Abstract
Background
Mesenchymal stem cells (MSCs) have emerged as promising candidates for immune modulation in various diseases that are associated with dysregulated immune responses like Graft-versus-Host-Disease (GVHD). MSCs are pleiotropic and the fate of MSCs following administration is a major determinant of their therapeutic efficacy.
Methods
Human MSCs were derived from bone marrow (BM) and Wharton’s Jelly (WJ) and preconditioned through exposure to hypoxia and induction of apoptosis, either sequentially or simultaneously. The immune programming potential of preconditioned MSCs was evaluated by assessing their effects on T cell proliferation, induction of Tregs, programming of effector T-cell towards Th2 phenotype, macrophage polarization in the direct co-culture of MSCs and aGVHD patients-derived PBMNCs. Additionally, efferocytosis of MSCs and relative change in the expression of immunomodulatory soluble factors were examined.
Results
Our study demonstrated that hypoxia preconditioned apoptotic MSCs (BM-MSCs, WJ-MSCs) bear more immune programming ability in a cellular model of acute Graft-versus-Host-Disease (aGVHD). Our findings revealed that WJ-MSCsHYP+APO were superior to BM-MSCsHYP+APO for immune regulation. These induced the differentiation of CD4+T-cell into Tregs, enhanced Th2 effector responses, and simultaneously mitigated Th1- and Th17 responses. Additionally, this approach led to the polarization of M1 macrophages toward an M2 phenotype.
Conclusion
Our study highlights the potential of WJ-MSCs conditioned with hypoxia and apoptosis concurrently, as a promising therapeutic strategy for aGVHD. It underscores the importance of considering MSC apoptosis in optimizing MSCs-based cellular therapy protocols for enhanced therapeutic efficacy in aGvHD.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-024-03947-2.
Keywords: Mesenchymal stem cells, Apoptosis, Hypoxia, Immunomodulation, Acute graft-versus-host-disease, Wharton’s Jelly, Bone marrow
Background
Mesenchymal stem cells (MSCs) exert their immunoregulatory effects in Graft-versus-Host-Disease (GVHD) [1] through the secretion of various soluble factors such as chemokines, cytokines, and extracellular vesicles/exosomes [2], resulting in modulation of the immune response by suppressing the activation of immune cells (T-cell, natural killer cells, dendritic cells, B-cell) in an inflammatory milieu [3].
Several preclinical studies reported that transplanted MSCs have a limited lifespan in recipients as they undergo apoptosis by various immune effector cells (cytotoxic T-cell, natural killer cell, granulocytes) [4, 5] within the host circulation and apoptotic MSCs are subsequently efferocytosed by macrophages which in turn licensed to disseminate soluble factors to regulate the activated immune response [6]. Therefore, this mechanism has been explored in a few studies by either inducing or inhibiting apoptosis in MSCs by silencing the apoptotic effector molecules BAK and BAX. These studies validated the therapeutic potential of MSCs [7] and demonstrated that reducing apoptosis in MSCs diminished their immunomodulatory capabilities in a viable form when administered in an asthmatic mice model. This finding underscores the necessity for MSCs to undergo apoptosis to exert their functional effect [7, 8]. Moreover, these apoptotic MSCs have been found to possess the ability to induce immunosuppressive effects in animal models of GVHD and various organ injuries like lung, liver, and spleen [4, 9, 10], suggesting that phagocytes are mediators of MSC-induced adaptive responses [11].
MSCs reside in a hypoxic microenvironment that can promote apoptosis which leads to therapeutic potential under in vivo conditions [12] by secreting water-soluble immunomodulatory factors [13, 14].
Allogeneic Hematopoietic Stem Cell Transplantation (Allo-HSCT) is a curative treatment for various hematological disorders [15]. The primary objectives of Allo-HSCT include promoting the engraftment of donor cells [16], restoring a healthy immune system, and eliminating residual tumor cells from the host [17]. However, the success of Allo-HSCT is often compromised by GVHD, wherein donor immunocompetent cells recognize and attack the host’s tissues in immunocompromised allogeneic recipients, significantly affecting transplant-related mortality [18]. Acute GVHD (aGVHD) occurs within the first 100 days post-Allo-HSCT in 40–60% of transplant recipients [19] and is primarily characterized by damage to the skin, liver, and gastrointestinal tract [20]. This condition is marked by strong pro-inflammatory cytokine responses, mediated by Th1 and Th17 immune responses [21, 22], involving donor T cell-mediated cytotoxic (CD8+) attacks on host tissues due to cell surface molecules and secreted factors [21].
Mesenchymal stem cells (MSCs) have shown promise in preventing and treating GVHD in preclinical and small-scale clinical studies through their immunomodulatory properties [11, 23]. Despite these advantages, translating the success of MSC therapy to larger clinical settings has been challenging, yielding mixed results [11, 22]. The variable immunomodulatory effect of MSCs in aGVHD can be attributed to differences in patient characteristics [11] and insufficient apoptosis of MSCs in the recipients [4].
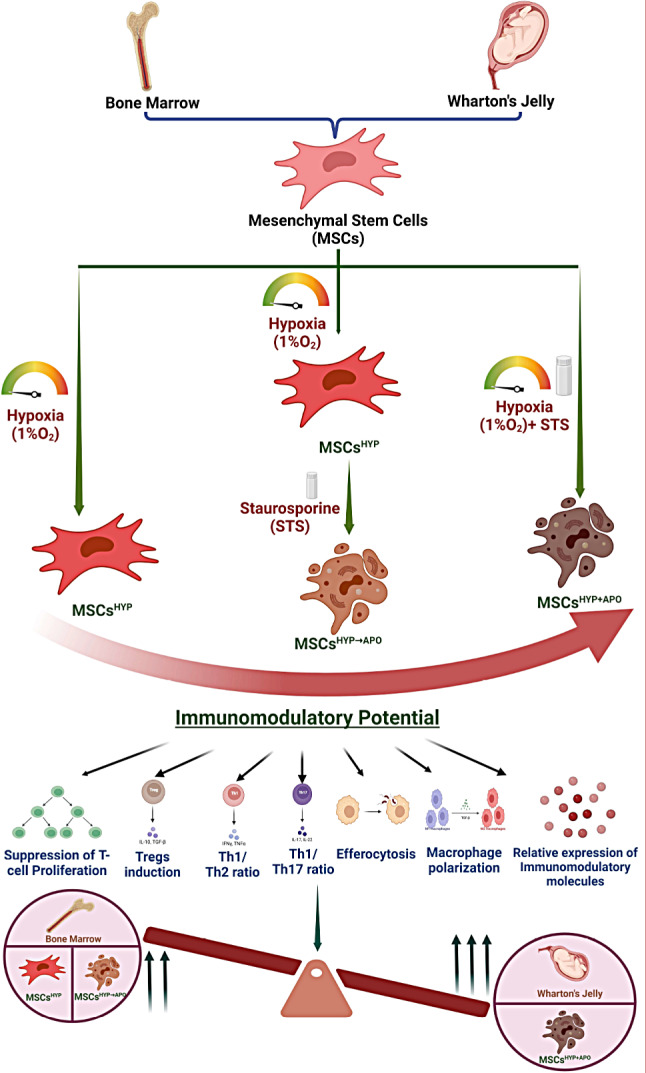
There is an urgent need to augment the efficacy of MSCs-based cellular therapy by tinkering apoptosis in conjunction with hypoxia appears to be the most effective conditions that impact immunomodulation potential, particularly for aGVHD patients who do not respond favorably to viable MSCs. In view of this prerequisite, our study provides experimental evidence that apoptotic in conjunction with hypoxia enhances the immune regulatory potential of tissue-specific human MSCs (BM-MSCs, WJ-MSCs) in a cellular model of aGVHD.
Methods
Isolation and characterization of MSCs from human BM and WJ
Human BM aspirates were collected from healthy donors (n = 5) of allogeneic stem cell transplant recipients at the Department of Medical Oncology, Dr. B. R. Ambedkar Institute Rotary Cancer Hospital, AIIMS, New Delhi, and MSCs were isolated using our previously established protocol [24]. The study involved the use of human mesenchymal stem cells and was approved by the Institutional Committee for Stem Cell Research, (Ref. No.: IC-SCR/110/20(R), All India Institute of Medical Sciences, New Delhi, India. Informed written consents were obtained from all participating subjects and all procedures were performed as per the guidelines and regulations approved by the ethics committee.
Briefly, the aspirate was seeded in a 60 mm culture dish (Nunc™, Thermo Fisher Scientific, USA) with 1X Low Glucose-Dulbecco’s Modified Eagle Media (LG-DMEM) (Thermo Fisher Scientific, USA) supplemented with 10% FBS (Thermo Fisher Scientific, USA), 1% antibiotic-antimycotic solution (Thermo Fisher Scientific, USA), and incubated in a humidified chamber at 37֯C with 5% CO2, 21% O2. After 72 h, the media was replaced with LG-DMEM complete media with Stem Pro™ MSC Serum-Free Media (SFM) (Thermo Fisher Scientific, USA) in a 3:1 ratio, and the media was changed every third day until the cell confluency reached 80%. Adherent cells were trypsinized using 0.05% Trypsin-EDTA and reseeded for further experiments.
Human umbilical cord (UC) (n = 5) was collected in a sterile transport media from the Department of Obstetrics and Gynaecology, AIIMS, New Delhi, and MSCs were isolated using our previously established protocol [24]. The study involved the use of human mesenchymal stem cells and was approved by the Institutional Committee for Stem Cell Research, (Ref. No.: IC-SCR/110/20(R), All India Institute of Medical Sciences, New Delhi, India. Informed written consents were obtained from all participating subjects and all procedures were performed as per the guidelines and regulations approved by the ethics committee.
Briefly, the explant culture method was used to obtain MSCs from WJ and approximately 2 cm*2 cm size of explant were seeded in a 35 mm culture dish (Nunc™, Thermo Fisher Scientific, USA) and the coverslips were placed over explants and 1X LG-DMEM complete medium was added dropwise. The culture dish was placed in a humidified chamber at 37֯C, 5% CO2 for 2–3 h for the attachment of explants followed by the addition of 1X LG-DMEM with Stem Pro MSC™ SFM in a 3:1 ratio. The cells were harvested using 0.05% Trypsin-EDTA after the attainment of 80% cell confluency and the cells were expanded for further experiments.
Characterization of MSCs
Both BM-MSCs and WJ-MSCs (Passage-3) were characterized according to the International Society for Cellular Therapy (ISCT) guidelines [25] using our established protocols [26]. Briefly, MSCs were characterized for their plastic adherence, surface marker profile, and trilineage differentiation potential. Uninduced MSCs were expanded in basal media, used as a control for trilineage differentiation assay and images were acquired using an inverted phase contrast microscope (Nikon, Japan). Further, the metabolic rate of MSCs was determined on Days 1, 3, 5, and 7 using an MTT assay, and the population doubling time (PDT) was enumerated after 72 h using the following formula [27]:
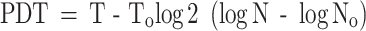 |
where, T: Time of harvesting, To: Time of seeding, N: Number of cells harvested, No: Number of cells seeded.
MSCs were pooled to attain homogeneity and passages 3–5 were used for subsequent in vitro experiments.
Generation and characterization of hypoxia-preconditioned apoptotic MSCs
Hypoxia-preconditioned apoptotic MSCs were generated either sequentially or concurrently. In the sequential method, MSCs were first preconditioned with 1% O2 for 24 h, followed by treatment with 1µM staurosporine (STS) (Sigma, USA) for 24 h in 1X LG-DMEM complete media in a tri-gas incubator (Thermo Fisher Scientific, USA), termed as MSCsHYP→APO. In the concurrent method, MSCs were exposed to 1% O2 and 1µM STS for 24 h in a tri-gas incubator, termed MSCsHYP+APO. Additionally, MSCs were exposed to 1% O2 alone for 24 h, termed hypoxia preconditioned MSCs (MSCsHYP).
The respective cell suspensions were collected, washed, and stained with Annexin V/7AAD for the enumeration of apoptotic cells using a DxFlex flow cytometer (Beckmann Coulter), and the data was analyzed using Kaluza software version 2.1 (Beckmann Coulter). Additionally, the apoptosis was confirmed by cleaved caspase-3 (Cell Science Technology, USA) using western blotting [7].
T-cell proliferation assay
Peripheral blood (PB) was collected from patients with grade II-IV acute graft-versus-host-disease (aGvHD) (n = 5) in a sterile sodium heparin-coated vacutainer (BD Biosciences, US) and the peripheral blood mononuclear cells (PBMNCs) were isolated using a standardized protocol [28]. The study involved the use of human subjects and was approved by the Institute Ethics Committee for Post Graduate Research, All India Institute of Medical Sciences, New Delhi, India (Ref. No.: IECPG-542/23.09.2020). Informed written consents were obtained from all participating subjects and all procedures were performed as per the guidelines and regulations approved by the ethics committee.
For each co-culture experimental condition, independent experiments were performed using PBMNCs derived from each donor, resulting in a total of 5 biological replicates. Within each biological replicate, experiments were conducted in triplicate (technical replicates) to ensure reproducibility and accuracy of the results.
CD3+ T cells were isolated from PBMNCs by negative selection using a Pan T cell isolation kit, human (Miltenyi Biotec, USA) as per the manufacturer’s instructions. CD3+ T cells were labeled with 1µM Cell Trace™ CFSE dye (BD Biosciences, USA) for 20 min at 37֯C and followed by activation with PHA (1 µg/ml) (Sigma, USA) and IL-2 (50IU/ml) (Thermo Fisher Scientific, USA) in 1X Rosewell Park’s Memorial Institute (RPMI)-1640 medium supplemented with L-glutamine (Thermo Fisher Scientific, USA), 10% FBS, 1% antibiotic-antimycotic solution at 37֯C, 5% CO2 for 48 h.
For co-culture, hypoxia-preconditioned MSCs (MSCsHYP, MSCsHYP→APO, MSCsHYP+APO) were treated with mitomycin-C (15 µg/ml) (Thermo Fisher Scientific, USA) for 1 h in 1X LG-DMEM incomplete medium. The mitomycin-treated MSCs were co-cultured with CFSE-labelled activated T cells in a 1:10 ratio in 1X RPMI-1640 complete medium with 1X LG-DMEM complete medium in a 1:1 ratio for 3 days. After 3 days of co-culture, the proliferation of T cells was assessed using a DxFlex flow cytometer (Beckmann Coulter, USA), and the data was analyzed using Kaluza software version 2.1 (Beckmann Coulter, USA). CFSE-labelled activated T-cell in the absence of MSCs were used as a control for the normalization of the proliferation of T-cell in the direct co-culture [28, 29].
Induction of Tregs
The mitomycin-treated MSCs and activated T cells were co-cultured in a 1:10 ratio for 5 days. After 5 days of co-culture, cells were collected, washed with 1X PBS, and stained with fluorochrome-conjugated anti-human monoclonal antibodies against CD3, CD4, CD8, CD25, CD45 (Beckmann Coulter, USA). For intracellular staining, cells were fixed and permeabilized with a FOXP3 buffer set (BD Biosciences, USA) followed by staining with anti-FOXP3 monoclonal antibody for 30 min at room temperature. A minimum of 50,000 events were acquired by a DxFlex flow cytometer (Beckmann Coulter) and the data was analyzed using Kaluza software version 2.1 (Beckmann Coulter). Activated T-cell in the absence of MSCs was used as a control for the baseline expression of CD3+ CD4+ CD25+ FOXP3+ Tregs [30, 31].
Enumeration of effector memory helper T cell subtypes (Th1, Th2, and Th17)
The proportion of Th1, Th2, and Th17 were enumerated in the 5-day co-culture of mitomycin-treated MSCs and activated T-cell by staining with fluorochrome-conjugated antihuman monoclonal antibodies against CXCR3, CXCR5, CCR10, CCR4, CCR7, CCR6 at 37֯C for 30 min followed by staining anti-human CD3, CD4, CD8, CD45RA monoclonal antibodies and Th1, Th2, Th17 was enumerated using DxFlex flow cytometer (Beckmann Coulter) [30, 32].
Macrophage polarization
CD14+ monocytes were isolated from PBMNCs using a pan-monocyte isolation kit, human (Miltenyi Biotec, USA) following the manufacturer’s guidelines. The isolated monocytes were treated with 25ng/ml GM-CSF for 5 days to induce differentiation into M0 macrophages followed by treatment with 10ng/ml LPS and 50ng/ml IFN-γ for 24 h to generate M1 macrophages. The mitomycin-treated MSCs were co-cultured with M1 macrophages in a 1:10 ratio for 3 days. The polarization of macrophages from M1 to M2 phenotype was assessed by staining the cells with fluorochrome-conjugated anti-CD206, iNOS, Arginase-1 (BD Biosciences, USA), and the cells were acquired using a DxFlex flow cytometer (Beckmann Coulter) to enumerate the M1 and M2 macrophages [33].
In vitro MSCs clearance assay
CFSE labelled aGvHD patients-derived M1 macrophages were treated with pH Rhodo red succinimidyl ester labelled MSCs (Thermo Fisher Scientific, USA) in a 1:2 ratio for 24 h. The percentage of M1 MФCFSE+ was also positive for the pH Rhodo red signal used for the calculation of uptake of MSCs by M1 macrophages using a DxFlex flow cytometer (Beckmann Coulter) [6, 34].
Gene expression analysis of immunomodulatory molecules
Total RNA was extracted from the co-culture of MSCs and PBMNCs using the phenol-chloroform extraction method by TRIzol™ reagent (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. Briefly, the cell suspension was collected from the co-culture and it was centrifuged at 800 rpm for 5 min at room temperature. After centrifugation, the supernatant consists of dead cells and debris which can be removed by decanting the supernatant while the viable cells form a pellet at the bottom of the tube. The pellet was resuspended in Trizol (Thermo Fisher Scientific, USA) and RNA was isolated from the pellet (viable cells). 2 µg total RNA was reverse transcribed to give cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Invitrogen, USA). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in triplicates using SYBR Green Master Mix (Promega, US) according to the manufacturer’s instructions using a CFX96 Real-Time System (Bio-Rad). Glyceraldehyde-3 phosphate dehydrogenase (GAPDH) was used as the housekeeping gene to normalize the gene expression. The comparative 2 − ΔΔCt method was performed to evaluate the mRNA expression of IDO, PGE2, IL-10, IFN-γ, IL-6, TNF-α, IL-12β, IL-1β and the sequence of primers were listed in Table S1.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism version 8.4.3. One-way and Tukey’s post hoc tests compared three or more groups. Data was shown as Mean ± S.D. and a p-value of ≤ 0.05 was considered statistically significant.
Results
Hypoxia preconditioning maintained characteristics of MSCs
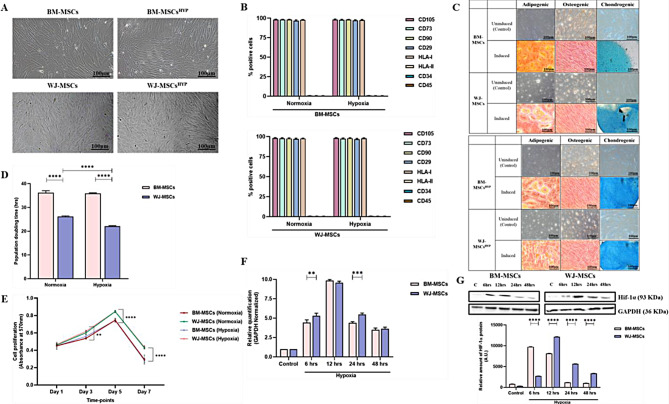
Initially, we characterized naïve and hypoxia-preconditioned MSCs (BM-MSCs, WJ-MSCs) according to the ISCT guidelines. We observed that hypoxia maintained the parental characteristics of MSCs like plastic adherence, fibroblast-like spindle-shaped morphology, exhibited ≥ 95% expression of CD105, CD73, CD90, CD29, HLA I, and the ≤ 2% expression of HLA II, CD34, and CD45, and differentiation into mesodermal lineages (adipogenic, osteogenic, and chondrogenic) (Fig. 1A, B, C, S1). While, we did not observe a significant variation in the doubling time of BM-MSCs between normoxia and hypoxia culture conditions (36.21 ± 0.852, 35.93 ± 0.226), however, WJ-MSCs exhibited a significant reduction in doubling time under hypoxia compared to normoxia conditions (22.123 ± 0.227 vs. 26.16 ± 0.284; p ≤ 0.0001) (Fig. 1D). Moreover, we observed that both MSCs exhibited exponential growth from days 1 to 5, followed by a decline in proliferation from day 5 to day 7 under normoxia and hypoxia culture conditions (Fig. 1E). Overall, WJ-MSCs demonstrated higher proliferation rates than BM-MSCs under both normoxia and hypoxia conditions, as evidenced by both PDT and metabolic rate. Low oxygen level during hypoxia is sensed by the Von Landau Hipple factor and manifested by the upregulation of the HIF-1α transcription factor which orchestrates hypoxia-induced changes in the cells. HIF-1α gene which undergoes ubiquitination in normoxic conditions but becomes stabilized following exposure to hypoxia. Consequently, the expression levels of this gene serve as an indicator of the cellular response to hypoxic conditions [35]. Interestingly, both MSCs (BM-MSCs, WJ-MSCs) had higher expression of HIF-1α from 6 h (5.085 ± 0.0495, 8.349 ± 0.454-fold change) to 12 h (9.65 ± 0.161, 14.420 ± 0.665 fold change) followed by a decline in their expression from 24 h (6.382 ± 0.059, 10.324 ± 0.119 fold change) to 48 h (4.288 ± 0.656, 5.470 ± 0.334 fold change) at the gene and protein level (Fig. 1F, G).
Fig. 1.
Characterization of human MSCs (BM-MSCs, BM-MSCsHYP, WJ-MSCs, and WJ-MSCsHYP) (A) Morphological images. (B) A bar graph depicts surface marker profiling using flow cytometry. (C) Trilineage differentiation into adipocytes (28 days of induction), osteocytes (21 days of induction), and chondrocytes (14 days of induction). (D) The bar graph represents the population doubling time in hours. (E) The line diagram represents the growth kinetics. (F) The bar graph depicts the relative expression of the HIF-1α gene using qPCR before and after exposure to 1% O2 at various time intervals (6 h, 12 h, 24 h, 48 h). (G) Western blot images represent the expression of Hif-1α protein and the bar graph represents the relative expression of Hif-1α protein before and after exposure to 1% O2 at various time intervals (6 h, 12 h, 24 h, 48 h). Full-length blots are presented in Supplementary Figure S9. Data are shown as Mean ± S.D. The data shown are from independent experiments performed with MSCs derived from three different donors (biological replicates) and conducted in triplicates (technical replicates)
Statistical analysis: Tukey’s multiple comparisons test; ***≤0.001; ****≤0.0001. All experiments were done in triplicates. Scale bar: 10X = 100 μm. Abbreviations: BM: Bone marrow; WJ: Wharton’s Jelly; MSCs: Mesenchymal stem cells; HYP: Hypoxia-preconditioned
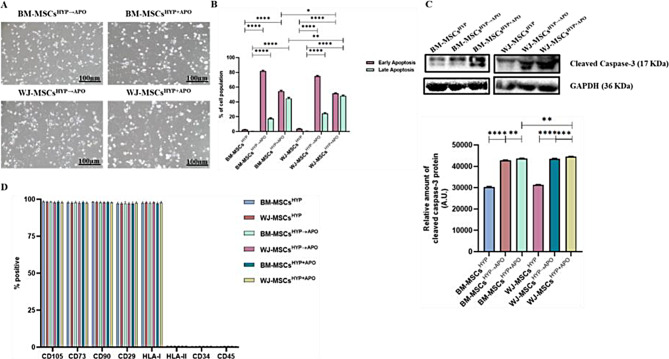
Immunomodulation of MSCs is linked to its elimination via apoptosis followed by efferocytosis post-administration [4, 5, 36]. Pre-conditioning with hypoxia enhances the apoptosis of MSCs which might augment the immunomodulation properties of MSCs [13]. Therefore, we induced apoptosis in hypoxia-preconditioned MSCs (BM-MSCs, WJ-MSCs) using STS in both successive and simultaneous ways. Both approaches resulted in a notable alteration in the morphology of MSCs, characterized by cellular shrinkage, blebbing, and fragmentation (Fig. 2A) with the proportion of apoptotic MSCs (≥ 98%), confirmed by Annexin-V/7AAD staining (Fig. 2B, S2) and the expression of cleaved caspase-3 was enhanced in apoptotic MSCs (Fig. 2C). Interestingly, despite apoptosis, the phenotype of MSCs in both approaches, remained unchanged (Fig. 2D, S3) indicating the stability of attributes of hypoxia-preconditioned MSCs following apoptosis.
Fig. 2.
Characterization of apoptotic human MSCs (BM-MSCsHYP→Apo, BM-MSCsHYP+APO, WJ-MSCsHYP→APO, and WJ-MSCsHYP+APO. (A) Microscopic images. (B) A bar graph represents the percentage of apoptotic cells (early and late apoptotic cells). (C) Western blot images depict the expression of cleaved caspase-3 and the bar graph represents the relative expression of cleaved caspase-3. (D) The bar graph represents the surface expression profile using flow cytometry. Full-length blots are presented in Supplementary Figure S10. Data are shown as Mean ± S.D. The data shown are from independent experiments performed with MSCs derived from three different donors (biological replicates) and conducted in triplicates (technical replicates).Statistical analysis: Tukey’s multiple comparisons test; ****≤0.0001. All experiments were done in triplicates. Scale bar: 10X = 100 μm. Abbreviations: BM: Bone marrow; WJ: Wharton’s Jelly; MSCs: Mesenchymal stem cells; APO: Apoptosis; HYP: Hypoxia-preconditioned
WJ-MSCsHYP+APO induced immune programming of effector T-cell
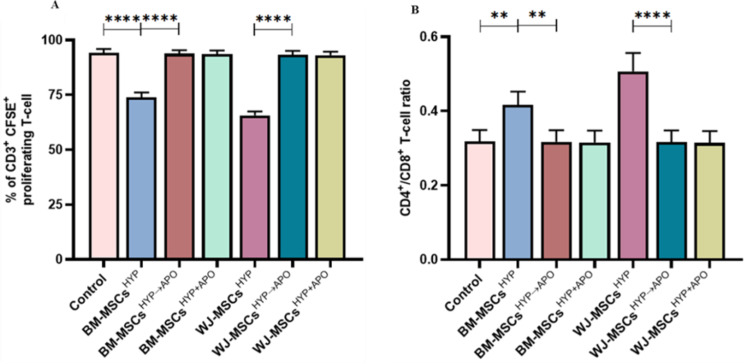
MSCs not only manifest regenerative potential but also play a crucial role in immunomodulation, contributing significantly to the maintenance of immune homeostasis and preventing inflammation in the host [37]. To address this, we assessed whether hypoxia-preconditioned viable or apoptotic MSCs (MSCsHYP, MSCsHYP→APO, MSCsHYP+APO) would be able to polarize effector T cells and macrophages. Initially, we co-cultured CD3+ T cells with MSCs, and T-cell proliferation, induction of Tregs, and polarization of helper T-cell from pro-inflammatory to anti-inflammatory phenotype was assessed using flow cytometry. We observed that both MSCsHYP (BM-MSCs, WJ-MSCs) inhibited the proliferation of T cells significantly, wherein, WJ-MSCsHYP were more effective in suppressing T-cell proliferation than BM-MSCsHYP (65.62% vs. 73.964%; p ≤ 0.0001). Interestingly, hypoxia-preconditioned apoptotic MSCs (MSCsHYP→APO, MSCsHYP+APO) did not effectively inhibit T cells proliferation, regardless of the tissue source [Fig. 3A, S4 (I-IV)]. Similarly, MSCsHYP (BM-MSCs, WJ-MSCs) increased the CD4/CD8 ratio indicating the inhibitory impact of MSCs on CD8+ T-cell populations (Fig. 3B).
Fig. 3.
Effect of hypoxia-preconditioned MSCs (MSCsHYP, MSCsHYP→APO, MSCsHYP+APO) on the proliferation of aGVHD patients derived T cells. The bar graph represents (A) the percentage of CD3+ CFSE+ proliferating T cells (n = 5). (B) the ratio of CD4+/CD8+ T cells (n = 5) in the direct co-culture of MSCs and T cells. Data shown represent the Mean ± S.D of 5 independent experiments performed with PBMNCs derived from 5 different donors (biological replicates), with each experiment conducted in triplicate (technical replicates). Statistical analysis: Tukey’s multiple comparisons test; **≤0.01; ****≤0.0001. Abbreviations: BM: Bone marrow; WJ: Wharton’s Jelly; MSCs: Mesenchymal Stem Cells; HYP: Hypoxia-preconditioned; APO: Apoptosis
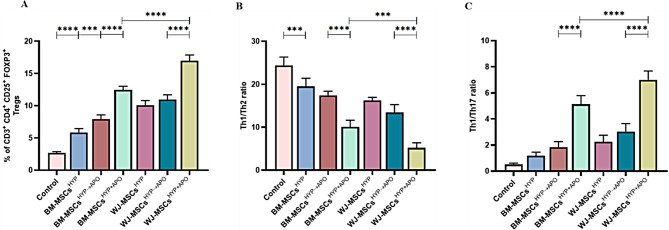
Based on the MSCHYP-mediated loss of the CD8+ T-cell population and concomitant increase in CD4+ T-cell, we anticipated that MSCs might favor either the proliferation of CD4+ T cell or promote their differentiation into Tregs as one of the underlying immune polarization mechanisms. To demonstrate this, we also analyzed the Tregs population in the direct co-culture, as described above. The co-culture of both MSCsHYP (BM-MSCs, WJ-MSCs) enhanced the differentiation of CD4 + T-cell to Tregs, more so for WJ-MSCsHYP than BM-MSCsHYP (10.08% vs. 5.856%; p: ≤0.0001). In contrast and unlike T cell proliferation, hypoxia-preconditioned apoptotic MSCs (MSCsHYP→APO, MSCsHYP+APO) enhanced the differentiation of CD4+ T cells into Tregs synergistically over MSCsHYP, regardless of tissue source. The percentage of Tregs induced by WJ-MSCsHYP+APO was significantly higher than WJ-MSCsHYP→APO (16.988% vs. 10.954%; p ≤ 0.0001). A similar trend was observed in BM-MSCs (12.442% vs. 7.929%; p ≤ 0.0001). This indicates that simultaneous exposure of hypoxia and apoptosis to MSCs is more effective than the successive approach (Fig. 4A, S5 (I-II)]. These results, supporting our hypothesis, suggested that MSCs are effective in modulating T cells phenotypically in co-cultures.
Fig. 4.
Effect of hypoxia-preconditioned MSCs (MSCsHYP, MSCsHYP→APO, MSCsHYP+APO) on the induction of Tregs and polarization of helper T cells from pro-inflammatory (Th1, Th17) to anti-inflammatory (Th2) phenotype. The Th1 phenotype is marked by CD45RA− CCR7− CXCR3+ CCR4− CCR6− CXCR5− CCR10−, Th2 is marked by CD45RA− CCR7− CXCR3− CCR4+ CCR6− CXCR5− CCR10−, and Th17 is marked by CD45RA− CCR7− CXCR3+/− CCR4+ CCR6+ CXCR5− CCR10−.The bar graph represents (A) the induction of CD3+ CD4+ CD25+ FOXP3+ Tregs (n = 5). (B) the ratio of Th1/Th2 (n = 5). (C) Th1/Th17 ratio (n = 5) in the direct co-culture of MSCs and T cells. Data shown represent the Mean ± S.D of 5 independent experiments performed with PBMNCs derived from 5 different donors (biological replicates), with each experiment conducted in triplicate (technical replicates). Statistical analysis: Tukey’s multiple comparisons test; ***≤0.001; ****≤0.0001. Abbreviations: BM: Bone marrow; WJ: Wharton’s Jelly; MSCs: Mesenchymal stem cells; HYP: Hypoxia-preconditioned; APO: Apoptosis, Tregs: Regulatory helper T cells, Th: Helper T cells
Based on this, we anticipated that these MSCs might tweak T cells functionally as well. To substantiate this, we analyzed both Th1/Th2 and Th1/Th17 ratios as indicators of overall immune responses. In line with this and following our hypothesis, co-culture of MSCsHYP/MSCsHYP→APO/MSCsHYP+APO with T-cell reduced the Th1/Th2 ratio and enhanced the Th1/Th17 ratio compared to control, suggesting that these MSCs can polarize Th1 to Th2 and inhibit Th17 response in the direct co-culture system. Similarly, we observed that both BM-MSCsHYP+APO and WJ-MSCsHYP+APO (10.056 vs. 5.214; p 0.0004) caused a significant decrease in Th1/Th2 ratio compared to sequentially generated counterparts (17.438 vs. 13.416; p 0.0038) (Fig. 4B, S6). Additionally, a significant increase in the Th1/Th17 ratio was observed with BM-MSCsHYP+APO and WJ-MSCsHYP+APO (7.008 vs. 5.132; p ≤ 0.0001) compared to their sequentially generated apoptotic MSCsHYP→APO (3.04 vs.1.836; p ≤ 0.0001), indicating a shift towards an anti-inflammatory state (Fig. 4C, S6).
These results showed that both MSCsHYP and MSCs HYP→APO/MSCsHYP+APO are capable of T-cell programming which indicates one of the potential mechanisms by which these cells can manipulate host immune responses.
WJ-MSCsHYP+APOundergo efferocytosis more efficiently and trigger macrophage polarization with a concurrent increase in the expression of immunomodulatory molecules.
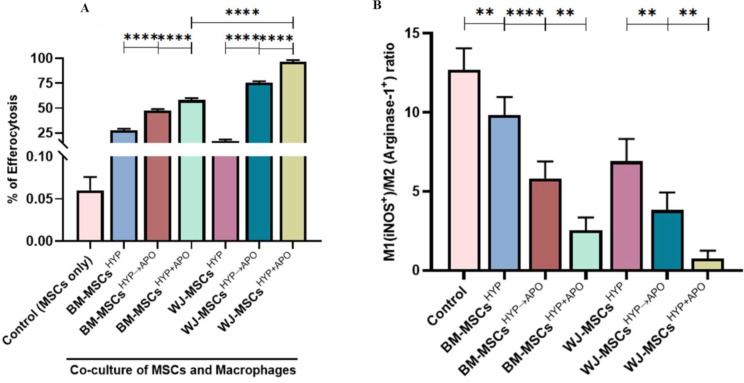
Indeed, T cells are major determinants of an effective immune response, but they rely on innate immune cells (macrophages) to maintain immune homeostasis. To address this, we assessed the phagocytosis of MSCsHYP, MSCsHYP→APO, and MSCsHYP+APO by macrophages by a process known as efferocytosis under in vitro settings. To mimic this in our model, we co-cultured MSCs with macrophages, as described above, and allowed them to phagocytize and assessed efferocytosis using flow cytometry. In line with the hypothesis, our data revealed that macrophages exhibited enhanced efficiency of efferocytosis of both MSCsHYP→APO and MSCsHYP+APO over MSCsHYP, irrespective of tissue origin. Our results demonstrated that BM-MSCs(HYP+APO) and WJ-MSCs(HYP+APO) (58.01% vs. 96.64%; p ≤ 0.0001) than BM-MSCs(HYP→APO) and WJ-MSCs(HYP→APO) (47.35% vs. 75.62%; p ≤ 0.0001), thereby explaining the maximum immunomodulation mediated by WJ-MSCs(HYP+APO) [Fig. 5A, S7 (I-II)]. Frequent apoptosis and uptake of various bacteria/dead cells carrying neutrophils and/or fibroblast and other cells by tissue-resident scavenging and CD68+ effector macrophages triggers the release of TGF-β, prostaglandins and other mediators which further promote in situ polarization of effector immune cells toward their refractory/anti-inflammatory phenotype. Since we have seen a Th2 bias in MSCsHYP+APO/MSCsHYP→APO co-cultured T cells and increased efferocytosis of MSCsHYP+APO/MSCsHYP→APO by macrophages, we anticipated a potent in situ reprogramming of M1 effector macrophages towards an M2 phenotype. To address this, we analyzed the M1/ M2 phenotype of the macrophages which were co-cultured with MSCsHYP/MSCsHYP+APO/MSCsHYP→APO, and a panel of Th1/Th2 effectors in the direct co-culture of MSCsHYP/MSCsHYP+APO/MSCsHYP→APO with CD3+ T cells.
Fig. 5.
Effect of hypoxia-preconditioned MSCs (MSCsHYP, MSCsHYP→APO, MSCsHYP+APO) on the efferocytosis of MSCs and macrophage polarization from proinflammatory (M1) to anti-inflammatory (M2) phenotype. The bar graph represents (A) efferocytosis of MSCs by macrophages (n = 5). (B) the ratio of M1 MФ/M2 MФ in the co-culture of MSCs and M1 MФ (n = 5). Data shown represent the Mean ± S.D of 5 independent experiments performed with PBMNCs derived from 5 different donors (biological replicates), with each experiment conducted in triplicate (technical replicates). Statistical analysis: Tukey’s multiple comparisons test; *≤0.05; **≤0.01; ***≤0.001; ****≤0.0001. Abbreviations: BM: Bone marrow; WJ: Wharton’s Jelly; MSCs: Mesenchymal stem cells; HYP: Hypoxia-preconditioned; APO: Apoptosis; MФ: Macrophages
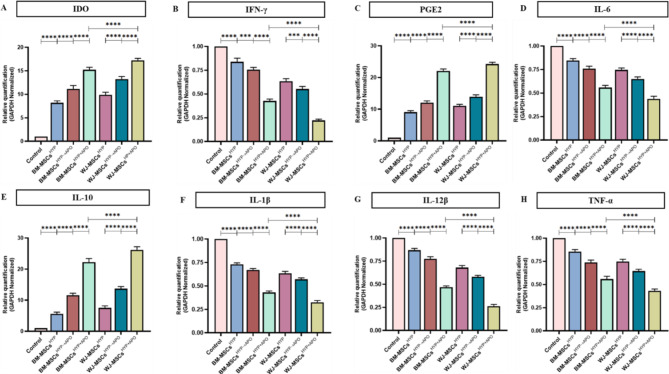
In line with our hypothesis, our data demonstrated a significant polarization of the M1 (iNOS+ Arginase-1−) macrophages toward the M2 (iNOS− Arginase-1+) phenotype with MSCs(HYP+APO) compared to MSCs(HYP→APO) in both BM-MSCs (2.536 vs. 5.818; p 0.0010) and WJ-MSCs (0.757 vs. 3.822; p 0.0024), evident by a decrease in the M1/M2 ratio [Fig. 5B, S8 (I-II)]. Furthermore, our experiments provided clear evidence that the co-culture of WJ-MSCsHYP+APO and T cells enhanced the expression of immunomodulatory molecules (IDO, PGE2) that counteract Th1 effector cytokines. This approach inhibited the expression of Th1 effectors (IL-1β, IL-12β, TNF-α, IL-6) and enhanced the expression of Th2 effector cytokines (IL-10), demonstrating immune metabolic programming of effector immune cells (Fig. 6A-H).
Fig. 6.
Effect of hypoxia-preconditioned MSCs (MSCsHYP, MSCsHYP→APO, MSCsHYP+APO) on the relative expression of immunomodulatory molecules and cytokines. The bar graphs represent the relative mRNA expression of (A) IDO. (B) IFN-γ. (C) PGE2. (D) IL-6. (E) IL-10. (F) IL-1β. (G) IL-12β. (H) TNF-α in the direct co-culture of MSCs and aPBMNCs derived from aGVHD patients (n = 5). Data shown represent the Mean ± S.D of 5 independent experiments performed with PBMNCs derived from 5 different donors (biological replicates), with each experiment conducted in triplicate (technical replicates). Statistical analysis: Tukey’s multiple comparisons test; *≤0.05; **≤0.01; ***≤0.001; ****≤0.0001. Abbreviations: BM: Bone marrow; WJ: Wharton’s Jelly; MSCs: Mesenchymal stem cells; HYP: Hypoxia-preconditioned; APO: Apoptosis; IDO: Indoleamine 2,3 dioxygenase; PGE2: Prostaglandin E2; IFN-γ: Interferon-γ; IL: Interleukin; TNF-α: Tumor necrosis factor-α
Discussion
Recent studies indicate that MSCs undergo apoptosis after administration due to interactions with the host’s immune effector cells which leads to the immune modulation of the host [4, 38]. In this context, our study shed new light on how the preconditioning of MSCs with short-term hypoxia (1% O2) and inducing apoptosis can enhance the immunomodulatory potential of MSCs (BM-MSCs, and WJ-MSCs).
Our results indicated that preconditioning of MSCs with hypoxia (1% O2) for 24 h does not affect the fibroblast-like spindle-shaped morphology of MSCs, which is consistent with the previous findings [39]. However, several studies demonstrated that hypoxia maintained the expression of negative markers (CD34, CD45, HLA-II) with a variable impact on the expression of positive markers (CD105, CD73, CD90, CD29, HLA-I) [40–42]. In contrast, few studies reported that a hypoxic environment did not confer a significant change in surface profile [43]. In this line, we also observed that a short exposure of MSC to hypoxia did not cause any change in surface markers on MSCs. Multiple studies revealed that hypoxia enhances the clonogenic potential of MSCs (WJ, adipose tissue) [43, 44]. However, our observations revealed differences in PDT with no variation in BM-MSCs while a significant difference was observed in WJ-MSCs, consistent with previous findings [35, 45]. The observed variations across the studies are probably due to the result of diverse protocols employed [RBC lysis [46], explant culture, enzymatic digestion [47]], variations in culture media compositions [48, 49], oxygen levels [43, 44], and the inherent heterogeneity among donors [50]. Notably, there was no difference in the differentiation of MSCs into mesodermal lineages (adipogenic, osteogenic, and chondrogenic) across both culture conditions (normoxia, hypoxia) in contrast to previous studies which demonstrated that hypoxia reduced the differentiation of MSCs into adipogenic, osteogenic lineages while increasing their differentiation into the chondrogenic lineage [51, 52]. Moreover, human osteogenic differentiation is regulated by complex molecular interactions, including epigenetic regulation by circRNAs and lncRNAs. CircRNAs modulate osteogenesis by acting as competitive endogenous RNAs to prevent miRNA inhibition of target genes [53], while lncRNAs interact with miRNAs to affect mRNA functionality [54].
Interestingly, MSCsHYP had higher expression of HIF-1α from 6 to 12 h followed by a decline in their expression from 24 to 48 h at both gene and protein levels consistent with our previous findings [35]. We observed a significant alteration in the morphology of hypoxia-preconditioned apoptotic MSCs similar to the others [7, 55, 56] without any change in their surface profile, which was not reported earlier.
Several studies reported that hypoxia-preconditioned MSCs had a profound effect on the suppression of T-cell proliferation [42, 57], induction of CD4+ regulatory T-cell [58], and modified T-cell polarization [22] toward the anti-inflammatory milieu in GVHD which is also related to an increase in the apoptosis of MSCs [59]. Similarly, we observed that MSCsHYP exhibited greater efficiency in modulating the immune system in an in vitro aGVHD model.
To the best of our knowledge, none of the studies have looked into the combined effect of hypoxia and apoptosis. Our study reports contrasting immunomodulation of MSCs preconditioned with hypoxia only versus both (hypoxia and apoptosis). Dual-preconditioned MSCs (MSCsHYP+APO/MSCsHYP→APO) induce the differentiation of effector CD4 + T cells into Tregs. This enhanced immunomodulatory potential is further evidenced by their ability to shift pro-inflammatory T-cell subsets towards an anti-inflammatory phenotype, as seen in changes to the Th1/Th2 and Th1/Th17 ratios, and to program macrophages toward an M2 phenotype. Nonetheless, the concurrent approach exhibits superior efficacy of dual preconditioned MSCs in immunomodulation than the sequential one. Similarly, preclinical studies have demonstrated that apoptotic MSCs are more effective in promoting immune suppression [5], It has been shown that the mode of administering apoptotic MSCs significantly reduces effector cell infiltration in aGVHD murine models without inhibiting T cell proliferation in vitro [4].
The impact of immunomodulation could also be driven by efferocytosis of MSCs. We observed that MSCsHYP+APO exhibit greater efficiency in their efferocytosis than MSCsHYP→APO. This process is often associated with the secretion of immunomodulatory molecules, although specific measurements were not performed in this study. Previous findings revealed that efferocytosis of the apoptotic MSCs by macrophages contributes to the programming of macrophages towards an M2 phenotype with increased secretion of immunomodulatory molecules such as PGE2, IDO, IL-10, PD-L1, and a concurrent decrease in IFN-γ, TNF-α, NO production, resulting in alleviation of inflammation with immune metabolic alterations [5–8, 10, 60, 61]. These findings point towards effective control of GVHD while preserving graft-versus-leukemia (GVL), although no direct evidence was provided in the study.
Interestingly, our observations revealed differences in PDT with no variation in BM-MSCs while a significant difference was observed in WJ-MSCs under both culture conditions. Preconditioned WJ-MSCs maintain their superiority for immune programming over BM-MSCs in all conditions (HYP, HYP→APO, HYP+APO). Furthermore, we observed that MSCs maintain their parental identity and immunomodulation potential despite their preconditioning, summarized in Table S2.
Despite the promising in vitro findings of our study, several limitations must be acknowledged. Firstly, the lack of in vivo validation is a significant constraint, as the complex interactions within a living organism cannot be fully captured in vitro. Future studies should utilize animal models of aGVHD or other immune-mediated conditions to confirm the efficacy and safety of hypoxia-preconditioned apoptotic MSCs (MSCsHYP→APO, MSCsHYP+APO). Secondly, while we hypothesized that efferocytosis leads to the secretion of immunomodulatory molecules, we did not perform direct measurements of these factors. Comprehensive profiling of cytokines and other soluble mediators in future studies will be essential to validate this aspect of our findings. Additionally, the specificity of our patient samples to aGVHD limits the generalizability of our results. Comparative studies involving healthy donors and other patient populations are necessary to determine the broader applicability of our conclusions. Although we observed enhanced immune programming abilities in hypoxia-preconditioned apoptotic MSCs, the precise molecular mechanisms underlying these effects remain unclear. Further research should focus on elucidating these mechanisms, including the role of hypoxia-inducible factors (HIFs), apoptosis-related pathways, and RNA molecules in modulating MSC function.
Moreover, an important limitation of our study is the lack of investigation into the plasticity of Tregs within our co-culture system. The majority of CD4 + T cells may have converted to Tregs under the described conditions, the observed Th1/Th2 and Th17 phenotypes could predominantly reflect Treg subtypes rather than conventional Th1, Th2, or Th17 cells [62–64]. Future studies should explore the plasticity of Tregs under these conditions to determine whether these phenotypes are attributable to Treg subtypes, thereby providing a more comprehensive understanding of the immune modulation observed.
Conclusion
Preconditioned MSCs have the potential to increase their therapeutic efficacy in GVHD. In previous clinical trials, non-conditioned BM-MSCs have been used that suffer the lack of responsiveness in certain patients which is linked to their inadequate apoptosis followed by efferocytosis, potentially stemming from a deficiency in immune cells capable of inducing apoptosis in MSCs [7]. This can be improved by dual (hypoxia and apoptosis) preconditioning of MSCs, preferably concurrently. Our findings highlight that WJ-MSCsHYP+APO is a promising therapeutic strategy for GVHD following BMT. However, further preclinical in vivo and clinical studies are warranted to validate these findings and optimize the protocols for MSCs-based cellular therapy in GVHD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors express their gratitude to the All India Institute of Medical Sciences (AIIMS), New Delhi, India for facilitating the execution of the study. A graphical abstract was created using Biorender.com.
Abbreviations
- Th
Helper T cell
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide
- CFSE
Carboxyfluorescein succinimidyl ester
- PHA
Phytohemagglutinin
- IL
Interleukin
- HYP
Hypoxia
- APO
Apoptosis
- Tregs
Regulatory T-cell
- LPS
Lipopolysaccharide
- IFN
Interferon
- SYBR
N’, N’-dimethyl-N-[4-[€-(3-methyl-1,3-benzothiazol-2-ylidene) methyl]-1-phenylquinolin-1-ium-2-yl]-N-propylpropane-1,3-diamine
- IDO
Indoleamine-2,3-dioxygenase
- PGE2
Prostaglandin E2
- TNF
Tumor Necrosis factor
- ANOVA
Analysis of Variance
- BMT
Bone Marrow Transplantation
Author contributions
MM and MM performed the experiments, acquisition, and analysis of data, results interpretation, and wrote the manuscript. SG, SR, RG, and BN were involved in data interpretation and analysis. LK, SB, VD, DP, PSM, RP, MA, AKG, RD, TS, and MM provided patient samples and their clinical details. TDS, SK, RAM, GK, HG, MS, and CPP were involved in data analysis. HP, SM, and RKS conceptualized the study, designed and supervised the experiments, data interpretation and analysis, and wrote the manuscript. All authors critically reviewed, and approved the final version of the manuscript.
Funding
The study has been supported by the Indian Council of Medical Research, New Delhi, India (Grant Id: 2021/14763).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study involved the use of human subjects only and was approved by the Institute Ethics Committee for Post Graduate Research, All India Institute of Medical Sciences, New Delhi, India (Title: Immunomodulatory properties of Mesenchymal Stem Cells in Graft-versus-host-disease; Ref. No.: IECPG-542/23.09.2020; 24.09.2020). The study involved the use of human mesenchymal stem cells and was approved by the Institutional Committee for Stem Cell Research, All India Institute of Medical Sciences, New Delhi, India (Title: Immunomodulatory properties of Mesenchymal Stem Cells in Graft-versus-host-disease; Ref. No.: IC-SCR/110/20(R); 31.12.2020). Informed written consents were obtained from all participating subjects and all procedures were performed as per the guidelines and regulations approved by the ethics committee
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hridayesh Prakash, Email: hprakash@amity.edu.
Sujata Mohanty, Email: drmohantysujata@gmail.com, Email: drmohantysujata@aiims.edu.
Ranjit Kumar Sahoo, Email: drranjitmd@aiims.edu, Email: drranjitmd@gmail.com.
References
- 1.Dunavin N, Dias A, Li M, McGuirk J. Mesenchymal Stromal Cells: What Is the Mechanism in Acute Graft-Versus-Host Disease? Biomedicines [Internet]. 2017 Jul 1 [cited 2020 Jul 8];5(3). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5618297/ [DOI] [PMC free article] [PubMed]
- 2.Yang G, Fan X, Liu Y, Jie P, Mazhar M, Liu Y, et al. Immunomodulatory mechanisms and therapeutic potential of mesenchymal stem cells. Stem Cell Rev Rep. 2023;19(5):1214–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53(1):e12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9(416). [DOI] [PubMed]
- 5.Giacomini C, Granéli C, Hicks R, Dazzi F. The critical role of apoptosis in mesenchymal stromal cell therapeutics and implications in homeostasis and normal tissue repair. Cell Mol Immunol. 2023;20(6):570–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung TS, Galleu A, von Bonin M, Bornhäuser M, Dazzi F. Apoptotic mesenchymal stromal cells induce prostaglandin E2 in monocytes: implications for the monitoring of mesenchymal stromal cell activity. Haematologica. 2019;104(10):e438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang SHM, D’Rozario J, Mendonca S, Bhuvan T, Payne NL, Zheng D, et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat Commun. 2021;12(1):6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss ARR, Dahlke MH. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front Immunol [Internet]. 2019 Jun 4 [cited 2024 Mar 17];10. https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2019.01191/full [DOI] [PMC free article] [PubMed]
- 9.He X, Hong W, Yang J, Lei H, Lu T, He C, et al. Spontaneous apoptosis of cells in therapeutic stem cell preparation exert immunomodulatory effects through release of phosphatidylserine. Sig Transduct Target Ther. 2021;6(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kholodenko IV, Kholodenko RV, Majouga AG, Yarygin KN. Apoptotic MSCs and MSC-Derived apoptotic bodies as New Therapeutic Tools. Curr Issues Mol Biol. 2022;44(11):5153–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadri N, Amu S, Iacobaeus E, Boberg E, Le Blanc K. Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Cell Mol Immunol. 2023;20(6):613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito A, Aoyama T, Yoshizawa M, Nagai M, Tajino J, Yamaguchi S, et al. The effects of short-term hypoxia on human mesenchymal stem cell proliferation, viability and p16INK4A mRNA expression: investigation using a simple hypoxic culture system with a deoxidizing agent. J Stem Cells Regen Med. 2015;11(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Ji XQ, Zhang SM, Bi RH. Hypoxia and inflammatory factor preconditioning enhances the immunosuppressive properties of human umbilical cord mesenchymal stem cells. World J Stem Cells. 2023;15(11):999–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarsenova M, Kim Y, Raziyeva K, Kazybay B, Ogay V, Saparov A. Recent advances to enhance the immunomodulatory potential of mesenchymal stem cells. Front Immunol [Internet]. 2022 Sep 23 [cited 2023 Jan 16];13. https://www.frontiersin.org/articles/10.3389/fimmu.2022.1010399/full [DOI] [PMC free article] [PubMed]
- 15.Trajkovska I, Georgievski B, Cevreska L, Gacovski A, Hasan T, Nedeska-Minova N. Early and late complications in patients with allogeneic transplantation of hematopoietic stem cell – case report. Open Access Maced J Med Sci. 2017;5(3):340–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnham AJ, Daley-Bauer LP, Horwitz EM. Mesenchymal stromal cells in hematopoietic cell transplantation. Blood Adv. 2020;4(22):5877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol. 2012;12(6):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dazzi F, Clay J, Galleu A. Immune Tolerance in Hemopoietic Stem Cell Transplantation. In: Ratcliffe MJH, editor. Encyclopedia of Immunobiology [Internet]. Oxford: Academic Press; 2016 [cited 2021 May 22]. pp. 241–7. https://www.sciencedirect.com/science/article/pii/B9780123742797150143
- 19.Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol. 2014;11(9):536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–52. [DOI] [PubMed] [Google Scholar]
- 21.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Jin N, Wang F, Chen B. Mesenchymal stem cells: a promising way in therapies of graft-versus-host disease. Cancer Cell Int. 2020;20(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35(2):e00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, Rawat S, Arora V, Kottarath SK, Dinda AK, Vaishnav PK, et al. An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. [DOI] [PubMed] [Google Scholar]
- 26.Mohanty S, Bose S, Jain KG, Bhargava B, Airan B. TGFβ1 contributes to cardiomyogenic-like differentiation of human bone marrow mesenchymal stem cells. Int J Cardiol. 2013;163(1):93–9. [DOI] [PubMed] [Google Scholar]
- 27.Rawat S, Srivastava P, Prabha P, Gupta S, Kanga U, Mohanty S. A comparative study on immunomodulatory potential of tissue specific hMSCs. Role of HLA-G; 2018.
- 28.De la Rosa-Ruiz MDP, Álvarez-Pérez MA, Cortés-Morales VA, Monroy-García A, Mayani H, Fragoso-González G, et al. Mesenchymal Stem/Stromal cells derived from Dental tissues: a comparative in vitro evaluation of their Immunoregulatory Properties against T cells. Cells. 2019;8(12):22. [DOI] [PMC free article] [PubMed]
- 29.Petrenko Y, Vackova I, Kekulova K, Chudickova M, Koci Z, Turnovcova K, et al. A comparative analysis of multipotent mesenchymal stromal cells derived from different sources, with a focus on neuroregenerative potential. Sci Rep. 2020;10:4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mareschi K, Castiglia S, Sanavio F, Rustichelli D, Muraro M, Defedele D, et al. Immunoregulatory effects on T lymphocytes by human mesenchymal stromal cells isolated from bone marrow, amniotic fluid, and placenta. Exp Hematol. 2016;44(2):138–e1501. [DOI] [PubMed] [Google Scholar]
- 31.Lee HJ, Kim SN, Jeon MS, Yi T, Song SU. ICOSL expression in human bone marrow-derived mesenchymal stem cells promotes induction of regulatory T cells. Sci Rep. 2017;7(1):44486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kara EE, Comerford I, Fenix KA, Bastow CR, Gregor CE, McKenzie DR, et al. Tailored Immune responses: Novel Effector helper T cell subsets in protective immunity. PLoS Pathog. 2014;10(2):e1003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peshkova M, Korneev A, Suleimanov S, Vlasova II, Svistunov A, Kosheleva N, et al. MSCs’ conditioned media cytokine and growth factor profiles and their impact on macrophage polarization. Stem Cell Res Ther. 2023;14(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharya P, Dhawan UK, Hussain MT, Singh P, Bhagat KK, Singhal A, et al. Efferocytes release extracellular vesicles to resolve inflammation and tissue injury via prosaposin-GPR37 signaling. Cell Rep. 2023;42(7):112808. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, Rawat S, Krishnakumar V, Rao EP, Mohanty S. Hypoxia preconditioning elicit differential response in tissue-specific MSCs via immunomodulation and exosomal secretion. Cell Tissue Res. 2022;388(3):535–48. [DOI] [PubMed] [Google Scholar]
- 36.Cheung TS, Giacomini C, Cereda M, Avivar-Valderas A, Capece D, Bertolino GM, et al. Apoptosis in mesenchymal stromal cells activates an immunosuppressive secretome predicting clinical response in Crohn’s disease. Mol Ther. 2023;31(12):3531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Fang J, Liu B, Shao C, Shi Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell. 2022;29(11):1515–30. [DOI] [PubMed] [Google Scholar]
- 38.Kholodenko IV, Konieva AA, Kholodenko RV, Yarygin KN. Molecular mechanisms of migration and homing of intravenously transplanted mesenchymal stem cells. J Regenerative Med Tissue Eng. 2013;2(1):4. [Google Scholar]
- 39.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358(3):948–53. [DOI] [PubMed] [Google Scholar]
- 40.Nowak-Stępniowska A, Osuchowska PN, Fiedorowicz H, Trafny EA. Insight in Hypoxia-Mimetic agents as potential tools for mesenchymal stem cell priming in Regenerative Medicine. Stem Cells Int. 2022;2022:8775591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antebi B, Rodriguez LA, Walker KP, Asher AM, Kamucheka RM, Alvarado L, et al. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roemeling-Van Rhijn M, Mensah F, Korevaar S, Leijs M, van Osch G, IJzermans J et al. Effects of Hypoxia on the Immunomodulatory Properties of Adipose Tissue-Derived Mesenchymal Stem cells. Frontiers in Immunology [Internet]. 2013 [cited 2022 Nov 28];4. https://www.frontiersin.org/articles/10.3389/fimmu.2013.00203 [DOI] [PMC free article] [PubMed]
- 43.Widowati W, Wijaya L, Bachtiar I, Gunanegara RF, Sugeng SU, Irawan YA, et al. Effect of oxygen tension on proliferation and characteristics of Wharton’s jelly-derived mesenchymal stem cells. Biomarkers Genomic Med. 2014;6(1):43–8. [Google Scholar]
- 44.Choi JR, Yong KW, Wan Safwani WKZ. Effect of hypoxia on human adipose-derived mesenchymal stem cells and its potential clinical applications. Cell Mol Life Sci. 2017;74(14):2587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwon A, Kim Y, Kim M, Kim J, Choi H, Jekarl DW, et al. Tissue-specific differentiation potency of mesenchymal stromal cells from perinatal tissues. Sci Rep. 2016;6:23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horn P, Bork S, Diehlmann A, Walenda T, Eckstein V, Ho AD, et al. Isolation of human mesenchymal stromal cells is more efficient by red blood cell lysis. Cytotherapy. 2008;10(7):676–85. [DOI] [PubMed] [Google Scholar]
- 47.Hendijani F. Explant culture: an advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017;50(2):e12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schallmoser K, Rohde E, Reinisch A, Bartmann C, Thaler D, Drexler C, et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods. 2008;14(3):185–96. [DOI] [PubMed] [Google Scholar]
- 49.Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205(2):228–36. [DOI] [PubMed] [Google Scholar]
- 50.Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, et al. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mas-Bargues C, Sanz-Ros J, Román-Domínguez A, Inglés M, Gimeno-Mallench L, El Alami M, et al. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. Int J Mol Sci. 2019;20(5):1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6(6):745–57. [DOI] [PubMed] [Google Scholar]
- 53.Mazziotta C, Badiale G, Cervellera CF, Tognon M, Martini F, Rotondo JC. Regulatory mechanisms of circular RNAs during human mesenchymal stem cell osteogenic differentiation. Theranostics. 2024;14(1):143–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lanzillotti C, De Mattei M, Mazziotta C, Taraballi F, Rotondo JC, Tognon M, et al. Long non-coding RNAs and MicroRNAs interplay in osteogenic differentiation of mesenchymal stem cells. Front Cell Dev Biol. 2021;9:646032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trivanovic D, Volkmann N, Stoeckl M, Tertel T, Rudert M, Giebel B, et al. Enhancement of immunosuppressive activity of mesenchymal stromal cells by platelet-derived factors is accompanied by Apoptotic Priming. Stem Cell Rev Rep. 2023;19(3):713–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le TM, Morimoto N, Ly NTM, Mitsui T, Notodihardjo SC, Ogino S, et al. Ex vivo induction of apoptotic mesenchymal stem cell by high hydrostatic pressure. Stem Cell Rev Rep. 2021;17(2):662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim Y, Jin HJ, Heo J, Ju H, Lee HY, Kim S, et al. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia. 2018;32(12):2672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kadle RL, Abdou SA, Villarreal-Ponce AP, Soares MA, Sultan DL, David JA, et al. Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion. PLoS ONE. 2018;13(3):e0193178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galipeau J, Sensébé L. Mesenchymal stromal cells: Clinical challenges and Therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS, et al. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by Monocytic cells. Stem Cells. 2018;36(4):602–15. [DOI] [PubMed] [Google Scholar]
- 61.Ghahremani Piraghaj M, Soudi S, Ghanbarian H, Bolandi Z, Namaki S, Hashemi SM. Effect of efferocytosis of apoptotic mesenchymal stem cells (MSCs) on C57BL/6 peritoneal macrophages function. Life Sci. 2018;212:203–12. [DOI] [PubMed] [Google Scholar]
- 62.Kitz A, Dominguez-Villar M. Molecular mechanisms underlying Th1-like Treg generation and function. Cell Mol Life Sci. 2017;74(22):4059–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao N, Cui W, Zhao LM, Li TT, Zhang JH, Pan LL. Contribution of Th2-like Treg cells to the pathogenesis of Takayasu’s arteritis. Clin Exp Rheumatol. 2020;38(Suppl 124):48–54. [PubMed] [Google Scholar]
- 64.Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3 + Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113(24):6102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.