Abstract
Background
Ureaplasma spp. can be classified into different serovars. It is unknown whether distinct serovars are associated with clinical signs and symptoms.
Methods
We conducted a multicentre cross-sectional study. U. parvum serovars were identified on the basis of their multiple-banded antigen (MBA) genes. After adjusting for demographic variables and other reproductive tract infections, the odds ratio (OR) and 95% confidence interval (CI) were calculated to determine the impact of U. parvum serovars on clinical symptoms.
Results
Among 5,277 individuals, U. parvum serovars 3 and 6 were the most prevalent serovars (17.9% and 16.0%, respectively). Potential confounders, such as age, body mass index (BMI), ethnicity, education level, contraceptive methods, number of sexual partners, gravidity, parity, and other sexually transmitted infections (STIs) that are associated with clinical symptoms (P < 0.1) were adjusted for in the univariate analysis. U. parvum serovar 14 was strongly positively associated with certain clinical symptoms, including redness and swelling of the vaginal wall (crude OR: 3.53, 95% CI: 1.92–6.49; adjusted OR: 5.21, 95% CI: 2.56–10.58), cervical bleeding and swelling (crude OR: 3.89, 95% CI: 2.38–6.36; adjusted OR: 7.37, 95% CI: 3.82–14.23), and cervical ectropion (crude OR: 2.08, 95% CI: 1.25–3.45; adjusted OR: 3.04, 95% CI: 1.60–5.74). In contrast, U. parvum serovar 3 was negatively associated with a variety of clinical symptoms, whereas no correlations were detected between U. parvum serovars 1and 6 with clinical symptoms.
Conclusions
Different U. parvum serovars exhibit distinct correlations with clinical symptoms, suggesting that U. parvum serovars are pathogenically heterogeneous and that further differentiation of serovars may be necessary.
Trial registration
The study was registered with ClinicalTrials.gov (https://www.clinicaltrials.gov; ID: NCT04694495; Registration Date: 2021–01-05).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10113-9.
Keywords: U. parvum serovars, Women of childbearing age, Clinical symptoms, Heterogeneity in pathogenicity
Background
Ureaplasma spp. is one of the most prevalent Mycoplasma species associated with urogenital tract infection in humans, and it can be classified into 2 biovars, Ureaplasma parvum (U. parvum) and Ureaplasma urealyticum (U. urealyticum) [1–3]. On the basis of multiple-banded antigen (MBA) genes, 4 serovars (serovars 1, 3, 6, and 14) belong to the U. parvum biovar, and the remaining serovars (serovars 2, 4, 5, 7–13) belong to the U. urealyticum biovar [4, 5]. U. urealyticum and U. parvum are prevalent in the reproductive tract of nonpregnant women of childbearing age, with estimated prevalence rates ranging from 7.6% to 28.5% for U. urealyticum and 38.3% to 60.6% for U. parvum [6–9]. U. urealyticum has been associated with a variety of medical problems, including spontaneous abortion and premature birth [10, 11]. In contrast, U. parvum is considered potentially harmless because of its high prevalence and weak correlation with disease [12]. Nevertheless, few studies have examined the possibility that U. parvum pathogenicity varies by serovar, which frequently leads to confusion among clinicians regarding the necessity of treating U. parvum-positive patients [11, 13]. Since distinct serovars may have varying degrees of pathogenicity, testing U. parvum as a whole rather than individual serovars may introduce confounding factors into the associations between U. parvum. and clinical complications.
The clinical presentation of symptoms and signs is the most straightforward indicator of pathological alterations. One study indicated that U. parvum was not associated with symptoms/signs in women [12]. Only a limited number of studies have identified diverse connections between different U. parvum serovars and symptoms [14, 15]. However, these studies focused only on U. parvum serovars, and no other sexually transmitted infections (STIs) were considered confounding variables. Furthermore, the sample size was restricted to only one centre. In addition, there is no clinical differentiation among serovars with respect to the treatment of U. parvum and U. urealyticum [16], but differences in drug susceptibility between U. parvum serovars have been demonstrated [17, 18]. Identifying variations in the pathogenicity of U. parvum serovars simultaneously assists clinicians in selecting the most appropriate antibiotic regimens.
Thus, we conducted a study to determine whether specific U. parvum serovars are related to specific symptoms and/or clinical indications in nonpregnant women, to explore the heterogeneity in the pathogenicity of U. parvum serovars.
Methods
Study design and population
For this cross-sectional study, we used baseline data (2020.11–2022.10) from the Chinese Association for cLinical Microbiome 2004 (CALM 2004) project. The CALM 2004 project is an ongoing prospective multicentre cohort study in China that aims to determine the relationship between the female genital tract microbiota and human papillomavirus (HPV) infection clearance, persistence, and progression to cervical intraepithelial neoplasia (CIN) or cervical cancer. All the participants who were routinely screened for cervical cancer were recruited from the gynaecological outpatient department of a class A tertiary hospital. The design was described previously [19]. The detailed inclusion, exclusion and elimination criteria of the study are outlined in Table S2. This analysis, which was based on baseline data from the CALM 2004 project, included participants who provided basic information, maintained exhaustive records of clinical signs and symptoms, and underwent thorough comprehensive testing for pathogens causing STIs. The final analysis included 38 centres and 5,277 participants. The sample sizes of each centre are presented in Table S3.
U. parvum serovar detection
Exfoliated cervical cells were collected from each individual by a clinician for U. parvum serovar testing, which was measured with an STIs detection kit (Hybribio, Guangdong, China) [20, 21]. In accordance with the manufacturer’s instructions, 1 mL of cell preservation solution containing cervical cells was centrifuged at 7,000 ×g for 5 min. Then, the supernatant was discarded, 0.5 mL of cell preservation mixture was added, and the cells were resuspended and centrifuged again at 7,000 ×g for 1 min. The supernatant was discarded, 50 µl of cell lysate was added, the mixture was boiled for 10 min, and centrifuged at 7,000 ×g for 10 min, the supernatant was kept for DNA amplification. The serovars of U. parvum were identified (Hybribio, Guangdong, China) via PCR and flow-through hybridization using specific probes. U. parvum was divided into U. parvum serovars 1, 3, 6 and 14 according to the following probe sequences.
U. parvum serovar 1: 5'-TTACACATATTAAATAAAGACAATAAA-3'.
U. parvum serovar 3: 5'-TATGTAAGATTACCAAATCTTAGTGTT-3'.
U. parvum serovar 6: 5'-ATTTTTTACTAGTATTAAATTAAAAACAAT-3'.
U. parvum serovar 14: 5'-TATTAATCTTACATAATTTCTAC-3'.
Outcomes
The eligibility of individuals for the study was determined and evaluated by a gynaecologist and research nurse. All participants completed a questionnaire on genital symptoms, including vaginal itching, lower abdominal pain, pain or bleeding during sexual intercourse and irregular vaginal bleeding. The clinical signs of purulent cervical discharge, redness and swelling of the vaginal wall, vaginal odour, cervical bleeding and swelling, and cervical ectropion were also recorded by a clinician during a genital examination.
Covariate collection
We selected covariates associated with clinical symptoms and U. parvum infections, including those identified by previous studies such as socioeconomic information (age, ethnicity, education level, contraceptive methods, number of sexual partners, gravidity, and parity), pathogens causing genital tract infections, including Neisseria gonorrhoeae (N. gonorrhoeae), U. urealyticum, Mycoplasma hominis (M. hominis), Mycoplasma genitalium (M. genitalium), Herpes simplex virus type 2 (HSV-2), Chlamydia trachomatis (C. trachomatis), HPV, Trichomonas vaginalis (T. vaginalis), Candida, and the Nugent and Donders scores [22, 23]. Socioeconomic information was compiled from a self-report questionnaire. Table S4 shows a detailed summary of the questionnaires. Ethnicity was a binary variable comprising Han or ethnic minorities, with ethnic minorities defined as ethnic groups other than the Han ethnic group. Education level was defined as below an undergraduate or an undergraduate or above. Contraceptive methods were divided into 4 categories: noncontraception, intrauterine device, contraceptives, and condom. Individuals using multiple contraceptive methods were excluded from the analysis. There were 2 categories for the number of sexual partners: 1 or ≥ 2 partners. We categorized gravidity and parity status into 4 groups accordingly: 0, 1, 2, and ≥ 3 pregnancies/births.
The methods used for the detection of N. gonorrhoeae, U. urealyticum, M. hominis, M. genitalium, HSV-2, and C. trachomatis were the same as those used for the detection of U. parvum serovars. Genotypes of HPV were determined according to the manufacturer's guidelines via an HPV GenoArray diagnostic kit (Hybribio, Guangdong, China). The presence or absence of T. vaginalis was determined via wet mount microscopy and Candida positivity was determined via both wet mount microscopy and culture methods for vaginal secretions [23]. The Nugent score was determined via Gram staining of the vaginal smear whereas the Donders score was determined via wet film microscopy of the vaginal secretions. The Nugent score was divided into 3 categories: bacterial vaginosis (BV), defined as a score ≥ 7; intermediate, defined as a score of 4–6; and normal, defined as a score of 3 [24]. Aerobic vaginitis (AV) was defined as a Donders score ≥ 3; normal was defined as a Donders score < 3 [25].
Data analysis
Continuous variables are reported as the means with standard deviations, and categorical variables are reported as frequencies and percentages. Univariate analyses were used to assess risk factors associated with clinical symptoms. The exposure factor was the U. parvum serovar, whereas the outcome variables consisted of numerous signs and symptoms of vaginal and cervical, for which data were collected via questionnaires and clinician examinations. The exposure and outcome variables were both classified as categorical variables. The correlation between U. parvum serovar and clinical symptoms were assessed using logistics regression. We constructed 3 models: 1) a crude model, which was unadjusted; 2) a minimally adjusted model, which was adjusted for basic demographic variables, including age, BMI and ethnicity; and 3) a fully adjusted model, which included the variables adjusted for in the minimally adjusted model and was additionally adjusted for potential confounders, such as education level, contraceptive methods, number of sexual partners, gravidity, parity and STIs with P < 0.1 in univariate analyses. If U. parvum serovars were the independent variables, other serovars were also adjusted for. Additional stratified analyses were conducted to evaluate the connection between U. parvum serovar 14 and cervical-related symptoms in different HPV infection statuses. Additionally, the P-value for the interaction between U. parvum serovar 14 and HPV was determined. Odds ratios (ORs) with 95% confidence intervals (CI) were calculated.
R 4.2.0 (http://www.R-project.org) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) were used for the statistical analysis. Statistical significance was set at a P value < 0.05.
Results
Description of the study population
A total of 5,277 participants whose U. parvum serovars test results and self-reported clinical symptoms were available were included in this cross-sectional study (Fig. 1). U. parvum serovar 3 was the most prevalent serovar (17.9%, n = 944), followed by U. parvum serovar 6 (16.0%, n = 845), and U. parvum serovar 14 was the least prevalent (1.6%, n = 87). U. parvum serovar 1 was detected in 423 participants (8.0%) (Table 1).
Fig. 1.
Flow chart of the study
Table 1.
Baseline characteristics of study participants
| Characteristic | U. parvum serovar 1 | U. parvum serovar 3 | U. parvum serovar 6 | U. parvum serovar 14 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Negative (n = 4854) |
Positive (n = 423) |
P |
Negative (n = 4333) |
Positive (n = 944) |
P |
Negative (n = 4432) |
Positive (n = 845) |
P |
Negative (n = 5190) |
Positive (n = 87) |
P | |
| Age, year | 35.96 ± 7.32 | 36.67 ± 7.40 | 0.057 | 36.00 ± 7.31 | 36.13 ± 7.40 | 0.618 | 35.95 ± 7.33 | 36.39 ± 7.27 | 0.106 | 36.03 ± 7.33 | 35.26 ± 6.81 | 0.332 |
| BMI, kg/m2 | 22.16 ± 3.14 | 22.33 ± 3.15 | 0.293 | 22.17 ± 3.13 | 22.19 ± 3.19 | 0.861 | 22.19 ± 3.13 | 22.08 ± 3.18 | 0.401 | 22.16 ± 3.14 | 22.83 ± 3.27 | 0.087 |
| Ethnicity | 0.220 | 0.899 | 0.075 | 0.248 | ||||||||
| Han | 4110 (94.24%) | 356 (92.71%) | 3679 (94.14%) | 787 (94.03%) | 3765 (94.38%) | 701 (92.72%) | 4407 (94.17%) | 59 (90.77%) | ||||
| Ethnic minorities | 251 (5.76%) | 28 (7.29%) | 229 (5.86%) | 50 (5.97%) | 224 (5.62%) | 55 (7.28%) | 273 (5.83%) | 6 (9.23%) | ||||
| Education level | 0.062 | 0.347 | 0.072 | 0.033 | ||||||||
| Below undergraduate | 2392 (64.70%) | 229 (69.82%) | 2159 (64.80%) | 462 (66.67%) | 2185 (64.53%) | 436 (68.23%) | 2577 (64.93%) | 44 (78.57%) | ||||
| Undergraduate and above | 1305 (35.30%) | 99 (30.18%) | 1173 (35.20%) | 231 (33.33%) | 1201 (35.47%) | 203 (31.77%) | 1392 (35.07%) | 12 (21.43%) | ||||
| Contraceptive methods | 0.002 | 0.192 | 0.009 | 0.236 | ||||||||
| Noncontraception | 692 (18.21%) | 74 (22.63%) | 618 (18.20%) | 148 (20.19%) | 624 (17.86%) | 142 (22.40%) | 758 (18.64%) | 8 (12.90%) | ||||
| Intrauterine device | 444 (11.68%) | 48 (14.68%) | 402 (11.84%) | 90 (12.28%) | 407 (11.65%) | 85 (13.41%) | 480 (11.81%) | 12 (19.35%) | ||||
| Contraceptives | 92 (2.42%) | 15 (4.59%) | 82 (2.42%) | 25 (3.41%) | 88 (2.52%) | 19 (3.00%) | 106 (2.61%) | 1 (1.61%) | ||||
| Condom | 2573 (67.69%) | 190 (58.10%) | 2293 (67.54%) | 470 (64.12%) | 2375 (67.97%) | 388 (61.20%) | 2722 (66.95%) | 41 (66.13%) | ||||
| Number of sexual partners | 0.590 | 0.113 | 0.865 | 0.125 | ||||||||
| 1 | 4343 (96.60%) | 367 (96.07%) | 3895 (96.75%) | 815 (95.66%) | 3958 (96.54%) | 752 (96.66%) | 4645 (96.51%) | 65 (100.00%) | ||||
| ≥ 2 | 153 (3.40%) | 15 (3.93%) | 131 (3.25%) | 37 (4.34%) | 142 (3.46%) | 26 (3.34%) | 168 (3.49%) | 0 (0.00%) | ||||
| Gravidity | 0.635 | 0.033 | 0.275 | 0.504 | ||||||||
| 0 | 609 (13.63%) | 49 (12.86%) | 518 (12.96%) | 140 (16.43%) | 554 (13.62%) | 104 (13.32%) | 652 (13.64%) | 6 (8.82%) | ||||
| 1 | 1015 (22.72%) | 85 (22.31%) | 920 (23.02%) | 180 (21.13%) | 929 (22.84%) | 171 (21.90%) | 1085 (22.70%) | 15 (22.06%) | ||||
| 2 | 1158 (25.92%) | 91 (23.88%) | 1045 (26.15%) | 204 (23.94%) | 1063 (26.14%) | 186 (23.82%) | 1227 (25.67%) | 22 (32.35%) | ||||
| ≥ 3 | 1685 (37.72%) | 156 (40.94%) | 1513 (37.86%) | 328 (38.50%) | 1521 (37.40%) | 320 (40.97%) | 1816 (37.99%) | 25 (36.76%) | ||||
| Parity | 0.621 | 0.015 | 0.230 | 0.130 | ||||||||
| 0 | 784 (17.76%) | 69 (18.16%) | 674 (17.06%) | 179 (21.18%) | 701 (17.41%) | 152 (19.79%) | 847 (17.90%) | 6 (9.38%) | ||||
| 1 | 1974 (44.71%) | 157 (41.32%) | 1759 (44.53%) | 372 (44.02%) | 1784 (44.30%) | 347 (45.18%) | 2096 (44.30%) | 35 (54.69%) | ||||
| 2 | 1441 (32.64%) | 134 (35.26%) | 1313 (33.24%) | 262 (31.01%) | 1344 (33.37%) | 231 (30.08%) | 1553 (32.83%) | 22 (34.38%) | ||||
| ≥ 3 | 216 (4.89%) | 20 (5.26%) | 204 (5.16%) | 32 (3.79%) | 198 (4.92%) | 38 (4.95%) | 235 (4.97%) | 1 (1.56%) | ||||
Abbreviations: U. parvum Ureaplasma parvum, BMI body mass index
Means ± SDs for continuous variables, n (%) for categorical variables
Statistics with a P-value of 0.05 or less are shown in bold text
Baseline characteristics of the study population
There were no significant differences in age or BMI between individuals infected with U. parvum serovars and those who were not infected (Table 1). The participants infected with U. parvum serovar 14 were less educated than those who were not infected (Table 1). Compared with those who did not experience pregnancy and childbirth, women who had experienced pregnancy and childbirth were significantly less likely to have U. parvum serovar 3 infection (Table 1). In addition, various contraceptive techniques were associated with different U. parvum serovars, and infected women were also less likely to use condoms than uninfected women (Table 1). Additionally, comparisons of baseline characteristics were conducted between symptomatic and asymptomatic females (Table S5). Infections with U. urealyticum, M. hominis, C. trachomatis and HPV were more prevalent among symptomatic women, who also presented greater Nugent and Donder scores (Table S5).
Coinfection with U. parvum serovars and other STIs pathogens
Individuals with other genital infections, such as M. hominis, HSV-2, HPV and C. trachomatis, were more likely to be infected with specific U. parvum serovars simultaneously, whereas individuals with T. vaginalis and U. urealyticum infections had a lower incidence of U. parvum serovar 3 infection than those uninfected individuals did (Table 2). Individuals with U. parvum serovar 6 infection had higher Nugent scores but did not differ from negative U. parvum serovar 6-individuals in terms of Candida infection (Table 2). Additionally, we evaluated coinfection among different U. parvum serovars. Individuals infected with U. parvum serovars 1, 3 and 6 were more susceptible to infection with a single serovar than to coinfection with multiple serovars, but individuals infected with U. parvum serovar 14 had a greater probability of coinfection with U. parvum serovars 1, 3, and 6 than noninfected individuals did (Table S6).
Table 2.
Baseline microbiological factors of the study participants
| Characteristic | U. parvum serovar 1 | U. parvum serovar 3 | U. parvum serovar 6 | U. parvum serovar 14 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Negative (n = 4854 |
Positive (n = 423) |
P |
Negative (n = 4333) |
Positive (n = 944) |
P |
Negative (n = 4432) |
Positive (n = 845) |
P |
Negative (n = 5190) |
Positive (n = 87) |
P | |
| N. gonorrhoeae | 0.746 | 0.543 | 0.397 | 0.001 | ||||||||
| Negative | 4838 (99.67%) | 422 (99.76%) | 4320 (99.70%) | 940 (99.58%) | 4419 (99.71%) | 841 (99.53%) | 5175 (99.71%) | 85 (97.70%) | ||||
| Positive | 16 (0.33%) | 1 (0.24%) | 13 (0.30%) | 4 (0.42%) | 13 (0.29%) | 4 (0.47%) | 15 (0.29%) | 2 (2.30%) | ||||
| U. urealyticum | 0.057 | < 0.001 | 0.005 | 0.162 | ||||||||
| Negative | 4345 (89.51%) | 391 (92.43%) | 3860 (89.08%) | 876 (92.80%) | 3955 (89.24%) | 781 (92.43%) | 4654 (89.67%) | 82 (94.25%) | ||||
| Positive | 509 (10.49%) | 32 (7.57%) | 473 (10.92%) | 68 (7.20%) | 477 (10.76%) | 64 (7.57%) | 536 (10.33%) | 5 (5.75%) | ||||
| M. hominis | < 0.001 | 0.092 | 0.021 | 0.237 | ||||||||
| Negative | 4480 (92.30%) | 371 (87.71%) | 3996 (92.22%) | 855 (90.57%) | 4091 (92.31%) | 760 (89.94%) | 4774 (91.98%) | 77 (88.51%) | ||||
| Positive | 374 (7.70%) | 52 (12.29%) | 337 (7.78%) | 89 (9.43%) | 341 (7.69%) | 85 (10.06%) | 416 (8.02%) | 10 (11.49%) | ||||
| M. genitalium | 0.295 | 0.804 | 0.397 | 0.303 | ||||||||
| Negative | 4801 (98.91%) | 416 (98.35%) | 4283 (98.85%) | 934 (98.94%) | 4384 (98.92%) | 833 (98.58%) | 5132 (98.88%) | 85 (97.70%) | ||||
| Positive | 53 (1.09%) | 7 (1.65%) | 50 (1.15%) | 10 (1.06%) | 48 (1.08%) | 12 (1.42%) | 58 (1.12%) | 2 (2.30%) | ||||
| HSV-2 | 0.565 | 0.010 | 0.656 | 0.086 | ||||||||
| Negative | 4790 (98.68%) | 416 (98.35%) | 4283 (98.85%) | 923 (97.78%) | 4371 (98.62%) | 835 (98.82%) | 5122 (98.69%) | 84 (96.55%) | ||||
| Positive | 64 (1.32%) | 7 (1.65%) | 50 (1.15%) | 21 (2.22%) | 61 (1.38%) | 10 (1.18%) | 68 (1.31%) | 3 (3.45%) | ||||
| C. trachomatis | 0.807 | 0.004 | 0.863 | 0.636 | ||||||||
| Negative | 4635 (95.49%) | 405 (95.74%) | 4155 (95.89%) | 885 (93.75%) | 4232 (95.49%) | 808 (95.62%) | 4956 (95.49%) | 84 (96.55%) | ||||
| Positive | 219 (4.51%) | 18 (4.26%) | 178 (4.11%) | 59 (6.25%) | 200 (4.51%) | 37 (4.38%) | 234 (4.51%) | 3 (3.45%) | ||||
| HPV | 0.091 | 0.007 | < 0.001 | 0.912 | ||||||||
| Negative | 3896 (80.26%) | 325 (76.83%) | 3496 (80.68%) | 725 (76.80%) | 3644 (82.22%) | 577 (68.28%) | 4151 (79.98%) | 70 (80.46%) | ||||
| Positive | 958 (19.74%) | 98 (23.17%) | 837 (19.32%) | 219 (23.20%) | 788 (17.78%) | 268 (31.72%) | 1039 (20.02%) | 17 (19.54%) | ||||
| T. vaginalis | 0.401 | 0.001 | 0.213 | 0.347 | ||||||||
| Negative | 4693 (98.80%) | 405 (99.26%) | 4187 (98.61%) | 911 (99.89%) | 4287 (98.76%) | 811 (99.27%) | 5024 (98.82%) | 74 (100.00%) | ||||
| Positive | 57 (1.20%) | 3 (0.74%) | 59 (1.39%) | 1 (0.11%) | 54 (1.24%) | 6 (0.73%) | 60 (1.18%) | 0 (0.00%) | ||||
| Nugent score | 0.988 | 0.408 | 0.007 | 0.779 | ||||||||
| ≤ 3 | 3651 (76.72%) | 313 (76.90%) | 3265 (76.75%) | 699 (76.64%) | 3338 (76.75%) | 626 (76.62%) | 3907 (76.73%) | 57 (77.03%) | ||||
| 4–6 | 830 (17.44%) | 71 (17.44%) | 749 (17.61%) | 152 (16.67%) | 775 (17.82%) | 126 (15.42%) | 887 (17.42%) | 14 (18.92%) | ||||
| ≥ 7 | 278 (5.84%) | 23 (5.65%) | 240 (5.64%) | 61 (6.69%) | 236 (5.43%) | 65 (7.96%) | 298 (5.85%) | 3 (4.05%) | ||||
| Donders score | 0.227 | 0.846 | 0.145 | 0.730 | ||||||||
| < 3 | 3916 (84.67%) | 346 (86.93%) | 3516 (84.80%) | 746 (85.06%) | 3606 (85.17%) | 656 (83.14%) | 4205 (84.83%) | 57 (86.36%) | ||||
| ≥ 3 | 709 (15.33%) | 52 (13.07%) | 630 (15.20%) | 131 (14.94%) | 628 (14.83%) | 133 (16.86%) | 752 (15.17%) | 9 (13.64%) | ||||
| Candidaa | 0.964 | 0.810 | 0.240 | 0.285 | ||||||||
| Negative | 4173 (90.38%) | 360 (90.45%) | 3742 (90.34%) | 791 (90.61%) | 3810 (90.18%) | 723 (91.52%) | 4475 (90.44%) | 58 (86.57%) | ||||
| Positive | 444 (9.62%) | 38 (9.55%) | 400 (9.66%) | 82 (9.39%) | 415 (9.82%) | 67 (8.48%) | 473 (9.56%) | 9 (13.43%) | ||||
Abbreviations: U. urealyticum Ureaplasma urealyticum, U. parvum Ureaplasma parvum, N. gonorrhoeae Neisseria gonorrhoeae, M. hominis Mycoplasma hominis, M. genitalium Mycoplasma genitalium, HSV-2 herpes simplex virus type 2, C. trachomatis Chlamydia trachomatis, HPV human papilloma virus, T. vaginalis Trichomonas vaginalis
N (%) for categorical variables
Statistics with a P-value of 0.05 or less were shown in bold text
aCandida positivity was based on the presence of visible pseudohyphae and/or budding yeasts via microscopy and culture positive
Self-reported clinical symptoms associated with U. parvum serovars
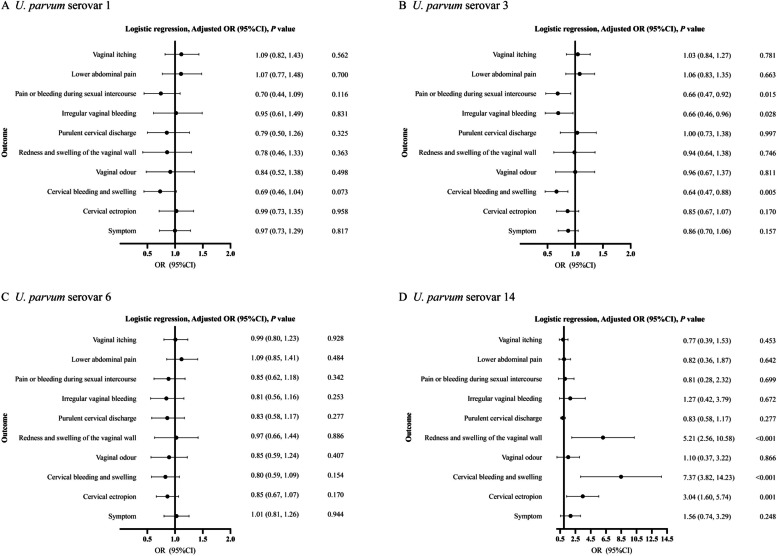
Potential risk variables associated with clinical symptoms were determined via univariate analysis (Table S7). To identify the independent effects of different U. parvum serovars on clinical symptoms, we constructed a crude model, a minimally adjusted model (Table S8) and a fully adjusted model (Fig. 2). According to the fully adjusted model, U. parvum serovar 14 was strongly positively associated with redness and swelling of the vaginal wall (OR: 5.21, 95% CI: 2.56–10.58), cervical bleeding and swelling (OR: 7.37, 95% CI: 3.82–14.23) and cervical ectropion (OR: 3.04, 95% CI: 1.60–5.74). In contrast, the relationship between U. parvum serovar 3 and clinical symptoms produced opposite effects. Compared with uninfected women, U. parvum serovar 3-infected women were less likely to experience pain or bleeding during sexual intercourse (OR: 0.66, 95% CI: 0.47–0.92), irregular vaginal bleeding (OR: 0.66, 95% CI: 0.46–0.96), and cervical bleeding and swelling (OR: 0.64, 95% CI: 0.47–0.88). Additionally, we observed that U. parvum serovar 1 and 6 infection was not associated with any clinical symptoms.
Fig. 2.
Association of clinical symptoms with U. parvum serovars. Age, BMI, ethnicity, education level, contraceptive methods, number of sexual partners, gravidity, parity, and other STIs that were associated with clinical symptoms were adjusted for (P < 0.1 in univariate analysis)
Stratified analysis
U. parvum serovar 14 is strongly correlated with symptoms associated with cervicitis, and HPV serves as a significant etiological factor for cervical cancer, which can manifest as cervical symptoms. We analysed the associations between U. parvum serovar 14 and cervicitis symptoms in individuals with or without HPV infection to further examine the correlations among U. parvum serovar 14, HPV, and symptoms of cervicitis. Among HPV-infected individuals, U. parvum serovar 14-infected women were more prone to cervical ectropion (OR: 2.59, 95% CI: 1.48–4.54), than HPV- uninfected women. U. parvum serovar 14-infected patients presented a significant positive association with cervical bleeding and swelling, regardless of their HPV status (OR: 3.95, 95% CI: 2.29–6.82 for HPV negative participants; OR: 3.74, 95% CI: 1.18–11.88 for HPV positive participants) (Table 3).
Table 3.
Effects of U. parvum serovar 14 on cervicitis-related symptoms in participants with or without HPV infection
| Stratified | Cervical bleeding and swelling | Cervical ectropion | |||
|---|---|---|---|---|---|
| OR (95% CI) | Test for interactiona | OR (95% CI) | Test for interaction | ||
| HPV | Negative | 3.95 (2.29, 6.82)** | 0.935 | 2.59 (1.48, 4.54)** | 0.085 |
| Positive | 3.74 (1.18, 11.88)* | 0.81 (0.22, 2.94) | |||
Abbreviations: U. parvum Ureaplasma parvum, HPV human papilloma virus
The data are presented as ORs (95% CIs), *P < 0.05, **P < 0.01
aTest for interaction between U. parvum serovar 14 (negative or positive) and HPV (negative or positive) on cervicitis-related symptoms
Discussion
In this study, we investigated the associations of U. parvum serovars with clinical symptoms in 5,277 Chinese women of reproductive age. Our results revealed that U. parvum serovars 3 and 6 were the most prevalent U. parvum serovars in Chinese women (17.9% and 16.0%, respectively). Even after adjusting for pathogens causing genital tract infection, such as BV, U. parvum serovar 14 was significantly positively related to clinical symptoms associated with cervicitis, including cervical bleeding and swelling and cervical ectropion independent of HPV infection. Notably, the findings indicated that U. parvum serovar 3 was significantly negatively associated with specific clinical symptoms, whereas no correlations were detected between U. parvum serovars 1, 6 and clinical symptoms. These findings suggest the relationships between different U. parvum serovars and clinical symptoms are heterogeneous.
Owing to the high detection rate of U. parvum and its presence in women with and without signs of genitourinary tract infections, it has been hypothesized that U. parvum is a natural part of the female genital tract flora [26–28]. In 2018, the Editorial Board of the European STIs issued a statement suggesting that there was no evidence of benefit in relation to routine testing and the treatment of M. hominis, U. parvum and U. urealyticum in adults based on existing available research [29]. The same conclusion was drawn in a cross-sectional investigation [12]. Notably, the recent relevant investigations did not adjust for genital pathogens and serovars of U. parvum were not considered. Recent research on the correlations between U. parvum serovars and clinical symptoms is conflicting and limited. Another study that compared U. parvum serovar infections in women with and without symptoms of genital tract infection reported that U. parvum serovar 3 or U. parvum serovar 14 infection was related to clinical symptoms, but that U. parvum serovar 6 infection was associated with asymptomatic women [15]. However, the sample size was limited in this study, and the U. parvum serovar 3/14 variable was used in the analysis instead of the individual serovar. Moreover, other genital tract pathogens were not adjusted for in this study. We found that the association between U. parvum serovar 3 and clinical symptoms was opposite that between U. parvum serovar 14 and clinical symptoms, suggesting heterogeneity in the pathogenicity of various U. parvum serovars.
Tetracyclines (particularly doxycycline) continue to be the first-line treatment for mycoplasma infections [16, 30]. As a consequence of the abuse of antibiotics, a separate study revealed that multidrug resistance among Ureaplasma spp. isolates has shown an increasing trend [31]. Compared with U. urealyticum, U. parvum has substantially higher drug resistance rates to ciprofloxacin and roxithromycin [17, 32]. In addition, different U. parvum serovars exhibit varying antibiotic sensitivities [18, 33]. According to our study, infection with various U. parvum serovars may result in various clinical signs. Therefore, it may be important to differentiate the serovars of U. parvum.
Notably, our study revealed a strong positive association between U. parvum serovar 14 and cervicitis-related symptoms, such as cervical bleeding and swelling and cervical ectropion. This contrasts with many studies and guidelines that have found no clear evidence of an association between U. parvum and cervicitis [7, 23, 29, 34, 35]. The differences observed could be because no discrimination between U. parvum serovars was performed in previous studies, potentially masking the significant association between U. parvum serovar 14 and the clinical symptoms. In contrast to U. parvum serovar 14, U. parvum serovar 3 was significantly negatively correlated with cervical bleeding, indicating that U. parvum might be a protective factor against cervical bleeding and swelling when the independent correlations between specific U. parvum serovars and symptoms were evaluated. The diversity of U. parvum serovars could be explained by genetic variations. Nevertheless, research comparing genomic differences across different U. parvum serovars is scarce. Undertaking comparative genomic analyses of U. parvum serovars could represent a fruitful avenue for future research.
Our study has several strengths, including its geographically diverse multicentre design with a large sample size. In this study, we were able to assess the associations between individual serovars of U. parvum and different clinical signs and symptoms. To evaluate the independent correlations between U. parvum serovars and clinical symptoms, we also adjusted for additional genital tract infections that could have confounded the results, which led to more robust results.
There are several limitations of this study. First, owing to the cross-sectional design, we were not able to assess the causal associations between U. parvum serovars and clinical symptoms in this study. Second, because the majority of participants in our study were Chinese women, the findings may not be applicable to women outside China. Third, as with any observational study, there may be other unobserved confounders that influence the results, despite adjusting for a considerable number of relevant variables known to affect clinical symptoms in our study. Fourth, we identified the serovar-specific pathogenicity of U. parvum in this study, and further investigation of U. urealyticum is needed. Finally, there may be selection bias, as our sample was recruited from hospital outpatient clinics. We aspire to mitigate this bias in future research.
Conclusion
We identified heterogeneous associations between different U. parvum serovars and various clinical symptoms and that the lack of discrimination of U. parvum serovars could result in a percentage of infected patients not exhibiting clinical symptoms when patients are tested only U. parvum. These findings highlight the importance of testing for different U. parvum serovars. We also found that in contrast to U. parvum serovar 3, U. parvum serovar 14 had a strong positive association with cervicitis-related symptoms, indicating a specific pathogenic mechanism of U. parvum serovar 14 that needs further investigation. Owing to the cross-sectional nature of our study and the lack of applicability to women outside, further investigations need to be conducted with a prospective cohort consisting of multiple ethnic groups to verify the results of our study.
Supplementary Information
Acknowledgements
We thank for all participants, staff and collaborators who participated in the CALM 2004 project. We thank for all members of CALM 2004 study group.
Abbreviations
- U. urealyticum
Ureaplasma urealyticum
- U. parvum
Ureaplasma parvum
- MBA
Multiple-banded antigen
- STIs
Sexually transmitted infections
- HPV
Human papillomavirus
- CIN
Cervical intraepithelial neoplasia
- N. gonorrhoeae
Neisseria gonorrhoeae
- M. hominis
Mycoplasma hominis
- M. genitalium
Mycoplasma genitalium
- HSV-2
Herpes simplex virus type 2
- C. trachomatis
Chlamydia trachomatis
- T. vaginalis
Trichomonas vaginalis
- BV
Bacterial vaginosis
- AV
Aerobic vaginitis
Authors’ contributions
Muxuan Chen, Hongwei Zhou and Yifeng Wang conceptualized and conducted the study. Rongdan Chen, Zuyi Zhou and Yi Hou were in charge of data collection. Longxu Xie, Wenyu Mo, Yiya Shi and Jinxia Ou contributed to the interpretation of data. Yingxuan Zhang, Wei Qing and Cancan Qi performed statistical analysis. Yingxuan Zhang wrote the original draft. Yingxuan Zhang, Wei Qing and Wenyu Mo reviewed and edited the manuscripts. All authors are members of CALM 2004 project and reviewed and approved the final version of the manuscript.
Funding
The study was supported by the National Natural Science Foundation of China (82205157, 81925026 and 82302610), the Guangdong Basic and Applied Basic Research Foundation (2021B1515230007), the China Postdoctoral Science Foundation (2022M721538) and the Guizhou High-level (BAI) Innovative Talents Project (QIANKehe Platform & Talents-GCC [2022]042–1).
Data availability
This study was a substudy of the ongoing CALM2004 project. Upon reasonable request, all the data and materials used in this study are available from the corresponding author.
Declarations
Ethics approval and consent to participate
This study was a substudy of the CALM2004 project, and all the individuals who participated in CALM2004 project provided written informed consent. The study was approved by the ethics committee of ZhuJiang Hospital of Southern Medical University (NO. 2020-KY-0711–02) and the ethics committees of the other collaborating research centres. The study has been registered with ClinicalTrials.gov (https://www.clinicaltrials.gov; ID: NCT04694495; Registration Date: 2021–01-05).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yingxuan Zhang, Wei Qing and Wenyu Mo contributed equally to this work.
Contributor Information
Muxuan Chen, Email: muxuanchen@126.com.
CALM 2004 Study Group:
Bingbing Xiao, Shuyi Han, Xuefeng Wang, Feng Fang, Weiguang Luo, Jing Zhao, Bo Wang, Xiaojuan Li, Kewei Zhao, Guofeng Xue, Hong Chen, Shuhua Li, Liangzhi Cai, Pengming Sun, Yingxiu Chen, Wei Liang, Yan Han, Xiaoyan Li, Yanan Zhang, Chunxia Guo, Zhiyu Pang, Qunxiang Liu, Liping Huang, Jinbo Liu, Ping Zhan, Fan Lu, Hualei Cai, Ming Li, Xianjin Wu, Maocheng Li, Yi Zhang, Ruizhe Wang, Xuesu He, Jing Sha, Kaifeng Wu, Chengmin Deng, Guijie Zhang, Beibei Sun, Dehua Sun, Yufeng Xiong, Liang Peng, Zhijuan Liu, Shuzhong Yao, Meng Xia, Haitao Yu, Xiaojuan Gao, Xiuming Zhang, Fen Lin, Yonghao Wu, Meiling Luo, Changzhong Li, Zhaofan Luo, Xue Guo, Chaoxin Jiang, Guoqing Hao, Guanghui Chen, Hui Chen, Lianhua Wei, and Zhemei Zhang
References
- 1.Beeton ML, Payne MS, Jones L. The role of Ureaplasma spp. in the development of nongonococcal urethritis and infertility among men. Clin Microbiol Rev. 2019;32(4):e00137–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimizu T, Kida Y, Kuwano K. Ureaplasma parvum lipoproteins, including MB antigen, activate NF-κB through TLR1, TLR2 and TLR6. Microbiology. 2008;154(5):1318–25. [DOI] [PubMed] [Google Scholar]
- 3.Razin S, Yogev D. Genetic relatedness among Ureaplasma urealyticum serotypes (serovars). Pediatr Infect Disease. 1986;5(6 Suppl):S300–4. [DOI] [PubMed] [Google Scholar]
- 4.Fanrong K, James G, Zhenfang M, Gordon S, Wang B, Gilbert GL. Phylogenetic analysis of Ureaplasma urealyticum–support for the establishment of a new species, Ureaplasma parvum. Int J Syst Evol MicroBiol. 1999;49(4):1879–89. [DOI] [PubMed] [Google Scholar]
- 5.Stellrecht KA, Woron AM, Mishrik NG, Venezia RA. Comparison of multiplex PCR assay with culture for detection of genital mycoplasmas. J Clin Microbiol. 2004;42(4):1528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y, Kim J, Lee KA. Prevalence of sexually transmitted infections among healthy Korean women: implications of multiplex PCR pathogen detection on antibiotic therapy. J Infect Chemother. 2014;20(1–2):74–6. [DOI] [PubMed] [Google Scholar]
- 7.Leli C, Mencacci A, Latino MA, Clerici P, Rassu M, Perito S, et al. Prevalence of cervical colonization by Ureaplasma parvum, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in childbearing age women by a commercially available multiplex real-time PCR: an Italian observational multicentre study. J Microbiol Immunol Infect. 2018;51(2):220–5. [DOI] [PubMed] [Google Scholar]
- 8.Lobao TN, Campos GB, Selis NN, Amorim AT, Souza SG, Mafra SS, et al. Ureaplasma urealyticum and U. Parvum in sexually active women attending public health clinics in Brazil. Epidemiol Infect. 2017;145(11):2341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doroftei B, Ilie OD, Armeanu T, Anton E, Scripcariu I, Maftei R. The prevalence of Ureaplasma Urealyticum and Mycoplasma Hominis Infections in Infertile patients in the Northeast Region of Romania. Med (Kaunas). 2021;57(3):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonduo ME, Vallely LM, Wand H, Sweeney EL, Egli-Gany D, Kaldor J, et al. Adverse pregnancy and birth outcomes associated with Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum: a systematic review and meta-analysis. BMJ open. 2022;12(8):e062990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittenschober-Böhm J, Waldhoer T, Schulz SM, Pimpel B, Goeral K, Kasper DC, et al. Vaginal Ureaplasma parvum serovars and spontaneous preterm birth. Am J Obstet Gynecol. 2019;220(6):594. e1-. e9. [DOI] [PubMed] [Google Scholar]
- 12.Plummer EL, Vodstrcil LA, Bodiyabadu K, Murray GL, Doyle M, Latimer RL, et al. Are Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum Associated with specific genital symptoms and clinical signs in Nonpregnant women? Clin Infect Dis. 2021;73(4):659–68. [DOI] [PubMed] [Google Scholar]
- 13.Zhao N, Li KT, Gao YY, Xu JJ, Huang DS. Mycoplasma Genitalium and Mycoplasma Hominis are prevalent and correlated with HIV risk in MSM: a cross-sectional study in Shenyang, China. BMC Infect Dis. 2019;19(1):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MS, Lee DH, Kim TJ, Oh JJ, Rhee SR, Park DS, Yu YD. The role of Ureaplasma parvum serovar-3 or serovar-14 infection in female patients with chronic micturition urethral pain and recurrent microscopic hematuria. Transl Androl Urol. 2021;10(1):96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Francesco M, Negrini R, Pinsi G, Peroni L, Manca N. Detection of Ureaplasma biovars and polymerase chain reaction-based subtyping of Ureaplasma parvum in women with or without symptoms of genital infections. Eur J Clin Microbiol Infect Dis. 2009;28(6):641–6. [DOI] [PubMed] [Google Scholar]
- 16.Krausse R, Schubert S. In-vitro activities of tetracyclines, macrolides, fluoroquinolones and clindamycin against Mycoplasma hominis and Ureaplasma ssp. isolated in Germany over 20 years. Clin Microbiol Infect. 2010;16(11):1649–55. [DOI] [PubMed] [Google Scholar]
- 17.Ma H, Zhang X, Shi X, Zhang J, Zhou Y. Phenotypic antimicrobial susceptibility and genotypic characterization of clinical ureaplasma isolates circulating in Shanghai, China. Front Microbiol. 2021;12:724935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghofran K, Al-khafaji S. Antimicrobial Resistance of Genital Ureaplasma Parvum. Nano Biomed Eng. 2017;9(3):236–41. [Google Scholar]
- 19.Zhou Z, Hou Y, Qing W, Shi Y, Zhang Y, Chen R et al. The association of HPV infection and vaginal microbiota of reproductive women in China: a multicenter cohort study protocol. 2023;15:100072.
- 20.Bai S, Li Y, Wan Y, Guo T, Jin Q, Liu R, et al. Sexually transmitted infections and semen quality from subfertile men with and without leukocytospermia. Reproductive Biology Endocrinol. 2021;19(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao N, Li KT, Gao Y-y, Xu J-j, Huang D-S. Mycoplasma Genitalium and Mycoplasma Hominis are prevalent and correlated with HIV risk in MSM: a cross-sectional study in Shenyang, China. BMC Infect Dis. 2019;19:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang N, Wang R, Li X, Liu X, Tang Z, Liu Y. Are Ureaplasma spp. a cause of nongonococcal urethritis? A systematic review and meta-analysis. PLoS ONE. 2014;9(12):e113771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, et al. Sexually Transmitted Infections Treat Guidelines 2021 MMWR Recomm Rep. 2021;70(4):1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donders GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG: Int J Obstet Gynecol. 2002;109(1):34–43. [DOI] [PubMed] [Google Scholar]
- 26.Robertson JA, Stemke GW, Davis JW, Harasawa R, Thirkell D, Kong F, et al. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticumShepard (1974) Robertson 2001. Int J Syst Evol Microbiol. 2002;52(Pt 2):587–97. [DOI] [PubMed] [Google Scholar]
- 27.Wetmore CM, Manhart LE, Lowens MS, Golden MR, Jensen NL, Astete SG, et al. Ureaplasma urealyticum is Associated with Nongonococcal Urethritis among men with fewer lifetime sexual partners: a case-control study. J Infect Dis. 2011;204(8):1274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kletzel HH, Rotem R, Barg M, Michaeli J, Reichman O. Ureaplasma urealyticum: the role as a Pathogen in Women’s Health, a systematic review. Curr Infect Dis Rep. 2018;20(9):1–12. [DOI] [PubMed] [Google Scholar]
- 29.Horner P, Donders G, Cusini M, Gomberg M, Jensen J, Unemo M. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women?–a position statement from the European STI guidelines Editorial Board. J Eur Acad Dermatol Venereol. 2018;32(11):1845–51. [DOI] [PubMed] [Google Scholar]
- 30.Wada K, Hamasuna R, Sadahira T, Araki M, Yamamoto S. UAA-AAUS guideline for M. genitalium and non-chlamydial non-gonococcal urethritis. J Infect Chemother. 2021;27(10):1384–8. [DOI] [PubMed] [Google Scholar]
- 31.Song T, Ye A, Xie X, Huang J, Ruan Z, Kong Y, et al. Epidemiological investigation and antimicrobial susceptibility analysis of ureaplasma species and Mycoplasma hominis in outpatients with genital manifestations. J Clin Pathol. 2014;67(9):817–20. [DOI] [PubMed] [Google Scholar]
- 32.Zhu C-t, Hu Z-y, Dong C-l, Zhang C-s, Wan M-z, Ling Y. Investigation of Ureaplasma urealyticum biovars and their relationship with antimicrobial resistance. Ind J Med Microbiol. 2011;29(3):288–92. [DOI] [PubMed] [Google Scholar]
- 33.Boujemaa S, Mlik B, Ben Allaya A, Mardassi H, Mardassi BB. Spread of multidrug resistance among Ureaplasma serovars. Tunisia Antimicrob Resist Infect Control. 2020;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lusk MJ, Garden FL, Rawlinson WD, Naing ZW, Cumming RG, Konecny P. Cervicitis aetiology and case definition: a study in Australian women attending sexually transmitted infection clinics. Sex Transm Infect. 2016;92(3):175–81. [DOI] [PubMed] [Google Scholar]
- 35.Lillis RA, Martin DH, Nsuami MJ. Mycoplasma genitalium infections in women attending a sexually transmitted Disease Clinic in New Orleans. Clin Infect Dis. 2019;69(3):459–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study was a substudy of the ongoing CALM2004 project. Upon reasonable request, all the data and materials used in this study are available from the corresponding author.