Abstract
Optimizing extraction conditions can help maximize the efficiency and yield of the extraction process while minimizing negative impacts on the environment and human health. For the purpose of the current study, an artificial neural network (ANN) combined with a genetic algorithm (GA) was utilized for that the extraction conditions of Hypericum spectabile were optimized. In this particular investigation, the main objective was to get the highest possible levels of total antioxidant status (TAS) for the extracts that were obtained. In addition to this, conditions of the extract that exhibited the maximum activity have been determined and the biological activity of the extract that was obtained under these conditions was analyzed. TAS values were obtained from extracts obtained using extraction temperatures of 30–60 °C, extraction times of 4–10 h, and extract concentrations of 0.25-2 mg/mL. The best model selected from the established ANN models had a mean absolute percentage error (MAPE) value of 0.643%, a mean squared error (MSE) value of 0.004, and a correlation coefficient (R) value of 0.996, respectively. The genetic algorithm proposed optimal extraction conditions of an extraction temperature of 59.391 °C, an extraction time of 8.841 h, and an extraction concentration of 1.951 mg/mL. It was concluded that the integration of ANN-GA can successfully be used to optimize extraction parameters of Hypericum spectabile. The total antioxidant value of the extract obtained under optimum conditions was determined as 9.306 ± 0.080 mmol/L, total oxidant value as 13.065 ± 0.112 µmol/L, oxidative stress index as 0.140 ± 0.001. Total phenolic content (TPC) was 109.34 ± 1.29 mg/g, total flavonoid content (TFC) was measured as 148.34 ± 1.48 mg/g. Anti-AChE value was determined as 30.68 ± 0.77 µg/mL, anti-BChE value was determined as 41.30 ± 0.48 µg/mL. It was also observed that the extract exhibited strong antiproliferative activities depending on the increase in concentration. As a result of LC-MS/MS analysis of the extract produced under optimum conditions in terms of phenolic content. The presence of fumaric, gallic, protocatechuic, 4-hydroxybenzoic, caffeic, 2-hydoxycinamic acids, quercetin and kaempferol was detected. As a result, it was determined that the H. spectabile extract produced under optimum conditions had significant effects in terms of biological activity.
Keywords: Extraction conditions, Genetic algorithm, Hypericum spectabile, Optimization
Introduction
Free and reactive oxygen radicals (ROS) are formed during metabolic and physiological activities to use oxygen in cyclical reactions and biochemical reactions in living things [1]. In addition, external factors such as pollution, exposure to chemicals, radiation, and drugs can accelerate the formation of ROS in cells [2]. These unstable radicals can interact with biomolecules (such as lipids, proteins, nucleic acids) and other reagents in cells, initiate chain reactions and cause oxidation [3]. ROS accumulated in cells over time pave the way for oxidative stress and related metabolic disorders, aging, chronic heart diseases, cancer, and various degenerative diseases [4]. Antioxidants are substances that eliminate the harmful effects of ROS in cells [5, 6]. Polyphenols, some vitamins, vitamin precursors, carotenoids, minerals, pigments and enzymes are compounds with antioxidant properties [7]. Antioxidants may be found in many different plants like genus Hypericum.
The flowering plant genus Hypericum is widely dispersed over the globe. Many species of Hypericum are classified as pests and nuisances because of their rapid spread. Trees, shrubs, annuals, and perennials all belong to the genus Hypericum. More than one trunk can grow from the same root system in several tree species. Shrubs are plants with upright or trailing stems. Roughly 490 species may be found across the globe. Hypericum spectabile used in our study is an endemic species. It generally spreads in the eastern and southeastern regions of Turkey. Antioxidant activity potential of bioactive compounds of this plant has been reported in previous studies [8–10].
The amount of bioactive compounds that may be extracted from various natural plant sources is dependent on such a wide variety of parameters that play a role in the process of extraction. In addition to other characteristics, these considerations include the nature of the plant material, the temperature and duration of the extraction process, as well as the properties of the solvent.
Optimization is a necessary step for any processing activities since there are a huge number of possible combinations between the process parameters. Among various optimization tools, an effective tool in the optimization of multivariate systems is the response surface methodology (RSM), which models the relationship between a set of independent variables and a set of dependent variables as a quadratic equation with a set of coefficients. The application of RSM based models for non-linear behavior is constrained, however, because they are precise only within a small window of input parameters. The learning process of an artificial neural network (ANN) using experimental data enables it to handle multivariate non-linear variables with indefinite interactions, exhibit significant generalization capability, and demonstrate tolerance towards noise and missing data. Hence, Artificial Neural Networks (ANN) are a superior approach for representing intricate non-linear correlations among variables compared to alternative fitting techniques like Response Surface Methodology (RSM). The genetic algorithm (GA) is a search algorithm that emulates the adaptive phenomena observed in natural biological systems. It has demonstrated its efficacy in addressing many optimization problems. In the field of food processing, a number of researchers have used ANNs in combination with GAs to generate predictions and optimize models of complicated processes [11–13]. But there is currently no research available on optimization extraction parameters of H. spectabile using ANN-GA combination.
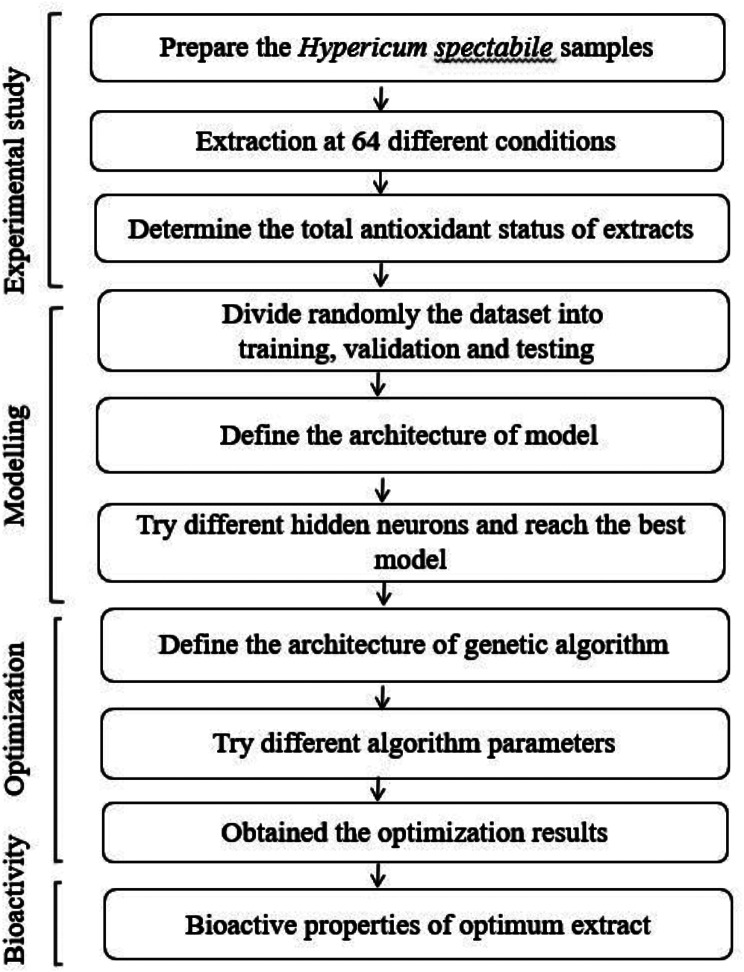
The bioactive properties of Hypericum spectabile are important because of its potential for use in modern and traditional medicine. Studies proving the antioxidant, antimicrobial, anti-inflammatory and antitumor properties of this plant have revealed its medicinal use and industrial potential [14]. It has also been revealed in previous studies that the bioactive properties of Hypericum species plants vary according to extraction conditions [15]. From this perspective, it was aimed the optimization of the extraction parameters of H. spectabile using artificial intelligence techniques. ANN coupled with GA was used to modelling and optimization of data obtained from experimental study. The flowchart of the study is presented in Fig. 1.
Fig. 1.
Flowchart of study
Material and method
Material
Plant material was collected from Duhok (Iraq) in 2022. The plant was identified by Dr. Falah Saleh Mohammed. Herbarium specimens are held at Zakho University, Department of Biology.
Extraction
30 grammes of dry materials were weighed and pulverised into powder using a mechanical grinder. A Gerhardt EV 14 Soxhlet extractor was used to derive the water extracts from the plant samples. A rotary evaporator (Heidolph Laborator 4000 Rotary Evaporator) was employed to concentrate the biological extracts. The extraction parameters were established as a temperature inside the range of 30–60 °C, a duration of 4–10 h, and an extraction concentration ranging from 0.25 to 2 mg/mL. A total of 64 extracts were extracted by studying 3 different values of each extraction parameter.
Antioxidant activity for optimization
In the study, total antioxidant status (TAS) values were determined as antioxidant activity of the extracts. The TAS values of each water extract obtained from the H. spectabile plant were calculated using commercial Rel Assay kits. The findings of the TAS studies were expressed as mmol Trolox equivalent/L using hydrogen peroxide as calibrators [16, 17].
Modelling
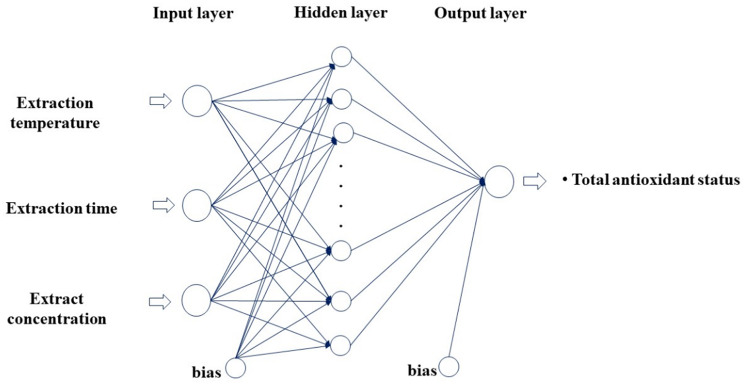
Artificial Neural Networks (ANNs) is an algorithm that is inspired by the biological nerve cell (neuron) structure, establishes a model and can learn by itself in the process. ANN have a wide range of applications, including but not limited to robot technology, pattern recognition, medicine, power systems, signal processing, prediction, and system modeling. ANNs are comprised of interconnected layers operating in parallel. These layers are a simulated representation of the neural structure found in the human brain. Neural networks are composed of three layers: the input layer, one or more hidden layers, and the output layer. The network’s primary purpose is to establish connections between different layers. The network is trained through the adjustment of the weight values associated with the connections between the layers of the network. The layers of the study were presented in Fig. 2.
Fig. 2.
The layers of study
The main objective of training a neural network is to minimize errors or inaccuracies by adjusting the weights inside the network. This repeated procedure persists until the desired output is achieved. To assess the efficacy of the training procedure, the neural network is subjected to testing using data that was not utilized during the training phase. The performance level of the neural network is defined by the outcomes of this testing procedure.
In ANN, there are no predetermined rules or guidelines that must be followed to solve the specific situation. Because of this, the best way to decide how many layers the model should have, as well as the learning algorithm and activation functions it should include is to use the practice of trial and error.
The data obtained from experimental study was randomly divided into three groups: (a) training sets (80%), (b) validation sets (10%), and (c) test sets (10%). The models with a single hidden layer were trained with the Levenberg-Marquardt learning algorithm. Twenty various (from 1 to 20) numbers of hidden neurons were tested throughout the investigation to find the optimal value. The error value was set to 1 × 10− 6; the maximum number of iterations was set to 100, the number of validation checks was set to 50, and the learning coefficient and momentum coefficient were both set to 0.5. Additionally, the maximum number of iterations was set at 100.
Error term statistics that are collected from prediction outputs are utilized as a reference point to determine the accuracy of the established models’ predictions. Evaluation of model performance was conducted using the mean square error (MSE) and the mean absolute percentage error (MAPE), calculated according to Eqs. 1 and 2, respectively,
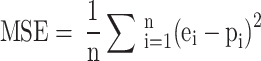 |
1 |
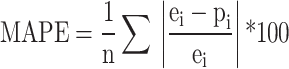 |
2 |
where e represents the experimental outcome, p represents the predicted outcome, and n is the sample size.
Optimization
The process of natural selection serves as the inspiration for genetic algorithms, also known as GAs. These algorithms are a form of optimization algorithm. It is common practice to utilize them to simulate the process of evolution to discover the most effective possible solution to a problem.
GA is a powerful optimization technique and have been applied to a wide range of problems in various fields, such as machine learning, computer science, engineering, and biology. In machine learning, GAs is used to optimize the parameters of a model to improve its performance. In computer science, they are used to solve problems such as the traveling salesman problem and the knapsack problem. In engineering, GAs is used to optimize the design of structures and systems.
The basic process of a GA involves the following steps:
Initialize the population.
Evaluate the fitness of each individual.
Select individuals for reproduction.
Create the next generation.
Repeat steps 2–4.
In this study, GA method was used to optimize the extraction parameters to find maximum total antioxidant status value. The optimization procedure can be expressed in the following:
Decision variables: Extraction temperature (Ete), Extraction time (Eti), Extract concentration (Ec).
Objective function: Maximum TAS (Ete, Eti, Ec).
Limitations of decision variables: 30 °C ≤ Ete ≤ 60 °C.
4 h ≤ Eti ≤ 10 h.
0.25 mg/mL ≤ Ec ≤ 2 mg/mL.
The size of the population was set at 30, and the roulette approach was utilized as the selection method. In order to generate a new generation, an implementation of one crossover point operator and a crossover rate of 0.95 was designed. The mutation rate, which is what allows for genetic variety, was set at 0.05%. The iteration number, which represents the greatest possible number of generations, was set to 60. In addition, 21 iterations were carried out to assess the efficiency of the optimization technique.
Extraction processes for biological activities
The goal of the optimization study was to identify the extraction parameters that would be most efficient in maximizing the biological activity of the plant sample. A temperature of 59.391 °C, an extraction duration of 8.841 h, and a concentration of 1.951 mg/mL were found to be the ideal conditions for extraction, according to the optimization process of the study. Optimized extraction conditions were accomplished by utilizing the Gerhardt SOX-414 equipment in a computerized environment. Throughout the course of the research, every single analysis was carried out on the extract that was collected under the optimized extraction conditions. After the extraction procedure was completed, the solvent that was used, which was water, was allowed to evaporate with the help of a Buchi R100 Rotary Evaporator, which ultimately led to the generation of crude extracts.
Antioxidant parameter tests
A total antioxidant status (TAS) and a total oxidant status (TOS) of plant extract were determined with the use of the Rel Assay kits. Using the methodology that was included in the package, experiments were carried out in line with the instructions. The TAS values are calculated using millimoles of trolox equivalent per liter as the unit of measurement. Micromoles of hydrogen peroxide equivalent per liter are the units of measurement that are used to quantify the TOS measurements [16]. To get the OSI value, the TOS data were divided by the TAS values, and the resulting percentage was expressed as the result [18].
Anticholinesterase activity test
To determine whether or not the plant extract possessed anticholinesterase action, the Ellman method was modified. It was determined that galantamine was the standard that was employed. To prepare stock solutions, plant extracts were diluted to quantities varied between 200 and 3.125 µg/mL. Subsequently, the microplate was filled with 130 µl of a phosphate buffer (0.1 M concentration, pH 8). Furthermore, 10 µl of the stock solution and 20 µl of an enzyme solution containing either acetylcholinesterase (AChE) at a concentration of 5.32 × 10^−3 U or butyrylcholinesterase (BChE) at a concentration of 6.85 × 10^−3 U were introduced. The sample was then placed in an inert environment for ten minutes at a temperature of twenty-five degrees Celsius, devoid of any electromagnetic radiation. Subsequently, a solution containing DTNB (5,5-dithiobis-(2-nitrobenzoic acid)) and a substrate consisting of either acetylcholine iodide (0.71 mM) or butyrylcholine iodide (0.2 mM) were each added in quantities of twenty microliters. Subsequently, the calibration was conducted at a wavelength of 412 nanometers. Three independent experiments were conducted on the material. The IC50 values, which quantify the degree of inhibition the substances exhibit, were reported in micrograms per milliliter (µg/mL) [19].
Antiproliferative activity tests
The potential of the plant extract to suppress cell proliferation was investigated using the A549 lung cancer cell line. Concentrations of 25, 50, 100, and 200 µg/mL of extracts were used to produce the solutions. After the cells had reached a confluence of 70–80%, they were seeded after being dissociated with a solution of trypsin-EDTA (Sigma-Aldrich, Missouri, United States) that was 3.0 milliliters in volume. Following that, the sample was put into an incubator for a period of twenty-four hours. After the incubation period had passed, the stock solutions were made available to the participants. A further twenty-four hours were added to the incubation period after it had already been brought forward. After that, the supernatants were combined with the growing medium, and another solution that included one milligram per milliliter of MTT (Sigma) was added to the mixture. Following that, the mixture was placed in an incubator set at 37 degrees Celsius until a purple-colored precipitate was observed. Afterwards, the MTT solution was diluted with dimethyl sulfoxide (DMSO), a chemical solvent obtained from Sigma-Aldrich in Missouri, United States of America. After that, the reading was taken with an Epoch spectrophotometer that was produced by BioTek Instruments and was located in Winooska, Vermont. The wavelength of the reading was 570 nm [20].
Phenolic analysis
In this study, we employed the Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) equipment to examine twenty-four frequently produced compounds in the plants. The experimental procedure was conducted using a C-18 Intersil ODS-4 analytical column with dimensions of 3.0 mm x 100 mm and a particle size of 2 μm. A temperature of 40 °C was established for the column. The mobile phase consists of two distinct components: solution A is a blend of water and 0.1% formic acid, while solution B is a blend of ethanol and 0.1% formic acid. The mobile phase is used interchangeably to refer to each of these solutions. The flow rate for the mobile phase is 0.3 milliliters per minute, whereas the volume of the injected sample is 2 µl [21].
Result and discussion
Antioxidant activity
The antioxidant properties of plants offer potential benefits in their use [22]. The antioxidant properties of H. spectabile can be considered as an alternative treatment method for its use. Antioxidants neutralize harmful free radicals in the body, preventing harmful effects. They also provide protection against cancer and slow down the aging process [23].
The total antioxidant status (TAS) value is a measure that indicates the total quantity of chemicals that are efficient antioxidants that are found in raw materials [24]. In the present investigation, the TAS values of plant samples of H. spectabile were measured (see Table 1). The samples were extracted at temperatures of 30, 40, 50, and 60 °C for 4, 6, 8, and 10 h at concentrations of 0.25, 0.50, 1, and 2 mg/mL. Within the confines of this scope, the temperature, extraction time, and concentration of the plant that proved to be the most effective were studied. According to the findings of the experimental tests, the TAS value of the plant that was found to be the highest among the extraction circumstances that were investigated was determined to be 9.646 mmol/L.
Table 1.
TAS values of obtained extracts
| Extraction temperature (°C) |
Extraction time (h) |
Extract concentration (mg/mL) |
TAS (mmol/L) |
|---|---|---|---|
| 30 | 4 | 0.25 | 6.188 ± 0.064 |
| 0.5 | 6.445 ± 0.052 | ||
| 1 | 6.759 ± 0.033 | ||
| 2 | 7.160 ± 0.078 | ||
| 6 | 0.25 | 6.367 ± 0.039 | |
| 0.5 | 6.727 ± 0.045 | ||
| 1 | 7.017 ± 0.067 | ||
| 2 | 7.336 ± 0.100 | ||
| 8 | 0.25 | 6.744 ± 0.042 | |
| 0.5 | 7.350 ± 0.106 | ||
| 1 | 7.631 ± 0.028 | ||
| 2 | 7.867 ± 0.056 | ||
| 10 | 0.25 | 6.850 ± 0.051 | |
| 0.5 | 7.573 ± 0.048 | ||
| 1 | 7.827 ± 0.035 | ||
| 2 | 8.094 ± 0.066 | ||
| 40 | 4 | 0.25 | 6.613 ± 0.098 |
| 0.5 | 6.963 ± 0.071 | ||
| 1 | 7.320 ± 0.095 | ||
| 2 | 7.670 ± 0.131 | ||
| 6 | 0.25 | 7.027 ± 0.071 | |
| 0.5 | 7.441 ± 0.071 | ||
| 1 | 7.789 ± 0.038 | ||
| 2 | 8.051 ± 0.050 | ||
| 8 | 0.25 | 7.292 ± 0.054 | |
| 0.5 | 7.805 ± 0.007 | ||
| 1 | 8.076 ± 0.056 | ||
| 2 | 8.512 ± 0.076 | ||
| 10 | 0.25 | 7.548 ± 0.055 | |
| 0.5 | 7.707 ± 0.015 | ||
| 1 | 8.002 ± 0.034 | ||
| 2 | 8.326 ± 0.070 | ||
| 50 | 4 | 0.25 | 6.705 ± 0.158 |
| 0.5 | 7.180 ± 0.149 | ||
| 1 | 7.785 ± 0.157 | ||
| 2 | 8.346 ± 0.103 | ||
| 6 | 0.25 | 7.335 ± 0.155 | |
| 0.5 | 7.741 ± 0.069 | ||
| 1 | 8.095 ± 0.073 | ||
| 2 | 8.585 ± 0.039 | ||
| 8 | 0.25 | 7.658 ± 0.064 | |
| 0.5 | 8.142 ± 0.124 | ||
| 1 | 8.406 ± 0.087 | ||
| 2 | 8.824 ± 0.048 | ||
| 10 | 0.25 | 8.032 ± 0.070 | |
| 0.5 | 8.233 ± 0.105 | ||
| 1 | 8.620 ± 0.100 | ||
| 2 | 8.942 ± 0.049 | ||
| 60 | 4 | 0.25 | 7.078 ± 0.112 |
| 0.5 | 7.454 ± 0.050 | ||
| 1 | 8.002 ± 0.029 | ||
| 2 | 8.539 ± 0.090 | ||
| 6 | 0.25 | 7.502 ± 0.131 | |
| 0.5 | 7.898 ± 0.031 | ||
| 1 | 8.361 ± 0.146 | ||
| 2 | 8.804 ± 0.090 | ||
| 8 | 0.25 | 7.691 ± 0.047 | |
| 0.5 | 8.235 ± 0.087 | ||
| 1 | 9.646 ± 0.088 | ||
| 2 | 9.240 ± 0.217 | ||
| 10 | 0.25 | 8.030 ± 0.148 | |
| 0.5 | 8.756 ± 0.144 | ||
| 1 | 9.013 ± 0.098 | ||
| 2 | 9.222 ± 0.026 |
It has been previously reported that an ethanol extract of H. spectabile was obtained at 50 °C for 6 h, and the TAS value was calculated as 4.215 mmol/L [10]. Our analysis reveals that the computed TAS value exceeds the value documented in the literature. Within this particular framework, it has been established that those extracts acquired under appropriate circumstances, such as in our investigation, will provide a more precise and effective representation of the antioxidant capabilities of the plants. Furthermore, TAS values of several plant species in literature has reported Alcea kurdica (3.298 mmol/L), Mentha longifolia subsp. longifolia (3.628 mmol/L), Rhus coriaria var. zebaria (7.342 mmol/L), and Rumex scutatus (8.556 mmol/L) [25–28]. By comparison to these investigations, it can be asserted that the H. spectabile used in our work exhibits a greater TAS value when subjected to ideal conditions. Based on the available evidence, it has been established that H. spectabile can serve as a significant reservoir of antioxidants.
Modelling
Artificial neural networks (ANNs) partition data into three distinct components: training, validation, and testing data. Such partitioning is essential for the purpose of training and assessing the precision of the model.
This division is important for evaluating the accuracy of the model and avoiding problems such as overfitting (performing well on the same data). In this study, among the developed ANN models, the model with 7 hidden neurons showed the best performance.
The prediction ability of ANN model is typically measured by the accuracy of its predictions on a dataset. The accuracy is usually expressed as a percentage and a high accuracy, close to 100%, is desired. Performance metrics of selected ANN model were presented in Table 2.
Table 2.
Performance metrics of selected ANN model
| Metric | Training | Validation | Test | All |
|---|---|---|---|---|
| MAPE (%) | 0.558 | 1.321 | 0.698 | 0.643 |
| MSE | 0.003 | 0.012 | 0.003 | 0.004 |
| R | 0.997 | 0.991 | 0.993 | 0.996 |
Mean absolute percentage error (MAPE) is a statistic used to assess the performance of artificial neural network (ANN) models. The MAPE is a metric used to quantify the proportion of inaccuracy between the predicted values and the true values. This number quantifies the level of comparability between the estimated values and the actual values. A low MAPE value indicates that the model is performing at a satisfactory level. While achieving a MAPE score of zero would be optimal, it is not always feasible in practice. A high MAPE score suggests that the model exhibits a poor degree of affinity to the real data. The study found that the MAPE values for training, validation, test, and by all data were 0.558%, 1.321%, 0.698%, and 0.643%, respectively.
The mean squared error (MSE) is another metric used to assess the performance of artificial neural network (ANN) models. The MSE is a statistical measure that calculates the mean of the squared discrepancy between the predicted values and the real values. These estimated values are compared to the actual values, and this metric reflects the degree of similarity between them. According to the MSE value, a low value suggests that the model is doing better. In a perfect world, the MSE value would be zero, but in practice, this is not always feasible. When the MSE score is high, it shows that the model is not very comparable to the values that are really being used. According to the findings of the research, (MSE) values for training, validation, test, and by all data were found to be 0.003, 0.012, 0.003, and 0.004 accordingly.
The Pearson correlation coefficient (R) is an extra statistic used to assess the prediction capability of artificial neural network (ANN) models. The R value is a numerical parameter ranging from − 1 to 1, which represents the level of correlation between the estimated values and the actual values. A figure of 1 is the maximum feasible value that indicates a perfect positive correlation between the estimated values and the actual values. A score of -1, which is the largest possible value, indicates a 100% negative correlation between the estimated values and the actual values. Thus, a score of 0 indicates that there is no correlation between the estimated values and the actual values.
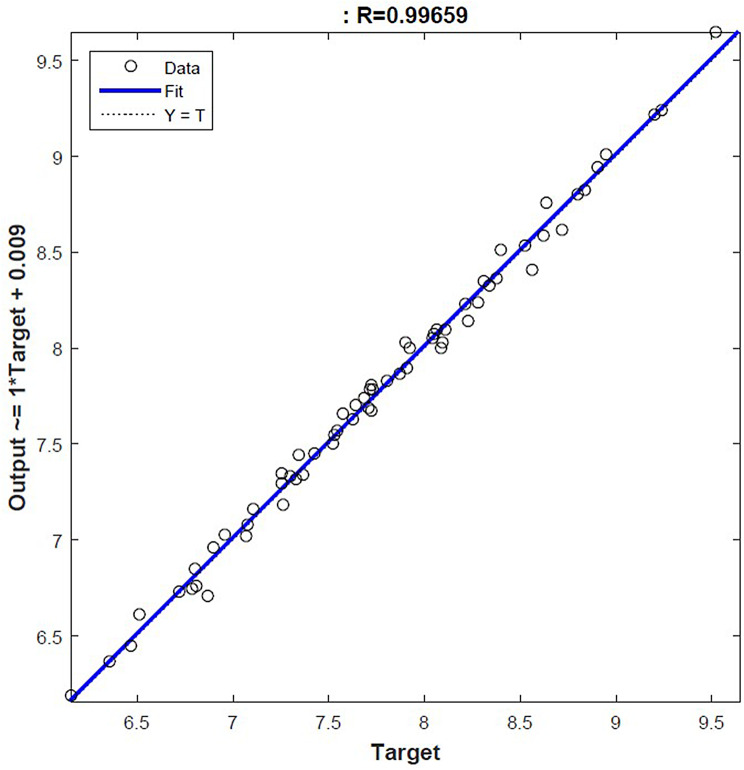
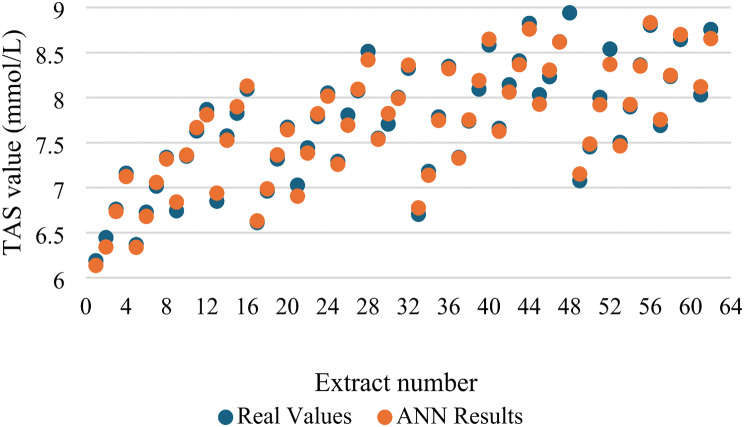
A high coefficient of determination (R value) suggests outstanding performance of the model. Preferred, the R value should be 1, although this is sometimes unattainable. A low R value suggests that the model exhibits least resemblance to the real values. In the study, R values of training, validation, test and all data were determined as 0.997, 0.991, 0.993, and 0.996 respectively (Fig. 3). As a result, it is seen how close the real values and estimated values are to each other (Fig. 4).
Fig. 3.
Correlation coefficient of ANN model
Fig. 4.
Real and estimated values
Optimization
The aim of this work is to determine the precise extraction parameters that yield the highest total antioxidant status (TAS) value among the extracted samples. An optimization technique based on genetic algorithms was employed to accomplish this objective.
In this study, the optimum extraction parameters obtained objective function were found as 59.391 extraction temperature (°C) and 8.841 extraction time (h) and 1.951 extraction concentration (mg/mL). The TAS value of optimum extraction parameters was estimated as 9.906 mmol/L (Table 3).
Table 3.
The optimum extraction parameters obtained optimization process
| Sample | Extraction temperature (°C) | Extraction time (h) |
Extract concentration (mg/mL) |
Estimated TAS value (mmol/L) |
|---|---|---|---|---|
| H. spectabile | 59.391 | 8.841 | 1.951 | 9.906 |
In literature, the suitability of ultrasound-assisted deep eutectic solvent extraction (UADESE) as a novel technique for extracting anthocyanins and other valuable chemicals from raspberries has been reported. An approach to optimize the extraction process was devised by combining response surface techniques with a genetic algorithm. An optimal set of extraction conditions was determined to maximize the yield of anthocyanins from raspberry powder. In the experiment, the greatest yield of anthocyanins (1.378 ± 0.009 mg/g) was achieved with a water content of 29%, ultrasonic power of 210 W, extraction temperature of 51 °C, and extraction time of 32 min [29].
A previous study examined the impact of ultrasonication on the extraction of phenolic components from cashew apple bagasse (CAB). Conclusive findings of this investigation were published. Several treatment durations, ultrasound amplitudes, and bagasse to solvent ratios were used to improve the extraction process for maximizing the production of total phenolic content, total tannin content, and β-carotene concentrate. Response surface process methodology (RSM) and artificial neural network (ANN) were employed to model and optimize the process parameters. At a treatment duration of 12 min, an ultrasonic amplitude of 55%, and a bagasse to solvent ratio of 45 grammes per milliliter, the optimal conditions for RSM were achieved. The optimal parameters recommended by the artificial neural network (ANN) were a treatment duration of 15 min, an ultrasonic amplitude of 60%, and a bagasse to solvent ratio of 50 g/mL. With the lowest statistical parameters and the highest coefficient of determination (R2), the artificial neural network (ANN) model outperformed the response surface methodology (RSM) model. Upon optimization of the settings, the measured values of TPC, TTC, and β-carotene content exhibited concordance with the projected values obtained from both models [11].
In a study, the extraction of general flavones from celery leaves was accomplished through the utilization of ultrasound-assisted extraction. The Box-Behnken design was utilized in order to assess the manner in which the extraction procedure was affected by a number of variables, including temperature, duration, the ratio of solids to liquids, and the concentration of ethanol. The response surface methodology and the BP neural network model with a genetic algorithm (GA) were utilized to implement additional optimization. A temperature of 67.2 °C, an ethanol concentration of 70.31%, and an extraction period of 26.6 min were found to be the ideal parameters for the extraction process, according to the findings at hand [13].
Because of of this, one can draw the conclusion that every optimization technique is distinct and one-of-a-kind, dependent on the parameters that are being investigated and the material that is being examined.
Antioxidant potential
Free radicals serve as a major source of oxidative stress in biological systems. Metabolic processes systematically produce them, and excessive amounts can result in substantial damage. A crucial function of the antioxidant defense system is to safeguard the body from the harmful impact of free radicals. Oxidative stress can arise when the antioxidant defense system is not operating with optimal efficiency [30]. The presence of oxidative stress in humans can significantly contribute to the pathogenesis of severe conditions including cancer, diabetes, cardiovascular illnesses, and neurological diseases. Antioxidants included in dietary supplements are used to mitigate the adverse impacts of oxidative stress [31]. Plants, within the context of this discourse, are significant natural resources that have a crucial function. An investigation was undertaken to assess the antioxidant capacity of the extract of H. spectabile, which was prepared under ideal conditions. The results are presented in Table 4.
Table 4.
Antioxidant pamareters of Hypericum Spectabile
| Sample | TAS (mmol/L) | TOS (µmol/L) | OSI (TOS/(TAS*10)) | TPC (mg/g) | TFC (mg/g) |
|---|---|---|---|---|---|
| H. spectabile | 9.306 ± 0.080 | 13.065 ± 0.112 | 0.140 ± 0.001 | 109.34 ± 1.29 | 148.34 ± 1.48 |
In our study, the antioxidant potential of Hypericum spectabile extracts produced under optimum conditions was determined. In the literature, the TAS value of the ethanol extract of Hypericum spectabile obtained at 50 °C for 6 h was reported as 4.215, TOS value as 23.421 and OSI value as 0.556 [10]. Compared to this study, Hypericum spectabile used in our study was extracted at higher temperature and for a longer time. As a result, it was determined to have a higher TAS value and a lower TOS value. This result shows that an extract produced under optimum conditions will exhibit more effective biological activity. In addition, there are TAS, TOS and OSI studies on different plants in the literature. In this context, TAS values of Helianthemum salicifolium, Glaucium alakirensis, Alcea kurdica and Rumex scutatus were reported as 9.490, 3.496, 3.298 and 8.656 mmol/L, respectively. TOS values were reported as 14.839, 2.204, 8.312 and 4.951 µmol/L, respectively. OSI values were reported as 0.157, 0.063, 0.252 and 0.057, respectively [23, 25, 32, 33]. Compared to these studies, the TAS value of Hypericum spectabile used in our study was measured as higher than Glaucium alakirensis, Alcea kurdica and Rumex scutatus, and lower than Helianthemum salicifolium. One way to measure the overall amount of antioxidant chemicals that are produced by the plant is by analyzing the TAS value. The TAS value of H. spectabile extracts that were produced under optimal conditions was taken into consideration in our research, and it was ultimately found that these extracts possessed a large amount of antioxidant capacity. The TOS value of H. spectabile, which was utilized in our research, was shown to be lower than that of Helianthemum salicifolium, higher than that of Glaucium alakirensis, Alcea kurdica, and Rumex scutatus, and lower than that of Helianthemum salicifolium. The total oxidant species (TOS) parameter is a measure of the overall oxidant compounds generated by the plant. Based on the scope of this inquiry, it was determined that H. spectabile had limited capacity for synthesising oxidizing compounds. An oxidative stress index (OSI) value quantifies the percentage of oxidizing substances that are inhibited by antioxidant molecules in a plant. According to the results of our research, the OSI value of H. spectabile was found to be lower than that of Helianthemum salicifolium and Alcea kurdica, but it was found to be greater than that of Glaucium alakirensis and Rumex scutatus. Because of this, it was found that the H. spectabile extract that was produced in our research experiment under ideal conditions possessed a significant amount of antioxidant potential.
Within their bodies, plants produce phenolics and flavonoids, each of which is associated with an exclusive group of biological activities. In the course of our research, we had the opportunity to ascertain the total phenolic and flavonoid contents of H. spectabile that had been obtained under ideal conditions. The results are presented in Table 4. A study in literature reported that the overall phenolic content of H. spectabile was 23.1 mg/g, while the total flavonoid content was 22.4 mg/g [34]. Both of these values were found in the published literature. The total phenolic and flavonoid contents of the H. spectabile that was employed in our investigation were found to be higher when compared to the results of this study. In this setting, it has been discovered that the plant extract, when prepared under optimal conditions, possesses higher levels of phenolic and flavonoid compounds.
Anticholinesterase activity
Alzheimer’s disease is a disease with a high prevalence in the elderly population worldwide. Although different treatment methods have been tried for this disease in recent years, the most common method is acetylcholine inflammation. Acetylcholine is hydrolyzed by acetylcholinesterase and butyrylcholinesterase enzymes. In this context, the use of natural products that exhibit these activities is important in the treatment of the disease [35]. In our study, the anticholinesterase activity of Hypericum spectabile was determined. The findings are shown in Table 5.
Table 5.
Anticholinesterase activity of Hypericum Spectabile
| Sample | AChE µg/mL | BChE µg/mL |
|---|---|---|
| Hypericum spectabile | 30.68 ± 0.77 | 41.30 ± 0.48 |
| Galantamine | 5.68 ± 0.21 | 13.77 ± 0.17 |
During our research, it was possible to ascertain the anti-AChE and anti-BChE activities of H. spectabile that was grown under ideal conditions. After doing the analyses, it was discovered that the antiproliferative activity of the substance was lower than that of galantamine, which was utilized as the reference. The presence of acetylcholinesterase activity in H. spectabile has been documented in the scientific literature. During the course of our research, we discovered that it possessed both acetyl and butyrylcholinesterase qualities. When it comes to the treatment of diseases, the existence of enzymes that are responsible for the etiology of diseases plays a very significant role. When seen in this light, the suppression of these enzymes can lead to substantial advancements in the fight against illnesses [36]. According to the findings of our research, H. spectabile possesses significant levels of acetyl and butyrylcholinesterase activities, and because of this, the plant has the potential to be utilized as a natural agent in this particular setting.
Antiproliferative activity
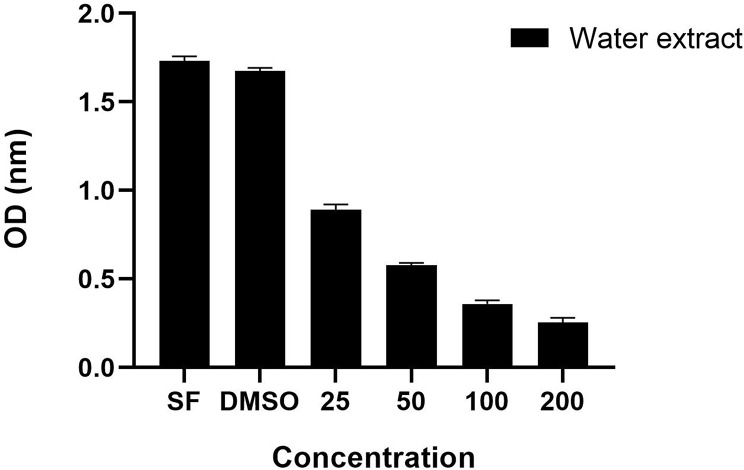
Nowadays, different methods are applied as a solution to widespread cancer cases. Herbal treatments are widely used by people [37]. In our study, the antiproliferative activity of Hypericum spectabile extract produced under optimum conditions was determined. In this context, the extracts were tested against the A549 cell line. The findings are shown in Fig. 5.
Fig. 5.
Antiproliferative activity of Hypericum spectabile extract
There is no finding in the literature regarding the effect of H. spectabile against A549 cancer cell line. However, in literature, H. spectabile has been shown to be ineffective against human cervical adenocarcinoma (HeLa) and normal rat kidney epithelial (NRK-52E) cell lines [34]. The results of our research showed that the extract of H. spectabile, which was extracted under ideal conditions, had powerful effects against the A549 cancer cell line. Through observation, it was found that the effects of the plant extract became more pronounced as the concentration of the extract increased. Upon completion of the test, it was shown that the concentration of 200 µg/mL had the most pronounced impact compared to all subsequent values examined. Considering this, it was determined that H. spectabile has the inherent capacity to be employed in pharmaceutical formulations as a candidate for anticancer agents.
Phenolic contents
Plants can synthesize a diverse range of biologically active compounds. The compounds are secondary metabolites of great importance in the medical domain. In this study, we used an LC/MS-MS instrument to examine the phenolic chemicals found in the H. spectabile extract prepared under optimal circumstances. The findings are shown in Table 6.
Table 6.
Phenolic contents of Hypericum Spectabile
| Phenolic compounds | Ret. time | Values (mg/kg) | Potential health benefit |
|---|---|---|---|
| Fumaric acid | 0.859 | 1249.53 ± 0.82 | Anti-inflammatory and analgesic activity [38] |
| Gallic acid | 1.483 | 5196.31 ± 0.86 | Anticancer activity [39] |
| Protocatechuic acid | 2.060 | 2097.95 ± 1.14 | Antioxidant and antihyperlipidaemic activity [40] |
| 4-hydroxybenzoic acid | 2.550 | 698.61 ± 1.11 | Antiproliferative and proapoptotic activity [41] |
| Caffeic acid | 2.850 | 3549.96 ± 1.38 | Anti-hepatitis B virus activity [41] |
| 2-hydoxycinnamic acid | 3.553 | 546.50 ± 0.75 | Antioxidant activity [42] |
| Quercetin | 4.000 | 2587.52 ± 1.24 | Anti-inflammatory activity [43] |
| Kaempferol | 4.222 | 390.91 ± 0.73 | Antitumor, antioxidant and anti-inflammatory [44] |
Twenty-four different phenolic compounds were examined in the H. spectabile extract that was prepared under ideal conditions for the purpose of our research. Upon the conclusion of the investigation, a grand total of eight distinct chemicals were discovered. Quercetin, kaempferol, fumaric acid, gallic acid, protocatechuic acid, 4-hydroxybenzoic acid, caffeic acid, and 2-hydoxycinamic acid are among the compounds mentioned. Among the identified compounds, gallic acid exhibited the highest level of abundance. The dosage of kaempferol was the least. Previous scientific literature has documented the presence of hypericin, pseudohypericin, chlorogenic acid, neochlorogenic acid, 2,4-dihydroxybenzoic acid, hyperoside, isoquercitrin, quercitrin, quercetin, avicularin, rutin, I3, II8-biapigenin, amentoflavone, mangiferin, (+)-catechin, and (−)-epicatechin in the body of H. spectabile [45]. A separate investigation revealed that H. spectabile comprised several unique chemicals such as pseudohypericin, hypericin, chlorogenic acid, rutin, hyperoside, isoquercitrin, quercitrin, kaempferol, quercetin, amentoflavone, and hyperforin [32]. The phenolic components of the extract of H. spectabile that were produced under optimal conditions and had the maximum biological activity were determined in our study. These investigations were conducted in addition to the ones that were mentioned above. As a result of the findings that were acquired, it was observed that it has the potential to be a significant natural source in terms of the compounds that were contained inside it. Furthermore, it is believed that the extract that is produced under optimal conditions and has the highest level of biological activity can be utilized in the development of pharmacological designs. Specifically, one limitation is that the extraction methods were restricted to certain conditions (temperature, time, and solvent) which may not encompass all variables that could affect the extraction yield and quality. Additionally, while our findings are promising, the results are based on in vitro studies, which may not fully represent the efficacy in vivo.
Conclusions
This study utilized artificial intelligence techniques to optimize the extraction conditions for H. spectabile for the first time. It was conducted experimental studies and developed a model using artificial neural networks (ANN) based on the obtained data. The model’s prediction error for output was below 0.7%. Next, it was applied optimization process using the GA algorithm. The study found that the ANN-GA integration was highly accurate and effective for optimization procedures. Future studies may explore optimization using different objective functions and analyze algorithm performance using a range of optimization techniques. Additionally, the antioxidant, anticholinesterase, and antiproliferative properties of the extracts generated under ideal conditions were assessed. Upon analysis, it was shown that they had noteworthy biological activities. Furthermore, the extract was shown to contain phenolic chemicals associated with significant biological activity. It is seen that it may be an important source in terms of the detected phenolic compounds.
Acknowledgements
None.
Abbreviations
- AChE
Acetylcholinesterase
- ANN
Artificial Neural Network
- BChE
Butyrylcholinesterase
- CAB
Cashew Apple Bagasse
- DMSO
Dimethyl Sulfoxide
- GA
Genetic Algorithm
- MAPE
Mean Absolute Percentage Error
- MSE
Mean Squared Error
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide
- R
Correlation Coefficient
- ROS
Reactive Oxygen Radicals
- RSM
Response Surface Methodology
- TAS
Total Antioxidant Status
- TFC
Total Flavonoid Content
- TOS
Total Oxidant Status
- TPC
Total Phenolic content
- UADESE
Ultrasound-Assisted Deep Eutectic Solvent Extraction
Author contributions
M.S. and O.U. supervised the research. A.G. and M.S. performed the experiments, analyzed the data, and wrote the manuscript. A.G. and T.K. designed the experiments. I.U. provided assistance during the experiments. A.G., M.S., T.K., and O.U. revised the manuscript. All authors read and approved the final manuscript.
Funding
None.
Data availability
On reasonable request, the datasets utilized and analysed in the present work can be obtained from the corresponding author.
Declarations
Ethics approval and consent to participate
This research does not involve any ethical issues.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morrissey P, O’brien N. Dietary antioxidants in health and disease. Int Dairy J. 1998;8(5–6):463–72. [Google Scholar]
- 2.Aseervatham GSB, Sivasudha T, Jeyadevi R, Arul Ananth D. Environmental factors and unhealthy lifestyle influence oxidative stress in humans—an overview. Environ Sci Pollut Res. 2013;20:4356–69. [DOI] [PubMed] [Google Scholar]
- 3.Gulcin İ. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020;94(3):651–715. [DOI] [PubMed] [Google Scholar]
- 4.Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, Vujacic-Mirski K, Helmstädter J, Kröller-Schön S, Münzel T. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxidative Med Cell Longev. 2019;2019(1):7092151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majumder D, Nath P, Debnath R, Maiti D. Understanding the complicated relationship between antioxidants and carcinogenesis. J Biochem Mol Toxicol. 2021;35(2):e22643. [DOI] [PubMed] [Google Scholar]
- 6.Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini E, Peluso I. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020;11:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zehiroglu C, Ozturk Sarikaya SB. The importance of antioxidants and place in today’s scientific and technological studies. J Food Sci Technol. 2019;56:4757–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozkan EE, Ozsoy N, Ozden TY, Ozhan G, Mat A. Evaluation of chemical composition and in-vitro biological activities of three endemic Hypericum species from Anatolia (H. Thymbrifolium, H. Spectabile and H. pseudolaeve). Iran J Pharm Research: IJPR. 2018;17(3):1036. [PMC free article] [PubMed] [Google Scholar]
- 9.Boga M, Ersoy E, Ozkan EE, Cinar E, Kara EM, Canturk YY, Zengin G. Volatile and phenolic profiling of a traditional medicinal plant, Hypericum empetrifolium with in vitro biological activities. J Ethnopharmacol. 2021;272:113933. [DOI] [PubMed] [Google Scholar]
- 10.Mohammed FS, Şabik AE, Doğan M, Selamoğlu Z, Sevindik M. Antioxidant potential of Hypericum Spectabile JAUB. ET SPACH. Bull Biotechnol. 2020;1(2):43–5. [Google Scholar]
- 11.Patra A, Abdullah S, Pradhan RC. Application of artificial neural network-genetic algorithm and response surface methodology for optimization of ultrasound‐assisted extraction of phenolic compounds from cashew apple bagasse. J Food Process Eng. 2021;44(10):e13828. [Google Scholar]
- 12.Rakshit M, Srivastav P. Optimization of pulsed ultrasonic-assisted extraction of punicalagin from pomegranate (Punica granatum) peel: a comparison between response surface methodology and artificial neural network‐multiobjective genetic algorithm. J Food Process Preserv. 2021;45(1):e15078. [Google Scholar]
- 13.Zhang Z, Guo S, Chen C, Lin J, Chen L, Wang D, Hu J, Chen X, Yang J, Li Y. Optimization of extraction and bioactivity detection of celery leaf flavonoids using BP neural network combined with genetic algorithm and response. Prep Biochem Biotechnol. 2022;52(6):648–56. [DOI] [PubMed] [Google Scholar]
- 14.Marrelli M, Statti G, Conforti F. Hypericum spp.: an update on the biological activities and metabolic profiles. Mini Rev Med Chem. 2020;20(1):66–87. [DOI] [PubMed] [Google Scholar]
- 15.Özkan Eroğlu E, Çelik Özbek B, Afife M. Antimicrobial activities of five endemic Hypericum species from Anatolia compared with Hypericum perforatum. J Res Pharm. 2019;23:114–9. [Google Scholar]
- 16.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37(4):277–85. [DOI] [PubMed] [Google Scholar]
- 17.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–11. [DOI] [PubMed] [Google Scholar]
- 18.Sevindik M. The novel biological tests on various extracts of Cerioporus Varius. Fresenius Environ Bull. 2019;28(5):3713–7. [Google Scholar]
- 19.Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88–95. [DOI] [PubMed] [Google Scholar]
- 20.Bal C, Akgul H, Sevindik M, Akata I, Yumrutas O. Determination of the anti-oxidative activities of six mushrooms. Fresenius Envir Bull. 2017;26(10):6246–52. [Google Scholar]
- 21.Sevindik M, Gürgen A, Khassanov VT, Bal C. Biological activities of ethanol extracts of Hericium erinaceus obtained as a result of optimization analysis. Foods. 2024;13(10):1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uysal I, Koçer O, Mohammed FS, Lekesiz Ö, Doğan M, Şabik AE, Sevindik E, Gerçeker FÖ, Sevindik M. Pharmacological and nutritional properties: Genus Salvia. Adv Pharmacol Pharm. 2023;11(2):140–55. [Google Scholar]
- 23.Karaltı İ, Eraslan EC, Sarıdoğan BGÖ, Akata I, Sevindik M. Total antioxidant, Antimicrobial, antiproliferative potentials and element contents of Wild Mushroom candolleomyces candolleanus (Agaricomycetes) from Turkey. Int J Med Mushrooms 2022, 24(12). [DOI] [PubMed]
- 24.Krupodorova T, Barshteyn V, Sevindik M. Antioxidant and antimicrobial potentials of mycelial extracts of Hohenbuehelia myxotricha grown in different liquid culture media. BioTechnologia. 2022;103(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unal O, Eraslan EC, Uysal I, Mohammed FS, Sevindik M, Akgul H. Biological activities and phenolic contents of Rumex scutatus collected from Turkey. Fresenius Environ Bull. 2022;31(7):7341–6. [Google Scholar]
- 26.Mohammed FS, Akgul H, Sevindik M, Khaled BMT. Phenolic content and biological activities of Rhus coriaria var. Zebaria. Fresenius Environ Bull. 2018;27(8):5694–702. [Google Scholar]
- 27.Mohammed FS, Kına E, Uysal İ, Mencik K, Dogan M, Pehlivan M, Sevindik M. Antioxidant and antimicrobial activities of ethanol extract of Lepidium Spinosum. Turkish J Agriculture-Food Sci Technol. 2022;10(6):1116–9. [Google Scholar]
- 28.Sevindik M, Akgul H, Pehlivan M, Selamoglu Z. Determination of therapeutic potential of Mentha longifolia ssp. Longifolia. Fresen Environ Bull. 2017;26(7):4757–63. [Google Scholar]
- 29.Xue H, Tan J, Li Q, Tang J, Cai X. Optimization ultrasound-assisted deep eutectic solvent extraction of anthocyanins from raspberry using response surface methodology coupled with genetic algorithm. Foods. 2020;9(10):1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alfei S, Marengo B, Zuccari G. Oxidative stress, antioxidant capabilities, and bioavailability: ellagic acid or urolithins? Antioxidants. 2020;9(8):707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Caparros P, De Filippis L, Gul A, Hasanuzzaman M, Ozturk M, Altay V, Lao MT. Oxidative stress and antioxidant metabolism under adverse environmental conditions: a review. Bot Rev. 2021;87:421–66. [Google Scholar]
- 32.Mohammed FS, Kına E, Sevindik M, Doğan M, Pehlivan M. Antioxidant and antimicrobial activities of ethanol extract of Helianthemum salicifolium (Cistaceae). Indian J Nat Prod Resour (IJNPR)[Formerly Nat Prod Radiance (NPR). 2021;12(3):459–62. [Google Scholar]
- 33.Özcandır A, Mohammed FS, Sevindik M, Aykurt C, Selamoglu Z, Akgül H. Phenolic composition, total antioxidant, antiradical and antimicrobial potential of endemic Glaucium Alakirensis. Sigma J Eng Nat Sci. 2024;42(1):42–8. [Google Scholar]
- 34.Eroglu Ozkan E, Ozsoy N, Ozden TY, Ozhan G, Mat A. Evaluation of chemical composition and in-vitro biological activities of three endemic Hypericum species from Anatolia (H. Thymbrifolium, H. Spectabile and H. pseudolaeve). Iran J Pharm Research: IJPR. 2018;17(3):1036. [PMC free article] [PubMed] [Google Scholar]
- 35.Thakral S, Yadav A, Singh V, Kumar M, Kumar P, Narang R, Sudhakar K, Verma A, Khalilullah H, Jaremko M. Alzheimer’s disease: molecular aspects and treatment opportunities using herbal drugs. Ageing Res Rev. 2023;88:101960. [DOI] [PubMed] [Google Scholar]
- 36.Świątek Ł, Sieniawska E, Sinan KI, Maciejewska-Turska M, Boguszewska A, Polz-Dacewicz M, Senkardes I, Guler GO, Bibi Sadeer N, Mahomoodally MF, LC-ESI-QTOF-MS/MS, Analysis. Cytotoxic, antiviral, antioxidant, and enzyme Inhibitory properties of four extracts of Geranium pyrenaicum Burm. f.: a good gift from the natural treasure. Int J Mol Sci. 2021;22(14):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hossain MS, Kader MA, Goh KW, Islam M, Khan MS, Harun-Ar Rashid M, Ooi DJ, Melo Coutinho HD, Al-Worafi YM, Moshawih S. Herb and spices in colorectal cancer prevention and treatment: a narrative review. Front Pharmacol. 2022;13:865801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shakya A, Singh GK, Chatterjee SS, Kumar V. Role of fumaric acid in anti-inflammatory and analgesic activities of a Fumaria indica extracts. J Intercultural Ethnopharmacol. 2014;3(4):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramanian A, John A, Vellayappan M, Balaji A, Jaganathan S, Supriyanto E, Yusof M. Gallic acid: prospects and molecular mechanisms of its anticancer activity. RSC Adv. 2015;5(45):35608–21. [Google Scholar]
- 40.Harini R, Pugalendi KV. Antioxidant and antihyperlipidaemic activity of protocatechuic acid on streptozotocindiabetic rats. Redox Rep. 2010;15(2):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seidel C, Schnekenburger M, Dicato M, Diederich M. Antiproliferative and proapoptotic activities of 4-hydroxybenzoic acid-based inhibitors of histone deacetylases. Cancer Lett. 2014;343(1):134–46. [DOI] [PubMed] [Google Scholar]
- 42.Teixeira J, Gaspar A, Garrido EM, Garrido J, Borges F. Hydroxycinnamic acid antioxidants: an electrochemical overview. Biomed Res Int. 2013;2013(1):251754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boots AW, Wilms LC, Swennen EL, Kleinjans JC, Bast A, Haenen GR. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition. 2008;24(7–8):703–10. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Fang X, Ge L, Cao F, Zhao L, Wang Z, Xiao W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE. 2018;13(5):e0197563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cirak C, Radusiene J, Jakstas V, Ivanauskas L, Yayla F, Seyis F, Camas N. Secondary metabolites of Hypericum species from the Drosanthe and Olympia sections. South Afr J Bot. 2016;104:82–90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
On reasonable request, the datasets utilized and analysed in the present work can be obtained from the corresponding author.