Abstract
Objectives
To evaluate the effect of daily zinc supplementation in children on the incidence of acute lower respiratory tract infections and pneumonia.
Design
Double masked, randomised placebo controlled trial.
Setting
A slum community in New Delhi, India.
Participants
2482 children aged 6 to 30 months.
Interventions
Daily elemental zinc, 10 mg to infants and 20 mg to older children or placebo for four months. Both groups received single massive dose of vitamin A (100 000 IU for infants and 200 000 IU for older children) at enrolment.
Main outcome measures
All households were visited weekly. Any children with cough and lower chest indrawing or respiratory rate 5 breaths per minute less than the World Health Organization criteria for fast breathing were brought to study physicians.
Results
At four months the mean plasma zinc concentration was higher in the zinc group (19.8 (SD 10.1) v 9.3 (2.1) μmol/l, P<0.001). The proportion of children who had acute lower respiratory tract infection during follow up was no different in the two groups (absolute risk reduction −0.2%, 95% confidence interval −3.9% to 3.6%). Zinc supplementation resulted in a lower incidence of pneumonia than placebo (absolute risk reduction 2.5%, 95% confidence interval 0.4% to 4.6%). After correction for multiple episodes in the same child by generalised estimating equations analysis the odds ratio was 0.74, 95% confidence interval 0.56 to 0.99.
Conclusions
Zinc supplementation substantially reduced the incidence of pneumonia in children who had received vitamin A.
What is already known on this topic
Mild to moderate zinc deficiency is common in children in developing countries and increases the risk of respiratory morbidity
What this study adds
A third of children from low socioeconomic classes in India have low plasma concentrations of zinc
Routine zinc supplementation of such children aged 6 months to 3 years substantially reduced the incidence of pneumonia
Introduction
Zinc deficiency is common in children in developing countries because of low food intake, particularly from animal sources, limited zinc bioavailability from local diets, and losses of zinc during recurrent diarrhoeal illnesses.1,2 Zinc deficiency leads to impairment in immunological and other defences that increases rates of serious infections.3–9 Trials of zinc supplements are a reliable method of assessing the health consequences of zinc deficiency. In developing countries a significantly lower incidence and prevalence of diarrhoea has consistently been observed in children given zinc supplements.2,10,11
Lower respiratory tract infections are a common cause of death in childhood. The effect of zinc supplementation on such infections is still unclear. Two of the published trials that found no significant effect were small.2 Another relatively larger trial reported a 45% reduction in the incidence of pneumonia,12 but the criteria for its diagnosis included high fever, a sign that is not diagnostic for pneumonia nor an established indicator of its severity.
We evaluated the impact of daily zinc supplementation in preschool children who had received a large dose of vitamin A, a routine practice in this setting, on the incidence of acute lower respiratory tract infections and pneumonia.
Methods
Study setting
The trial took place in the urban slum of Dakshinpuri in New Delhi, India, comprising 15 000 dwellings and 75 000 inhabitants. Recent data from a neighbouring community indicated that childhood malnutrition, zinc deficiency, diarrhoea, and lower respiratory tract infections were common.3,11,12
Randomisation and masking
We included children if their parents gave informed consent. Eligible children were individually randomised by a simple randomisation scheme in blocks of eight. The randomisation scheme was generated by a statistician at Statens Serum Institut, not otherwise involved with this study, using SAS software. Zinc or placebo syrups were prepared and packaged in unbreakable bottles by GK Pharma ApS, Koge, Denmark, who also labelled bottles with a unique child identification number according to the randomisation scheme. Six bottles, one for each month and two extra, for each child were produced and labelled before enrolment commenced. The supplies for each child were kept separately in labelled plastic bags. The zinc and placebo syrups were similar in appearance, taste, and packaging. Masking was maintained during analyses by coding the groups as A or B.
Enrolment and intervention delivery
Enrolment commenced on 15 February 1999. All children aged 6 to 30 months in the community were identified through a survey. We excluded children if consent was refused, if they were likely to move out of the study area within the next four months, and if they needed urgent admission to hospital on the enrolment day or had received massive dose of vitamin A (100 000 IU for infants and 200 000 for older children) within the two months before enrolment. The follow up of the last child was completed on 30 September 2000.
Doses of elemental zinc were 10 mg for infants and 20 mg for children (twice the recommended daily doses) as zinc gluconate. Zinc or placebo was taken daily for four months. An attendant administered the syrup daily for four months except on Sundays, when the mother administered it. One bottle containing 250 ml was kept in the child's home and replaced monthly. Immunisations and treatment for acute illnesses were provided as per World Health Organization guidelines.13 Children with acute lower respiratory tract infections received co-trimoxazole. Amoxicillin was substituted if the child did not respond within three days. Children were sent to hospital if they had signs and symptoms that warranted referral according to WHO guidelines.13
We used Seca Salter scales and locally manufactured length boards that read to the nearest 0.1 kg and 0.1 cm respectively to assess growth at enrolment. The study was approved by the All India Institute of Medical Sciences ethics committee. Informed written consent was obtained from community leaders and parents. Signatures or thumb impressions were obtained on consent forms and a copy left with the family.
Measurement of outcomes
Field workers visited each child every seventh day during the entire study. At each visit, mothers were asked about fever, cough, other characteristics of illness, and whether they had sought treatment for the child in the previous seven days. Respiratory rates were counted twice for one minute each and the temperature recorded. Children with cough and respiratory rates ⩾35/min at age ⩾12 months and ⩾45/min at <12 months or lower chest indrawing were brought to study physicians for assessment. We used the cut offs of 5 breaths/min below the WHO criteria for fast breathing to maximise detection of acute lower respiratory tract infections and pneumonia. Two study physicians examined and repeated measurements of respiratory rate, clinical indicators of hypoxaemia, and auscultation. In cases of disagreement, a senior paediatrician assessed the child. Mothers were encouraged to bring their children to the study clinic whenever they were ill and they were similarly assessed.
Acute lower respiratory tract infections were defined by cough and fast breathing or lower chest indrawing as assessed by the physician; other clinical signs were not taken into account.13 Fast breathing was defined as two counts of ⩾50 breaths/min for infants and ⩾40 breaths/min for older children. The day of onset of infection was the first day when this combination was detected. The day of recovery was the day after the last day when this combination was present. Pneumonia was diagnosed either by a combination of cough with crepitations or bronchial breathing by auscultation or as an episode of acute lower respiratory tract infection associated with at least one of lower chest indrawing, convulsions, not able to drink or feed, extreme lethargy, restlessness or irritability, nasal flaring, or abnormal sleepiness. For episodes to be counted as individual, there had to be at least 14 intervening days.
Sample size
We calculated sample sizes for 80% power with 95% level of confidence. We took values for the control group from a previous study in an adjacent slum, which reported 0.41 episodes of acute lower respiratory tract infection and 0.12 episodes of severe acute lower respiratory tract infections or pneumonia per child per four months.12 We required 907 children per group to detect a 20% or greater reduction in incidence of acute lower respiratory tract infections. We required a larger sample size of 1234 per group to detect a 30% or greater reduction in incidence of pneumonia.
Plasma zinc
At enrolment and at the end of the study we collected samples of non-fasting venous blood (about 5 ml) in zinc-free heparinised polypropylene tubes (Sarstedt, Nümbrecht, Germany) between 9 am and 12 noon. The samples were centrifuged and plasma transferred to zinc-free polypropylene vials (Eppendorf, Hinz, Germany) for storage at −20°C. About half of the plasma samples were analysed for zinc by a standard flame furnace atomic absorption spectrophotometer technique (GBC Avanta, Dandenong, Victoria, Australia) and the other half by inductively coupled plasma atomic emission spectrometry (Ash IRIS/AP, Thermo Jarell, MA, USA) using SERONORM (Sero AS, Billingstad, Norway) as the reference standard in every batch of 20 samples in both methods. The two methods were calibrated to give the same results before we started to analyses study samples.14,15
Standardisation and quality control
We used a study manual describing standard operating procedures. Standardisation exercises were done to achieve agreement within and between study personnel for measurement of respiratory rate and anthropometry every three months. All physicians had some experience in paediatrics and were retrained in the recognition of crepitations, bronchial breathing, and wheezing by a senior paediatrician every three months.
Supervisors monitored field workers' activities. Independent supervisory checks to inquire about daily dispensing of the syrup (0.5% of total visits) and to authenticate the data collected at morbidity visits (1% of total visits) were made. Field workers were additionally observed at home visits (1% of visits) to assess counting of respiratory rate, measurement of temperature, and assessment of lower chest indrawing.
Data management and analysis
The forms for the study were designed with FoxPro for Windows (Microsoft Corporation, Redmond, Washington, USA) and range and consistency checks incorporated. Double data entry by two data clerks followed by validation was completed within 48 hours. We compared incidence of acute lower respiratory tract infections or pneumonia in the two groups and calculated absolute risk reductions with their 95% confidence intervals.16 For the person time analyses, the denominator was the days for which reliable information about illness was available.
To account for the correlation of multiple episodes in the same child, we used generalised estimating equations for longitudinal data analysis. The surveillance data for each child were divided into eight child periods of 15 days each. To be included in this analysis a child had to contribute at least 15 days of follow up to the denominator. In the generalised estimating equations model, occurrence of a new episode of acute lower respiratory tract infection or pneumonia in a child period was modelled as a binomial dependent variable and group allocation (zinc or placebo) as the independent variable. The model used a logit link, binomial variance, and exchangeable correlation.
Statistical analysis was performed with Stata, version 6 (StataCorp, College Station, TX).
Results
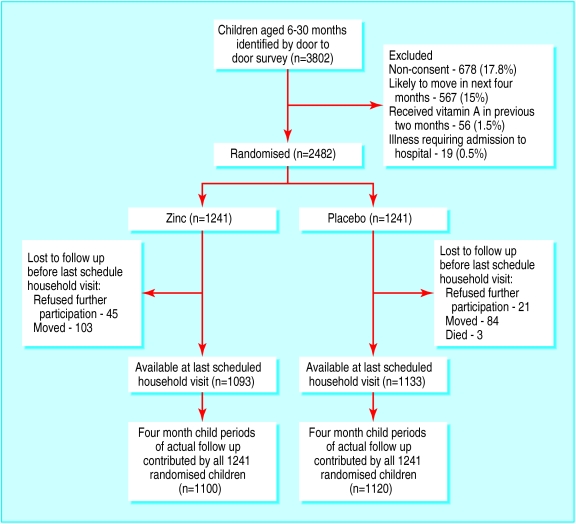
We identified 3802 children and randomised 2482. The children in the two groups were comparable for age, anthropometry, child feeding practices, morbidity in the previous 24 hours, socioeconomic characteristics, and plasma zinc concentration (table 1). The figure shows the flow of the participants through the study. Of those randomised, 1093 (88%) children in the zinc group and 1133 (91%) in the placebo group remained in the trial at the last scheduled household visit.
Table 1.
Baseline characteristics of children aged 6-30 months randomised to receive daily twice recommended daily allowance of zinc and included in analyses. Figures are number (percentage) of children unless stated otherwise
|
Characteristic
|
Zinc group n=1241
|
Placebo group n=1241
|
|---|---|---|
| Mean (SD) age (months) at enrolment | 15.6 (7.5) | 15.0 (7.4) |
| Boys | 618 (49.8) | 682 (55.0) |
| Breast fed | 858 (69.1) | 865 (69.7) |
| Prevalence in previous 24 hours: | ||
| Cough | 374 (30.1) | 375 (30.2) |
| Fever | 108 (8.7) | 91 (7.3) |
| Fast breathing | 58 (4.7) | 54 (4.4) |
| Reported diarrhoea | 142 (11.4) | 154 (12.4) |
| Literate mother | 804 (65.1) | 788 (63.5) |
| Median (interquartile range) annual family income (rupees) | 36 000* (24 000-54 000) | 36 000* (24 000-54 000) |
| Mean (SD) weight (kg) | 8.1 (1.6) | 8.0 (1.6) |
| Mean (SD) length (cm) | 73.0 (7.1) | 72.4 (7.2) |
| Plasma zinc†: | ||
| Mean (SD) concentration (μmol/l) | 9.5 (2.2) | 9.51 (1.7) |
| <8.4 μmol/l | 364 (29.8) | 317 (25.8) |
| <9.2 μmol/l | 572 (46.8) | 528 (43.1) |
£510 (€829, $738).
Available for 1222 children in zinc group and 1226 children in placebo group.
Ninety per cent of intended intervention doses were administered to the trial children. There was a small but significant increase in the average number of days with vomiting in the zinc group (4.3 (SD 5.8) v 2.6 (SD 3.9); difference in means 1.7, 95% confidence interval 1.3 to 2.1). Only eight children in the zinc group and none in the placebo group discontinued the intervention because of vomiting.
Intervention efficacy
The mean plasma zinc concentration was significantly higher at the end of the study in the children who received zinc supplementation (19.8 (SD 10.1) v 9.3 (SD 2.1) μmol/l, P<0.001 non-parametric test). The change from enrolment was also substantially higher in the zinc group (10.4 (SD 10.0) μmol/l) compared with the placebo group (−0.3 (SD 2.2) μmol/l, P<0.0001 non-parametric test).
Acute lower respiratory tract infections
In the child based analyses, 425 children in the zinc group and 423 in the placebo group experienced at least one episode of acute lower respiratory tract infection (absolute risk reduction −0.2%, 95% CI −3.9% to 3.6%).
In the person time analyses, the incidence of acute lower respiratory tract infections was 0.53 and 0.54 episodes per four month child period of actual follow up in zinc and placebo groups respectively (absolute risk reduction 1%, −2.9% to 5%). As multiple episodes of acute lower respiratory tract infections in a child may not be independent we performed a generalised estimating equations analysis to correct for this correlation. The results of this analysis were similar to those of the uncorrected analysis (odds ratio 0.98, 0.86 to 1.13, table 2).
Table 2.
Impact of zinc supplementation on acute lower respiratory infections, pneumonia, and admissions to hospital for all causes in children aged 6-35 months during four months of follow up
|
Zinc
|
Placebo
|
Absolute risk reduction (95% CI)
|
Odds ratio (95% CI)*
|
|
|---|---|---|---|---|
| Child based analysis | ||||
| No of children | 1241 | 1241 | — | — |
| No (%) with ⩾1 episodes of acute lower respiratory tract infections | 425 (34.2) | 423 (34.1) | −0.2% (−3.9% to 3.6%) | — |
| No (%) with ⩾1 episodes of pneumonia | 81 (6.5) | 112 (9.0) | 2.5% (0.4% to 4.6%) | — |
| No (%) admitted to hospital for any cause | 21 (1.8) | 30 (2.4) | 0.6% (−0.5% to 1.8%) | — |
| Person time based analysis† | ||||
| No of four month child periods | 1100 | 1120 | — | |
| Acute lower respiratory tract infections: | ||||
| Total episodes | 581 | 594 | — | |
| Episodes per four month period | 0.53 | 0.54 | 1% (−2.9% to 5%) | 0.98 (0.86 to 1.13) |
| Pneumonia: | ||||
| Total episodes | 88 | 118 | — | |
| Episodes per four month period | 0.08 | 0.105 | 2.4% (0.2% to 4.6%) | 0.74 (0.56 to 0.99) |
After correction for correlation of data by generalised estimating equations.
Person time for each child was calculated as total follow up days when reliable informant was available.
Pneumonia
Eighty one children in the zinc group and 112 in the placebo group had at least one episode of pneumonia (absolute risk reduction 2.5%, 0.4% to 4.6%). The number needed to treat was therefore 40—that is, supplementation of 40 children would be expected to prevent one child from having from pneumonia.
In the person time analyses, the incidence of pneumonia for the four month child period was similarly lower in the zinc group (absolute risk reduction 2.4%, 0.2% to 4.6%). The findings were similar in the generalised estimating equations analysis (odds ratio 0.74, 0.56 to 0.99, table 2).
The number of children admitted to hospital for any cause was lower in the zinc group (absolute risk reduction 0.6%, −0.5% to 1.8%). Three children, all in the placebo group, died.
Discussion
Daily zinc supplementation of infants and young children in a relatively deprived population prevented one quarter of the episodes of pneumonia in children, all of whom had received the recommended dose of vitamin A. Zinc supplementation had no effect on acute lower respiratory tract infections defined by cough and fast breathing or lower chest indrawing. Routine administration of zinc may therefore be effective in preventing severe rather than mild illness. Previous trials have shown that zinc supplementation has a large effect on the incidence of severe but not mild diarrhoeal illness.2,10,11
The WHO definition of acute lower respiratory tract infections that is commonly used in primary care programmes aims at high sensitivity for detection of true pneumonia at the expense of some loss in specificity. In our study, acute lower respiratory tract infections by WHO criteria had 80% sensitivity but only 69% specificity using physician diagnosis by auscultation as gold standard. Lack of specificity in the diagnostic criteria for measuring study outcomes biases the risk or rate ratio towards null values.17 This may be an alternate explanation for the observed impact on pneumonia but not on acute lower respiratory tract infections. We defined pneumonia by findings on auscultation or as acute lower respiratory tract infections associated with one or more previously validated indicators of severity.18–21 Notably, the inclusion of high fever as one of the criteria for pneumonia in addition to cough and fast breathing or chest indrawing, as used in a previous zinc supplementation trial,12 did not change the effect sizes (data not shown).
In a recent study in Bangladesh there was a significant reduction in the incidence and prevalence of acute lower respiratory tract infections in children supplemented with both zinc and vitamin A. In children who received zinc alone, however, incidence and prevalence increased.22 We did not examine this issue because vitamin A was given to all the children for ethical reasons. However, in a pooled analysis two of the four studies used zinc without vitamin A but did not report any increased respiratory morbidity.2
We chose twice the daily recommended dose for supplementation to allow for possible impairment in absorption of ingested zinc among children because of bacterial overgrowth and protozoal or parasitic infestations and to compensate for excessive intestinal zinc losses during diarrhoeal illnesses.23–25 Comparison of the effect sizes in the our study with those in earlier trials that used the daily recommended dose is difficult because of differences in the diagnostic criteria used for pneumonia.
Prevention of pneumonia by optimising zinc intakes is biologically plausible. The low mean plasma zinc concentrations at enrolment show that deficiency was common in these children. Supplementation improves immune functions, including delayed cutaneous hypersensitivity, and increases the number of CD4 (helper) lymphocytes.26,27 In experimental models zinc deficiency has been shown to impair cellular and humoral immune function.27,28
Possible limitations of study
As the children in our trial were a representative sample of a well defined low income community, our findings are generalisable to populations with similar dietary and morbidity patterns. We did not include young infants, but recent reports of a decline in mortality in infants aged 1-9 months who were small for gestational age and received zinc supplements suggests benefits at this age, particularly in areas where low birth weight is common.2 As all children in our study received a large dose of vitamin A, the effects reported can only be generalised to such children. Most developing countries supplement children aged 6 months to 5 years with large doses of vitamin A every four to six months.
Radiology, the ideal method for diagnosis of pneumonia, was impractical to use under field conditions. Nevertheless, rigorous training was provided to the study physicians to achieve acceptable levels of variability within and between observers in arriving at the diagnosis. Furthermore, sufficient supplies of syrup were produced at the outset, and all the bottles for a particular child were labelled by a unique child identification number to ensure complete masking of the observers.
Conclusions
The findings of current and previous studies show that improving zinc status in deficient populations should substantially reduce serious morbidity. Prompt measures to improve zinc status are therefore warranted, given the substantial and consistent reduction in severe diarrhoea and pneumonia in supplementation trials. The possible approaches include food fortification, dietary diversification, cultivation of plants that are zinc-dense or have a decreased concentration of zinc absorption inhibitors or supplementation of selected subgroups.
Figure.
Trial profile
Acknowledgments
We thank Dr Martin Frigg, Task Force SIGHT AND LIFE, Basle, Switzerland, for providing the vitamin A and placebo capsules. We acknowledge the core support of the Indian Council of Medical Research. We also thank Geeta Trilok Kumar and the Laboratory of Clinical Biochemistry, University of Bergen, Norway, for help in analysis of plasma zinc specimens and Sandeep Saxena for the statistical analysis.
Footnotes
Funding: European Union (Contract No IC18-CT96-0045), Norwegian Council of Universities' Committee for Development Research and Education (PRO 53/96), Department of Child and Adolescent Health and Development (CAH), World Health Organization.
Competing interests: None declared.
References
- 1.Black RE. Therapeutic and preventive effects of zinc on serious childhood infectious diseases in developing countries. Am J Clin Nutr. 1998;68(suppl 2):S476–S479. doi: 10.1093/ajcn/68.2.476S. [DOI] [PubMed] [Google Scholar]
- 2.Bhutta ZA, Black RE, Brown KN, Gardner JM, Gore S, Hidayat A, et al. Zinc Investigators' Collaborative Group. Prevention of diarrhoea and pneumonia by supplementation in children in developing countries: pooled analysis of randomized controlled trials. J Pediatr. 1999;155:689–697. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari N, Bahl R, Hambidge KM, Bhan MK. Increased diarrhoeal and respiratory morbidity in association with zinc deficiency—a preliminary report. Acta Paediatr. 1996;85:148–150. doi: 10.1111/j.1651-2227.1996.tb13981.x. [DOI] [PubMed] [Google Scholar]
- 4.Prasad AS. Clinical, biochemical, and pharmacological role of zinc. Annu Rev Pharmacol Toxicol. 1979;19:393–426. doi: 10.1146/annurev.pa.19.040179.002141. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes G, Nair M, Onoe K, Tanaka T, Floyd R, Good RA. Impairment of cell-mediated immunity functions by dietary zinc deficiency in mice. Proc Natl Acad Sci USA. 1979;76:457–461. doi: 10.1073/pnas.76.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chvapil M. New aspects in the biological role of zinc: a stabilizer of macromolecules and biological membranes. Life Sci. 1973;13:1041–1049. doi: 10.1016/0024-3205(73)90372-x. [DOI] [PubMed] [Google Scholar]
- 7.Roy SK, Tomkins AM. Impact of experimental zinc deficiency on growth, morbidity and ultrastructural development of intestinal tissue. Bangladesh J Nutr. 1989;2:1–7. [Google Scholar]
- 8.Roy SK, Behrens RH, Haider R, Akrumuzzaman SM, Mahalanabis D, Wahed MA, et al. Impact of zinc supplementation on intestinal permeability in Bangladeshi children with acute diarrhoea and persistent diarrhoea syndrome. J Pediatr Gastroenterol Nutr. 1992;15:289–296. doi: 10.1097/00005176-199210000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68 (suppl 2):S447–S463. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 10.Sazawal S, Black RE, Bhan MK, Jalla S, Bhandari N, Sinha A, et al. Zinc supplementation reduces the incidence of persistent diarrhoea and dysentery among low socioeconomic children in India. J Nutr. 1996;126:443–450. doi: 10.1093/jn/126.2.443. [DOI] [PubMed] [Google Scholar]
- 11.Sazawal S, Black RE, Bhan MK, Jalla S, Sinha A, Bhandari N. Efficacy of zinc supplementation in reducing the incidence and prevalence of acute diarrhoea—a community-based, double-blind, controlled trial. Am J Clin Nutr. 1997;66:413–418. doi: 10.1093/ajcn/66.2.413. [DOI] [PubMed] [Google Scholar]
- 12.Sazawal S, Black RE, Jalla S, Mazumdar S, Sinha A, Bhan MK. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: a double-blind, controlled trial. Pediatrics. 1998;102:1–5. doi: 10.1542/peds.102.1.1. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization; Division of Child Health and Development. Integrated management of childhood illness. Geneva: World Health Organization; 1997. (WHO/CHD/97.3E). [PMC free article] [PubMed] [Google Scholar]
- 14.Hambidge KM, King JC, Kern DL, English-Westcott JL, Stall C. Pre-breakfast plasma zinc concentrations: the effect of previous meals. J Trace Elem Electrolytes Health Dis. 1990;4:229–231. [PubMed] [Google Scholar]
- 15.Melton LA, Tracy ML, Moller G. Screening trace elements and electrolytes in serum by inductively-coupled plasma emission spectrometry. Clin Chem. 1990;36:247–250. [PubMed] [Google Scholar]
- 16.Gardner MJ, Altman DG, editors. Statistics with confidence: confidence intervals and statistical guidelines. London: BMJ Publishing; 1989. [Google Scholar]
- 17.Smith PG, Morrow RH, editors. Field trials of health interventions in developing countries: a toolbox. London: Macmillan Education; 1996. [Google Scholar]
- 18.Smyth A, Carty H, Hart CA. Clinical predictors of hypoxaemia in children with pneumonia. Ann Trop Paediatr. 1998;18:31–40. doi: 10.1080/02724936.1998.11747923. [DOI] [PubMed] [Google Scholar]
- 19.Usen S, Weber M, Mulholland K, Jaffar S, Oparaugo A, Omosigho C, et al. Clinical predictors of hypoxaemia in Gambian children with acute lower respiratory tract infection: prospective cohort study. BMJ. 1999;318:86–91. doi: 10.1136/bmj.318.7176.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shann F, Hart K, Thomas D. Acute lower respiratory tract infections in children: possible criteria for selection of patients for antibiotic therapy and hospital admission. Bull World Health Organ. 1984;62:749–753. [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell H, Byass P, Lamont AC, Forgie IM, O'Neill KP, Lloyd-Evans N, et al. Assessment of clinical criteria for identification of severe acute lower respiratory tract infections in children. Lancet. 1989;i:297–299. doi: 10.1016/s0140-6736(89)91308-1. [DOI] [PubMed] [Google Scholar]
- 22.Rahman MM, Vermund SH, Wahed MA, Fuchs GJ, Baqui AH, Alvarez JO. Simultaneous zinc and vitamin A supplementation in Bangladeshi children: randomised double blind controlled trial. BMJ. 2001;323:314–318. doi: 10.1136/bmj.323.7308.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo-Duran C, Vial P, Uauy R. Trace mineral balance during acute diarrhoea in infants. J Pediatr. 1988;113:452–457. doi: 10.1016/s0022-3476(88)80627-9. [DOI] [PubMed] [Google Scholar]
- 24.Zinc and copper wastage during acute diarrhoea. Nutr Rev. 1990;48:19–22. doi: 10.1111/j.1753-4887.1990.tb02874.x. [DOI] [PubMed] [Google Scholar]
- 25.Ruz M, Solomons NW. Fecal zinc of endogenous zinc during oral rehydration therapy for acute diarrhoea. J Trace Elem Exp Med. 1995;7:89–100. [Google Scholar]
- 26.Sazawal S, Jalla S, Mazumder S, Sinha A, Black RE, Bhan MK. Effect of zinc supplementation on cell-mediated immunity and lymphocyte subsets in preschool children. Indian Pediatr. 1997;34:589–597. [PubMed] [Google Scholar]
- 27.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68 (suppl 2):S447–S463. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 28.Sempertegui F, Estrella B, Correa E, Aguirre L, Saa B, Torres M, et al. Effects of short term zinc supplementation on cellular immunity, respiratory symptoms, and growth of malnourished Equadorian children. Eur J Clin Nutr. 1996;50:42–46. [PubMed] [Google Scholar]