Abstract
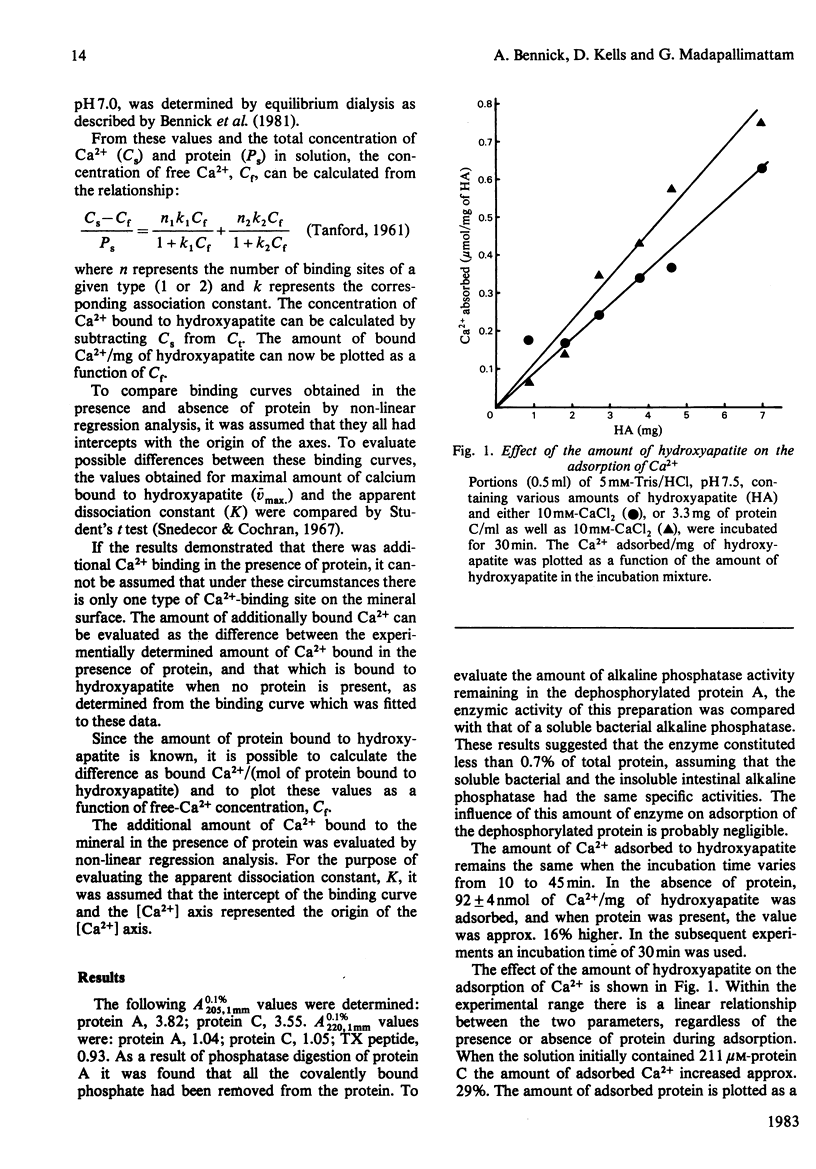
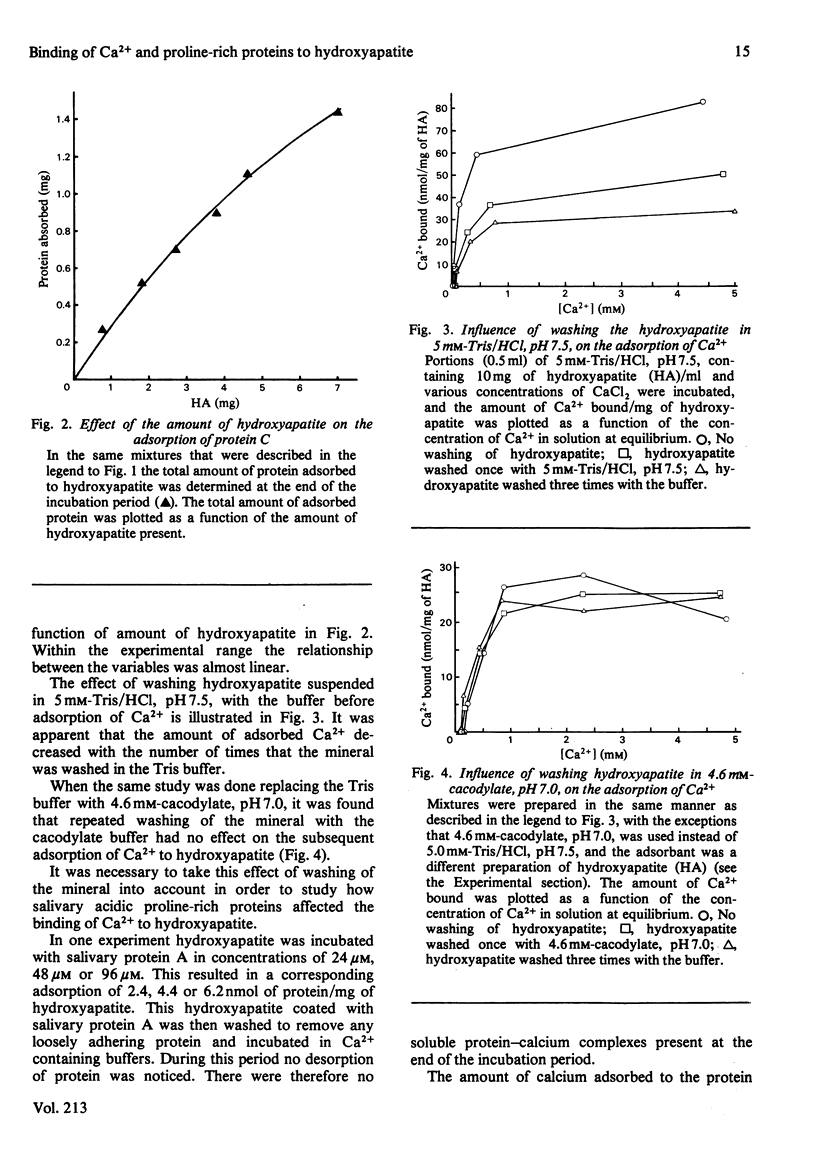
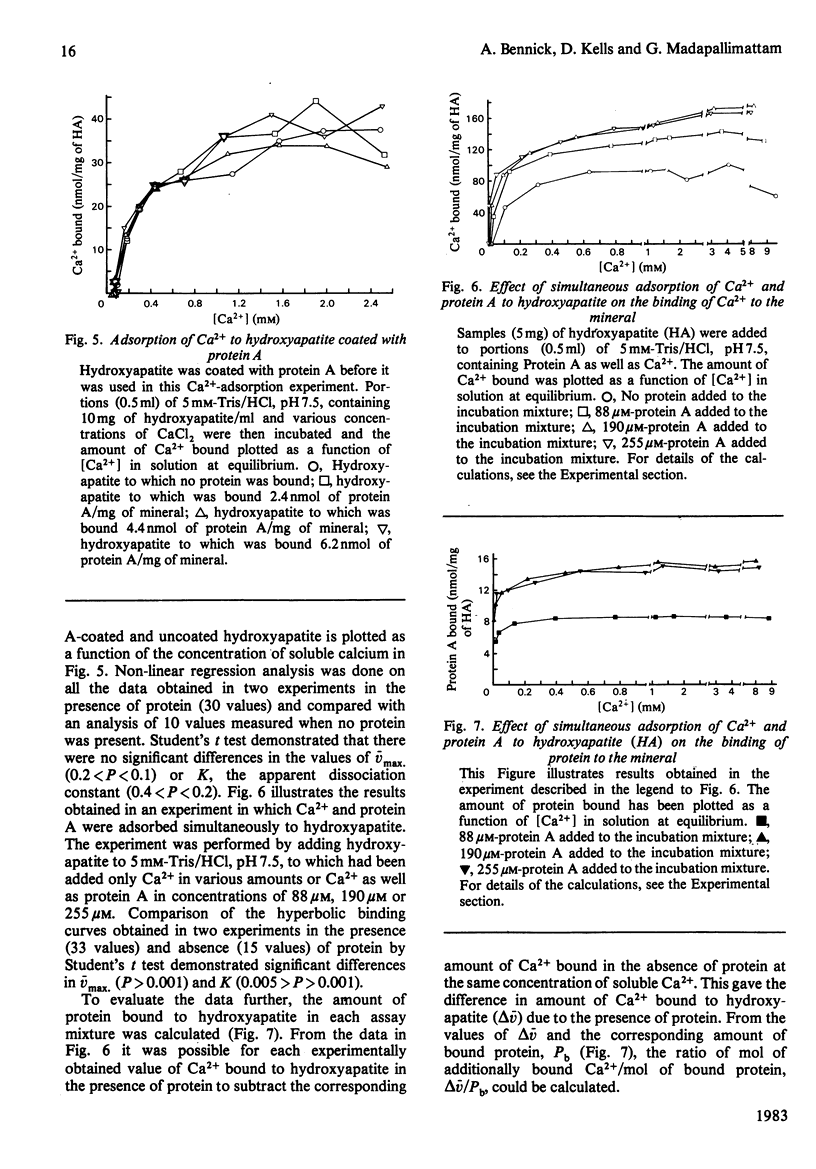
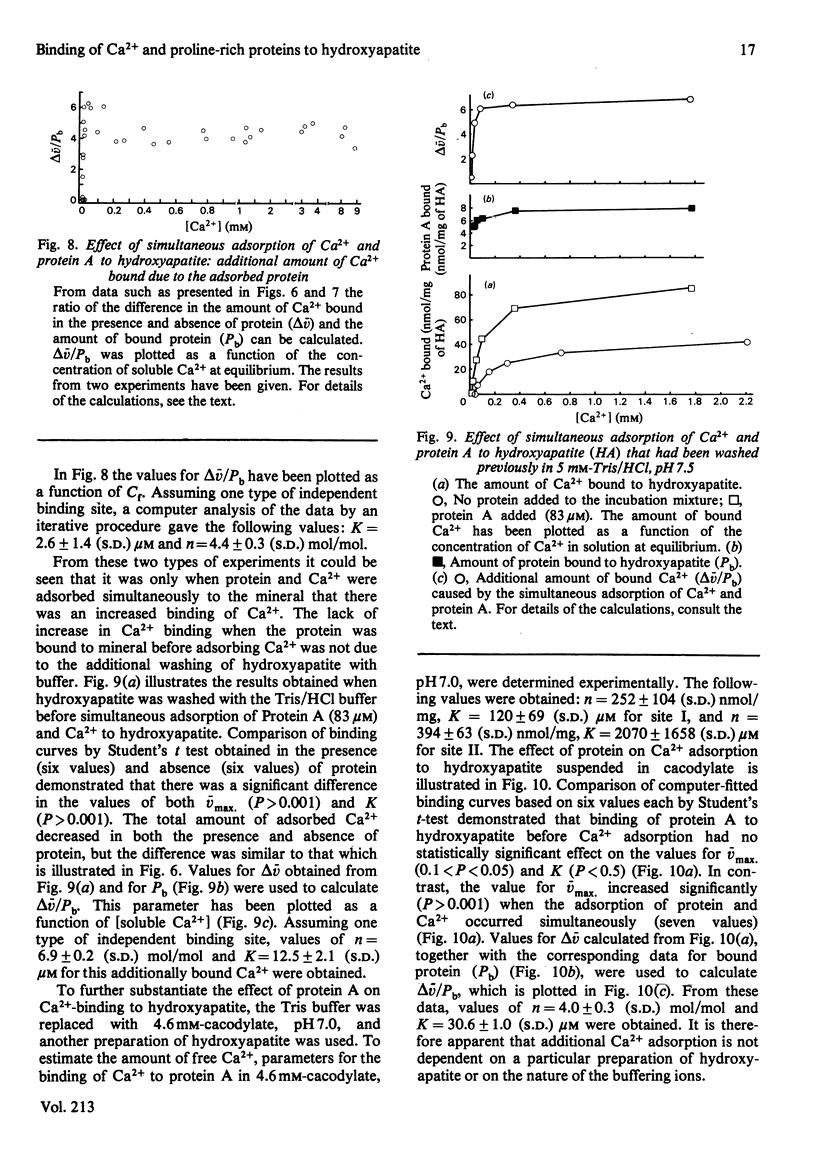
The relationship between Ca2+- and hydroxyapatite-binding sites in salivary acidic proline-rich phosphoproteins A and C was investigated. Coating of hydroxyapatite with protein before adsorption had no effect on Ca2+ binding to the mineral, but simultaneous adsorption of Ca+ and protein to hydroxyapatite caused additional Ca2+ binding to the solid. The additional amount of Ca2+ adsorbed, measured in mol of Ca2+/mol of protein adsorbed to hydroxyapatite, was approx. 2 for protein C, 4 for protein A, 9 for the N-terminal tryptic peptide and 2 for dephosphorylated protein A. It is suggested that the ability of the proteins to inhibit hydroxyapatite formation is related to the binding of the proteins to crystal growth sites on the mineral, which prevents access of Ca2+ from the surrounding liquid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bennick A., Cannon M., Madapallimattam G. The nature of the hydroxyapatite-binding site in salivary acidic proline-rich proteins. Biochem J. 1979 Oct 1;183(1):115–126. doi: 10.1042/bj1830115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A., Cannon M. Quantitative study of the interaction of salivary acidic proline-rich proteins with hydroxyapatite. Caries Res. 1978;12(3):159–169. doi: 10.1159/000260326. [DOI] [PubMed] [Google Scholar]
- Bennick A., Chau G., Goodlin R., Abrams S., Tustian D., Madapallimattam G. The role of human salivary acidic proline-rich proteins in the formation of acquired dental pellicle in vivo and their fate after adsorption to the human enamel surface. Arch Oral Biol. 1983;28(1):19–27. doi: 10.1016/0003-9969(83)90022-5. [DOI] [PubMed] [Google Scholar]
- Bennick A. Chemical and physical characteristics of a phosphoprotein from human parotid saliva. Biochem J. 1975 Mar;145(3):557–567. doi: 10.1042/bj1450557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A. Chemical and physical characterization of a phosphoprotein, Protein C, from human saliva and comparison with a related protein A. Biochem J. 1977 May 1;163(2):229–239. doi: 10.1042/bj1630229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A., Connell G. E. Purification and partial characterization of four proteins from human parotid saliva. Biochem J. 1971 Jul;123(3):455–464. doi: 10.1042/bj1230455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A., McLaughlin A. C., Grey A. A., Madapallimattam G. The location and nature of calcium-binding sites in salivary acidic proline-rich phosphoproteins. J Biol Chem. 1981 May 25;256(10):4741–4746. [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Hay D. I., Moreno E. C. Differential adsorption and chemical affinities of proteins for apatitic surfaces. J Dent Res. 1979 Mar;58(SPEC):930–942. doi: 10.1177/00220345790580024701. [DOI] [PubMed] [Google Scholar]
- Meyer J. L., Nancollas G. H. The influence of multidentate organic phosphonates on the crystal growth of hydroxyapatite. Calcif Tissue Res. 1973 Dec 31;13(4):295–303. doi: 10.1007/BF02015419. [DOI] [PubMed] [Google Scholar]
- Moreno E. C., Varughese K., Hay D. I. Effect of human salivary proteins on the precipitation kinetics of calcium phosphate. Calcif Tissue Int. 1979 Aug 24;28(1):7–16. doi: 10.1007/BF02441212. [DOI] [PubMed] [Google Scholar]
- Pearce E. I. Ion displacement following the adsorption of anionic macromolecules on hydroxyapatite. Calcif Tissue Int. 1981;33(4):395–402. doi: 10.1007/BF02409462. [DOI] [PubMed] [Google Scholar]
- Rölla G., Bowen W. H. Surface adsorption of fluoride and ionic exchange reactions on hydroxyapatite. Acta Odontol Scand. 1978;36(4):219–224. doi: 10.3109/00016357809004671. [DOI] [PubMed] [Google Scholar]
- Wong R. S., Bennick A. The primary structure of a salivary calcium-binding proline-rich phosphoprotein (protein C), a possible precursor of a related salivary protein A. J Biol Chem. 1980 Jun 25;255(12):5943–5948. [PubMed] [Google Scholar]
- Wong R. S., Hofmann T., Bennick A. The complete primary structure of a proline-rich phosphoprotein from human saliva. J Biol Chem. 1979 Jun 10;254(11):4800–4808. [PubMed] [Google Scholar]
- Zahradnik R. T., Moreno E. C., Burke E. J. Effect of salivary pellicle on enamel subsurface demineralization in vitro. J Dent Res. 1976 Jul-Aug;55(4):664–670. doi: 10.1177/00220345760550042101. [DOI] [PubMed] [Google Scholar]



