment (n = 41, 98%). Most HCPs (n = 40, 95%) “agreed” or “strongly agreed” that the treatment benefits of mirikizumab outweighed any dissatisfaction with its administration (Table 3). Subgroup findings of interest are as follows:
Subgroup analysis revealed that gastroenterologists were “very satisfied” (n = 9, 82%) with IV infusions, whereas the nurses were “satisfied” (n = 9, 53%; p = 0.0153).
Similar differences, although not statistically significant, were seen in “strongly agree” responses that treatment benefits outweighed any dissatisfaction with the administration: 82% among gastroenterologists (n = 9) vs 53% among nurses (n = 9), p = 0.2186.
Lastly, HCPs with longer experience providing care to patients with UC (≥16 years) were more frequently very satisfied (n = 9, 90%) with SC injections than the HCPs with less experience (n = 13, 72%) although this was not a statistically significant difference (p = 0.2090).
Regarding strategies recommended by HCPs to patients to ease the administration burden, only 79% of HCPs selected warm medication before injecting, despite the label recommending doing. Moreover, this suggestion was more frequently given by nurses than gastroenterologists (82% vs 73%; p = 0.0494). Choosing a different injection location was recommended by 69% of HCPs, but only 9% of patients noted choosing a different injection location as a method to ease the administration burden, suggesting an HCP-patient disconnect.
Discussion
This international web survey demonstrated that patients with moderately-to-severely active UC and their HCPs were predominantly satisfied and accepting of mirikizumab treatment administration, whether administered via IV infusion or SC injection including monthly SC dosing with 2 injections. Gastroenterologists were more likely than nurses to think patients were “very satisfied” with and “completely acceptable” of IV infusion and SC injections. HCPs overall were more likely to overestimate the proportion of patients with the highest degree of satisfaction or acceptance of IV infusions and SC injections. However, the overall satisfaction range responses (very satisfied + satisfied) and overall acceptability range responses (completely acceptable + acceptable) for IV infusion and SC injection were similar across patients’ experiences, HCPs perspectives of patients’ experiences, and HCPs’ personal experiences. HCPs and patients were closely aligned that the abdomen was the preferred site for injections. HCPs were less likely than patients to respond that patients fully understood administration instructions and indicate that the self-injection option was easy and convenient. There was also a disconnect between patients and HCPs regarding methods for helpfulness with SC injection in which HCPs recommended choosing a different injection site, but almost no patients selected this as an option.
Importantly, patients and HCPs believed that the benefits of mirikizumab therapy outweighed any potential burdens associated with the administration method. Patients were satisfied with and accepted both the IV and SC routes of administration because they felt mirikizumab treatment helped their UC. These results offer some insight into patients’ preferences regarding UC treatment attributes and the treatment administration burden they are willing to accept for therapeutic success. They suggest acceptance of the administration burden for therapies that offer clinically relevant therapeutic benefits. This is in alignment with previous studies showing that the administration route is of lesser concern to patients and their HCPs than treatment effectiveness and safety profile.27–31 For example, a conjoint analysis assessing biologics treatment preferences among patients with IBD showed that patients, either naïve or experienced in biologics, rated route of administration as the third important treatment characteristic after efficacy and safety. Another study on biologic naïve patients with moderate-to-severe UC found that patients primarily cared for long-lasting effectiveness, and they considered of “no real importance” or “completely irrelevant” the route of administration (25.3%) and the dosing frequency (32.3%).28 There are data available from 4 discrete choice experiments among patients with IBD that included route of administration as one of the treatment attributes assessed.29–32 Two of those studies found that the administration route was not among patients’ primary considerations.29,31 In the third study, some patients were willing to accept 10.3% (95% CI, 6.6%-14.0%) added risk to replace IV administration at a hospital with an injection at home.30 The most recently published study reported that patients with UC preferred oral or SC administration over IV (P < 0.001).32 The current data does suggest a similar patient preference for SC injection over IV infusion: satisfied or very satisfied (IV, 83%; SC, 91%) and completely acceptable or somewhat acceptable (IV, 87%; SC, 97%). Finally, in a structured interview with patients of a UK hospital with IBD, patients with prior biological therapy experience were more receptive to SC or IV therapies than bionaïve patients.33
For several survey questions, HCPs’ and patients’ responses were not aligned. For example, the HCP perspective of patient overall satisfaction with IV infusions was elevated compared with what was reported by patients. Similarly, HCPs believed that patients preferred the HCP to give the SC injections, whereas patients reported that they preferred to self-inject. In addition, the HCP survey demonstrated a disconnect between gastroenterologists and nurses. This was observed with patient satisfaction for IV infusion and SC injection, patient acceptability for IV infusion, and overall HCP satisfaction with the administration of study medication. While gastroenterologists’ responses tended to aggregate around “very satisfied” or “completely” acceptable,” nurses’ responses were divided between these 2 categories and were closer to patients’ responses. For example, gastroenterologists were “very satisfied” (IV, 82%; SC, 82%) more than nurses (IV, 29%; SC, 65%). This discrepancy appears to be influenced by the finding that nurses were less likely to respond that patients felt their treatment “helped my ulcerative colitis” (IV: gastroenterologist 100% vs nurse 81.3%; SC: gastroenterologist 100% vs nurse 87.5%). Nurses were also less likely to believe patients found the self-injection device easy to use (strongly agree: gastroenterologist 81.8% vs nurse 41.2%) or that patients felt confident using the SC self-injection device (strongly agree: gastroenterologist 81.8% vs nurse 58.8%). The similarity of nurses’ responses to patients’ responses was previously shown in a Spanish study assessing the satisfaction of patients with IBD with healthcare services received.34 Although not specific to treatment administration preferences like the current study, the Spanish study highlighted the essential role of nurses in the management of patients with IBD that brings them closer to patients and ultimately the understanding of their needs. A role has been suggested to involve them in IBD care management as patients’ educators.35
Considerations, Strengths, and Limitations
When interpreting these findings, the study’s limitations and strengths should be acknowledged. Strengths include that this web-based survey provides the first evidence regarding the level of patients’ and HCPs’ satisfaction and acceptability of mirikizumab treatment administration in UC. Moreover, beyond some reports on satisfaction with conventional therapies,28,36 there is no apparent research evidence on satisfaction with other biological treatments. This is also the first research to look at patient and HCP drug administration preferences for a specific medicine used to treat UC.
Among the study’s strengths is that its findings are not geographically limited, as participants were recruited from varying countries. The fact that the treatment under evaluation was administered within a clinical trial helps to overcome disparities in prescription patterns, pharmaceutical costs, reimbursement, and general therapeutic approaches between countries.
The main study limitation was its small sample size. Because of this, the study was underpowered, and all comparative results and subgroup analyses should be interpreted with caution. These findings may only apply to patients with UC who meet the eligibility criteria to enroll in a phase 3 study in UC; such criteria usually exclude many patients attending routine clinical practice. Patients’ participation in LUCENT-3 was voluntary so it was unlikely and expected that no patients in the survey would have found the study medication completely unacceptable. Since the questionnaire was administered a long time after the induction IV administration, patients who discontinued during or shortly after induction treatment may have had different opinions of mirikizumab administration than those who did not discontinue, and those insights thus may not have been captured. Thus, patients who found the study medication administration unacceptable might have discontinued study participation before LUCENT-3; however, study discontinuation data do not suggest this was an issue.18,20,37,38 Of note, only 3.8% of mirikizumab-treated patients discontinued the LUCENT-1 induction study due to any reason—1.7% due to adverse event; 0.6% due to lack of efficacy; 0.3% due to withdrawal by subject.20 During the LUCENT-2 maintenance study: amongst both mirikizumab induction responders and non-responders 25% discontinued due to any reason—1.7% due to adverse event, 19.0% due to lack of efficacy, and 1.6% due to withdrawal by subject.20
The currently described survey was added to an ongoing extension study (LUCENT-3). The patient population in LUCENT-3 represented a subset of patients who were originally in the induction and maintenance studies, LUCENT-1 and LUCENT-2. This survey was conducted as a protocol amendment offered to a subset of participating countries. Participation in the survey was voluntary. Because only a subset of eligible patients and HCPs completed the questionnaire, this could have introduced bias into the results. Similarly, since the questionnaire was administered to a patient population that successfully entered the extension study (LUCENT-3), the majority of patients were experiencing clinical benefit from treatment, introducing bias regarding acceptance of the administration route versus acceptance due to the clinical improvement presented. Many experienced patients had transitioned to a hybrid of on-site and remote study visits and dosing, which decreased their access to this site-based survey, and this may also have added bias. Additionally, the voluntary nature of survey participation could have introduced selection bias, which could have leaned toward more satisfied patients.
The survey study did not allow open-ended responses, and no adverse event questions were asked; therefore, there is no way to align adverse events and survey respondents nor ascertain how these might have influenced patient satisfaction and acceptability. Of note, 0.4% of mirikizumab-treated patients reported infusion-site reactions during IV induction, 8.7% reported injection-site reactions during maintenance treatment, and 5.5% during the first year of extension treatment.20,39
Future discrete choice experiment research designs may give more detailed information about patients’ preference drivers and the trade-offs they are willing to make between different treatment attributes.
Conclusion
For the administration of mirikizumab, this study revealed some aspects of discordance between patients’ experiences and HCPs’ perspectives of patients’ experiences, such as patient preference for self-administration, as well as some differences amongst HCP subgroups such as gastroenterologists and nurses. However, overall, both HCPs and patients reported satisfaction with and acceptance of mirikizumab IV and SC administration. Nevertheless, it may be important for HCPs to fully understand patients’ treatment administration preferences and perspectives. Importantly, most patients felt that UC improvement outweighed any administration dissatisfaction. These findings may aid patients and HCPs in their treatment choices if they are seeking information on prior patients’ experiences.
Supplementary Material
Acknowledgments
Stephanie Woerner (study execution), Richard Moses (medical peer review), Deborah Fisher (medical peer review), and Jordan Johns (stat peer review) from Eli Lilly and Company; Patrick Daniele (stat analysis, Evidera). Athanasia Benekou (Evidera) and Phil Leventhal (Evidera) provided medical writing services, which were funded by Eli Lilly and Company, in accordance with Good Publication Practice (GPP) guidelines (Good Publication Practice (GPP) Guidelines for Company-Sponsored Biomedical Research: 2022 Update. Ann Intern Med).
Contributor Information
David Clemow, Eli Lilly and Company, Indianapolis, IN, USA.
Christine Radawski, Eli Lilly and Company, Indianapolis, IN, USA.
Joe Milata, Eli Lilly and Company, Indianapolis, IN, USA.
Karla Alaka, Eli Lilly and Company, Indianapolis, IN, USA.
Theresa Hunter Gibble, Eli Lilly and Company, Indianapolis, IN, USA.
Adam Schaum, Eli Lilly and Company, Indianapolis, IN, USA.
Obi Ezennia, Eli Lilly and Company, Indianapolis, IN, USA.
Nicholas Martinez, Gastroenterology Research of America, San Antonio, TX, USA.
Tibor Szaloki, Javorszky Hospital, Vac, Hungary.
Yuka Ito, NHO Mito Medical Center, Ibaraki, Japan.
Danielle Rodriguez, Evidera, Bethesda, MD, USA.
Katherine Kirk, Evidera, Bethesda, MD, USA.
Authors’ Contributions
David Clemow: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing—review & editing; Christine Radawski: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing—review & editing; Joe Milata: Conceptualization; Investigation; Methodology; Validation; Writing—review & editing; Karla Alaka: Conceptualization; Investigation; Methodology; Writing—review & editing; Theresa Hunter Gibble: Conceptualization; Methodology; Writing—review & editing; Adam Schaum: Conceptualization; Investigation; Methodology; Supervision; Writing—review & editing; Obi Ezennia: Data curation; Investigation; Writing—review & editing; Nicholas Martinez: Writing—review & editing; Tibor Szaloki: Writing—review & editing; Yuka Ito: Writing—review & editing; Danielle Rodriguez: Formal analysis; Investigation; Methodology; Validation; Writing—original draft; Writing—review & editing; Katherine Kirk: Formal analysis; Investigation; Methodology; Validation; Writing—original draft; Writing—review & editing. All authors approved the final version of the manuscript.
Funding
Eli Lilly and Company was the current study sponsor, funded the original research as well as the medical writing and editing services.
Conflicts of Interest
David Clemow, Christine Radawski, Joe Milata, Karla Alaka, Theresa Hunter Gibble, Adam Schaum, and Obi Ezennia are employees of Eli Lilly at the time the study was conducted and the manuscript developed. Danielle Rodriguez and Katherine Kirk are employees of Evidera, which was contracted by Eli Lilly for work relating to this study. Tibor Szaloki is an employee of Javorszky Hospital and was contracted by Eli Lilly to assist as an external collaborator for this study. Nicholas Martinez is an employee of Gastroenterology Research of America and was contracted by Eli Lilly to assist as an external collaborator for this study. Yuka Ito is an employee of NHO Mito Medical Center and was contracted by Eli Lilly to assist as an external collaborator for this study. Medical writing support was provided by Athanasia Benekou, Principal Medical Writer, from Evidera’s Medical Writing and Healthcare Communications, and funded by Eli Lilly.
Data Availability
Eli Lilly and Company provides access to all individual participant data collected during the study, after anonymization. Data are available to request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptability, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, and study report will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at [www.vivli.org].
References
- 1. Koliani-Pace JL, Haron AM, Zisman-Ilani Y, Thompson KD, Siegel CA.. Patients’ perceive biologics to be riskier and more dreadful than other IBD medications. Inflamm Bowel Dis. 2020;26(1):141-146. doi: 10.1093/ibd/izz121 [DOI] [PubMed] [Google Scholar]
- 2. Bretto E, Ribaldone DG, Caviglia GP, Saracco GM, Bugianesi E, Frara S.. Inflammatory bowel disease: emerging therapies and future treatment strategies. Biomedicines. 2023;11(8):2249. doi: 10.3390/biomedicines11082249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD.. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384-413. doi: 10.14309/ajg.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 4. Guo M, Wang X.. Pathological mechanism and targeted drugs of ulcerative colitis: a review. Medicine (Baltim). 2023;102(37):e35020. doi: 10.1097/MD.0000000000035020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gros B, Kaplan GG.. Ulcerative colitis in adults: a review. JAMA. 2023;330(10):951-965. doi: 10.1001/jama.2023.15389 [DOI] [PubMed] [Google Scholar]
- 6. Aggarwal A, Sabol T, Vaziri H.. Update on the use of biologic therapy in ulcerative colitis. Curr Treat Options Gastroenterol. 2017;15(1):155-167. doi: 10.1007/s11938-017-0120-8 [DOI] [PubMed] [Google Scholar]
- 7. NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases: Treatment for Ulcerative Colitis 2020. Accessed October 5, 2023. https://www.niddk.nih.gov/health-information/digestive-diseases/ulcerative-colitis/treatment#:~:text=How%20do%20doctors%20treat%20symptoms%20and%20complications%20of,fluids%20and%20electrolytes%20to%20prevent%20and%20treat%20dehydration [Google Scholar]
- 8. Chao YS, Loshak H.. CADTH rapid response reports: biologics versus immunomodulators for the treatment of ulcerative colitis: a review of comparative clinical effectiveness and cost-effectiveness. Published April 17, 2029. Accessed February 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK549363/pdf/Bookshelf_NBK549363.pdf [PubMed] [Google Scholar]
- 9. Roda G, Jharap B, Neeraj N, Colombel JF.. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7(1):e135. doi: 10.1038/ctg.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ho GT, Chiam P, Drummond H, Loane J, Arnott IDR, Satsangi J.. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther. 2006;24(2):319-330. doi: 10.1111/j.1365-2036.2006.02974.x [DOI] [PubMed] [Google Scholar]
- 11. D’Haens G, Lindsay JO, Panaccione R, Schreiber S.. Ulcerative colitis: shifting sands. Drugs R D. 2019;19(2):227-234. doi: 10.1007/s40268-019-0263-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011(2):CD008794. doi: 10.1002/14651858.CD008794.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanzel J, Hulshoff MS, Grootjans J, D’Haens G.. Emerging therapies for ulcerative colitis. Expert Rev Clin Immunol. 2022;18(5):513-524. doi: 10.1080/1744666X.2022.2069562 [DOI] [PubMed] [Google Scholar]
- 14. Allocca M, Furfaro F, Fiorino G, Gilardi D, D'Alessio S, Danese S.. Can IL-23 be a good target for ulcerative colitis? Best Pract Res Clin Gastroenterol. 2018;32-33 (Epub 2018 May 23):95-102. doi: 10.1016/j.bpg.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 15. Sewell GW, Kaser A.. Interleukin-23 in the pathogenesis of inflammatory bowel disease and implications for therapeutic intervention. J Crohns Colitis. 2022;16(Supplement_2):ii3-ii19. doi: 10.1093/ecco-jcc/jjac034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verstockt B, Salas A, Sands BE, et al. ; Alimentiv Translational Research Consortium (ATRC). IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2023;20(7):433-446. doi: 10.1038/s41575-023-00768-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steere B, Beidler C, Martin A, Bright S, Kikly K, Benschop RJ.. Generation and characterization of Mirikizumab, a humanized monoclonal antibody targeting the p19 subunit of IL-23. J Pharmacol Exp Ther. 2023;387(2):180-187. doi: 10.1124/jpet.122.001512 [DOI] [PubMed] [Google Scholar]
- 18. Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology. 2020;158(3):537-549. doi: 10.1053/j.gastro.2019.08.043 [DOI] [PubMed] [Google Scholar]
- 19.Omvoh (mirikizumab): EPAR - product information. Accessed December 18, 2023.https://www.ema.europa.eu/en/documents/product-information/omvoh-epar-product-information_en.pdf [Google Scholar]
- 20. D’Haens G, Dubinsky M, Kobayashi T, et al. Mirikizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2023;388(26):2444-2455. doi: 10.1056/nejmoa2207940 [DOI] [PubMed] [Google Scholar]
- 21. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324-1338. doi: 10.1038/ajg.2015.233 [DOI] [PubMed] [Google Scholar]
- 22. Schreiber S, Panés J, Louis E, Holley D, Buch M, Paridaens K.. Perception gaps between patients with ulcerative colitis and healthcare professionals: an online survey. BMC Gastroenterol. 2012;12:108. doi: 10.1186/1471-230X-12-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of continued treatment with mirikizumab in a phase 2 trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20(1):105-115.e14. doi: 10.1016/j.cgh.2020.09.028 [DOI] [PubMed] [Google Scholar]
- 24. Irvine EJ. Development and subsequent refinement of the inflammatory bowel disease questionnaire: a quality-of-life instrument for adult patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1999;28(4):S23-S27. doi: 10.1097/00005176-199904001-00003 [DOI] [PubMed] [Google Scholar]
- 25. Irvine EJ, Zhou Q, Thompson AK.. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91(8):1571-1578. [PubMed] [Google Scholar]
- 26. Dubinsky M, Rice A, Yarlas A, et al. Systematic literature review: ability of the IBDQ-32 to detect meaningful change in ulcerative colitis health indicators. Inflamm Bowel Dis. 2023:izad282. doi: 10.1093/ibd/izad282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Almario CV, Keller MS, Chen M, et al. Optimizing selection of biologics in inflammatory bowel disease: development of an online patient decision aid using conjoint analysis. Am J Gastroenterol. 2018;113(1):58-71. doi: 10.1038/ajg.2017.470 [DOI] [PubMed] [Google Scholar]
- 28. Peyrin-Biroulet L, Van Assche G, Sturm A, et al. Treatment satisfaction, preferences and perception gaps between patients and physicians in the ulcerative colitis CARES study: a real world-based study. Dig Liver Dis. 2016;48(6):601-607. doi: 10.1016/j.dld.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 29. Wickramasekera N, Coates E, Barr A, et al. Patient preferences for treatment in steroid resistant ulcerative colitis - a discrete-choice experiment. Scand J Gastroenterol. 2022;57(7):797-806. doi: 10.1080/00365521.2022.2036808 [DOI] [PubMed] [Google Scholar]
- 30. Louis E, Siegel CA, James B, Heidenreich S, Krucien N, Ghosh S.. Patients with inflammatory bowel disease have heterogeneous treatment preferences that are largely determined by the avoidance of abdominal pain and side effects [P-POWER IBD Study]. J Crohns Colitis. 2023;17(2):231-239. doi: 10.1093/ecco-jcc/jjac130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schubert S, Picker N, Cavlar T, Knop J, Kahraman A, Mohl W.. Inflammatory bowel disease patients’ treatment preferences using a discrete choice experiment technique: the InPuT study. Adv Ther. 2022;39(6):2889-2905. doi: 10.1007/s12325-022-02143-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fiorino G, Bent-Ennakhil N, Varriale P, Braegger F, Hoefkens E.. Patient preferences for treatment attributes in inflammatory bowel disease: results from a large survey across seven european countries using a discrete choice experiment. Inflamm Bowel Dis. 2024. doi: 10.1093/ibd/izae015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Denesh D, Carbonell J, Kane JS, Gracie D, Selinger CP.. Patients with inflammatory bowel disease (IBD) prefer oral tablets over other modes of medicine administration. Expert Rev Gastroenterol Hepatol. 2021;15(9):1091-1096. doi: 10.1080/17474124.2021.1898944 [DOI] [PubMed] [Google Scholar]
- 34. Casellas F, Vera I, Ginard D, Torrejón A.. Inflammatory bowel disease patient’s satisfaction with healthcare services received. Physicians’ and nurses’ perceptions. Rev Esp Enferm Dig. 2013;105(7):385-391. doi: 10.4321/s1130-01082013000700003 [DOI] [PubMed] [Google Scholar]
- 35. Prasad SS, Potter M, Keely S, Talley NJ, Walker MM, Kairuz T.. Roles of healthcare professionals in the management of chronic gastrointestinal diseases with a focus on primary care: a systematic review. JGH Open 2020;4(2):221-229. doi: 10.1002/jgh3.12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coates E, Wickramasekera N, Barr A, et al. Patient preferences and current practice for adults with steroid-resistant ulcerative colitis: POPSTER mixed-methods study. Health Technol Assess. 2022;26(41):1-118. doi: 10.3310/rhxr5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sands BE, Peyrin-Biroulet L, Kierkus J, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology. 2022;162(2):495-508. doi: 10.1053/j.gastro.2021.10.050 [DOI] [PubMed] [Google Scholar]
- 38. Blauvelt A, Kimball AB, Augustin M, et al. Efficacy and safety of mirikizumab in psoriasis: results from a 52-week, double-blind, placebo-controlled, randomized withdrawal, phase III trial (OASIS-1). Br J Dermatol. 2022;187(6):866-877. doi: 10.1111/bjd.21743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sands BE, D’Haens G, Clemow DB, et al. Two-year efficacy and safety of mirikizumab following 104 weeks of continuous treatment for ulcerative colitis: results from the LUCENT-3 open-label extension study. Inflamm Bowel Dis. 2024;30(6):1044-1045. doi: 10.1093/ibd/izae024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Eli Lilly and Company provides access to all individual participant data collected during the study, after anonymization. Data are available to request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptability, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, and study report will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at [www.vivli.org].