Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is one of the most universal liver diseases with complicated pathogenesis throughout the world. Insulin resistance is a leading risk factor that contributes to the development of NAFLD. Vascular endothelial growth factor B (VEGFB) was described by researchers as contributing to regulating lipid metabolic disorders. Here, we investigated VEGFB as a main target to regulate insulin resistance and metabolic syndrome.
Methods
In this study, bioinformatics, transcriptomics, morphological experiments, and molecular biology were used to explore the role of VEGFB in regulating insulin resistance in NAFLD and its molecular mechanism based on human samples, animal models, and cell models. RNA-seq was performed to analyze the signal pathways associated with VEGFB and NAFLD; Palmitic acid and High-fat diet were used to induce insulin-resistant HepG2 cells model and NAFLD animal model. Intracellular glucolipid contents, glucose uptake, hepatic and serum glucose and lipid levels were examined by Microassay and Elisa. Hematoxylin-eosin staining, Oil Red O staining, and Periodic acid-schiff staining were used to analyze the hepatic steatosis, lipid droplet, and glycogen content in the liver. Western blot and quantitative real-time fluorescent PCR were used to verify the expression levels of the VEGFB and insulin resistance-related signals PI3K/AKT pathway.
Results
We observed that VEGFB is genetically associated with NAFLD and the PI3K/AKT signal pathway. After VEGFB knockout, glucolipids levels were increased, and glucose uptake ability was decreased in insulin-resistant HepG2 cells. Meanwhile, body weight, blood glucose, blood lipids, and hepatic glucose of NAFLD mice were increased, and hepatic glycogen, glucose tolerance, and insulin sensitivity were decreased. Moreover, VEGFB overexpression reduced glucolipids and insulin resistance levels in HepG2 cells. Specifically, VEGFB/VEGFR1 activates the PI3K/AKT signals by activating p-IRS1Ser307 expression, inhibiting p-FOXO1pS256 and p-GSK3Ser9 expressions to reduce gluconeogenesis and glycogen synthesis in the liver. Moreover, VEGFB could also enhance the expression level of GLUT2 to accelerate glucose transport and reduce blood glucose levels, maintaining glucose homeostasis.
Conclusions
Our studies suggest that VEGFB could present a novel strategy for treating NAFLD as a positive factor.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-024-05621-w.
Keywords: VEGFB, NAFLD, Insulin resistance, PI3K/AKT, GLUT2
Introduction
Non-alcoholic fatty liver disease (NAFLD) is manifested with overloaded lipids in the liver as pathological features [1]. Hepatic insulin resistance is tightly linked to the development of NAFLD, leading to steatosis and lipid storage in hepatocytes [2]. Increased lipids or free fatty acids flow into the liver and elevated de novo synthesis of lipids causes the excessive production of fat in the body, which gradually accumulates to form lipid droplets, exacerbating the progression of insulin resistance [3]. Hyperinsulinemia induced by insulin resistance caused inability to inhibit glucose production and reduced glucose metabolism in the liver, affecting systemic glucose metabolism [4]. Therefore, glucolipid metabolism disorders caused by insulin resistance predispose individuals to NAFLD, type 2 diabetes, and other metabolic diseases [5]. At present, a common therapeutic strategy for NAFLD is to reduce excessive lipids and change lifestyles. However, specific pharmacotherapy is expected the most.
Vascular endothelial growth factor B (VEGFB) belongs to the VEGF family, but its usual function is not manifested in angiogenesis [6]. A larger number of researches proved VEGFB plays a part in glucolipid metabolism and obesity-related diseases. VEGFB transduction in mice with obesity could ameliorate glucolipid metabolism conditions [7]. tPep-VEGFB treatment alleviated hyperlipidemia in high-fat diet (HFD) induced NAFLD mice [8]. VEGFB reduced lipid deposition by inhibiting the expression of fatty acid transport proteins in diabetic mice [9]. Hence, VEGFB was regarded to play a part in regulating lipid metabolism and insulin resistance in the body with obesity.
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signal pathway is vital for the development of insulin resistance [10]. The impaired PI3K/AKT signal exacerbated insulin resistance in tissues, leading to the expedited development of NAFLD [11]. PI3K/AKT signal activation in the liver alleviates whole-body insulin resistance and obesity [3]. Specifically, insulin-mediated insulin resistance was regulated by up- and downstream molecules involved in the PI3K/AKT pathway. Insulin receptor (INSR) and insulin receptor substrate (IRS), the upstream proteins of PI3K, were activated by the increase of insulin release [12]. The activation of INSR promotes the bioactivity of IRS, which upregulates the PI3K/AKT signals to stimulate the downstream signals [13]. Forkhead box O1 (FOXO1) and glycogen synthase kinase-3β (GSK3β), the downstream proteins of AKT, are respectively responsible for hepatic gluconeogenesis and glycogen synthesis [14]. Glucose uptake and output in hepatocytes is regulated by the glucose transporter 2 (GLUT2) [15]. Therefore, increasing PI3K/AKT signals could promote glucose uptake by activating GLUT2, and reduce the activity of FOXO1 and GSK3β to inhibit glucose production and increase glucose utilization in NAFLD [16].
Here, in our study, we analyzed the relationship between VEGFB and glycolipid metabolism, and NAFLD by using bioinformatic databases. By screening the transcriptome data of clinical samples, the differential signal pathways associated with NAFLD were analyzed. VEGFB knockdown and overexpression were used to verify the regulatory effect of VEGFB on palmitic acid (PA) induced insulin-resistant HepG2 cells and its molecular signal PI3K/AKT pathway. In addition, the NAFLD mouse model was successfully established by HFD in VEGFB+/+ and VEGFB−/− mice. The VEGFB overexpression model was constructed by injecting adeno-associated virus. The effects and molecular mechanisms of VEGFB on insulin resistance and liver injury in NAFLD were examined by molecular biology. Our findings provide the supportive basis and novel insights for the diagnosis and treatment of NAFLD.
Methods
Human subjects
All the experiments involving human subjects were approved by the ethics committee of Binzhou Medical University (IRB: 2023 − 318). All participants in the human study signed informed consent. The study was in accord with the declaration of Helsinki. The inclusion criteria for patients with NAFLD were: (1) No history of alcohol consumption or alcohol consumption less than 140 g per week (< 70 g for women); (2) Excluding specific diseases that can lead to fatty liver such as viral hepatitis, drug-induced liver disease, total parenteral nutrition, and hepatolenticular degeneration; (3) Ultrasound examination showed diffuse enhancement of echo in liver tissue with mild to moderate liver enlargement. Healthy Individuals without a diagnosis of NAFLD were regarded as the controls. The blood samples of participants (n = 60) were collected from Binzhou Medical University Hospital. All blood biochemical indexes of human subjects were measured by the fully automatic biochemical analyzer (Japan, SYSMEX XE5000) (see Table 1).
Table 1.
Main parameters of patients with NAFLD and control groups
| NAFLD | Control | p-value | |
|---|---|---|---|
| Ages (years) | 70.76 ± 12.242 | 65.07 ± 11.594 | 0.0579 |
| SBP (mmHG) | 158.0 ± 27.119 | 157.1 ± 4.631 | 0.1270 |
| DBP (mmHG) | 89.83 ± 15,297 | 82.67 ± 10.186 | 0.0376 |
| Weight (kg) | 60.70 ± 9.920 | 65.28 ± 9.749 | 0.0765 |
| BMI (kg/m2) | 24.08 ± 3.654 | 20.85 ± 3.848 | < 0.01 |
| ALT (U/L) | 38.51 ± 10.210 | 19.80 ± 9.304 | < 0.01 |
| AST (U/L) | 31.90 ± 11.698 | 23.53 ± 7.948 | < 0.01 |
| ALP (U/L) | 98.21 ± 25.731 | 96.47 ± 21.272 | 0.7759 |
| Albumin (g/L) | 39.79 ± 3.795 | 41.31 ± 5.978 | 0.2454 |
| Globumin (g/L) | 27.36 ± 5.604 | 27.67 ± 4.300 | 0.8070 |
| A/G | 1.843 ± 1.705 | 1.567 ± 0.198 | 0.3857 |
| TG (mmol/L) | 1.395 ± 0.636 | 0.9693 ± 0.504 | < 0.01 |
| TC (uumol/L) | 4.489 ± 1.095 | 4.688 ± 0.975 | 0.4610 |
| HDL-C (mmol/L) | 1.164 ± 0.340 | 1.628 ± 0.649 | < 0.01 |
| LDL-C (mmol/L) | 6.615 ± 1.837 | 2.450 ± 0.819 | < 0.01 |
| FPG (mmol/L) | 8.903 ± 3.580 | 6.698 ± 1.049 | < 0.01 |
| HbA1C (%) | 10.803 ± 1.816 | 7.287 ± 1.390 | < 0.01 |
| Ins (ulU/mL) | 16.34 ± 7.374 | 11.25 ± 6.872 | < 0.01 |
Animal protocol
The experimental mice were provided by Jinan PengYue Experimental Animal Breeding Co., Ltd and housed in an environment of temperature at 24 °C, humidity at 50%, and light/dark duration of 12 h/12 h. One week of adjustable feeding with food and water was performed before the experiment was executed. All animal experiments were under the approval of the animal ethics committee of Binzhou Medical University (IACUC protocol number: 2023 − 317).
VEGFB+/+ and VEGFB−/− mice (catalog number: S-KO-05685) were designed in the previous study(17). VEGFB+/+ mice were named as WT mice and VEGFB−/− mice were named as KO mice. C57BL/6 mice were fed a standard diet (SD) from the 4th week. VEGFB+/+ and VEGFB−/− mice were fed a HFD and named HFD-WT and HFD-KO mice from the 8th week to the 28th week. Elevated TC, TG, and HOMA-IR indexes were used to select the mice of NAFLD with insulin resistance. The selected mice were given AAV injections and named AAV-Ctrl and AAV-B186 mice. All mice were sacrificed with anesthesia by being broken the neck in the 28th week (Supplemented Fig. 1A).
Cell line and reagent
The HepG2 cell line was purchased from Procell Life Science Technology Co. Ltd and cultured in the DMEM medium (Gibco, Brazil) with 10% FBS (Procell, China) and 1% P/S (Solarbio, China) at 5% CO2 atmosphere and 37 °C temperature. Palmitic acid (PA) was prepared to induce insulin resistance in HepG2 cells as described in the previous study(17). Cells were incubated in the 96-well plate for the detection of viability with the MTT reagent. 300 µM PA was the optimal concentration to induce the insulin-resistant cell model.
Bioinformatics and RNA-seq analysis
Genome-wide association Studies (GWAS) were used to analyze the association between the VEGFB gene and environmental factors on a few significant loci in the human genome database (https://www.ebi.ac.uk/gwas/genes/VEGFB). Phenome-wide association analysis (PheWAS), a supplementary study to GWAS, was used to assess the associations of VEGFB with a great number of different phenotypes in the mouse genome database (https://systems-genetics.org/phewas). To assess the correlation between VEGFB and PI3K, we used GeneCards dataset (https://www.genecards.org) to screen VEGFB and PI3K co-expressed genes and design the Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/). The KEGG signal pathway was analyzed by WebGestalt (https://www.webgestalt.org/) after co-expressed genes were enriched. The whole blood samples of human subjects (n = 5) were collected and transferred into the PAXgen blood collection tube (PreAnalytiX GmbH, USA) for the RNA-seq analysis.
Knockdown of VEGFB
VEGFB−/− mice were generated with CRISPR/Cas9 technology by Guangzhou Cyagen Biosciences. HepG2 cells were transfected with or without siRNA-mediated VEGFB silencing plasmid after being cultured in the 6-well plate. Jet PRIME and Jet buffer were the vectors used to transfer the plasmid into the cells. Replaced the medium after 4 h of transfection and added the fresh medium to continue to culture. The protein and mRNA expression of VEGFB in liver tissue and HepG2 cells were detected.
Overexpression of VEGFB
The lentivirus used for HepG2 cells and AAV used for mice were gengerated by OBIO Technology (Shanghai, China). The vectors carried on the VEGFB-control and VEGFB-overexpressed plasmid were labeled with green fluorescence protein. HepG2 cells were cultured in the 12-well plate before the infection. The optimal multiplicity of infection (MOI) was 20. Lentivirus with pcSLenti-EF1-EGFP-P2A-Puro-CMV-MCS-3xFLAG-WPRE and pcSLenti-EF1-EGFP-P2A-Puro-CMV-VEGFB-3xFLAG-WPRE was added to infect the HepG2 cells for 72 h. 4 µg/mL puromycin was used to select stable cell line. The virus volume for the AAV injection was calculated according to the total virus volume of 2.5 × 1011 vg per mouse and the virus titer of 2.5 × 1011 vg/mL. At the 20th week, VEGFB+/+ mice were injected with VEGFB overexpression plasmid and empty carrier plasmid respectively through the tail vein, and the livers were collected 4 weeks later to observe the fluorescence intensity. Extracted the mRNA and protein of HepG2 cells and the livers to determine the level of VEGFB expression.
Enzyme-linked immunosorbent assay (Elisa)
Plasma VEGFB content of human samples was detected with the Human VEGFB Elisa kit (No.ml1060753V) according to the instructions. The serum insulin of mice was examined in the 24th week with the mouse insulin Elisa kit (No.ml001983) under the instructions. The serum was acquired by centrifuging blood from eyeball blood after mice were exposed to anesthesia. OD value for the calculation was detected by a microplate reader.
Biochemical measurement of lipids
The content of TG, TC, HDL, and LDL were determined in the serum of mice and HepG2 cells. All detection kits were purchased from Nanjing Jiancheng Bioengineering Institute. The detection process followed the manufacturer’s instructions.
Glucose uptake assay
HepG2 cells were cultured in the 24-well plate with a density of 70%. Replaced the DMEM with the glucose-free medium containing 50 nM insulin for 30 min at 37 °C. 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose (2-NBDG) with the concentration of 10 µM was added into the glucose-free medium for 1 h at 37 °C. The fluorescence intensity of 2-NBDG was detected by a microplate reader at 485 nm excitation and 535 nm emission.
Glucose consumption assay
5 × 106 HepG2 cells were collected into the centrifuge tube and the supernatant was discarded after centrifugation. 1 mL distilled water was added into the centrifuge tube, the cells were broken by ultrasound in an ice bath under the power of 200 W, 5 s of ultrasonication, and 5 s of intervals. Repeated 15 times of ultrasonication and boiled in a 100 °C water bath for 10 min. After cooling, centrifuged cells with the speed of 8000 g at 25 °C for 10 min, and the supernatant was used to measure by a glucose-oxidase assay kit (BC2505, Solarbio Technologies Inc.)
The liver tissues were collected for the detection of glucose content by a glucose-oxidase assay kit (E1011, Applygen Technologies Inc.). Taken 50 mg of liver tissues and homogenized with 1000 µL lysis Buffer. The supernatant was collected to measure the protein content by the Bradford method kit (T9310A TaKaRa). Added the standard distilled water, substance, and samples respectively according to the manufacturer’s instruction. Measured the OD value at 550 operating wavelength. Calculated the glucose content with protein content of the liver tissues.
Glycogen assessment assay
1 × 107 HepG2 cells were collected into the centrifuge tube and centrifuged. Liver tissues were extracted and weighed at 200 mg. 0.75 mL lysate was added for the ultrasonication of cells (power 200 W, ultrasonicated 5s, 5s interval, repeated 20 times). Similarly, 0.75 mL lysate was added to the liver tissues. Both HepG2 cells and liver tissues were transferred into a 10 mL tube and boiled in a 100 °C water bath for 20 min in the tube. Shook the tube once every 5 min to mix thoroughly. Filled with distilled water to 5 mL after cooling. Mixed and centrifuged at 8000 g for 10 min, and the supernatant was taken to be measured by a glycogen content test kit (BC0345, Solarbio Technologies Inc.).
Hematoxylin-eosin (H&E) staining
H&E staining was performed to observe the morphological structure of the liver in mice as described previously(17).
Oil Red O staining
The frozen sections of the liver and HepG2 cells were aired and stained with Oil Red O for 10 min. 60% isopropanol was used to differentiate and wash. Then hematoxylin was added to re-stain the sections for 5 min. Sealed with glycerogelatin after washing to observe by using an optical microscope. Image J software was used to analyze the contents of lipids by calculating the area of red lipid droplets.
Periodic acid–Schiff (PAS) staining
The frozen sections were rewarmed and fixed for 15 min. Periodic acid was used to immerse the liver sections for 15 min. Washed the sections with tap water and distilled water twice respectively. Added Schiff liquid in the liver sections for 15 min in the dark. Rinsed with running water for 2 min and viewed under the microscope. Image J software was used to analyze the glycogen content by calculating the area of red glycogen particles.
Western blot
Proteins samples were produced from the liver tissue and HepG2 cells by using RIPA (Solarbio, R0010, Beijing) containing 1% PMSF (Solarbio, PO100, Beijing) and were boiled with loading buffer (Solarbio, D1020-5, Beijing) for 10 min. The protein molecules were departed by electrophoresis in SDS-PAGE and were transferred to the methyl alcohol-activated PVDF membrane.The membrane was incubated with primary antibodies after sealing in the milk or BSA. Mouse or Rabbit-sourced secondary antibodies were used to incubate the membrane after TBST washing. The bands of proteins were developed with ECL by an enhanced chemiluminescence reaction (Tanon5200, Tanon Science & Technology). The targeted proteins were normalized with β-actin controlled. Antibodies for human and mouse species used in this study were illustrated in Table 2.
Table 2.
Antibodies (human and mouse) used in this study
| Antibody | Dilution rate | Source | Company |
|---|---|---|---|
| INSR | 1:1000 | Rabbit | Abmart |
| p-INSRThr1375 | 1:1000 | Rabbit | Abmart |
| IRS1 | 1:1000 | Rabbit | Abmart |
| p-IRS1Ser307 | 1:1000 | Rabbit | Abmart |
| PI3K | 1:1000 | Rabbit | Abmart |
| p-PI3KTyr467 | 1:1000 | Rabbit | Abmart |
| AKT | 1:1000 | Rabbit | Abmart |
| p-AKTSer473 | 1:1000 | Rabbit | Abmart |
| GLUT2 | 1:1000 | Rabbit | Abmart |
| GSK3β | 1:1000 | Mouse | Abmart |
| p-GSK3βSer9 | 1:1000 | Rabbit | Abmart |
| FOXO1 | 1:1000 | Rabbit | Abmart |
| p-FOXO1pS256 | 1:1000 | Rabbit | Abmart |
| VEGFB | 1:1000 | Rabbit | Abcam |
| VEGFR1 | 1:1000 | Rabbit | Abmart |
| β-actin | 1:2000 | Mouse | GenScript |
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA in the liver and HepG2 cells were extracted by Trizol (R401-01-AA, Vazyme Biotech Co., Ltd.). Detection of RNA concentration was used to quantitate for reverse transcription. Removal of genomic DNA was performed by 4× DNA Wiper Mix, RNA, and DEPC water. Transcription reaction was initiated by 5× HiScript II qRT SuperMix II (R223-01, Vazyme Biotech Co., Ltd.). After cDNA synthesis, the quantitative PCR amplification reaction was performed with SYBR Green methods by QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific, Inc.). The relative expression of target genes was calculated by the 2−△△Ct method. The sequence of primers for human and mouse species used in this study were illustrated in Tables 3 and 4.
Table 3.
Sequence of primers (human) used in this study
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| VEGFR1 | TTTGCCTGAAATGGTGAGTAAGG | TGGTTTGCTTGAGCTGTGTTC |
| INSR | TAGTGACCAGCTATAATCAGAG | ACGCCAAGGTCTGAAGGTCC |
| IRS1 | ACAAACGCTTCTTCGTACTGC | AGTCAGCCCGCTTGTTGATG |
| PI3K | TATTTGGACTTTGCGACAAGACT | TCGAACGTACTGGTCTGGATAG |
| AKT | TCCTCCTCAAGAATGATGGCA | GTGCGTTCGATGACAGTGGT |
| GLUT2 | GCTGCTCAACTAATCACCATGC | TGGTCCCAATTTTGAAAACCCC |
| PEPCK | GCCATCATGCCGTAGCATC | AGCCTCAGTTCCATCACAGAT |
| G6Pase | GTGTCCGTGATCGCAGACC | GACGAGGTTGAGCCAGTCTC |
| β-actin | CATCCGTAAAGACCTCTATGCCAAC | ATGGAGCCACCGATCCACA |
Table 4.
Sequence of primers (mouse) used in this study
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| VEGFR1 | CTCAGGGTCGAAGTTAAAAGTGC | TTGCCTGTTATCCCTCCCACA |
| INSR | ATGGGCTTCGGGAGAGGAT | CTTCGGGTCTGGTCTTGAACA |
| IRS1 | CGATGGCTTCTCAGACGTG | CAGCCCGCTTGTTGATGTTG |
| PI3K | ACACCACGGTTTGGACTATGG | GGCTACAGTAGTGGGCTTGG |
| AKT | ATGAACGACGTAGCCATTGTG | TTGTAGCCAATAAAGGTGCCAT |
| GLUT2 | TCAGAAGACAAGATCACCGGA | GCTGGTGTGACTGTAAGTGGG |
| PEPCK | CTGCATAACGGTCTGGACTTC | CAGCAACTGCCCGTACTCC |
| G6Pase | CGACTCGCTATCTCCAAGTGA | GTTGAACCAGTCTCCGACCA |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
Statistical analysis
All data were analyzed by the SPSS 20.0 software. Means ± standard deviation was used to calculate the produced data. Two groups were analyzed with the two-tailed independent-samples t-test. Multiple groups were analyzed by one-way analysis of variance (ANOVA) when the data conformed to the normal distribution and the variance of data was homogenous; multiple groups were analyzed by Kruskal-Wallis (K-W) test when the data did not conform to the normal distribution and the variance of data was not homogenous. Pearson correlation analysis was used to perform the correlation between VEGFB and Hb1Ac and LDL levels when the data conformed to the normal distribution. Data followed by p < 0.05 were considered significantly different and labeled with symbols *, #, and Δ.
Results
VEGFB is genetically associated with the PI3K/AKT signal pathway in NAFLD
We examined plasma VEGFB levels by Elisa in the patients with NAFLD. The results found that plasma VEGFB content was much lower in the NAFLD group compared to the control group (n = 30) (Fig. 1A). Moreover, VEGFB was analyzed to have correlations with HbA1c (r = − 0.433, p = 0.0167), and LDL (r = − 0.5452, p = 0.0018) in patients with NAFLD (n = 30) (Fig. 1B, C). The Manhattan diagram showed that the VEGFB gene is associated with lipid-related indicators after bioinformatic GWAS analysis. Significant SNP dots were analyzed with colors, representing different characters in the GWAS catalog database (Fig. 1D). The PheWAS diagram results of phenotype correlation analysis illustrated that glucose metabolism-related phenotypes including LDL cholesterol level, and blood glucose level are associated with the VEGFB gene. Moreover, the liver injury phenotype also link to the VEGFB gene (Fig. 1E). Venn diagram demonstrated that 23 co-expressed VEGFB and PI3K genes were enriched in the PI3K/AKT, VEGF, and FOXO signal pathway (Fig. 1F, G). RNA-seq results of patients with NAFLD and control analyzed that the VEGF signals were enriched in downregulated genes in patients with the NAFLD groups and the control group (Fig. 1H). Moreover, total differentially expressed genes enriched in the negative regulation of the PI3K/AKT signal pathway between patients with the NAFLD groups and the control group (Fig. 1I).
Fig. 1.
VEGFB is genetically associated with the PI3K/AKT signal pathway in NAFLD. (A) Plasma VEGFB content (n = 30) (B) Correlation between VEGFB and HbA1c (n = 30) (C) Correlation between VEGFB and LDL (n = 30) (D) GWAS analysis (VEGFB location: Chromosome 11-NC_000011.10 (64225980.64248214)) (E) PheWAS analysis (F) Venn diagram of the interaction between PI3K and VEGFB gene (G) Bubble diagram of KEGG analysis in co-expressed genes of PI3K and VEGFB (H) Bubble diagram of KEGG analysis in top20 downregulated genes between the NAFLD group and the Control group (n = 5) (I) Bubble diagram of KEGG analysis in top20 all differentiated genes between the NAFLD group and Control group (n = 5) * indicates a significant difference p < 0.05
VEGFB improves glucolipid levels by activating the PI3K/AKT signal pathway in PA-induced HepG2 cells
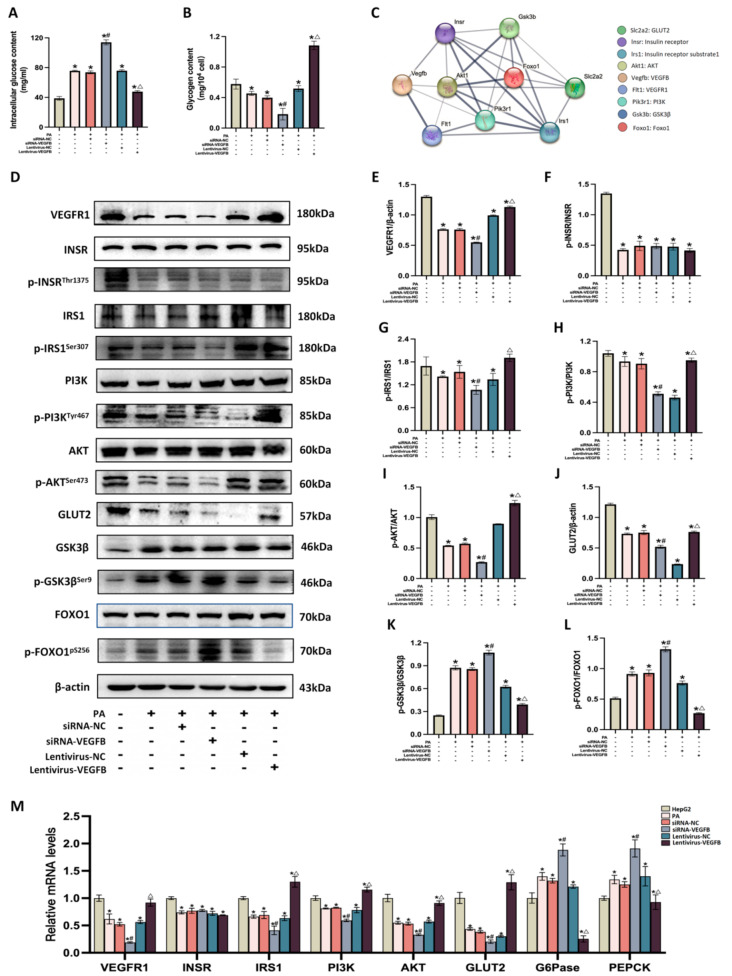
We assessed VEGFB gene was downregulated and overexpressed in HepG2 cells at protein and mRNA levels (Fig. 2A-D). MTT assay was used to detect the toxicity of PA by assessing cell viability, indicating that 300 µM PA was nontoxic to the cell viability (Fig. 2E). 2-NBDG a fluorescence probe, was used to confirm that the ability of glucose uptake in HepG2 cells reduced at 300 µM PA induction (Fig. 2F). Therefore, 300 µM PA was regarded as the optimal concentration to induce the insulin-resistant HepG2 cell. Results of the Oil Red O staining and quantitative analysis illustrated that numerous lipid droplets significantly accumulated in PA-induced HepG2 cells (Fig. 2G, H). Intracellular TC and TG contents were elevated after VEGFB was knocked down and were reduced by VEGFB overexpression (Fig. 2I, J). We assessed the ability of glucose uptake of HepG2 cells with 2-NBDG, indicating that glucose uptake was decreased with VEGFB down-regulation, and increased significantly after VEGFB was overexpressed (Fig. 2K).
Fig. 2.
Effects of VEGFB on glucolipid levels in PA-induced HepG2 cell (A) VEGFB expression at the protein level with siRNA transfection (n = 3) (B) VEGFB expression at the mRNA level with siRNA transfection (n = 3) (C) VEGFB expression at the protein level with lentivirus infection (n = 3) (D) VEGFB expression at the mRNA level with lentivirus infection (n = 3) (E) Cell viability was examined by MTT (n = 4) (F) Glucose uptake was assessed with 2-NBDG in HepG2 cell (n = 6) (G) Oil red O staining (H) Quantitative analysis of Oil red O staining (n = 3) (I) Intracellular TC content (n = 3) (J) Intracellular TG content (n = 3) (K) Glucose uptake in HepG2 cell (n = 3) * indicates to compare with HepG2 group, # indicates to compare with IR + siRNA-NC group, Δ indicates to compare with IR + LV-NC group with a significant difference p < 0.05
We detected the intracellular glucose and glycogen contents in insulin-resistant HepG2 cells. We found that VEGFB suppression caused an increase in glucose level and a decrease in glycogen level. In contrast, VEGFB overexpression reduced the levels of glucose and increased glycogen levels (Fig. 3A, B). We used PPI network analysis to confirm interactions between VEGFB and VEGFR1, INSR, IRS1, PI3K, AKT, GLUT2, GSK3β, and FOXO1 proteins (Fig. 3C). Mechanically, VEGFB knockout reduced the expression of VEGFR1, p-IRS1Ser307, p-PI3KTyr467, and p-AKTSer473 at protein levels, while VEGFB overexpression markedly increased their expressions in PA-induced HepG2. However, VEGFB knockout and overexpression did not affect INSR expression at protein level. Additionally, the variation of GLUT2 expression at the protein level was consistent with the VEGFB’s alterations. We further measured the GSK3β and FOXO1 proteins that control gluconeogenesis and glycogen synthesis. We found that the expression levels of p-GSK3βSer9 and p-FOXO1pS256 increased after VEGFB was suppressed, and decreased as VEGFB overexpression (Fig. 3D-L). Detection at the mRNA levels showed the expressions of VEGFR1, IRS1, PI3K, AKT, and GLUT2 were reduced with the VEGFB knockdown and were overactivated by VEGFB overexpression. Also, the INSR expression did not change with VEGFB suppression and overexpression. Gluconeogenesis-related genes PEPCK and G6Pase were dramatically elevated with siRNA-VEGFB and were decreased with lentivirus-VEGFB in HepG2 cells (Fig. 3M).
Fig. 3.
VEGFB activates PI3K/AKT signal pathway in insulin-resistant HepG2 cells (A) Intracellular glucose content (n = 3) (B) Intracellular glycogen content (n = 3) (C) PPI analysis (D) VEGFR1, INSR, p-INSRThr1375, IRS1, p-IRS1Ser307, PI3K, p-PI3KTyr467, AKT, p-AKTSer473, GLUT2, GSK3β, p-GSK3βSer9, FOXO1, and p-FOXO1pS256 proteins expression were detected by western blot (E) VEGFR1/β-actin expression (n = 3) (F) p-INSRThr1375/INSR expression (n = 3) (G) p-IRS1Ser307/IRS1 expression (n = 3) (H) p-PI3KTyr467/PI3K expression (n = 3) (I) p-AKTSer473/AKT expression (n = 3) (J) GLUT2/β-actin expression (n = 3) (K) p-GSK3βSer9/GSK3β expression (n = 3) (L) p-FOXO1pS256/FOXO1 expression (n = 3) (M) Effects of VEGFB on VEGFR1, INSR, IRS1, PI3K, AKT, GLUT2, G6Pase, and PEPCK genes expression at mRNA level (n = 3) * indicates to compare with HepG2 group, # indicates to compare with IR + siRNA-NC group, Δ indicates to compare with IR + LV-NC group with a significant difference p < 0.05
VEGFB ameliorates insulin resistance and hepatic steatosis in NAFLD mice
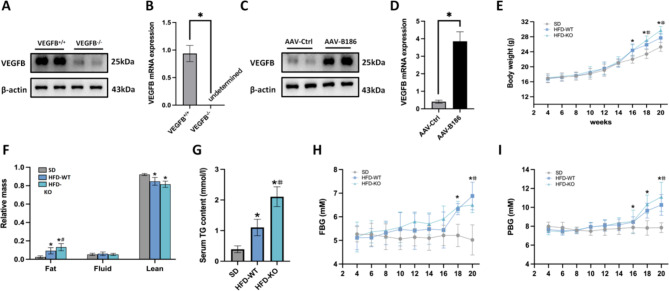
In animal experiments, we generated the VEGFB knockout and liver-specific VEGFB overexpression mouse models and detected the protein and mRNA levels of VEGFB (Fig. 4A-D). To verify the status of insulin resistance mice model, we determined the body weight, body fat mass, FBG, and PBG levels of mice induced by SD and HFD. In addition, the detection of TG was performed to assess NAFLD model with insulin resistance. The results showed that the body weight and PBG of VEGFB+/+ and VEGFB-/- mice induced by HFD were higher from the 16th week to the 20th week when compared to SD mice. The FBG of HFD-induced VEGFB+/+ and VEGFB-/- mice was higher from the 18th week to the 20th week. Moreover, the relative body fat mass and serum TG levels in HFD-induced VEGFB+/+ and VEGFB-/- mice increased significantly in the 20th week. Therefore, we considered that insulin resistance in NAFLD model was completed in the 20th week. Compared with the HFD-WT mice, the body weight of HFD-KO mice significantly increased from the 18th week to the 20th week, while the FBG and PBG of the HFD-KO mice increased dramatically in the 20th week. Likewise, the body fat mass and serum TG levels of the HFD-KO mice were higher than that of the HFD-WT mice in the 20th week (Fig. 4E-I).
Fig. 4.
The NAFLD mice model was completed. (A) VEGFB protein expression (n = 3) (B) Relative mRNA level of VEGFB (n = 3) (C) VEGFB protein expression (n = 3) (D) Relative mRNA level of VEGFB (n = 3) (E) Body weight (SD, n = 9; HFD-WT, n = 27; HFD-KO, n = 9) (F) Relative body fat mass, fluid mass, and lean mass (SD, n = 9; HFD-WT, n = 27; HFD-KO, n = 9) (G) Serum TG (SD, n = 9; HFD-WT, n = 27; HFD-KO, n = 9) (H) FBG (SD, n = 9; HFD-WT, n = 27; HFD-KO, n = 9) (I) PBG (SD, n = 9; HFD-WT, n = 27; HFD-KO, n = 9) * indicates to compare with SD group, # indicates to compare with HFD-WT group, Δ indicates to compare with HFD-KO group with a significant difference p < 0.05
Furthermore, we randomly classified VEGFB+/+ mice fed with HFD into 3 groups: HFD-WT, AAV-Ctrl, and AAV-B186 groups in the 20th week for further study. We observed that the body weight of the HFD-KO mice remarkably increased from the 20th week to the 24th week. However, after VEGFB overexpression, the AAV-B186 mice significantly lost weight in the 24th week (Fig. 5A). In terms of fat in the body, in the HFD-KO group, the fat of mice obviously increased and decreased after VEGFB overexpression in the 24th week (Fig. 5B). Afterward, we detected the serum lipid indicators and found TG, TC, and LDL were higher, and HDL was lower in the HFD-KO mice than in the HFD-WT mice (Fig. 5C-F). We also observed that the FBG and PBG of mice in HFD-KO group were remarkably higher than in the HFD-WT group from the 20th week to the 24th week, while blood glucose decreased in the 24th week after VEGFB injection (Fig. 5G, H). OGTT and IPITT analysis showed that the glucose tolerance and insulin sensitivity were lower in the HFD-KO mice than those in the HFD-WT, and increased as VEGFB was overexpressed (Fig. 5I-L) at the 24th week. The content of HbA1c in HFD-KO mice was higher than that in the HFD-WT mice and was lower in the AAV-B186 mice than in the AAV-Ctrl mice (Fig. 5M). The serum insulin was detected to calculate the HOMA-IR of mice, indicating that serum insulin decreased to the lowest level in the HFD-KO group and increased significantly after VEGFB was overexpressed (Fig. 5N, O). QUICKI was calculated to assess insulin sensitivity based on the levels of serum insulin and FBG. The results showed that QUICKI decreased dramatically in the HFD-KO group and increased with VEGFB overexpression (Fig. 5P).
Fig. 5.
Effects of VEGFB on insulin resistance of NAFLD mice. (A) Body weight (n = 9) (B) Relative body fat mass in the 24th week (n = 9) (C) Serum TG (n = 6) (D) Serum TC (n = 6) (E) Serum LDL (n = 6) (F) Serum HDL (n = 6) (G) FBG (n = 9) (H) PBG (n = 9) (I) OGTT (n = 9) (J) AUC of OGTT (n = 9) (K) IPITT (n = 9) (L) AUC of IPITT (n = 9) (M) HbA1c content (n = 6) (N) Serum insulin (n = 6) (O) HOMA-IR (n = 6) (P) QUICKI (n = 6) * indicates to compare with SD group, # indicates to compare with HFD-WT group, Δ indicates to compare with HFD-KO group with a significant difference p < 0.05
We observed from HE staining that after HFD, hydropic degeneration was shown in hepatocytes, and manifested in the central area of the liver lobule. The hepatocytes swelled and became larger, and the red-stained particles were assembled in the cytoplasm. Meanwhile, vacuolation was observed in the cytoplasm. After AVV injection, the hydrodegeneration of hepatocytes was alleviated (Fig. 6A). Oil-red O staining indicated that in the HFD mice, hepatocytes were densely surrounded with red-stained lipid droplets with different sizes, and the liver sinus dilated significantly compared to the SD group. The number of red lipid droplets in the liver of the HFD-KO mice was much more than in the HFD-WT mice, while the number of the lipid droplets decreased after VEGFB injection (Fig. 6B) (Supplemented Fig. 1B). PAS staining demonstrated that a large number of rose red glycogen particles were uniformly dispersed in the cytoplasm of the SD mice. Compared with HFD-WT mice, a lower small number of unevenly distributed glycogen particles were observed in the hepatocytes of HFD-KO mice. In the liver of the AAV-B186 mice, glycogen particles were increased than those in the AAV-Ctrl mice (Fig. 6C) (Supplemented Fig. 1C).
Fig. 6.
Effects of VEGFB on the morphology of the liver in NAFLD mice. (A) HE staining (B) Oil red O staining (C) PAS staining
VEGFB inhibits gluconeogenesis and promotes glycogen synthesis by activating PI3K/AKT signal pathway in NAFLD mice
Based on the validation of VEGFB on PI3K/AKT signals in vitro, we next performed western blot and qPCR to detect the function of VEGFB involved in insulin transduction, gluconeogenesis, and glycogen synthesis pathways in vivo. Similarly, we illustrated that VEGFR1, IRS1, PI3K, AKT, and GLUT2 expressions were downregulated in HFD-KO mice and were upregulated by VEGFB overexpression. Likewise, we observed the bioactivity of INSR has no obvious change due to the VEGFB gene modification. In addition, VEGFB knockout increased FOXO1 and GSK3β expressions and decreased them after VEGFB overexpression (Fig. 7A-J). Meanwhile, PEPCK and G6Pase served the highest mRNA expression levels in the HFD-KO mice and their expressions were reduced by the overexpression of VEGFB, contributing to a decrease in hepatic glucose production and an increase in glycogen synthesis (Fig. 7K, L).
Fig. 7.
Effects of VEGFB on hepatic insulin resistance via PI3K/AKT signal pathway (A) VEGFR1, INSR, p-INSRThr1375, IRS1, p-IRS1Ser307, PI3K, p-PI3KTyr467, AKT, p-AKTSer473, GLUT2, GSK3β, p-GSK3βSer9, FOXO1, and p-FOXO1pS256 proteins expression (B) VEGFR1/β-actin expression (C) p-INSRThr1375/INSR expression (D) p-IRS1Ser307/IRS1 expression (E) p-PI3KTyr467/PI3K expression (F) p-AKTSer473/AKT expression (G) GLUT2/β-actin expression (H) p-GSK3βSer9/GSK3β expression (I) p-FOXO1pS256/FOXO1 expression (J) Effects of VEGFB on VEGFR1, IRS1, PI3K, AKT, GLUT2, INSR, PEPCK, and G6Pase genes expression at mRNA level (K) Hepatic glucose content (L) Hepatic glycogen content * indicates to compare with SD group, # indicates to compare with HFD-WT group, Δ indicates to compare with HFD-KO group with a significant difference p < 0.05
Discussion
Vascular endothelial growth factor B, as well as VEGFA, are regarded as the pro-angiogenic factors in the VEGF family. VEGFB and VEGFA exert their biological functions by combining to the vascular endothelial growth factor receptor 1 (VEGFR1). VEGFA is mainly responsible for the neovascularization in tissues, while VEGFB shows limited performance in it [6]. VEGFB primarily participates in the formation of non-neovascularization and plays an important role in neuronal cells such as retinal nerves and cerebral cortex nerve [18]. VEGFB’s novel role in metabolic diseases has been investigated in recent years. Studies newly illustrated that VEGFB is regarded as an important regulatory molecular of glucolipid metabolism disorders such as diabetes and obesity [19]. Robciuc et al. confirmed VEGFB’s role in participating in insulin signal transduction and insulin function of obese mice [7]. Shang et al. proved that VEGFB can promote lipid metabolism level of myocardial cells in mice with diabetes [20]. An increasing number of researches illustrated that VEGFB was predicted to be a positive regulator in ameliorating metabolism diseases. Here, we confirmed by GWAS and PheWAS that the VEGFB gene is associated with glucolipid metabolism. Moreover, we investigated that the VEGFB gene is associated with liver injury. We observed the effects of VEGFB on NAFLD in the previous studies that VEGFB deficiency could aggravate lipid metabolism disorder in NAFLD, and also increase insulin resistance levels, which inspired our current hypothesis that VEGFB is involved in regulating insulin resistance in the pathogenesis of NAFLD. Research showed that low serum VEGFB levels could be regarded as an indicator in adults with obesity at early stage [21]. We explored the plasma VEGFB levels were decreased in patients with NAFLD. Moreover, we analyzed that VEGFB levels were associated negatively with HbA1c and LDL levels, which indicated that low VEGFB levels may accelerate insulin resistance in NAFLD to increase levels of the makers of insulin resistance such as HbA1c and LDL.
VEGFB has positive regulatory effects on lipid metabolism in obesity and NAFLD. More specifically, VEGFB’s role in regulating lipid metabolism depends on promoting lipid oxidation and inhibiting lipid synthesis [17, 22]. Robicuc et al. confirmed that the body weight of mice with obesity reduced significantly after AAV-VEGFB186 injection [7]. Lei Hu et al. found that VEGFB recombinant protein alleviated hyperlipidemia in NAFLD mice [8]. Our study also showed a similar regulatory effect of VEGFB that after 16 weeks of HFD, the body weight, body fat, serum TC, TG, and LDL content of mice were decreased, indicating that VEGFB ameliorates lipid metabolism levels in NAFLD. Long-term lipid metabolism disorders have negative impacts on glucose homeostasis, aggravating insulin resistance [23]. VEGFB is not only involved in improving lipid metabolism but also in ameliorating glucose metabolism. Robicuc et al. also found that VEGFB lowered insulin levels and promoted insulin signal transduction besides playing a lipid-reducing effect in obese mice [7]. In addition, it can also be observed that VEGFB overexpression effectively improves glucose tolerance and insulin sensitivity in NAFLD mice. Our study noticed that VEGFB deficiency elevated the FBG and PBG levels of NAFLD mice, and reduced the blood glucose after VEGFB overexpression. VEGFB is also regarded as a regulator to control glucose tolerance and insulin sensitivity by OGTT and IPITT in mice. Therefore, we concluded that VEGFB could restore impaired glucose tolerance and insulin sensitivity in NAFLD mice. An increased risk of insulin resistance with NAFLD could be assessed by the measures of HbA1c [24]. Our test showed that HbA1c was increased in VEGFB−/− mice, indicating that VEGFB could be targeted in biomarker HbA1c to regulate insulin resistance in NAFLD. Meanwhile, HOMA-IR and QUICKI are calculated to assess insulin resistance and insulin sensitivity in NAFLD as critical alternative parameters [25, 26]. In this study, we found that there was an increase in HOMA-IR and a decrease in QUICKI when VEGFB was knocked out, which revealed that VEGFB regulated hepatic insulin resistance in NAFLD mice as an insulin resistance sensitizer.
A range of histopathological features of NAFLD include simple steatosis, steatohepatitis, fibrosis, and cirrhosis [27]. The imbalance of lipid metabolism could trigger excessive lipid accumulation in hepatocytes, destroying normal glucose production and consumption [28]. Rongrong Li et al. proved that VEGFB had an ameliorative effect on hepatocyte steatosis and liver injury by assessing bullous steatosis, microtubular steatosis, hepatocyte hypertrophy, and inflammatory cell aggregation [17]. In our study, semblable results were observed that VEGFB could reduce the hydropic degeneration of hepatocytes in the stage of simple hepatic steatosis in NAFLD. Similarly, our results of Oil red O staining are consistent with that, the content of lipid droplets in hepatocytes increased after VEGFB knockout and decreased after VEGFB overexpression. In addition, we also observed the glycogen content in hepatocytes by PAS staining, and the results showed that VEGFB can enhance the glycogen synthesis capacity. In conclusion, VEGFB reduced lipid deposition, restored glycogen contents, and liver injury at morphologic levels.
To probe the regulatory mechanism of VEGFB on the PI3K/AKT signal, we generated PA-induced insulin-resistant HepG2 cell models. In vitro, we observed in cell homogenate that VEGFB ameliorated glucose and lipid levels by reducing TC, TG, and glucose levels and increasing glucose uptake and glycogen levels. Furthermore, we verified whether VEGFB exerts its effects through the PI3K/AKT signal pathway, classic insulin signal transduction. We first investigated VEGFB’s role in the PI3K/AKT signal pathway by bioinformatics, indicating that the VEGFB gene is associated with PI3K/AKT signals. What’s more, RNA-seq analysis demonstrated that negative PI3K/AKT signals were connected with NAFLD.
In the insulin signal PI3K transduction pathway, insulin first binds to the INSR on the membrane and promotes INSR phosphorylation, triggering the upregulation of IRS1 [29]. The activation of IRS1 combined with PI3K molecule containing SH2 domain to activate several downstream molecules of the signal pathway [30]. The abnormal decomposition, phosphorylation, and distribution of IRS protein is one of major mechanisms leading to trigger the impairment of insulin signal transduction and insulin resistance in cells [31, 32]. Our results suggested that phosphorylation of IRS1 increased with VEGFB overexpression. However, no changes in INSR expression with VEGFB knockdown or overexpression were observed. This is because INSR is embedded in a specific position on the membrane and INSR is highly specific and can only bind to insulin or proinsulin-containing insulin molecules [33]. VEGFB is required to perform its biological function in cells by activating the tyrosine kinase receptor VEGFR1 [34]. We analyzed that VEGFR1, as a tyrosine kinase receptor, can induce IRS activation through tyrosine residue phosphorylation, triggering a series of downstream events of metabolic processes. When VEGFB binds to VEGFR1, it changes the structure of the intracellular kinase region, improves the level of substrate protein phosphorylation by increasing the activity of the kinase, and activates the cascade signal to play a wide range of biological functions [35, 36]. VEGFB/VEGFR1 can activate intracellular cascade reaction via PI3K/AKT and cause multiple biological effects related to growth, differentiation, immunity, and tumor [36]. Jared C. Weddell et al. proved that VEGFR1 activates PI3K/AKT signaling to promote macrophage migration [37]. Guohu Di et al. indicated that VEGFB/VEGFR1 improved corneal health by upregulating PI3K/AKT signals in mice with diabetes [38]. In our study, we found that overexpression of VEGFB activated the impaired PI3K/AKT signals pathway and its downstream molecules. AKT phosphorylates its downstream protein FOXO1, resulting in cytoplasmic degradation, and down-regulates the rate-limiting enzymes PEPCK and G6Pase genes of gluconeogenesis, inhibiting gluconeogenesis [39]. Our results also showed that after VEGFB overexpression, AKT activity was enhanced, FOXO1 was inhibited, PEPCK and G6Pase expression levels were decreased, and hepatocyte gluconeogenesis was inhibited. Insulin resistance-induced NAFLD is not only involved in hepatic gluconeogenesis but also in glycogen synthesis [40]. GSK3β is another downstream AKT protein that controls glycogen synthesis. AKT kinase phosphorylates GSK3β, resulting in its inactivation to activate glycogen synthesis [41]. Our results show that VEGFB inhibits GSK3β activity by activating AKT and enhances glycogen synthesis in liver cells.
GLUT2 is an insulin-independent transmembrane carrier protein that promotes the movement of glucose across cell membranes [15]. GLUT2 is located in hepatocyte membranes and is responsible for transferring glucose between the hepatocyte and the blood [42]. PI3K/AKT can exert biological effects through the cascade reaction of protein kinase and phosphatase, including stimulating GLUT2 to promote glucose uptake, stimulating glycogen synthesis [43]. Yanjing Li et al. confirmed that hepatic PI3K and GLUT2 expression levels were significantly reduced in HFD mice [44]. Our results show that VEGFB can enhance GLUT2 expression, improve hepatic glucose uptake, and reduce blood glucose levels to maintain glucose homeostasis in the body.
Our findings showed that VEGFB is tightly linked to the onset of NAFLD, and regulates the insulin resistance through the PI3K/AKT signal pathway in the development of NAFLD. The experimental outcomes in vivo and in vitro revealed the clinical value of VEGFB gene in predicting the status of insulin resistance in NAFLD as a potential biomarker. Furthermore, VEGFB might be introduced to a novel strategy for the improvement of insulin resistance in the patients with NAFLD or other metabolic diseases as an insulin sensitizer.
However, our studies remain some limitations on rescue experiments of VEGFB knockout mice. In further studies, we will conduct the rescue experiments by adding VEGFB recombinant protein in VEGFB knockout animal and cell models, exploring whether VEGFB has the potential therapeutic effect to rescue the impaired insulin sensitivity and improved insulin resistance in the development of the NAFLD, which could provide more in-depth evidences of VEGFB’s effects on NAFLD.
Conclusion
In summary, our research illustrated that VEGFB ameliorates glycolipid metabolism levels and insulin resistance in NAFLD. Mechanically, VEGFB activates IRS1 by binding to VEGFR1, up-regulates PI3K/AKT signaling pathway, inhibits FOXO1 and GSK3β proteins, reduces the activities of PEPCK and G6Pase, inhibits glucose production, and promotes glycogen synthesis in hepatocytes. Our findings provide a new direction to study the occurrence and development of NAFLD and also provide a novel theoretical and experimental basis for the diagnosis and treatment for NAFLD and other metabolic diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- VEGFB
Vascular endothelial growth factor B
- VEGFR1
Vascular endothelial growth factor receptor 1
- NAFLD
Non-alcoholic fatty liver disease
- TC
Total cholesterol
- TG
Triglyceride
- LDL
Low-density lipoprotein
- HDL
High-density lipoprotein
- HE
Hematoxylin eosin
- PAS
Periodic acid-schiff
- INSR
Insulin receptor
- p-INSR
Phosphorylated insulin receptor
- IRS1
Insulin receptor substrate 1
- p-IRS1
Phosphorylated insulin receptor substrate 1
- PI3K
Phosphatidylinositol 3-kinase
- p-PI3K
Phosphorylated phosphatidylinositol 3-kinase
- AKT
Protein kinase B
- p-AKT
Phosphorylated protein kinase B
- GLUT2
Glucose transporter 2
- FOXO1
Forkhead box protein O1
- p-FOXO1
Phosphorylated forkhead box protein O1
- GSK3p
Glycogen synthase kinase 3β
- p-GSK3p
Phosphorylated glycogen synthase kinase 3β
- PEPCK
Phosphoenolpyruvate carboxykinase
- G6Pase
Glucose-6 phosphatase
- PA
Palmitic acid
- HFD
High fat diet
- SD
Standard diet
- AAV
Adeno-associated virus
- LV
Lentivirus
- FBG
Fasting blood glucose
- PBG
Postprandial blood glucose
- OGTT
Oral glucose tolerance test
- IPITT
Intraperitoneal insulin tolerance test
- HOMA-IR
Homeostasis model assessment of insulin resistance
- QUICKI
Quantitative insulin sensitivity check index
Author contributions
The study was designed by Y.Q.L and Y.N.L. Experiments have been performed by Y.Q.L, X.N.Z, and N.X, and supervised by F.X, and Y.N.L. Human material in this study was provided by W.H.L. Transcriptomics and data sets have been analyzed by W.H.L, Q.Y.M., M.Z.Y, W.G.J and L.Z. The manuscript was written by Y.Q.L and further input was given by all authors.
Funding
Support by The Basic Research Project of Yantai Science and Technology Innovation and Development Plan (grant number: 2022JCYJ026). The Natural Science Foundation of Shandong province (grant number: ZR202111250163). The Basic Research Project of Yantai Science and Technology Innovation and Development Plan (grant number: 2023JCYJ068). College Students’ innovation and entrepreneurship training program (grant number: S202310440040).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
All animal experiments were approved by the animal ethics committee of Binzhou Medical University.
Consent for publication
All authors have read and approved the content and agree to submit for consideration for publication in the journal.
Conflict of interest
The manuscript has been read and approved by all authors. The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuqi Li and Wenhao Li share co-first authorship.
Contributor Information
Fang Xu, Email: xufang1979@163.com.
Yana Li, Email: liyanuo@bzmc.edu.cn.
References
- 1.Lin Q, Huang Z, Cai G, Fan X, Yan X, Liu Z, et al. Activating Adenosine Monophosphate-activated protein kinase mediates fibroblast growth factor 1 Protection from nonalcoholic fatty liver disease in mice. Hepatology. 2021;73(6):2206–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 2017;49(2):197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr Rev. 2019;40(5):1367–93. [DOI] [PubMed] [Google Scholar]
- 4.Saltiel AR. Insulin signaling in health and disease. J Clin Invest. 2021;131(1). [DOI] [PMC free article] [PubMed]
- 5.Tilg H, Moschen AR, Roden M. NAFLD and Diabetes Mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Kumar A, Zhang F, Lee C, Tang Z. Complicated life, complicated VEGF-B. Trends Mol Med. 2012;18(2):119–27. [DOI] [PubMed] [Google Scholar]
- 7.Robciuc MR, Kivela R, Williams IM, de Boer JF, van Dijk TH, Elamaa H, et al. VEGFB/VEGFR1-Induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab. 2016;23(4):712–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu L, Shan Z, Wang F, Gao X, Tong Y. Vascular endothelial growth factor B exerts lipid-lowering effect by activating AMPK via VEGFR1. Life Sci. 2021;276:119401. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y, Chen W, Han L, Bian Q, Fan J, Cao Z, et al. VEGF-B antibody and interleukin-22 fusion protein ameliorates diabetic nephropathy through inhibiting lipid accumulation and inflammatory responses. Acta Pharm Sin B. 2021;11(1):127–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14(11):1483–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu D, Zhong P, Wang Y, Zhang Q, Li J, Liu Z, et al. Hydrogen Sulfide attenuates high-Fat Diet-Induced non-alcoholic fatty liver disease by inhibiting apoptosis and promoting autophagy via reactive oxygen Species/Phosphatidylinositol 3-Kinase/AKT/Mammalian target of Rapamycin Signaling Pathway. Front Pharmacol. 2020;11:585860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubota N, Kubota T, Kajiwara E, Iwamura T, Kumagai H, Watanabe T, et al. Differential hepatic distribution of insulin receptor substrates causes selective insulin resistance in diabetes and obesity. Nat Commun. 2016;7:12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin M, Feng H, Wang Y, Yan S, Shen B, Li Z, et al. Gentiopicroside ameliorates oxidative stress and lipid Accumulation through Nuclear factor erythroid 2-Related factor 2 activation. Oxid Med Cell Longev. 2020;2020:2940746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone. 2012;50(2):437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58(2):221–32. [DOI] [PubMed] [Google Scholar]
- 16.Shearer AM, Wang Y, Fletcher EK, Rana R, Michael ES, Nguyen N, et al. PAR2 promotes impaired glucose uptake and insulin resistance in NAFLD through GLUT2 and akt interference. Hepatology. 2022;76(6):1778–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Li Y, Yang X, Hu Y, Yu H, Li Y. Reducing VEGFB accelerates NAFLD and insulin resistance in mice via inhibiting AMPK signaling pathway. J Transl Med. 2022;20(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mould AW, Tonks ID, Cahill MM, Pettit AR, Thomas R, Hayward NK, et al. Vegfb gene knockout mice display reduced pathology and synovial angiogenesis in both antigen-induced and collagen-induced models of arthritis. Arthritis Rheum. 2003;48(9):2660–9. [DOI] [PubMed] [Google Scholar]
- 19.Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsater H, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490(7420):426–30. [DOI] [PubMed] [Google Scholar]
- 20.Rui S, Nathaniel L, Chae SL, Yajie Z, Karanjit P, Oscar S. Cardiac-specific VEGFB overexpression reduces lipoprotein lipase activity 2 and improves insulin action in rat heart. Am J Physiol Endocrinol Metab. 2021;321(6):E753–65. [DOI] [PubMed] [Google Scholar]
- 21.Lago-Sampedro A, Lhamyani S, Valdes S, Colomo N, Maldonado-Araque C, Gonzalez-Molero I, et al. Serum vascular endothelial growth factor b and metabolic syndrome incidence in the population based cohort Di@bet.es study. Int J Obes (Lond). 2022;46(11):2013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Xin L, Zhao YC, Li SQ, Li YN. Role of vascular endothelial growth factor B in nonalcoholic fatty liver disease and its potential value. World J Hepatol. 2023;15(6):786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zang Y, Fan L, Chen J, Huang R, Qin H. Improvement of lipid and glucose metabolism by Capsiate in Palmitic Acid-treated HepG2 cells via activation of the AMPK/SIRT1 Signaling Pathway. J Agric Food Chem. 2018;66(26):6772–81. [DOI] [PubMed] [Google Scholar]
- 24.DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, et al. Nonalcoholic fatty liver disease and serum lipoproteins: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2013;227(2):429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye X, Kong W, Zafar MI, Zeng J, Yang R, Chen LL. Plasma vascular endothelial growth factor B is elevated in non-alcoholic fatty liver disease patients and associated with blood pressure and renal dysfunction. EXCLI J. 2020;19:1186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holzinger U, Kitzberger R, Fuhrmann V, Funk GC, Madl C, Ratheiser K. Correlation of calculated indices of insulin resistance (QUICKI and HOMA) with the euglycaemic hyperinsulinaemic clamp technique for evaluating insulin resistance in critically ill patients. Eur J Anaesthesiol. 2007;24(11):966–70. [DOI] [PubMed] [Google Scholar]
- 27.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuel VT, Shulman GI. Nonalcoholic fatty liver disease as a Nexus of metabolic and hepatic diseases. Cell Metab. 2018;27(1):22–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molinaro A, Becattini B, Mazzoli A, Bleve A, Radici L, Maxvall I, et al. Insulin-driven PI3K-AKT signaling in the hepatocyte is mediated by redundant PI3Kalpha and PI3Kbeta activities and is promoted by RAS. Cell Metab. 2019;29(6):1400–9. e5. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Liu B, Han H, Yuan Q, Xue M, Xu F, et al. Acute hepatic insulin resistance contributes to hyperglycemia in rats following myocardial infarction. Mol Med. 2015;21(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng J, He L. IRS posttranslational modifications in regulating insulin signaling. J Mol Endocrinol. 2018;60(1):R1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honma M, Sawada S, Ueno Y, Murakami K, Yamada T, Gao J, et al. Selective insulin resistance with differential expressions of IRS-1 and IRS-2 in human NAFLD livers. Int J Obes (Lond). 2018;42(9):1544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.G M Ward. The insulin receptor concept and its relation to the treatment of diabetes. Drugs. 1987;33(2):156–70. [DOI] [PubMed] [Google Scholar]
- 34.Roskoski R. Jr. VEGF receptor protein-tyrosine kinases: structure and regulation. Biochem Biophys Res Commun. 2008;375(3):287–91. [DOI] [PubMed] [Google Scholar]
- 35.Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176(6):1248–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uemura A, Fruttiger M, D’Amore PA, De Falco S, Joussen AM, Sennlaub F, et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog Retin Eye Res. 2021;84:100954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weddell JC, Chen S, Imoukhuede PI. VEGFR1 promotes cell migration and proliferation through PLCgamma and PI3K pathways. NPJ Syst Biol Appl. 2018;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di G, Zhao X, Qi X, Zhang S, Feng L, Shi W, et al. VEGF-B promotes recovery of corneal innervations and trophic functions in diabetic mice. Sci Rep. 2017;7:40582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan S, Huang W, Liu X, Liu X, Chen N, Xu Q, et al. IMPDH2 promotes colorectal cancer progression through activation of the PI3K/AKT/mTOR and PI3K/AKT/FOXO1 signaling pathways. J Exp Clin Cancer Res. 2018;37(1):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13(10):572–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Li J, Di LJ. Glycogen synthesis and beyond, a comprehensive review of GSK3 as a key regulator of metabolic pathways and a therapeutic target for treating metabolic diseases. Med Res Rev. 2022;42(2):946–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leturque A, Brot-Laroche E, Le Gall M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am J Physiol Endocrinol Metab. 2009;296(5):E985–92. [DOI] [PubMed] [Google Scholar]
- 43.Schultze SM, Hemmings BA, Niessen M, Tschopp O. PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Expert Rev Mol Med. 2012;14:e1. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Tang Y, Shi S, Gao S, Wang Y, Xiao D, et al. Tetrahedral Framework nucleic acids ameliorate insulin resistance in type 2 diabetes Mellitus via the PI3K/Akt Pathway. ACS Appl Mater Interfaces. 2021;13(34):40354–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.