Abstract
Chimeric antigen receptor (CAR) T-cell therapy fails to achieve durable responses in over 60% of relapsed/refractory (R/R) large B-cell lymphoma (LBCL) patients in the third or later line setting. After CAR-T failure, survival outcomes are heterogeneous and a prognostic model in this patient population is lacking. A training cohort of 216 patients with progressive disease (PD) after CAR-T from 12 Spanish centers was used to develop the Post-CAR Prognostic Index (PC-PI); primary endpoint was overall survival (OS) from CAR-T progression. Validation was performed in an external cohort from three different European centers (n = 204). The prognostic score incorporated five variables, assessed at time of PD to CAR-T: ECOG (> 0), hemoglobin (< 10 g/dL), LDH (≥ 2xULN), number of extranodal sites (> 1) and time from CAR-T to PD (< 4 months). Patients were classified in four risk groups with distinct OS (p-value < 0.05 in all comparisons). In the validation cohort, median OS in the low (31%), intermediate-low (26%), intermediate-high (17%) and high risk (26%) were 15.7, 7.1, 1.8 and 1.0 months, respectively (p < 0.05 in all comparisons). Results were consistent following adjustment for subsequent treatment. In the external cohort, the PC-PI showed a C-statistic of 0.79 (95%CI 0.76–0.82), outperforming IPI and R-IPI. In conclusion, the PC-PI score is a novel tool for OS prediction and could facilitate risk-adapted management of LBCL patients relapsing after CAR T-cells. Additionally, these results will help stratification and interpretation of trials and real-world data incorporating CART-exposed patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-024-01608-8.
Keywords: CAR-T, Large B-cell lymphoma, Overall survival, Score, Disease progression
Key points
Key point #1
The Post-CAR Prognostic Index (PC-PI) is a tool to inform on overall survival after CAR T-cell progression in large B-cell lymphoma patients.
Key point #2
The PC-PI can help risk-stratification and interpretation of trials and real-world data of regimens incorporating CART-exposed patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-024-01608-8.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment landscape of patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL). Long term follow-up of the pivotal trials and real-world data have confirmed the curative potential of this treatment modality [1–10]. However, over 60% of infused patients will experience progressive disease (PD) after CAR T-cells, with lack of a standard subsequent treatment approach and heterogenous outcomes [11–14]. Data regarding prognostic factors at time of PD to CAR-T are scarce and real-world studies have mainly focused on pre-treatment variables [15–18].
This paradigm shift in the treatment schema of patients with R/R LBCL calls into question the validity of current prognostic scores, developed in the chemotherapy era. The International Prognostic Index (IPI) score arrived in the pre-Rituximab period [19] and was revisited after the advent of this anti-CD20 monoclonal antibody, introducing the Revised IPI (R-IPI) [20]. The potential of the IPI to predict CAR T-cell therapy outcomes with variables assessed at time of lymphodepleting chemotherapy has been explored [17], but none of the current prognostic scores have been examined after CAR T-cell progression.
Due to the regulatory approval of CAR T-cells in the second line setting [21–23], and with many clinical trials exploring this treatment in first line [24–26], the patient population with disease progression after CAR T-cell therapy is expected to substantially increase in the coming years. At the same time, there is a growing armamentarium of approved therapeutic options and trials for R/R LBCL, which will be mainly applied in the post-CART space. Therefore, a tool to stratify this patient population is warranted, allowing cross-trial comparisons and a better understanding of emergent real-world data in this context.
In this study, we explored the performance of IPI and R-IPI at time of CAR T-cell progression. In light of the suboptimal results, we developed and validated a novel tool to predict overall survival (OS) after CAR-T failure with easily-available parameters from routine clinical practice, obtained at time of PD to CAR T-cells.
Methods
Patients
For the training cohort (TC) of the post-CAR prognostic Index (PC-PI), we performed a retrospective data collection from patients with R/R LBCL who experienced PD after receiving CD19-targeted CAR T-cell therapy in the third or later line setting at 12 Spanish centers, from September 2018 until May 2022. Patients could be included if they were CART-refractory and if they relapsed at any timepoint after achieving an initial response (partial or complete) to CAR T-cells. We recorded a total of 15 clinical and laboratory parameters which were routinely available at time of CAR-T failure, taking into account predictors of other models in LBCL [19, 20, 27] and previously published prognostic factors in the peri-CART setting [5, 7, 11, 14, 18, 28]: age, sex, histology, prior hematopoietic cell transplant, number of prior treatment lines, history of chemo-refractory disease, best response to CAR T-cells, time from CAR-T infusion to PD, stage, number of extranodal sites (FDG-avid in the positron emission tomography [PET]), performance status (ECOG PS), hemoglobin, neutrophils, platelets and lactate dehydrogenase (LDH). All values were assessed at time of PD to CAR T-cells. Each participant provided informed consent and the study was approved by the ethics committee of the Vall d’Hebron Hospital Board (PR[AG]404/2020).
The validation cohort (VC) comprised patients treated at three European centers, namely CHU Lyon (Lyon, France), King’s College Hospital (London, United Kingdom) and LMU University Hospital (Munich, Germany) who also presented PD after CAR T-cell therapy administered as third or later line from June 2018 until May 2023. The selected variables were collected to validate the findings, after the final model for the TC was established.
Endpoints and study plan
The primary endpoint of this study was OS measured from date of PD to CAR T-cell therapy until death from any cause [29]. With the aim of developing a tool for this purpose, we first explored the performance of existing LBCL prognostic scores, namely IPI and R-IPI, with values collected at time of PD. In light of the results, we developed and validated a readily-accessible prognostic score with clinical utility to predict OS from time of CAR T-failure in patients with R/R LBCL.
Response evaluation to CAR T-cell therapy was based on local PET/CT assessment, following Lugano criteria [30]. The treatment approach after disease progression was distributed in 3 categories: (1) immunotherapy or targeted agents, (2) chemotherapy or radiotherapy and (3) palliative management or best supportive care.
Statistical analyses
A descriptive analysis was conducted for all collected variables. OS was estimated from time of PD to CAR T-cells using the Kaplan-Meier method. First, univariate Cox proportional hazard models were fitted in the training set. To facilitate the interpretability and utilization of the score, optimal discriminatory thresholds were identified using maximally selected log-rank statistics, considering as well clinical relevance [31, 32] (Figure S1). Second, to select variables with the highest prognostic impact on OS, the least absolute shrinkage and selection operator (LASSO) regression with lambda 1-standard error was used after cross validation for variable selection. Third, a multivariable stratified Cox model was constructed using the selected variables, with post-progression treatment modality as a stratification factor, allowing a different baseline hazard function for each treatment type. Hazard ratios (HR) with 95% confidence interval (95%CI) were estimated from the Cox model. And fourth, to improve the clinical applicability of the model, patients were categorized into risk groups based on the results of the variables used to build the score.
The score was evaluated in an external VC, comprising a patient population from three European countries. We compared the performance of the proposed score with other existing scores such as the IPI and R-IPI in both the TC and VC. The C-statistic was estimated using a bootstrap method, employing 1000 resamples, to correct for optimism and to calculate confidence intervals. We formally compared the C-statistics of all models by the compareC R package [33, 34]. Calibration plots with Harrel’s bias optimism correction [35] were estimated to evaluate the calibration of the model at 6 and 12 months. Missing at random values were imputed using the chained equations method [36]. The prevalence of missing data was < 5% in all variables. Following imputation, a sensitivity analysis was conducted to ensure that these imputations did not significantly alter the obtained results (Figure S2). In order to validate the possible bias, we used the PROBAST questionnaire (supplementary appendix-1) [37]. This study was in compliance with the TRIPOD guidelines (supplementary appendix-2) [38].
Results were considered statistically significant if p < 0.05. All statistical analyses were performed using R software version 4.2.2.
Results
Training cohort
Patient characteristics
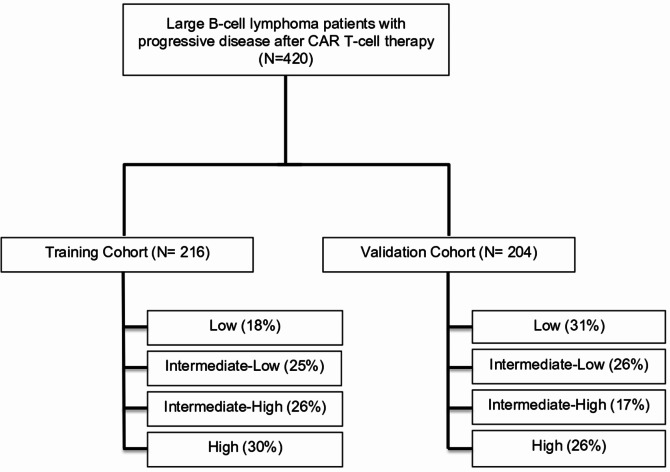
The training cohort comprised 216 patients with R/R LBCL who experienced disease progression after CAR T-cell therapy administered in the third or later line setting. Of these, 89% were commercial products (axicabtagene ciloleucel [axi-cel] and tisagenlecleucel [tisa-cel]) and 11% clinical trials with tisa-cel and lisocabtagene maraleucel (liso-cel) (Fig. 1). In terms of baseline characteristics, most were male (66%), had a diffuse large B-cell lymphoma (DLBCL) histology (68%), stage IV disease (70%) and a median of 1 extranodal site (IQR 0–2) involved at time of PD. Best response to prior CAR T-cells was complete response (CR) in 33 (15%) patients, partial response (PR) in 74 (34%), stable disease (SD) in 16 (8%) and PD in 93 (43%)(Table 1).
Fig. 1.
CONSORT diagram
Table 1.
Patient characteristics of the training cohort
| Variables | All patients N = 216 |
High N = 65 |
Int-high N = 57 |
Int-low N = 55 |
Low N = 39 |
|---|---|---|---|---|---|
| Age, median years (range) | 60 (20–81) | 62 (23–81) | 56 (20–78) | 63 (24–76) | 62 (27–80) |
| Male sex, n (%) | 143 (66) | 43 (66) | 34 (60) | 38 (69) | 28 (72) |
| Histology, n (%) | |||||
| - DLBCL | 148 (68) | 42 (65) | 38 (67) | 42 (76) | 26 (66) |
| - HGBL | 35 (16) | 12 (18) | 10 (18) | 9 (16) | 4 (10) |
| - tFL | 12 (6) | 3 (5) | 4 (7) | 2 (4) | 3 (8) |
| - THRLBCL | 11 (5) | 4 (6) | 2 (3) | 2 (4) | 3 (8) |
| - PMBL | 10 (5) | 4 (6) | 3 (5) | 0 | 3 (8) |
| Prior lines > 2, n (%)# | 93 (43) | 30 (46) | 25 (44) | 24 (44) | 14 (36) |
| Primary refractory, n (%)#* | 139 (64) | 41 (63) | 40 (70) | 33 (60) | 25 (64) |
| Previous HCT – n (%)# | 54 (25) | 15 (23) | 12 (21) | 12 (22) | 15 (38) |
| Construct, n (%) | |||||
| - Axi-cel | 84 (39) | 18 (28) | 28 (49) | 18 (33) | 20 (51) |
| - Tisa-cel | 113 (52) | 41 (63) | 24 (42) | 30 (56) | 17 (44) |
| - Liso-cel | 19 (9) | 6 (9) | 5 (9) | 6 (11) | 2 (5) |
| Best response to CART, n (%) | |||||
| - CR | 33 (15) | 1 (1) | 6 (11) | 11 (20) | 15 (38) |
| - PR | 74 (34) | 14 (22) | 27 (47) | 18 (33) | 15 (38) |
| - SD | 16 (8) | 2 (3) | 6 (11) | 5 (9) | 3 (8) |
| - PD | 93 (43) | 48 (74) | 18 (31) | 21 (38) | 6 (16) |
| CAR T-cell infusion to PD | |||||
| - Median months (IQR) |
2.5 (1.0-3.3) |
1.1 (0.9–2.5) |
2.6 (1.1–3.1) |
2.9 (1.1–3.3) |
5.7 (3.0-6.8) |
| - < 4 months, n (%) | 97 (81) | 65 (100) | 49 (86) | 47 (85) | 13 (33) |
| ECOG, n (%) | |||||
| - 0 | 52 (24) | 1 (2) | 3 (5) | 17 (31) | 31 (79) |
| - 1 | 104 (48) | 28 (43) | 36 (63) | 33 (60) | 7 (18) |
| - > 1 | 60 (28) | 36 (55) | 18 (32) | 5 (9) | 1 (3) |
| Stage, n (%) | |||||
| - I | 15 (7) | 1 (2) | 2 (3) | 2 (3) | 10 (26) |
| - II | 33 (15) | 1 (2) | 8 (14) | 18 (33) | 6 (15) |
| - III | 16 (8) | 1 (2) | 5 (9) | 6 (11) | 4 (10) |
| - IV | 152 (70) | 62 (94) | 42 (74) | 29 (53) | 19 (49) |
| Extranodal sites | |||||
| - Median (IQR) | 1 (0–2) | 3 (2–4) | 1 (1–2) | 1 (0–1) | 0 (0–1) |
| - ≥2, n (%) | 93 (43) | 58 (89) | 27 (47) | 7 (13) | 1 (3) |
| Hemoglobin | |||||
| - Median g/dL (IQR) |
10.3 (8.9–11.8) |
8.9 (8.2–9.5) |
10.1 (8.9–11.5) |
11.0 (10.3–12.1) |
12.4 (11.2–13.2) |
| - < 10 g/dL, n (%) | 97 (45) | 57 (88) | 26 (46) | 12 (22) | 2 (5) |
| Neutrophils | |||||
| - Median x109/L (IQR) | 1.7 (1.0-2.9) | 2.1 (0.8-4.0) | 1.3 (0.6–2.1) | 1.6 (1.0-2.6) | 2.1 (1.3–2.5) |
| - < 1.0 x109/L, n (%) | 54 (25) | 18 (28) | 19 (33) | 14 (25) | 3 (8) |
| Platelets | |||||
| - Median x109/L (IQR) |
88 (36–160) |
36 (20–114) |
72 (37–144) |
87 (60–140) |
156 (103–178) |
| - < 50 x109/L, n (%) | 66 (31) | 38 (58) | 17 (30) | 9 (16) | 2 (5) |
| LDH, n (%) | |||||
| - > 1 xULN | 140 (65) | 59 (91) | 36 (63) | 32 (58) | 13 (33) |
| - ≥2 xULN | 65 (30) | 43 (66) | 15 (26) | 6 (11) | 1 (3) |
| Subsequent strategy, n (%) | |||||
| - Immuno/Targeted | 92 (43) | 15 (23) | 28 (49) | 29 (53) | 20 (51) |
| - Chemo/Radiotherapy | 43 (20) | 9 (14) | 6 (11) | 14 (25) | 14 (36) |
| - Palliative care | 81 (38) | 41 (63) | 23 (40) | 12 (22) | 5 (13) |
| Allo-HCT, n (%) | 15 (7) | 1 (2) | 2 (4) | 5 (9) | 7 (18) |
Abbreviations: Int, intermediate; DLBCL, diffuse large B-cell lymphoma; HGBL, high-grade B-cell lymphoma; tFL, transformed follicular lymphoma; THRLBCL, T-cell/histiocyte-rich large B-cell lymphoma; PMBL, primary mediastinal B-cell lymphoma; HCT, hematopoietic cell transplant; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ECOG, Eastern Cooperative Oncology Group; IQR, Interquartile range, LDH, Lactate Dehydrogenase; ULN, Upper Limit of Normal; allo-HCT, allogeneic hematopoietic cell transplantation
Missing data in the following variables (N): LDH (10), neutrophils (8), hemoglobin (8), platelets (8), number of previous lines (10), Ann Arbor stage (5), ECOG (7), refractory lymphoma (1), best response to CAR T-cells (9)
# Prior to CAR T-cell therapy
* Never achieving a complete response
Median time from CAR T-cell infusion to PD in the full cohort was 2.5 months (95%CI 1.0-3.3) (Figure S3), and median follow-up from PD was 15 months (95% CI, 13–19). The first subsequent treatment approach included (1) immunotherapy or targeted agents in 92 (43%) patients (27/92 with bispecific antibodies), (2) chemotherapy or radiotherapy in 43 (20%) patients and (3) palliative care in 81 (38%) patients (Figure S4A); further description of the agents included in each treatment group are available in Table S1 and Table S2.
Outcomes after disease progression to CAR T-cells
Median OS after disease progression to CAR-T for the full cohort was 5.1 months (95% CI 4.1–6.2), with a 6- and 12-month OS of 44.1% and 26.7%, respectively. In the univariate analysis for OS including the 15 selected variables, we identified that ECOG, PD as best response to CAR T-cells, time from CAR-T infusion to PD, number of extranodal sites, LDH, hemoglobin, and neutrophil count values at progression had a significant impact on OS (Table S3).
Development of the Post-CAR Prognostic Index
Variable selection and defined prognostic subgroups
A stratified multivariate Cox model was fitted using the five variables selected in the feature selection procedure (Table 2 and Figure S5). Given the proximity in hazard ratios across these variables (ranging from 1.48 to 1.77), each risk factor was assigned 1 point, establishing a scoring range from 0 to 5 points. Finally, we identified 4 distinct risk groups, achieving balanced sample sizes across groups: a low-risk group for patients with 0 or 1 points, an intermediate-low risk group for patients with 2 points, an intermediate-high risk group for patients with 3 points and a high-risk group for patients with 4 or 5 points (Table 3).
Table 2.
Multivariate modeling in the training cohort
| Variable | 1 point | % pts | HR | 95% CI | p |
|---|---|---|---|---|---|
| ECOG | ≥ 1 | 76 | 1.77 | 1.11–2.81 | 0.02 |
| LDH (x ULN) | ≥ 2 | 30 | 1.56 | 1.10–2.22 | 0.01 |
| Hemoglobin (g/dL) | < 10 | 45 | 1.48 | 1.03–2.13 | 0.03 |
| Number extranodal sites | ≥ 2 | 43 | 1.54 | 1.09–2.19 | 0.02 |
| Months from CAR-T to PD | < 4 | 81 | 1.71 | 0.97–3.00 | 0.06 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; ULN, upper limit of normal; CAR-T, chimeric antigen receptor T-cells; PD, progressive disease; pts, patients; HR, hazard ratio; CI, confidence interval
Table 3.
PC-PI risk groups in the training cohort
| PC-PI risk group | Nº of factors | % patients | Median OS | 12-month OS, % |
|---|---|---|---|---|
| Low | 0,1 | 18 | NR | 63 |
| Intermediate-low | 2 | 26 | 7.3 | 30 |
| Intermediate-high | 3 | 26 | 5.0 | 22 |
| High | 4,5 | 30 | 1.9 | 8 |
Abbreviations: OS, overall survival
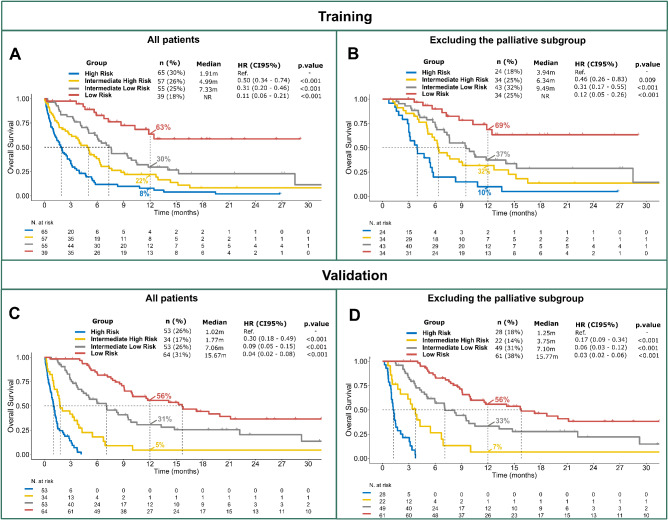
In the TC, the 4 risk groups showed statistically significant differences in terms of OS. Median OS was not reached in the low-risk group (n = 39 [18%]), 7.3 months in the intermediate-low risk (n = 55 [26%], HR = 0.11 [p < 0.01]), 5.0 months in the intermediate-high risk (n = 57 [26%], HR = 0.31 [p < 0.01]) and 1.9 months in the high-risk group (n = 65 [30%], HR = 0.50 [p < 0.01]) (Fig. 2A). The same significant differences in OS were observed across groups when the palliative subset was excluded (Fig. 2B).
Fig. 2.
- Overall survival of the training cohort and validation cohort for the full patient population (A, C). Overall survival of training cohort and validation cohort, excluding the palliative subset (B, D)
Performance of the Post-CAR Prognostic Index
Validation cohort
Patient characteristics of the validation cohort
The VC included 204 patients with R/R LBCL who experienced PD after CAR T-cell therapy at 3 different European centers. Most patients were male (62%), with a DLBCL histology (76%) and stage IV disease (73%) at time of CAR T-cell progression (Table S4). Median time from CAR T-cell infusion to PD was 2.7 months (IQR 1.0–3.6) and median follow-up from PD was 21.9 months (95% CI, 17.7–31.5). The first subsequent treatment included immunotherapy or targeted agents in 58% of patients (30/204 patients with bispecific antibodies), chemotherapy or radiotherapy in 20%, and palliative care in 22% (Figure S4A).
Post-CAR Prognostic Index in the validation cohort
The VC had a similar patient distribution in the 4 prognostic risk groups to the TC (low risk 35%, intermediate-low risk 25%, intermediate-high risk 12%, high risk 27%). The median OS for each of these risk groups was 15.2, 5.3, 2.9 and 0.9 months, respectively. Each group had distinct OS outcomes when compared with all the other risk groups (p < 0.05 for each comparison) (Fig. 2C). Excluding the palliative subgroup, and focusing only on the patient subsets who received subsequent treatment after progression, these differences were maintained (Fig. 2D, Figure S4B).
Comparison of the Post-CAR Prognostic Index with the IPI and R-IPI scores
Applying the IPI score to the TC, 22% were classified as low risk, 26% as low-intermediate, 25% as high-intermediate and 28% as high risk. Median OS for these risk groups was 8.3, 7.6, 4.1 and 2.1 months, respectively (Figure S6). According to the R-IPI, 47% of patients were classified in the poor risk group (mOS 3.0 months), 44% in the good risk (mOS 7.4 months) and 9% in the very good risk group (mOS 5.9 months) (Figure S6).
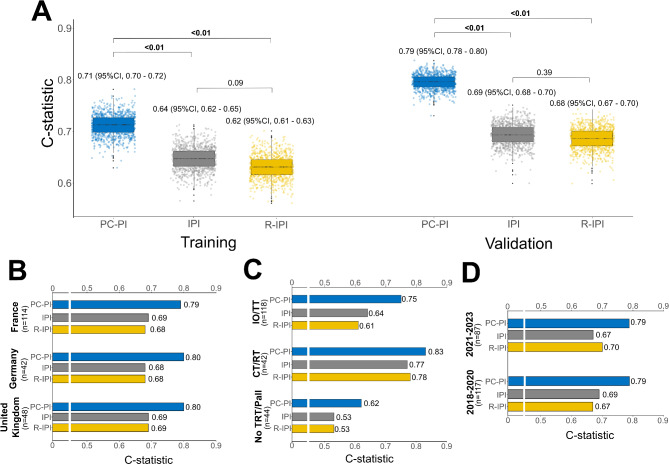
After the bootstrap analysis and optimism correction, as specified in Methods, the C-statistic for the PC-PI was 0.71 in TC and 0.79 in VC. For the IPI, values were 0.64 in the TC and 0.69 in the VC. Furthermore, the R-IPI values were 0.62 and 0.68 for the TC and VC, respectively (Fig. 3A). The same advantage for PC-PI vs. IPI and R-IPI was observed when the palliative subgroup was excluded (Figure S7). In the VC, the C-statistic values remained consistent across the different countries (Fig. 3B, Table S5). When stratifying by subsequent treatment modality in the VC, the PC-PI exhibited the highest C-statistic values compared to both the IPI and R-IPI prognostic scores (Fig. 3C, Table S6). Additionally, the PC-PI outperformed IPI when the analysis was restricted to the patients from the TC and VC who received bispecific antibodies as first subsequent treatment after PD to CAR-T (N = 57, Figure S8). Finally, the C-statistic of PC-PI remained higher than IPI and R-IPI across different time periods (2018–2020 and 2021–2023)(Fig. 3D).
Fig. 3.
- C-statistic values from 1000 bootstrap resampling data comparing the PC-PI score with the IPI and R-IPI (A). Comparative analysis of the 3 prognostic scores across countries (B), subsequent treatment strategies (C), and time periods (D) of the validation cohort
The calibration of the PC-PI score in the TC and VC for the 6 and 12-month post-progression time points demonstrated consistency across various scoring groups. Both the hazard regression method (with and without correction for optimism) and the Kaplan-Meier method confirmed this consistency (Figure S9).
Discussion
In light of the increasing patient population with PD after CAR T-cells and the incorporation of this immunotherapy strategy to second (and potentially first) line for patients with LBCL, a reliable tool to inform on life expectancy after disease progression and allow stratification of this patient population in subsequent clinical trials is warranted. To the best of our knowledge, this is the largest study to date focused on LBCL patient outcomes after PD to CAR T-cell therapy (N = 420). Based on the data collected from the 216 patients who constituted the TC, we identified 5 readily-available variables at time of CAR-T failure with the strongest impact on OS and developed a score to inform on patient prognosis in this particular setting. Then, we validated the score in 204 patients from a different multicenter, international cohort.
The patient characteristics were in line with published reports in this setting [11–14]. As expected, median time from CAR T-cell infusion to progression was short, usually within 3 months, and patients who were only candidates for best supportive care had a dismal short-term outcome. Focusing on patients who were candidates for subsequent treatment, survival was heterogeneous, highlighting the need for tools to aid OS prediction in this difficult-to-treat patient population.
The first aim of our study was to examine the results of available prognostic tools in LBCL, when applied to the post-CART setting. Both IPI and R-IPI underperformed, identifying only 2 risk groups each. Focusing on the former, the high and low groups melded with their intermediate counterparts, showing a lower discriminative potential in comparison to the 4 risk groups of the original study developed in the chemotherapy era [19]. Concerning R-IPI, we observed an uneven patient distribution (only 9% had a very good prognosis) and the very good and good risk groups presented a similar outcome (p = 0.95) [20]. This highlighted the need for a novel tool to predict OS after progression to CAR T-cells in R/R LBCL patients.
In the second part of this study, we analyzed the impact of each variable on OS and through LASSO modelling selected the combination which yielded a better predictive capacity. As opposed to other prognostic scores for B-cell lymphoma [19, 20, 27, 39], the PC-PI score did not include age. The advent of CAR T-cells as a therapeutic option for patients of (practically) any age has potentially abrogated the prognostic relevance this variable had in previous scores, when patients’ options in the R/R setting were limited by autologous stem cell transplant eligibility criteria [40, 41]. On the other hand, we incorporated hemoglobin in the PC-PI score, a marker of hematopoietic reserve with a confirmed prognostic role in the pre-CAR-T setting [18, 42, 43] and previously included in other prognostic scores for B-cell lymphoma [39], together with time from CAR T-cell infusion to PD, which has already shown on multiple analyses to play a key role in the outcome of this patient population [11, 12, 14, 18, 42] and is, in fact, one of the variables which yielded a higher prognostic relevance in our score. Interestingly, hemoglobin and time from CAR-T to PD showed a significant interaction (p = 0.03), and incorporating both variables in the model enhanced its predictive capacity.
In terms of the PC-PI risk groups, patients with 0–1 point had a good prognosis, with a 12-month OS of 63% in the TC. The favorable outcome for this low-risk cohort was confirmed in the VC and in each subsequent treatment group, maintaining a 12-month OS > 50% in all cases. Noteworthy, this was the best outcome achieved after CAR-T failure in the third or later line, underlying the large room for improvement in this setting. With the recent second-line indication for refractory or early relapsing (< 12 months) patients, we may observe better outcomes after PD to CAR-T, but this is yet to be confirmed. Patients with 2 risk factors, in the intermediate-low risk group, had a significantly worse OS in comparison with the low-risk patients (12-month 30% in TC, p = 0.002), warranting closer attention when planning future steps after PD. Patients with 3 points, in the intermediate-high risk group, leaned toward the intermediate-low risk in the TC (12-month OS of 22%), but were closer to the high-risk in the VC (12-month OS of 5%); this could be due to the underlying differences in patient characteristics between both subsets. Finally, patients with 4 or 5 points, in the high-risk group, had a dismal prognosis (12-month OS 8% in the TC and 0% in the VC); adapting this score to the pre-CART treatment phase in future studies could try to identify this latter group of patients beforehand, steering them towards other treatment modalities. After disease progression to CAR-T, both the high and intermediate-high risk groups would benefit from implementing early subsequent treatment, at the first sign of PD, favoring clinical trials in light of the discouraging outcomes observed with available regimens. Meeting trial inclusion criteria could, however, be a potential barrier for some of these early progressing patients who, often, have not fully recovered cytopenias and ECOG PS at time of PD. Overall, the patient distribution among the 4 risk groups in the PC-PI was balanced, both in the TC and VC, ranging from 17 to 31%. The superior concordance index of the PC-PI vs. IPI and R-IPI was not only observed for the full patient population, but also among treatment subgroups, time periods and across each of the 3 European countries participating in the VC, confirming the robustness of the score. Noteworthy, the PC-PI also outperformed IPI when the analysis was restricted to patients receiving subsequent bispecific antibodies in the TC and VC, despite the limited number of patients which underpowered this subgroup analysis.
This study has several limitations, starting with the retrospective nature of data collection and the heterogeneity of treatments following CAR-T progression across participating centers. The use of bispecific antibodies as first subsequent treatment strategy seemed lower than expected, probably due to the timing of study development. In light of the rapidly evolving post-CART treatment landscape, the PC-PI will need to be further evaluated in prospective cohorts. However, both the training and validation patients were treated uniformly with CD19-directed CAR T-cells in the third or later line setting, and met standard patient and disease criteria, potentially allowing our results to be generalized to other patients in the same context; if the PC-PI score will perform as well for patients progressing after second-line treatment with axi-cel or liso-cel remains to be evaluated [21, 44]. Additionally, even though a more complex approach to build the prognostic model could have yielded a more accurate OS prediction on an individual patient level, the aim of our study was to rely on a deliberately limited number of routinely evaluated parameters to allow a straightforward score assessment. The large number of included patients in the TC and VC allowed us to confirm across treatment groups and participating countries its potential worldwide applicability in this setting.
In conclusion, the Post-CAR Prognostic Index (PC-PI) is a clinically useful score for OS prediction and risk-adapted planning in LBCL patients relapsing after CAR T-cell therapy. Given the medical need for more effective treatments post CAR-T, our results will aid in the stratification of clinical trials that include patients with prior CAR T-cell exposure. These findings should be validated prospectively in an independent patient population, and in earlier lines of therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
AcknowledgementsThe authors thank the patients and their families for their participation in this study.
Author contributions
Conception and design: G.I., V.N., P.B., G.V., P. A. Provision of study materials or patients: G.I., P.S., K.R., M.B-O., F.S., A-A. M-L., J.D., A.P., M. G., A. C-C., N. M-C., H.L.H., J-M. S-P., J-M.S., H.G., A.M., L.L.C., R.H., J-L.R., A.S., F.B., A.M.G-S., M.K., M.S., A.K., E.B., P.B., P.A.Collection and assembly of data: G.I., J.I-T., V.N., P.B., G.V., P.A.Data analysis and interpretation: G.I., J.I-T., V.N., P.B., G.V., P.A.Manuscript writing: All authorsFinal approval of manuscript: All authorsAccountable for all aspects of the work: All authors.
Data availability
Data are available upon reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Competing interests
Conflicts of Interest G.I. Consultancy and Honoraria: Novartis, Roche, Kite/Gilead, Bristol-Myers Squibb, Abbvie, Janssen, Sandoz, Miltenyi, AstraZeneca.P.S. Honoraria, Advisory/Consultancy from Janssen, Roche, BMS, Abbvie, AstraZeneca, Chugai; Novartis and Kite/Gilead.K.R. Kite/Gilead: Research Funding, Consultancy, Honoraria and travel support; Novartis: Honoraria; BMS/Celgene: Consultancy, Honoraria; Pierre-Fabre: travel support.M.G. Consultancy/advisory: Novartis, Kite/Gilead, BMS, MSD, Pierre Fabre.AC.C. Honoraria: Novartis, Gilead, Bristol-Myers, Abbvie.A.M. Consulting fees: Takeda, BMS, Merck, Jazz Pharma, Sanofi. Research fundings: Gilead/Kite.R.H. Honoraria (Gilead, Janssen, Novartis, Celgene, MSD), Travel expenses (Gilead, Janssen).JL.R-H: Honoraria for lectures, consulting and advisory boards from Johnson&Johnson, Kite/Gilead, Novartis, BMS and Sanofi. Travel grants from Johnson&Johnson, BMS, Amgen, Kite/Gilead. Served on a Speakers’ Bureau for BMS, Johnson&Johnson, Kite/Gilead, Novartis, BMS, Amgen and Pfizer.A.S. Honoraria: Takeda, BMS/Celgene, MSD, Janssen, Amgen, Novartis, Gilead Kite, Sanofi, Roche, GenMab, Abbvie, Jazz Pharmaceuticals. Consultancy: Takeda, BMS/Celgene, Novartis, Janssen, Gilead, Sanofi, GenMab, Abbvie. Speaker’s Bureau: Takeda. Research Support: Takeda. Non-profit organizations: President of the EBMTA.M.G-S. Honoraria and/or consulting fees: Roche, BMS, Takeda, Janssen, Kyowa Kirin, Gilead / Kite, Incyte, Lilly, Miltenyi, Ideogen, Genmab, Abbvie, Sobi, Astra-Zeneca, GSK.M.K. Consulting and lectures: Gilead, Jazz, Pfizer.M.S. receives industry research support from Amgen, Bristol-Myers Squibb/Celgene, Gilead, Janssen, Miltenyi Biotec, Morphosys, Novartis, Roche, Seattle Genetics, and Takeda, and serves as a consultant/advisor to AvenCell, CDR-Life, Ichnos Sciences, Incyte Biosciences, Janssen, Molecular Partners, and Takeda. She serves on the speakers’ bureau at Amgen, AstraZeneca, BMS/Celgene, Gilead, GSK, Janssen, Novartis, Pfizer, Roche, and Takeda.A.K. Honoraria/ad board from Kite/Gilead, Roche, BMS, Novartis, Abbvie.E.B. Honorarium/Ad Board/Safety Review Committee: Kite/Gilead, Bristol Myers Squibb, Novartis, Pfizer, Incyte, ADC Therapeutics, Roche, Takeda, Daiichi Sankyo, Cellectis, Innate Pharma. Personal fees/Travel support: Kite/Gilead, Bristol Myers Squibb, Novartis, Pfizer, Takeda, Abbvie, Roche. Research funding: Amgen, BMS.P.B. Allogene: Honoraria; Amgen: Honoraria; BMS: Honoraria; Kite/Gilead: Honoraria; Janssen: Honoraria; Jazz Pharmaceuticals: Honoraria; Miltenyi: Honoraria; Novartis: Honoraria; Nektar: Honoraria.P.A. Consulting/advisory: Roche, Genmab, Janssen, BMS, AbbVie, AstraZeneca, BeiGene; Honoraria: Roche, Genmab, Janssen, BMS, AbbVie, AstraZeneca, Gilead, Incyte.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neelapu SS, Jacobson CA, Ghobadi A, Miklos DB, Lekakis LJ, Oluwole OO et al. 5-Year Follow-Up supports curative potential of Axicabtagene Ciloleucel in Refractory large B-Cell lymphoma (ZUMA-1). Blood. 2023. [DOI] [PMC free article] [PubMed]
- 2.Schuster SJ, Tam CS, Borchmann P, Worel N, McGuirk JP, Holte H, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(10):1403–15. [DOI] [PubMed] [Google Scholar]
- 3.Abramson JS, Palomba ML, Gordon LI, Lunning M, Wang M, Arnason J, et al. Two-year follow-up of lisocabtagene maraleucel in relapsed or refractory large B-cell lymphoma in TRANSCEND NHL 001. Blood. 2024;143(5):404–16. [DOI] [PubMed] [Google Scholar]
- 4.Pasquini MC, Hu ZH, Curran K, Laetsch T, Locke F, Rouce R, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-hodgkin lymphoma. Blood Adv. 2020;4(21):5414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iacoboni G, Villacampa G, Martinez-Cibrian N, Bailén R, Lopez Corral L, Sanchez JM, et al. Real-world evidence of tisagenlecleucel for the treatment of relapsed or refractory large B-cell lymphoma. Cancer Med. 2021;10(10):3214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson CA, Locke FL, Ma L, Asubonteng J, Hu ZH, Siddiqi T, et al. Real-world evidence of Axicabtagene Ciloleucel for the treatment of large B cell lymphoma in the United States. Transpl Cell Ther. 2022;28(9):581.e1-.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon M, Iacoboni G, Reguera JL, Corral LL, Morales RH, Ortiz-Maldonado V et al. Axicabtagene ciloleucel compared to tisagenlecleucel for the treatment of aggressive B-cell lymphoma. Haematologica. 2022. [DOI] [PMC free article] [PubMed]
- 8.Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022;28(10):2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bethge WA, Martus P, Schmitt M, Holtick U, Subklewe M, von Tresckow B, et al. GLA/DRST real-world outcome analysis of CAR T-cell therapies for large B-cell lymphoma in Germany. Blood. 2022;140(4):349–58. [DOI] [PubMed] [Google Scholar]
- 10.Kuhnl A, Roddie C, Kirkwood AA, Tholouli E, Menne T, Patel A, et al. A national service for delivering CD19 CAR-Tin large B-cell lymphoma - the UK real-world experience. Br J Haematol. 2022;198(3):492–502. [DOI] [PubMed] [Google Scholar]
- 11.Di Blasi R, Le Gouill S, Bachy E, Cartron G, Beauvais D, Le Bras F et al. Outcomes of patients with aggressive B-Cell lymphoma after failure of anti-CD19 CAR T-Cell therapy: a DESCAR-T analysis. Blood. 2022. [DOI] [PubMed]
- 12.Zurko JC, Nizamuddin I, Epperla N, David KA, Cohen JB, Moyo T et al. Peri-CAR-T practice patterns and survival predictors for all CAR-T patients and post-CAR-T failure in aggressive B-NHL. Blood Adv. 2022. [DOI] [PMC free article] [PubMed]
- 13.Alarcon Tomas A, Fein JA, Fried S, Flynn JR, Devlin SM, Fingrut WB et al. Outcomes of first therapy after CD19-CAR-T treatment failure in large B-cell lymphoma. Leukemia. 2022. [DOI] [PMC free article] [PubMed]
- 14.Iacoboni G, Iraola-Truchuelo J, O’Reilly M, Navarro V, Menne T, Kwon M, et al. Treatment outcomes in patients with large B-cell lymphoma after progression to chimeric antigen receptor T-cell therapy. Hemasphere. 2024;8(5):e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-care Axicabtagene Ciloleucel for relapsed or refractory large B-Cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38(27):3119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K, et al. Axicabtagene Ciloleucel in the Non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38(27):3095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Recio M, Wudhikarn K, Pennisi M, Alonso-Trillo R, Flynn J, Shouval R, et al. The International Prognostic Index is Associated with outcomes in diffuse large B cell lymphoma after Chimeric Antigen Receptor T Cell Therapy. Transpl Cell Ther. 2021;27(3):233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rejeski K, Perez A, Iacoboni G, Penack O, Bücklein V, Jentzsch L et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J Immunother Cancer. 2022;10(5). [DOI] [PMC free article] [PubMed]
- 19.Project IN-HLPF. A predictive model for aggressive non-hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–94. [DOI] [PubMed] [Google Scholar]
- 20.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–61. [DOI] [PubMed] [Google Scholar]
- 21.Westin JR, Oluwole OO, Kersten MJ, Miklos DB, Perales MA, Ghobadi A, et al. Survival with Axicabtagene Ciloleucel in large B-Cell lymphoma. N Engl J Med. 2023;389(2):148–57. [DOI] [PubMed] [Google Scholar]
- 22.Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene Ciloleucel as Second-Line therapy for large B-Cell lymphoma. N Engl J Med. 2022;386(7):640–54. [DOI] [PubMed] [Google Scholar]
- 23.Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–308. [DOI] [PubMed] [Google Scholar]
- 24.Neelapu SS, Dickinson M, Munoz J, Ulrickson ML, Thieblemont C, Oluwole OO, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022;28(4):735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.https://www.clinicaltrials.gov/study/NCT03960840?cond=NCT03960840&rank=1#study-plan
- 26.Westin J, Jacobson CA, Chavez JC, Sureda A, Morschhauser F, Glaß B, CONTROLLED STUDY OF AXICABTAGENE CILOLEUCEL VERSUS STANDARD OF CARE AS FIRST-LINE THERAPY IN PATIENTS WITH HIGH-RISK LARGE B-CELL LYMPHOMA. ZUMA-23: A GLOBAL, PHASE 3, RANDOMIZED. Hematol Oncol. 2023;41(S2):171–3. [Google Scholar]
- 27.Maurer MJ, Jakobsen LH, Mwangi R, Schmitz N, Farooq U, Flowers CR, et al. Relapsed/Refractory International Prognostic Index (R/R-IPI): an international prognostic calculator for relapsed/refractory diffuse large B-cell lymphoma. Am J Hematol. 2021;96(5):599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buecklein VL, Rejeski K, Perez A, Iacoboni G, Jurinovic V, Kharboutli S, et al. Impact of sex on clinical outcomes after CD19 CAR T-Cell therapy for large B-Cell lymphoma: response and survival are significantly Superior in Female compared to male patients. Blood. 2023;142(Supplement 1):3787. [Google Scholar]
- 29.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86. [DOI] [PubMed] [Google Scholar]
- 30.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lausen B, Schumacher M. Maximally selected Rank statistics. Biometrics. 1992;48(1):73–85. [Google Scholar]
- 32.https://cran.r-project.org/web/packages/survminer/index.html
- 33.Kang L, Chen W, Petrick NA, Gallas BD. Comparing two correlated C indices with right-censored survival outcome: a one-shot nonparametric approach. Stat Med. 2015;34(4):685–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.https://cran.r-project.org/web/packages/compareC/index.html
- 35.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. [DOI] [PubMed] [Google Scholar]
- 36.van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 37.Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: A Tool to assess the risk of Bias and Applicability of Prediction Model studies. Ann Intern Med. 2019;170(1):51–8. [DOI] [PubMed] [Google Scholar]
- 38.TRIPOD. www.tripod-statement.org.
- 39.Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–65. [DOI] [PubMed] [Google Scholar]
- 40.Berning P, Shumilov E, Maulhardt M, Boyadzhiev H, Kerkhoff A, Call S, et al. Chimeric antigen receptor-T cell therapy shows similar efficacy and toxicity in patients with diffuse large B-cell lymphoma aged 70 and older compared to younger patients: a multicenter cohort study. Hemasphere. 2024;8(3):e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ram R, Grisariu S, Shargian-Alon L, Amit O, Bar-On Y, Stepensky P, et al. Toxicity and efficacy of chimeric antigen receptor T-cell therapy in patients with diffuse large B-cell lymphoma above the age of 70 years compared to younger patients - a matched control multicenter cohort study. Haematologica. 2022;107(5):1111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rejeski K, Perez A, Sesques P, Hoster E, Berger C, Jentzsch L, et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138(24):2499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rejeski K, Perez A, Iacoboni G, Blumenberg V, Bücklein VL, Völkl S, et al. Severe hematotoxicity after CD19 CAR-T therapy is associated with suppressive immune dysregulation and limited CAR-T expansion. Sci Adv. 2023;9(38):eadg3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abramson JS, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood. 2023;141(14):1675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request to the corresponding author.