Abstract
Cuproptosis is characterized by the aggregation of lipoylated enzymes of the tricarboxylic acid cycle and subsequent loss of iron-sulfur cluster proteins as a unique copper-dependent form of regulated cell death. As dysregulation of copper homeostasis can induce cuproptosis, there is emerging interest in exploiting cuproptosis for cancer therapy. However, the molecular drivers of cancer cell evasion of cuproptosis were previously undefined. Here, we found that cuproptosis activates the Wnt/β-catenin pathway. Mechanistically, copper binds PDK1 and promotes its interaction with AKT, resulting in activation of the Wnt/β-catenin pathway and cancer stem cell (CSC) properties. Notably, aberrant activation of Wnt/β-catenin signaling conferred resistance of CSCs to cuproptosis. Further studies showed the β-catenin/TCF4 transcriptional complex directly binds the ATP7B promoter, inducing its expression. ATP7B effluxes copper ions, reducing intracellular copper and inhibiting cuproptosis. Knockdown of TCF4 or pharmacological Wnt/β-catenin blockade increased the sensitivity of CSCs to elesclomol-Cu-induced cuproptosis. These findings reveal a link between copper homeostasis regulated by the Wnt/β-catenin pathway and cuproptosis sensitivity, and suggest a precision medicine strategy for cancer treatment through selective cuproptosis induction.
Subject terms: Cancer stem cells, Tumour biomarkers, Cell biology, Signal transduction
Introduction
Copper is an essential micronutrient used by cells for critical biological processes [1]. However, excess intracellular copper can also be highly toxic. Cancer cells tend to accumulate abnormally high copper levels to meet their increased nutritional demands [2–5]. Thus, these cells must precisely regulate copper homeostasis to ensure adequate copper supply while avoiding toxicity [6]. Human cells have evolved complex mechanisms to maintain systemic and cellular copper balance [7]. Dietary copper is absorbed in the intestine via the SLC31A1 transporter and regulated by the liver [8]. Cellular copper uptake also involves SLC31A1 along with metalloreductases that generate the preferred Cu(I) state [9, 10]. The ATP7A/B transporters mediate cellular copper efflux as needed [7, 11]. Cytosolic chaperones and glutathione (GSH) bind copper to prevent free toxicity [7]. In cancer cells, heightened copper demand can overwhelm normal buffers, allowing excess accumulation, particularly in mitochondria. This mitochondrial copper overload was found to trigger a form of regulated cell death termed cuproptosis [12].
Cuproptosis is distinct from other death modalities like apoptosis or ferroptosis [12–14]. Cuproptosis is mediated by mitochondrial proteotoxic stress rather than oxidative stress. Specifically, copper ions impair proper lipoylation of tricarboxylic acid (TCA) cycle enzymes like DLAT, causing their oligomerization. The ferredoxin FDX1 acts upstream to regulate this lipoylation pathway. Copper also binds and destabilizes iron-sulfur cluster proteins through FDX1 [13, 15]. The combined dysregulation of lipoylated and iron-sulfur proteins leads to severe metabolic disruption and proteotoxic stress, culminating in cuproptosis [12, 13, 16]. Uncovering this evolutionarily conserved cell death mechanism provides insight into why stringent copper homeostasis mechanisms evolved. It also reveals a potential vulnerability in some cancer cells exhibiting copper addiction [17]. Recently, a study exploring head and neck squamous cell carcinoma (HNSCC) has systematically assessed the differential expression and genetic modifications in cuproptosis-related genes (CRGs), devising CRG risk models to forecast patient outcomes [18]. This investigation enriches our understanding of potential drug therapies targeting CRG. Additionally, the association between elesclomol and cuproptosis has also led to renewed interest in the clinical anticancer potential of elesclomol as a copper ionophore [12].
Aberrant activation of Wnt/β-catenin signaling is common in many cancers and promotes tumor growth and progression [19, 20]. Concurrently, Wnt signaling enhances the stem cell-like properties of cancer cells, enabling self-renew, and resistance to cytotoxic and targeted therapies [21, 22]. However, the link between cuproptosis and cancer phenotype and the precise mechanism by which the Wnt/β-catenin pathway regulates cuproptosis remain elusive. Additional research can facilitate refinement of cuproptosis-targeting therapeutic approaches.
In this study, we found cuproptosis process is accompanied by robust activation of the Wnt/β-catenin pathway. Mechanistically, copper promotes PDK1/AKT/GSK3β/β-catenin pathway activation and cancer stem cell (CSC) properties. The β-catenin/TCF4 complex regulates intracellular copper homeostasis and confers cuproptosis resistance by transcriptionally activating ATP7B. Moreover, knocking down TCF4 or pharmacologically inhibiting the Wnt/β-catenin pathway increased cancer cell sensitivity to the cuproptosis in vitro and in vivo. Our data elucidates a mechanism of cancer cell evasion of cuproptosis and provides new insights into inducing cuproptosis for cancer treatment.
Results
Cuproptosis is accompanied by the activation of the Wnt/β-catenin pathway
To comprehensively analyze the changes in transcriptome levels during cuproptosis in cancer cells, CAL27 cells were treated with elesclomol and copper in a 1:1 ratio (elesclomol-Cu, ES-Cu), and the transcriptome was sequenced and analyzed (Fig. S1a). Gene ontology (GO) and KEGG enrichment analysis revealed a significant enrichment with the biological process terms “cellular response to chemical stress”, “response to unfolded protein”, “response to oxidative stress”, “negative regulation of growth”, “heat shock protein binding”, and “protein processing in endoplasmic reticulum” (Fig. 1a, b). In addition, significant downregulation of gene expression was observed for Fe-S cluster proteins (e.g., NFU1, FXN), TCA cycle and respiratory electron transport (e.g., DBT, NDUSF1, IDH3B), cell cycle (e.g., CDK1, E2F2, CCND2), and DNA repair-related (e.g., PCNA, KDM1A, RAD51) pathways (Fig. 1c). We hypothesize that excessive copper leads to irreversible cell death of cancer cells by inhibiting the TCA cycle and mitochondrial electron transport chain, cell cycle, and decreasing nucleotide excision repair capacity.
Fig. 1. Cuproptosis activates Wnt/β-catenin signaling.
a Schematic diagram illustrating the mechanism of cuproptosis. b Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis conducted on CAL27 cells treated with 100 nM elesclomol-Cu (ES-Cu) for 24 h compared to control cells treated with DMSO. c A hierarchical clustering analysis comparing the expression of downregulated genes in control and ES-Cu treated CAL27 cells. d WB analysis of indicated proteins of CAL27 cells after 24 h of ES-Cu treatment. e DLAT oligomerization was analyzed in CAL27 and FaDu cells (ES-Cu, 50 nM, 24 h). Gene set enrichment analysis (GSEA) highlight enrichment of “TCF-dependent pathway in response to Wnt” pathways in ES-Cu treated CAL27 cells (f) and liver tissues of Atp7b-/- mice (g), respectively. h The heatmap depicts changes in expression of Wnt pathway genes in ES-Cu treated CAL27 cells (24 h). i WB analysis of Wnt pathway proteins in CAL27 cells following treatment with TTM (40 μM) and specified concentrations of ES-Cu for 24 h. Histone H3 was loaded as a nuclear marker. j Immunofluorescence staining shows increased β-catenin translocation in CAL27 cells after 24 h of 10 nM ES-Cu treatment. White arrowheads indicate β-catenin translocation into the nucleus. k Representative tumor images and growth curves demonstrate reduced tumor volumes in ES-Cu (0, 5, 10 mg/kg) treated CAL27 tumors. l Representative IHC staining and quantitative analysis of Ki67, β-catenin, and p-GSK3β in ES-Cu treated tumors. Data were expressed as mean ± SD of ≥ 3 independent experiments. The p-values in k, l were calculated by Student’s t test.
Further results showed that ES-Cu promoted cell death in HNSCC (CAL27, FaDu) and melanoma (A375) in a dose-dependent manner (Fig. S1b–d). In addition, ES-Cu-induced cell death could be significantly blocked by the copper chelator tetrathiomolybdate (TTM), but not by the apoptosis inhibitor Z-VAD-FMK, the necroptosis inhibitor necrostatin-1 (Nec-1), or the ferroptosis inhibitor ferrostatin-1 (Fer-1) (Fig. S1b–d). We noted that ES-Cu significantly upregulated the abundance of HSP70 and downregulated the expression of the Fe–S cluster protein FDX1 (Fig. 1d). Copper directly binds and promotes oligomerization of lipoylated DLAT protein [12]. Consistent with this, ES-Cu efficiently induced oligomerization of DLAT (Fig. 1d, e), validating the underlying mechanism by which cuproptosis occurs [12].
The Wnt/β-catenin signaling plays a crucial role as cancer cells adapt to therapeutic perturbations and changing biological stresses, developing resistance to cell death [23, 24]. Interestingly, the “TCF-dependent signaling in response to Wnt” was significantly enriched in ES-Cu-treated CAL27 cells compared to the control group (Fig. 1f). Knockdown of ATP7B leads to tissue copper overload, reduced levels of Fe-S cluster proteins, and onset of cuproptosis [12]. We found that the “TCF-dependent pathway in response to Wnt” was also significantly enriched in liver tissues of Atp7b−/− mice (a Wilson’s disease model) (Fig. 1g) [25]. In addition, the expression of some critical Wnt pathway genes was upregulated after ES-Cu treatment (e.g., CTNNB1, MYC, CCND1) (Fig. 1h). Bioinformatics analysis guided us to further explore the changes in the Wnt pathway during cuproptosis. Treatment with ES-Cu promoted accumulation of copper in tumor cells in a dose-dependent manner, while the copper chelator TTM led to a reduction in copper levels within tumor cells (Fig. S2a). Western blot analysis revealed that ES-Cu treatment induced phosphorylation of AKT (Thr308) and GSK3β (Ser9), and expression of nuclear β-catenin in cancer cells. These effects were abrogated by TTM (Figs. 1i and S2b). Neither elesclomol alone nor supplementation with low dose CuCl2 alone caused significant changes in Wnt pathway-associated markers (Fig. S2d, e). We subsequently confirmed that ES-Cu promoted the translocation of β-catenin from cytosol into the nucleus, which could be blocked by the copper chelator TTM (Fig. 1j). Additionally, ES-Cu significantly inhibited tumor growth in vivo (Fig. 1k). Compared to the control group, ES-Cu treatment resulted in decreased Ki67 expression and increased p-GSK3β and β-catenin expression (Fig. 1l). Altogether, these results suggest that cuproptosis is accompanied by significant activation of the canonical Wnt/β-catenin signaling pathway.
The SLC31A1-copper axis plays an important role in activating the Wnt/β-catenin pathway
We further investigated the potential effects of copper on the activation of the Wnt/β-catenin pathway in cancer cells. Exposing cancer cells with 100 micromoles of copper resulted in a time-dependent increase in cellular copper levels without affecting cell viability (Fig. S3a–c). Silencing SLC31A1, a transmembrane pump facilitating copper uptake in mammalian cells, significantly reduced copper content in tumor cells (Fig. S3c) [26]. However, subcellular fractionation reveals distinct copper distributions between ES-Cu and CuCl2 treatments alone. In ES-Cu-treated CAL27 cells, notable copper accumulation is primarily observed in the mitochondrial fraction (Fig. S3d). Conversely, in cells treated with CuCl2 alone, most of the copper remains in the cytosolic fraction (Fig. S3e). To investigate the impact of these different copper distributions on mitochondrial stress, we compared the effects of various treatments on mitochondrial reactive oxygen species (mtROS) production. ES-Cu treatment significantly induces mtROS generation, whereas CuCl2 treatment alone does not result in a significant alteration of mtROS levels (Fig. S3f). This difference in mitochondrial stress elucidates why CuCl2 treatment alone, unlike ES-Cu treatment, fails to significantly induce cell death.
Elevating CuCl2 levels in the culture medium triggered phosphorylation of AKT and GSK3β, and enhanced β-catenin expression, which was reversible upon SLC31A1 silencing (Figs. 2a–c and S4a). Further analysis indicated that inhibiting copper uptake by SLC31A1 knockdown notably diminished activation of the p-AKT/p-GSK3β/β-catenin pathway, decreased sphere formation capacity, and reduced the percentage of ALDH1+ stem-like cells, implying suppressed CSC properties (Figs. 2d–f and S4b–e). Compared to the control group, SLC31A1 knockdown sphere cells showed lower in vivo tumor initiation frequency (TIF), implying that CSC properties were suppressed (Figs. 2g and S4f, g). Low-dose ES-Cu supplementation to SLC31A1-deficient cells effectively restored sphere-forming ability and upregulated critical Wnt pathway components, indicating that ES-Cu can bypass the SLC31A1-mediated copper uptake pathway to directly influence Wnt signaling and cancer stem cell-like characteristics, even in the absence of SLC31A1 (Figs. 2h, I and S5a, b). Moreover, the copper chelator TTM diminished β-catenin expression regardless of Wnt3a’s presence (Fig. S5c). Overall, these results demonstrate the crucial role of copper in Wnt/β-catenin pathway activation under physiological conditions.
Fig. 2. The SLC31A1-copper axis regulates PDK1/AKT/GSK3β/β-catenin signaling and stemness.
a WB results show time-dependent copper (CuCl2, 100 μM) induction of Wnt pathway protein expression in CAL27 cells with SLC31A1 knockdown (sgSLC31A1) versus control cells (sgNC). Immunofluorescence images reveal increased nuclear β-catenin localization in CAL27 (b) and A375 (c) cells after 10 μM copper treatment for 24 h. White arrowheads indicate β-catenin translocation into the nucleus. d WB analysis of the indicated proteins in sgNC and sgSLC31A1 CAL27 cells. e Stemness of copper-treated CAL27 and FaDu cells was examined by tumor sphere assay. f Flow cytometry analysis show different ALDH activity in copper-treated CAL27 and FaDu cells (100 μM, 12 h). g Limiting dilution assays demonstrate decreased tumor initiation frequency of sgSLC31A1 sphere cells in nude mice. h Stemness of CAL27 and FaDu cells with SLC31A1 knockdown following low-dose ES-Cu treatment (10 nM) was examined by tumor sphere assay. i WB analysis of Wnt pathway proteins in CAL27 cells with the treatment of ES-Cu (10 nM, 24 h). j Representative IHC images show increased SLC31A1 expression in primary (n = 210) and recurrent (n = 25) HNSCC versus normal mucosa (n = 42). k Proportional differences in clinicopathological factors in the SLC31A1high and SLC31A1low expression groups from our patient cohort. The χ2 test was used to evaluate the correlation between SLC31A1 and clinical characteristics. l Kaplan–Meier curve of SLC31A1 expression in HNSCC patients (using median cutoff). m Representative IHC images of SLC31A1 and β-catenin in our tissue microarrays. n Statistical analyses indicate positive correlations between SLC31A1 and β-catenin, p-AKT, PD-L1, CD44, and CD133 in HNSCC. o GSEA plot highlights correlation between SLC31A1 expression and Wnt/β-catenin signaling in HNSCC patients from TCGA. p Copper content in recombinant PDK1 proteins as determined by ICP-MS. q Ultraviolet–visible absorption spectra of 22 µM Cu(I) with 200 μM BCS in 50 mM HEPES, 200 mM NaCl, 1 mM GSH, pH 7.4 during titrations with apo-PDK1. r The proposed model summarizes the roles of SLC31A1-copper signaling in driving Wnt/β-catenin activation and CSC properties. Data were expressed as mean ± SD of ≥3 independent experiments. The p-values in panels e, f, h were calculated by one-way ANOVA. The p-value in panel j was calculated by Kruskal–Wallis test.
Next, immunohistochemistry staining and tissue microarray (TMA) were performed to investigate the expression of SLC31A1 in human HNSCC. As shown in Fig. 2j, there was a notable increase in SLC31A1 expression in HNSCC tissues when compared to normal mucosa. Moreover, patients with recurrence demonstrated a tendency toward elevated SLC31A1 expression than primary HNSCC patients (Fig. 2j). Further investigation was carried out to assess the correlation between SLC31A1 expression and clinicopathological parameters (Table S1). Moreover, patients with older age, lymph node metastasis, and higher pathological grades showed significantly higher expression of SLC31A1 (Fig. 2k). Kaplan-Meier curve in Fig. 2l demonstrated that high SLC31A1 expression in HNSCC was correlated with significantly worse clinical outcomes. We also analyzed the relationship between SLC31A1 and Wnt pathway-related proteins by HNSCC TMA. The findings revealed a positive correlation between SLC31A1 expression and β-catenin, p-AKT, PD-L1, CD44, and CD133 (Fig. 2m, n). This strong association between SLC31A1 expression and Wnt pathway markers was further validated in the TCGA pan-cancer database (Fig. S5d–f). The Wnt signaling pathway was significantly enriched in the SLC31A1high expression subgroup compared to the SLC31A1low expression subgroup (Fig. 2o). Thus, the cellular copper axis serves as an essential physiological modulator for Wnt pathway activation and stemness maintenance.
Copper binds PDK1 to activate Wnt/β-catenin pathway
Recent studies indicate that copper can bind directly to PDK1, thereby enhancing the interaction between PDK1 and its downstream effectors, particularly AKT [27]. Based on this, we hypothesized that copper could stimulate the p-AKT-p-GSK3β-β-catenin pathway by directly binding to PDK1. To test this hypothesis, we employed a recombinant PDK1 protein for in vitro experiments to determine copper binding stoichiometry and affinity. Given the reducing intracellular environment maintained by elevated glutathione levels, where copper predominantly exists as Cu(I), we incubated the recombinant PDK1 with ten molar equivalents of Cu(I) in the presence of 1 mM glutathione [12, 28, 29]. This experimental condition mimics physiological settings. After removing excess copper, ICP-MS analysis revealed that approximately 2.4 moles of Cu(I) bind per mole of PDK1 protein (Fig. 2p). This finding suggests the existence of multiple Cu(I) binding sites on PDK1.
To determine the binding affinity of Cu(I) for PDK1, we performed competitive binding assays, adopting methodologies previously used to study the Cu(I)-bathocuproinedisulfonic acid (BCS) complex [30]. The Cu(I)-BCS complex displays a characteristic absorption peak at approximately 483 nm [30]. We observed a dose-dependent decrease in this peak as apo-PDK1 was added to a Cu(I)-(BCS)2 mixture, indicating competitive binding between PDK1 and the Cu(I)-(BCS)2 complex (Fig. 2q). By applying the formation constant of the Cu(I)-BCS complex and fitting the UV titration curve, we determined the average affinity (KCu) of Cu(I) for PDK1 to be 7.08 × 1016 M−1 at pH 7.4 (Fig. 2q) (Table S5). To corroborate our findings, we conducted control experiments with apo-CRIP2 protein, which has a known affinity for Cu(I), under the same pH and ionic strength conditions (Fig. S6a, b) (Table. S6) [31]. These tests confirmed the expected stoichiometry and competitive binding behavior, emphasizing PDK1’s effectiveness as a Cu(I)-binding protein and its potential involvement in copper-regulated signaling cascades (Table S7). Nevertheless, our study did not demonstrate the direct transfer of copper from ES-Cu complexes to PDK1. Further exploration into whether ES-Cu complexes can transfer Cu(II) to cytosolic proteins like PDK1 remains an important scientific question worthy of further investigation.
Cysteine and histidine residues are essential for copper binding. Prior research indicates that the H117 and H203 residues of PDK1 are involved in this process [27]. Cells expressing PDK1 mutants H117A and H203A exhibited decreased p-AKT-p-GSK3β-β-catenin signaling when compared to those expressing wild-type (WT) PDK1 (Fig. S6c). Furthermore, copper enhances the binding affinity of PDK1 to AKT (Fig. S6d). Notably, the double mutation of PDK1 (termed H117/H203A) significantly reduces the intracellular interaction between PDK1 and AKT, emphasizing the importance of the H117 and H203 residues in copper-induced activation of the Wnt pathway (Fig. S6e). These findings suggest that these residues function as primary copper binding sites, thereby influencing the activation of the Wnt pathway. These findings suggest that these residues function as primary copper binding sites, thereby influencing the activation of the Wnt pathway. However, our study does not clearly distinguish between the mechanisms by which Cu(I) and Cu(II) binding sites on PDK1 activate the Wnt/β-catenin pathway. The exact role of Cu(II) in this pathway still needs to be explored.
To compare the effects of copper treatment on cancerous and non-cancerous cells, we included the normal mucosal cell line HIOEC in our studies. Our results show that neither copper supplementation nor ES-Cu treatment notably affects the expression of Wnt pathway-related proteins in HIOEC cells (Fig. S6f). Consistently, PDK1 expression is considerably higher in CAL27 than in HIOEC (Fig. S6g). This disparity provides an explanation for the preferential activation of the Wnt pathway by copper treatment in cancerous cells, as opposed to normal mucosal cells. Intriguingly, compared to 4MOSC1 cells with a lower propensity for metastasis, copper supplementation significantly upregulated nuclear β-catenin levels in highly metastatic 4MOSC2 cells (Fig. S6h, i) [32]. Meanwhile, 4MOSC1 cells exhibited greater sensitivity to ES-Cu treatment, suggesting inherent differences in sensitivity to cuproptosis between metastatic and non-metastatic cancer cells (Fig. S6j). Collectively, these observations implicate copper’s direct binding to PDK1 as a critical factor in maintaining and activating the Wnt/β-catenin pathway, as well as enhancing CSC properties in cancer cells (Fig. 2r).
Activation of Wnt/β-catenin signaling drives resistance to cuproptosis in cancer stem cells
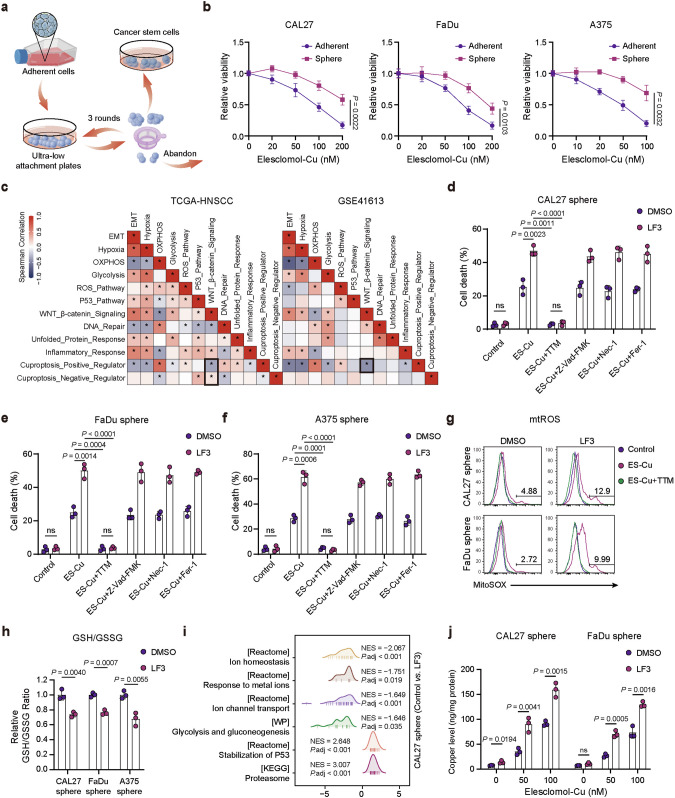
The interplay between stemness and cuproptosis remains unknown. To further investigate the role of cuproptosis in CSCs, we used a suspension culture assay to isolate CSCs [33–35]. Primary adhesion cells were characterized as “parental cells”, and isolated CSCs were characterized as “sphere cells” (Fig. 3a). Interestingly, we found that suspension-cultured CSCs were more resistant to ES-Cu-induced cuproptosis than parental adherent cells (Fig. 3b). Thirteen cuproptosis-related genes (ATP7A, ATP7B, CDKN2A, DLAT, DLD, FDX1, GLS, LIAS, LIPT1, MTF1, PDHA1, PDHB, SLC31A1) were categorized into cuproptosis positive regulation (CPR) signature and cuproptosis negative regulation (CNR) signature (Fig. S7a). Results from two independent datasets, TCGA-HNSCC and GSE41613, indicate that the Wnt/β-catenin pathway was negatively correlated with CPR signature that promote cuproptosis, and positively correlated with CNR signature that suppress cuproptosis. Furthermore, CPR signature were positively correlated with oxidative phosphorylation (OXPHOS) and ROS pathways and negatively correlated with hypoxia and glycolysis pathways.
Fig. 3. Activation of Wnt/β-catenin pathway promotes resistance to cuproptosis in cancer stem cells.
a Schematic diagram illustrates the isolation of CSCs. b Cell viability of CAL27, FaDu, and A375 sphere cells compared to parental cells after 24 h of ES-Cu treatment. c Spearman correlation analyses reveal associations between Wnt/β-catenin signaling, cuproptosis regulation signatures, and hallmark gene signatures in TCGA and GSE41613 dataset. Cell death measurement of CAL27 (d), FaDu (e), and A375 (f) sphere cells following treatment with ES-Cu (100 nM) and LF3 (CAL27 and FaDu sphere for 10 μM, A375 sphere for 5 μM) in the absence or presence of TTM (40 μM), Z-VAD-FMK (20 μM), Nec-1 (20 μM), or Fer-1 (10 μM) for 24 h. g Mitochondrial ROS levels of CAL27 and FaDu sphere cells treated with ES-Cu (100 nM) for 24 h. h Relative GSH/GSSG ratio was evaluated in CAL27, FaDu, and A375 sphere cells. i GSEA results of the indicated pathways in CAL27 sphere cells treated with the Wnt inhibitor LF3 (10 μM). j Copper content measured by ICP-MS in CAL27 and FaDu sphere cells treated with LF3 (10 μM) and indicated concentrations of ES-Cu for 4 h. Cellular uptake results were corrected by protein levels. Data were expressed as mean ± SD of ≥3 independent experiments. The p-values in b, d–f, h, j were calculated by Student’s t test.
We hypothesized that the activated Wnt/β-catenin pathway may confer resistance to cuproptosis in cancer cells, especially CSCs. LF3 inhibits Wnt/β-catenin signaling by disrupting β-Catenin/TCF4 interaction without inducing cell death [36]. Interestingly, LF3 treatment selectively enhanced the sensitivity of sphere cells to ES-Cu-induced cuproptosis, which was rescued by the copper chelator TTM, but not by the apoptosis inhibitor Z-VAD-FMK, the necroptosis inhibitor Nec-1, and ferroptosis inhibitor Fer-1 (Figs. 3d–f, S7b, and S8a–c). Excessive copper leads to mitochondrial dysfunction and triggers an elevated level of mtROS [1, 37]. We found that LF3 treatment enhanced cuproptosis-induced mtROS production, which could be blocked by TTM (Fig. 3g). GSH serves as an intracellular chelator of copper ions, binding copper and preventing cellular damage [12, 38]. Our results showed that the intracellular GSH/GSSG ratio was significantly decreased upon LF3 treatment, and this reduction also contributed to an increase in intracellular copper concentration, ultimately leading to cell death (Fig. 3h). We utilized RNA-seq to investigate transcriptomic changes in CAL27 sphere cells treated with LF3. Gene set enrichment analysis (GSEA) results indicated that LF3 treatment may influence biological processes such as ion homeostasis, p53 stability, and glycolysis, which are important factors affecting cuproptosis sensitivity (Fig. 3i). Inductively coupled plasma mass spectrometry (ICP-MS) confirmed that LF3 treatment increased intracellular copper levels in cancer sphere cell lines, implying a role for the Wnt/β-catenin pathway in regulating copper homeostasis (Fig. 3j). Collectively, these data suggest that inhibition of Wnt/β-catenin signaling increases intracellular copper levels, decreases GSH levels, and promotes cuproptosis sensitivity of CSCs.
TCF4 acts as a repressor of cuproptosis through ATP7B
Canonical Wnt/β-catenin pathway activation is characterized by the relocation of β-catenin into the nucleus, where it binds to the transcription factor TCF4/LEF1 and subsequently promotes transcription of Wnt target genes and CSC properties [39]. TCF4 is a major transcription factor in the Wnt/β-catenin signaling pathway. To further explore the potential oncogenic role of TCF4, we investigated its expression, clinicopathological significance, and prognostic value of TCF4 in HNSCC (Table S2). TCF4 expression was significantly upregulated in HNSCC compared to adjacent normal tissues in our patient cohort and TCGA-HNSCC database (Figs. 4a and S9a, b). Moreover, TCF4 expression positively correlated with pathological grades (Figs. 4a and S9c). Survival analysis suggested that HNSCC patients with high TCF4 expression had significantly shorter prognostic outcomes compared to individuals with low TCF4 expression (Fig. 4b). Therefore, we postulated that elevated expression of TCF4 in HNSCC likely promotes the progression of this malignancy.
Fig. 4. TCF4 protects cancer stem cells from cuproptosis by regulating ATP7B.
a Representative immunohistochemistry results show diminished TCF4 expression in normal mucosa (n = 42) in contrast to HNSCC tissues with different pathological grades (I, 53; II, 121; III, 36). b Kaplan–Meier analysis reveals worse overall survival for HNSCC patients with high TCF4 expression (median cutoff). Cell death measurement of CAL27 (c) and A375 (d) sphere cells following treatment with ES-Cu (100 nM) in the absence or presence of TTM (40 μM), Z-VAD-FMK (20 μM), Nec-1 (20 μM), or Fer-1 (10 μM) for 24 h. e Cell death measurement of CAL27 and A375 sphere cells transfected with control or TCF4-coding plasmid. f Relative GSH/GSSG ratio was evaluated in CAL27 and A375 sphere cells. g Copper content measured by ICP-MS in CAL27 and A375 sphere cells treated with indicated concentrations of ES-Cu for 4 h. Cellular uptake results were corrected by protein levels. h Cuproptosis marker FDX1 expression was detected in CAL27 sphere cells treated with ES-Cu for 24 h. i Schematic diagram illustrating the CUT&Tag assay in CAL27 sphere cells. j Venn diagram illustrating genes at the intersection of TCF4 bound promoters that were identified by CUT&Tag, RNA-seq data from LF3-treated CAL27 sphere cells, and cuproptosis-regulated genes. k The heatmap depicting the expression levels of genes in CAL27 sphere cells treated with or without LF3. l Bar graphs showing fold change of the cuproptosis-related genes expression in sgTCF4 CAL27 sphere cells. m Cell death measurement of ES-Cu (100 nM, 24 h) treated sgTCF4 CAL27 and A375 sphere cells transfected with ATP7B-coding plasmid. n DLAT oligomerization was analyzed of ES-Cu (50 nM, 24 h) treated sgTCF4 CAL27 sphere cells transfected with ATP7B-coding plasmid. Data were expressed as mean ± SD of ≥ 3 independent experiments. The p-value in panel a was calculated by Kruskal–Wallis test. The p-values in c–f, m were calculated by one-way ANOVA. The p-value in g was calculated by Student’s t test.
To validate the role of TCF4 in tumor cuproptosis, TCF4-targeting sgRNA (sgTCF4) or corresponding controls (sgNC) were introduced into CAL27 and A375 cell lines (Fig. S9d). Knockdown of TCF4 enhanced the sensitivity of sphere cells to ES-Cu-induced cuproptosis. The copper chelator TTM inhibited ES-Cu-induced death in sgTCF4 sphere cells, while inhibitors of apoptosis, necroptosis, and ferroptosis failed to maintain cell viability (Figs. 4c, d and S10a, b). In contrast, sphere cells overexpressing TCF4 were more resistant to ES-Cu-induced cuproptosis (Figs. 4e and S11a). Furthermore, the knockdown of TCF4 significantly reduced the intracellular GSH/GSSG ratio and increased copper concentration (Fig. 4f, g). Together, these results indicate that TCF4 depletion promotes ES-Cu-induced cuproptosis in human CSCs.
To elucidate the specific mechanism by which TCF4 regulates cuproptosis, we performed high-throughput CUT&Tag analysis to map the genomic distribution of TCF4 (Fig. 4i). A total of 7,597 TCF4 binding sites near transcription start sites (TSSs) were identified in CAL27 sphere cells. Notably, by integrating the CUT&Tag results, RNA-seq data from LF3-treated CAL27 sphere cells, and cuproptosis-regulated genes, ATP7B and CDKN2A were identified as potential TCF4 targets (Fig. 4j, k). ATP7B maintains copper homeostasis and was the most significantly changed cuproptosis-related gene in TCF4 knockdown sphere cells (Figs. 4l and S11b). Inactivating mutations in CDKN2A occur frequently in HNSCC and melanoma [40, 41]. Although CDKN2A mRNA levels were downregulated in TCF4 knockdown cells, CDKN2A protein expression was barely detectable in CAL27, FaDu, and A375 cells (Fig. S11c). Therefore, we focused on exploring ATP7B’s role in TCF4-mediated cuproptosis inhibition. Enforced ATP7B expression limited cuproptosis induction in sgTCF4 cells, with decreased cell death, DLAT oligomerization, and mtROS generation (Figs. 4m–n, and S12a–c).
We conducted an analysis of the correlation between ATP7B and Wnt pathway-associated proteins using the TCGA-HNSCC dataset, which demonstrated a positive correlation between ATP7B expression and levels of TCF4 and β-catenin (Fig. S13a, b). Notably, the RNA levels of TCF4 and ATP7B were significantly higher in HNSCC cell lines (CAL27, FaDu, SCC4, and SCC9) compared to normal mucosal cells, HIOEC (Fig. S13c). Additionally, expressions of TCF4 and ATP7B were significantly upregulated in CAL27 sphere cells, characterized by an enhanced stem-like phenotype, in contrast to the parental cells (Fig. S13d). Furthermore, TCF4 and ATP7B expressions were notably upregulated in CAL27 sphere cells, which possess enhanced stem-like features, compared to parental cells (Fig. S13d). Under basal conditions, copper concentrations in CAL27 sphere cells were slightly lower than those in their parental tumor cells, although the difference was not statistically significant (Fig. S13e). However, after supplementation with exogenous copper, CAL27 sphere cells displayed a significant reduction in copper levels compared to parental CAL27 cells (Fig. S13e). This observation supports our hypothesis that CSCs have developed effective mechanisms to regulate copper homeostasis, possibly maintaining lower intracellular copper levels to evade cuproptosis. Thus, our findings demonstrate that TCF4 suppresses cuproptosis by regulating ATP7B-mediated copper homeostasis.
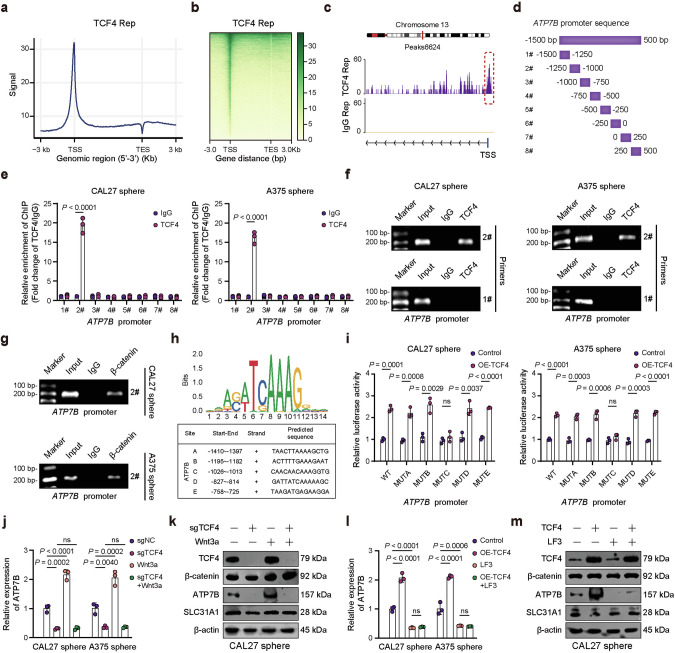
The β-catenin/TCF4 transcription complex promotes ATP7B expression
Considering that CUT&Tag results suggested the presence of TCF4 binding peak in the ATP7B promoter region, we hypothesized that the β-catenin/TCF-4 complex transcriptionally activates ATP7B (Figs. 5a–c and S14a). We then assessed potential TCF4 binding sites in the ATP7B promoter region by chromatin immunoprecipitation (ChIP) assay (Fig. 5d). Among the regions enriched with TCF4 antibody, the ATP7B promoter region 2# (−1250 to −1000 bp before the TSS) had the highest abundance (Fig. 5e–g). Analysis using the JASPAR database identified the TCF4 motif and five TCF4 binding sites in the ATP7B promoter region (between −1500 and 500 bp) (Fig. 5h). To further validate the specific interaction sites, we performed a dual luciferase reporter assay. Promoter activities of WT, MUTA, MUTB, MUTD, and MUTE constructs were upregulated in TCF4-overexpressing sphere cells. However, mutation of the MUTC site prevented this effect, implying the deletion of exact binding elements significantly reduced the relative luciferase activity (Figs. 5i and S14b). These results indicate that TCF4 directly binds to ATP7B promoter regions and promotes its transcriptional activity.
Fig. 5. The β-catenin/TCF4 complex activates ATP7B transcription.
a TCF4 CUT&Tag signal height and position relative to transcription start sites (TSSs) for all genes in CAL27 sphere cells. b CUT&Tag density heatmap of TCF4 enrichment in CAL27 sphere cells within 3 kb around TSS. c IGV tracks reveal TCF4 occupancy peaks in the ATP7B promoter region. d Primers spanning the ATP7B promoter for ChIP analyses. e Enrichment of ATP7B promoter fragments using TCF4 antibody was assessed by ChIP-qPCR analysis. Agarose gels results confirm amplification of ATP7B promoter regions in TCF4 (f) and β-catenin (g) ChIP samples. h The binding motif of TCF4 and potential binding sites in ATP7B promoter region. i Dual-luciferase reporter assay was carried out to verify the binging sequence of TCF4 in the ATP7B promoter region. Q-PCR (j) and WB (k) analysis of the indicated markers in TCF4 knockout CAL27 and A375 sphere cells by Wnt3a treatment (50 ng/ml, 24 h). Q-PCR (l) and WB (m) analysis of the indicated markers in CAL27 and A375 sphere cells followed by TCF4-coding plasmid and LF3 treatment (CAL27 sphere, 10 μM; A375 sphere, 5 μM) for 24 h. Data were expressed as mean ± SD of ≥3 independent experiments. The p-values in e, i were calculated by Student’s t test. The p-values in j, l were calculated by one-way ANOVA.
Additionally, we investigated whether ATP7B induction by TCF4 was associated with Wnt/β-catenin signaling. TCF4 knockdown eliminated the ATP7B expression increase induced by Wnt3a (Figs. 5j, k, and S14c). ATP7B expression was remarkably elevated by TCF4 overexpression, and this was abolished by LF3 treatment (Figs. 5l, m and S14d). However, expression of the copper transporter SLC31A1 did not significantly change after Wnt3a treatment or TCF4 knockdown. Thus, the β-catenin/TCF4 transcription complex promotes ATP7B expression, conferring resistance to cuproptosis.
Knocking out TCF4 or inhibiting Wnt pathway promotes cuproptosis in vivo
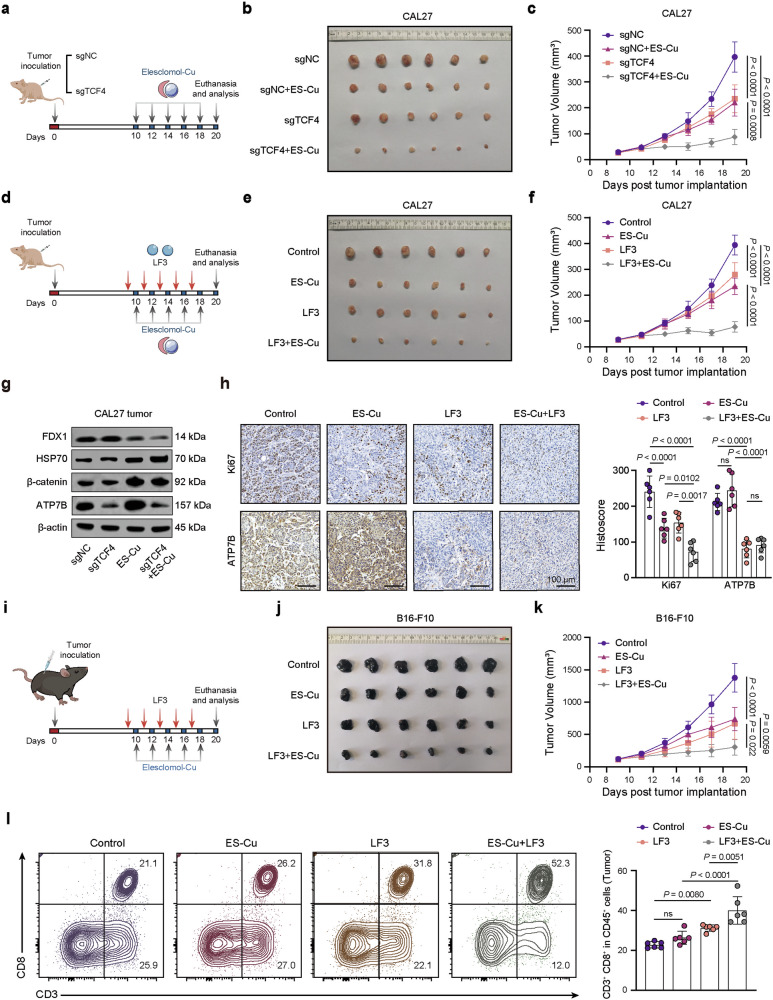
Since our findings suggest that the TCF4-ATP7B axis protects CSCs from cuproptosis, we investigated whether TCF4 deficiency enhances the anticancer activity of ES-Cu in vivo (Fig. 6a). Knockdown of TCF4 or ES-Cu treatment alone inhibited CAL27 tumor growth. Combining TCF4 knockdown with ES-Cu treatment further decreased tumor size (Fig. 6b, c). Additionally, the Wnt pathway inhibitor LF3 showed synergistic antitumor effects in vivo when combined with ES-Cu (Fig. 6d–f). ES-Cu treatment decreased FDX1 expression and increased HSP70 expression, which was more pronounced following TCF4 knockdown (Fig. 6g).
Fig. 6. TCF4 deficiency or Wnt signaling inhibition enhances the anticancer activity of elesclomol-Cu in vivo.
a The scheme outlines subcutaneous implantation of sgNC or sgTCF4 CAL27 cells in BALB/c nude mice followed by ES-Cu treatment (10 mg/kg). Representative tumor images (b) and tumor volume growth curves (c) in different groups. d Scheme illustrating combined treatment with the Wnt inhibitor LF3 (50 mg/kg) and ES-Cu (10 mg/kg) in CAL27 tumor-bearing nude mice. Representative tumor images (e) and tumor volume growth curves (f) in different groups. g WB analysis of indicated markers expression in sgTCF4 and ES-Cu treated CAL27 tumors. h Representative IHC staining and quantification of Ki67 and ATP7B in different groups. i Scheme illustrating combined LF3 and ES-Cu therapy in B16-F10 melanoma allografts. Representative tumor images (j) and growth curves (k) of B16-F10 tumor treated with LF3 in combination with ES-Cu. l Representative flow cytometry plots and quantification analysis of CD8+ T cell populations in different groups. Data were expressed as mean ± SD of ≥ 3 independent experiments. The p-values in panels c, f, h, k, l were calculated by one-way ANOVA.
Consistent with in vitro findings, Ki-67 staining result showed that the combination of LF3 and ES-Cu effectively suppressed tumor cell proliferation (Fig. 6h). Moreover, LF3 significantly inhibited ATP7B expression, validating that Wnt signaling inhibition sensitizes cancer cells to ES-Cu-induced cuproptosis by exacerbating ATP7B pathway activation in vivo.
To determine if cuproptosis is a promising target for amplifying tumor-specific immunity, we performed in vivo experiments using an immunocompetent B16-F10 melanoma model (Fig. 6i). The combination of ES-Cu and LF3 significantly inhibited tumor growth compared to single treatments (Fig. 6j, k). Previous studies show that activated Wnt/β-catenin signaling inhibits dendritic cell infiltration in melanoma, consequently mediating T cell exclusion and a “cold” tumor phenotype [42, 43]. Consistent with this, LF3 alone promoted T cell homing to tumors, while ES-Cu-induced cuproptosis did not significantly increase CD8+ T cell infiltration (Fig. 6l). Since ES-Cu treatment activates Wnt/β-catenin signaling, the specific β-catenin transcriptional program may limit the effect of cuproptosis on inducing T-cell infiltration [39, 44]. However, the ES-Cu and LF3 combination increased CD8+ T cell infiltration in the tumor microenvironment (TME) to a greater extent (Figs. 6l and S15). In summary, we demonstrate that the Wnt/β-catenin pathway is a key regulator of ES-Cu-induced cuproptosis in cancer cells (Fig. 7).
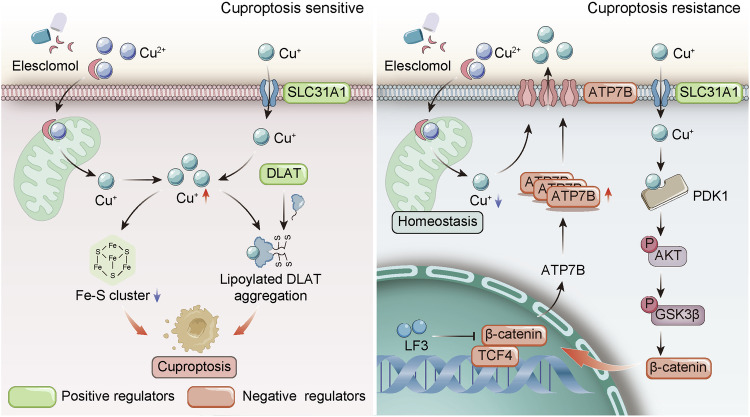
Fig. 7. Model for regulation of cuproptosis sensitivity by Wnt/β-catenin signaling in cancer cells.
Copper directly binds and activates PDK1, stimulating its interaction with AKT to trigger downstream AKT-GSK3β-β-catenin pathway activation and cancer stem cell (CSC) properties. Importantly, aberrant Wnt/β-catenin activation confers CSC resistance to cuproptosis through transcriptional upregulation of the copper efflux transporter ATP7B. Genetic or pharmacological inhibition of Wnt/β-catenin signaling restores cuproptosis sensitivity, enhancing the anticancer efficacy of the copper-binding compound elesclomol.
Discussion
Emerging evidence implies that copper homeostasis plays an essential role in tumor proliferation, metastasis, angiogenesis, and immune evasion [2, 3, 5, 45, 46]. The high-affinity transporter SLC31A1 is responsible for the majority of copper uptake into cells [10]. Knockdown of SLC31A1 inhibits copper-mediated ULK1 activation, suggesting a predominant role of the SLC31A1-copper axis in tumorigenesis [4]. The study revealed that copper transporter SLC31A1 is upregulated in HNSCC tissues and was associated with lymph node metastasis, pathologic stage, and patient survival. Functionally, we identified an important role for the SLC31A1-copper axis in maintaining CSC properties. Copper directly binds to PDK1 and enhances the interaction between PDK1 and AKT under both physiological conditions or copper supplementation, which in turn maintains and activates the downstream AKT-GSK3β-β-catenin pathway, explaining the mechanism by which copper treatment induces activation of the Wnt/β-catenin pathway.
Cuproptosis refers to a mitochondrial cell death pathway that is initiated by an excessive accumulation of copper, which subsequently leads to proteotoxic stress [12, 13, 47]. Elesclomol, a copper ionophore, efficiently binds to copper and facilitates its transport into mitochondria [47, 48]. Within this process, mitochondrial copper directly interacts with lipoylated proteins like DLAT, causing their aggregation [12]. Our findings reveal a notable accumulation of copper primarily within the mitochondrial fraction of CAL27 cells exposed to ES-Cu treatment. In contrast, cells treated with CuCl2 alone retain most of the copper in the cytosolic fraction. This intracellular copper distribution correlates with our observations regarding mtROS generation. Specifically, ES-Cu treatment markedly boosts mtROS production, whereas CuCl2 treatment has minimal effect on mtROS levels. Consistent with our results, previous studies have shown that elesclomol, unlike other chelators such as disulfiram, selectively transports copper to mitochondria and promotes mitochondrial stress [48]. These results suggest that ES-Cu exerts significant mitochondrial stress, whereas CuCl2 does not. These disparities in mitochondrial stress explain why CuCl2, unlike ES-Cu, does not significantly induce cell death. This underscores the distinct mechanisms through which these treatments affect cell viability and modulate signaling pathways linked to copper homeostasis and cellular stress responses [47].
How to induce cuproptosis using high copper ion content in malignant tumors such as HNSCC is a promising strategy for cancer therapy. Our results showed that CSCs are more resistant to cuproptosis compared to parental cells. CSCs exhibit aberrant activation of the Wnt/β-catenin pathway, which facilitates their adaptation to therapeutic perturbations and changing biological stresses in the TME [22, 39, 49, 50]. This phenomenon led us to link the Wnt/β-catenin pathway to the CSC resistance to cuproptosis. LF3 effectively inhibited the interaction between β-catenin and TCF4 without inducing cell death [36]. Our results suggest that LF3-treated CSCs have higher copper levels and lower GSH levels, which favored the occurrence of cuproptosis. Consistent with this, CSCs with TCF4 knockdown exhibited increased sensitivity to cuproptosis. These findings indicate that the activated Wnt/β-catenin pathway modulates copper homeostasis and promotes resistance to cuproptosis in CSCs.
ATP7B primarily operates by transporting copper into the TGN lumen to support copper-dependent enzyme biosynthesis and to enable the efflux of excess copper from the cell by containing it into extracellular vesicles [7]. ATP7B is significantly elevated in platinum-resistant cells and contributes to platinum resistance in cancer cells by promoting platinum pumping, suggesting its role in promoting therapy resistance [51]. More importantly, recent studies have shown that deletion of ATP7B leads to the development of copper overload and cuproptosis in liver tissues of Wilson’s disease mouse models, linking ATP7B-mediated copper homeostasis to Wilson’s disease pathogenesis [12, 52]. Our results show that ATP7B expression is transcriptionally upregulated in CSCs by the β-catenin/TCF4 transcriptional complex, which inhibits the onset of cuproptosis. TCF4 is highly expressed in HNSCC tissues and is closely associated with tumor progression. Our findings propose TCF4 as a plausible biomarker for predicting the sensitivity of cancer cells to cuproptosis, indicating that a combination therapy targeting TCF4 inhibition and cuproptosis induction represents an effective therapeutic strategy for treating cancer patients.
In summary, the present study elucidates how aberrant Wnt/β-catenin activation promotes cuproptosis evasion in cancer cells, especially CSCs. Cuproptosis process is accompanied by robust activation of the Wnt/β-catenin pathway. Mechanistically, copper directly binds PDK1, which activates the downstream AKT-GSK3β-β-catenin pathway and CSC properties. TCF4 deficiency or Wnt signaling inhibition promotes CSC sensitivity to cuproptosis by regulating ATP7B transcriptional activation and copper homeostasis. Overall, our study reveals a potential tumor-promoting role of the Wnt/β-catenin pathway in resisting cuproptosis. The crosstalk between gene transcription and copper metabolism may have broad implications for the regulation of cuproptosis under pathological conditions such as cancer.
Materials and methods
Patient cohorts
Tissue microarrays (TMAs) containing normal oral mucosa, epithelial hyperplasia, and head and neck squamous cell carcinoma (HNSCC) were collected from patients at the Hospital of Stomatology of Wuhan University between 2011 and 2016. The TMAs included 42 cases of normal oral mucosa, 69 cases of epithelial dysplasia, 210 cases of primary HNSCC, and 25 cases of recurrent HNSCC (Tables S1 and S2). Approval for sample collection was granted by the Medical Ethics Committee of the School and Hospital of Stomatology, Wuhan University (2016LUNSHENZI62), following the guidelines of Declaration of Helsinki. All patients provided written informed consent. Tumors were histopathologically classified per World Health Organization criteria.
Cells and reagents
The human HNSCC cell lines (CAL27, FaDu), the human melanoma cell line A375, the mouse melanoma cell line B16-F10, and the human embryonic kidney cell line HEK293 were sourced from the American Type Culture Collection (ATCC) and Chinese Academy of Sciences Cell Bank (Shanghai, China). These cell lines were maintained in DMEM medium (Gibco, Carlsbad, CA, USA) supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum. Additionally, human oral epithelial cells (HIOEC) were cultured in Keratinocyte Growth Medium-Gold (KGM-Gold; Lonza, Switzerland) with the addition of essential growth factors. All cell lines were used at low passages and identity confirmed with short tandem repeat (STR) DNA profiling.
Elesclomol (HY-12040), LF3 (HY-101486), tetrathiomolybdate (TTM, HY-128530), Z-VAD-FMK (HY-16658B), necrostatin-1 (Nec-1, HY-15760), ferrostatin-1 (Fer-1, HY-100579), Wnt3a (HY-P70453B), PDK1 recombinant protein (HY-P701738), and CRIP2 recombinant protein (HY-P76290) were obtained from MedChemExpress (MCE, NJ, USA).
Immunohistochemistry (IHC) and immunofluorescence
Formalin-fixed, paraffin-embedded specimens were evaluated by H&E staining. IHC was performed using antibodies against Ki67 (#9129, CST), β-catenin (#8480, CST), p-GSK3β (T40070, Abmart), SLC31A1 (T510261, Abmart), TCF4 (ab217668, Abcam), and ATP7B (T58616, Abmart). Signals were detected using enzyme-labeled secondary antibodies and DAB regents, with hematoxylin counterstaining. Slides were scanned using a Pannoramic Midi scanner (3DHISTECH) and analyzed by QuantCenter software (3DHISTECH). The histoscore was normalized to 0–300.
For immunofluorescence, cells grown on confocal dishes were subjected to fixation using paraformaldehyde, permeabilizion with Triton X-100, and subsequent blocking with goat serum. Primary antibodies β-catenin (#8480, CST) or DLAT (#12362, CST) were applied to cells, followed by the appropriate fluorescent secondary antibodies (Abbkine). Nuclei were counterstained with DAPI (Beyotime, Shanghai, China). Utilizing a confocal microscope (FV1200, OLYMPUS), images were captured.
Western blotting (WB) and PCR
Tumor cells or tissues were lysed in RIPA lysis buffer containing phosphatase and protease inhibitors (Beyotime), and protein concentrations were quantified using a BCA Protein Assay Kit (Beyotime). For immunoprecipitation, cell lysates were incubated with target-specific primary antibodies followed by protein A/G sepharose beads (HY-K0202, MCE) overnight. The immunocomplexes were washed thoroughly with RIPA lysis buffer to remove nonspecifically bound proteins. Equal protein amounts were separated by SDS-PAGE and analyzed by WB using antibodies against DLAT(#12362, CST), β-catenin (#8480, CST), Histone H3 (#4499, CST), ALDH1A1 (#36671, CST), Lipoic Acid (ab58724, Abcam), TCF4 (ab217668, Abcam), GSK3β (ab32391, Abcam), FDX1 (T510671, Abmart), HSP70 (M20033, Abmart), AKT (T55561, Abmart), p-AKT (Thr308, T40068, Abmart), p-GSK3β (Ser9, T40070, Abmart), PDK1 (T55834, Abmart), SLC31A1 (T510261, Abmart), ATP7B (T58616, Abmart), HA-Tag (M20003, Abmart), Myc-Tag (M20002, Abmart), with β-actin (Proteintech) as loading control. Blots were imaged on an Odyssey Imager (LI-COR Biosciences). All original images are provided in the supplementary data file.
Total RNA was extracted using an RNA isolation kit (Axygen, NY, USA) and reverse transcribed (Vazyme, Nanjing, China). Quantitative PCR was performed on a Bio-Rad CFX96 system. Gene expression was calculated from Ct values. Primers were synthesized by Sangon Biotech (Shanghai, China, Table S3).
Cell viability and sphere formation assay
CAL27, FaDu, and A375 cells were seeded overnight in 96-well plates (5 × 103 cells/well). Cells were treated with ES-Cu and/or other reagents for 24 h. Viability was assessed by CCK-8 kit (Beyotime). The absorbance (OD) was measured at 450 nm using a BioTek plate reader (BioTek).
For sphere formation, cells were cultured in ultra-low adherence plates (103 cells/well for sphere formation and 104 cells/well for passing) in serum-free DMEM/F12 with 1% N2, 2% B27, 20 ng/mL EGF, and 20 ng/mL bFGF. Spheres were filtered through 70 μm cell filters, dissociated with trypsin, and re-cultured for three rounds to obtain cancer stem cells (CSCs).
Plasmids and transfection
CRISPR-Cas9 was performed following the established protocol as previously described [53]. Lentiviruses carrying SLC31A1 or TCF4 single guide RNAs (sgRNAs) were from Ubigene Biosciences (Guangzhou, China). Knockout lines were generated by infection and puromycin (5 μg/ml) selection. Cells were maintained in culture medium containing puromycin. The specific targeting sequences were listed in Table S4. TCF4 or ATP7B complementary DNAs (cDNAs) in pcDNA3.1 vectors (Tsingke, Beijing, China) were overexpressed by Lipofectamine 3000 transfection (Invitrogen, Carlsbad, CA, USA). pcDNA3-Myc-PDK1 and pcDNA3-HA-AKT1 were obtained from Tsingke Biotechnology Co., Ltd. Various PDK1 mutants were produced utilizing the QuikChange XL Site-Directed Mutagenesis Kit (Agilent, Stockport, UK). Transfection efficiency was validated by qPCR and WB.
Flow cytometry
Cell death was detected using an Annexin V/Propidium Iodide (PI) Apoptosis kit (Multi Sciences, Hangzhou, China). Cells were digested without EDTA, washed, and stained with annexin V-FITC and PI. PI+ cells were quantified as dead cells. Aldehyde dehydrogenase (ALDH) enzyme activity was detected by ALDEFLUORTM assay (Stemcell Technologies, MA, USA) with diethylaminobenzaldehyde (DEAB) as negative control. MitoSOXTM indicator (Invitrogen) detects superoxide produced by mitochondria and is used to quantify mtROS production. The mtROS in sphere cells were measured with MitoSOX in accordance with the guidelines provided by the manufacturer.
Tumor infiltrating lymphocytes were identified by flow cytometry with antibodies against CD45 (APC/Cy7, eBiosciences), CD3 (FITC, eBiosciences), CD8 (PerCP/Cy5.5, eBiosciences), and Fixable Viability Dye. Data were acquired on a Beckman Coulter cytometer and analyzed by FlowJo software. Gating strategy is in Supplementary Fig. 12.
Glutathione (GSH) measurement
For GSH measurement, cells were seeded overnight in 12-well plates (2 × 105 cells/well). GSH and oxidized glutathione (GSSG) levels were calculated using the GSH/GSSG assay kit (Beyotime) by measuring absorbance (OD) at 412 nm according to the instructions.
Subcellular fractionation and isolation of mitochondria
The tumor cells were lysed using a hypotonic buffer (pH 7.0) consisting of 10 mM HEPES and 0.05% digitonin. This mild lysis permitted the disruption of the cell membrane, thereby releasing the cytoplasmic contents. To reinstate isotonic conditions, we adjusted the lysate by adding sucrose to achieve a final concentration of 0.5 M. Afterward, a centrifugation step at 12,000 × g for 10 min was performed, allowing us to harvest the supernatant enriched in cytosolic fractions.
Mitochondria were isolated from the tumor cells using a Mitochondria Isolation Kit (Thermofisher, Waltham, MA, USA), following the manufacturer’s instructions. Briefly, 2 × 107 cells were pelleted, lysed with Isolation Reagent A, vortexed, incubated on ice, and homogenized. Differential centrifugation was employed to separate mitochondrial components, removing unbroken cells and collecting the mitochondrial pellet. The mitochondrial pellet was washed and purified. Finally, the protein concentration in the cytosolic and mitochondrial fractions was determined using the BCA method.
Inductively coupled plasma-mass spectrometry (ICP-MS)
ICP-MS was utilized to assess intracellular copper distribution. Following the treatment with copper, cancer cells were harvested, rinsed with PBS, and their number quantified via an automatic cell counter. Copper content was determined by ICP-MS on an Aglient 7800 instrument with the assistance of Shiyanjia lab (www.shiyanjia.com). Cellular uptake results were corrected by protein levels.
In vitro Cu-binding experiments
Size exclusion chromatography was utilized to accurately remove unbound Cu(I) from the Cu-protein complex [28, 31]. This process was facilitated by a Superdex Increase 75 10/300 analytical column integrated into an AKTA purification system maintained at 4 °C. Before use, the column was equilibrated with a buffer comprising 50 mM HEPES, 200 mM NaCl, and 1 mM GSH, adjusted to a pH of 7.4. To generate Cu-bound proteins, apo-PDK1 and apo-CRIP2 were incubated with ten equivalents of Cu(I) for 60 min. Following incubation, a desalting process was performed at 4 °C to efficiently remove any unbound Cu(I). This separation ensured the elimination of unbound copper ions. Post-desalting, ICP-MS was used to quantify copper levels in PDK1. Prior to ICP-MS analysis, samples underwent digestion with 50% HNO3 at 65 °C to ensure accurate measurement by completely liberating bound copper ions.
BCS competition assay
The average Cu(I) affinity of PDK1 was determined using a Bathocuproinedisulfonic acid (BCS) competition assay [30, 31]. BCS forms a highly stable and easily detectable complex with Cu(I), characterized by an affinity constant (β2) of 1019.8 and a molar extinction coefficient of 13000 M−1 cm−1 at 483 nm. To prevent oxidation, the BCS stock solution was meticulously prepared under argon and mixed with Cu(I) solutions in specific molar ratios of 1:9.1 or 1:11.1. The mixture was then diluted using a buffer composed of 50 mM HEPES, 200 mM NaCl, and 1 mM GSH at pH 7.4, which had previously been degassed under argon. For the PDK1 titration experiments, the concentrations were standardized to 22 µM Cu(I) and 200 µM BCS, whereas for the CRIP2 titration experiments, they were adjusted to 18 µM Cu(I) and 200 µM BCS.
Absorbance measurements were executed using a Nanodrop spectrometer (Thermofisher), scanning between 400 and 650 nm at 25 °C. Prior to measurements, the solutions were incubated for 1 min to facilitate equilibration, ensuring precise detection of competitive binding occurrences. Baseline adjustments were performed with argon-degassed buffer. The recombinant PDK1 protein (HY-P701738) and recombinant CRIP2 protein (HY-P76290) were procured from MCE. To evaluate the Cu(I) binding affinities to apo-PDK1 or apo-CRIP2, Cu(BCS)23- solution was titrated against the corresponding proteins.
Luciferase reporter gene assay
CAL27 and A375 cells were cotransfected with firefly luciferase reporter plasmids containing WT or mutant ATP7B promoter regions along with pRL-TK control plasmid (GenePharma, Shanghai, China) using Lipofectamine 3000 (Invitrogen). Luciferase activity was measured by dual luciferase assay (Promega, Madison, WI, USA) and determined as the ratio of firefly luciferase to Renilla luciferase activity.
High-throughput CUT&Tag
CUT&Tag was performed as previously described by Jiayin Biotechnology Ltd. (Shanghai, China) [54]. Purified nuclei were incubated with concanavalin A-coated magnetic beads and TCF4 antibody or IgG overnight. After secondary antibody, samples were tagged with Tn5 transposase and sequenced. Libraries were sequenced on an Illumina NovaSeq 6000. Data are available on Gene expression Omnibus (GEO, GSE248246).
Chromatin immunoprecipitation (ChIP)
ChIP was performed using the ChIP-IT kit (Active Motif, Carlsbad, CA, USA). Cells were crosslinked, lysed, and sonicated. Chromatin was immunoprecipitated with TCF4 (ab217668, Abcam), β-catenin (#8480, CST), or IgG antibodies, and protein-DNA complexes isolated using protein A/G beads. Purified DNA was analyzed by qPCR with indicated primers (Table S3).
RNA sequencing and bioinformatics analysis
Total RNA was extracted with TRIzol (Invitrogen) and treated with DNase I. Libraries were prepared and sequenced by BGI Genomics (Shenzhen, China). The next-generation sequencing experiments were run on an MGISEQ-2000 sequencer. Data are available on GSE248083 and GSE248084. Gene Set Enrichment Analysis (GSEA) was done on logFC-ranked genes. DAVID tool was used to conduct Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. Public data were obtained from GEO (GSE41613, GSE5348) and TCGA database. Hallmark gene sets (h.all.v7.5.1.symbols. gmt) were from the Molecular Signatures Database (MSigDB). Enrichment scores were calculated as previously described by ssGSEA in R 3.6.3 software [49, 55].
Animals
All animal experiments were carried out with the approval of the center for Animal Experiment of Wuhan University (WP20230467) and performed according to institutional guidelines. 6-8 weeks old female BALB/c nude and C57BL/6 mice were purchased from Hubei Provincial Academy of Preventive Medicine (Wuhan, China). For CAL27 HNSCC xenograft models, CAL27 cells (4 × 106) resuspended in PBS/Matrigel (100 μl, 1:1) were injected subcutaneously into BALB/c nude mice. For B16-F10 melanoma syngeneic models, B16-F10 cells (0.5 × 106) resuspended in DMEM (100 μl, serum-free) were injected subcutaneously into C57BL/6 mice. Mice were randomly grouped after tumor formation (six mice per group) and treated with intratumoral ES-Cu (10 mg/kg) and/or intraperitoneal LF3 (50 mg/kg). Tumor size was measured using calipers and its volume was estimated using the following formula: (length × width2)/2. Mice were euthanized at the end of treatment, and tumors were excised and preserved. The collection and analysis of tumor samples were strictly conducted in accordance with the double-blind principle to ensure the objectivity and reliability of the experimental results. No sample or animal was excluded.
Statistics
Statistical tests were performed with GraphPad Prism 8.0 and R 3.6.3 software. Data are represented as mean ± SD of ≥3 independent experiments. Two-tailed Student’s t-test (2 groups), one-way ANOVA and Tukey’s multiple comparison test (>2 groups) were used to estimate the statistical significance. χ2 test was used to analyze categorical variables. Kaplan–Meier curves were used for survival analysis. Correlation analysis was performed using Spearman correlation analysis. Statistically significance was considered as p-value < 0.05.
Supplementary information
Author contributions
Y.T. Liu: Data curation, conceptualization, methodology, formal analysis, investigation, writing-original draft. L. Chen: Data curation, methodology, formal analysis, writing-original draft. S.J. Li: Data curation, methodology. W.Y. Wang: Investigation, methodology. Y.Y. Wang: Data curation. Q.C. Yang: Data curation. A. Song: Methodology. M.J. Zhang: Formal analysis. W.T. Mo: Formal analysis. H. Li: Supervision. C.Y. Hu: Writing-review and editing, funding acquisition. Z.J. Sun: Conceptualization, supervision, funding acquisition, writing-original draft, project administration, resources, writing-review and editing.
Funding
This work was financially supported by National Natural Science Foundation of China 82273202, 82072996, 82002893, 82103670, National Key Research and Development Program 2022YFC2504200, Interdisciplinary innovative foundation of Wuhan University XNJC202303, and the Fundamental Research Funds for the Central Universities (2042022dx0003).
Data availability
RNA-seq and CUT&Tag data have been deposited in GEO under the following accession numbers: GSE248083, GSE248084, and GSE248246.
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Approval for human tissue samples utilization was granted by the Medical Ethics Committee of the School and Hospital of Stomatology, Wuhan University (2016LUNSHENZI62), following the guidelines of Declaration of Helsinki. All patients provided written informed consent. All animal experiments were carried out with the approval of the center for Animal Experiment of Wuhan University (WP20230467) and performed according to institutional guidelines.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yuan-Tong Liu, Lei Chen.
Contributor Information
Chuan-Yu Hu, Email: chuanyuhu@hust.edu.cn.
Zhi-Jun Sun, Email: sunzj@whu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-024-01341-2.
References
- 1.Ge E, Bush A, Casini A, Cobine P, Cross J, DeNicola G, et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer. 2022;22:102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature. 2014;509:492–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das A, Ash D, Fouda A, Sudhahar V, Kim Y, Hou Y, et al. Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis. Nat Cell Biol. 2022;24:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsang T, Posimo J, Gudiel A, Cicchini M, Feldser D, Brady D. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat Cell Biol. 2020;22:412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramchandani D, Berisa M, Tavarez D, Li Z, Miele M, Bai Y, et al. Copper depletion modulates mitochondrial oxidative phosphorylation to impair triple negative breast cancer metastasis. Nat Commun. 2021;12:7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Mo W, Hang Z, Huang Y, Yi H, Sun Z, et al. Cuproptosis: harnessing transition metal for cancer therapy. ACS Nano. 2023;17:19581–99. [DOI] [PubMed] [Google Scholar]
- 8.Xie J, Yang Y, Gao Y, He J. Cuproptosis: mechanisms and links with cancers. Mol Cancer. 2023;22:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohgami R, Campagna D, McDonald A, Fleming M. The Steap proteins are metalloreductases. Blood. 2006;108:1388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su Y, Zhang X, Li S, Xie W, Guo J. Emerging roles of the copper-CTR1 axis in tumorigenesis. Mol Cancer Res. 2022;20:1339–53. [DOI] [PubMed] [Google Scholar]
- 11.La Fontaine S, Mercer J. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch Biochem Biophys. 2007;463:149–67. [DOI] [PubMed] [Google Scholar]
- 12.Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahlson M, Dixon S. Copper-induced cell death. Science. 2022;375:1231–32. [DOI] [PubMed] [Google Scholar]
- 14.Xiong C, Ling H, Hao Q, Zhou X. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ. 2023;30:876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz V, Basu S, Freibert S-A, Webert H, Boss L, Mühlenhoff U, et al. Functional spectrum and specificity of mitochondrial ferredoxins FDX1 and FDX2. Nat Chem Biol. 2023;19:206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, Zhang Y, Yang B, Sun S, Zhang P, Luo Z, et al. Lactylation of METTL16 promotes cuproptosis via mA-modification on FDX1 mRNA in gastric cancer. Nat Commun. 2023;14:6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cobine P, Brady D. Cuproptosis: cellular and molecular mechanisms underlying copper-induced cell death. Mol Cell. 2022;82:1786–87. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X, Ke J, Jia L, An X, Ma H, Li Z, et al. A novel cuproptosis-related gene signature of prognosis and immune microenvironment in head and neck squamous cell carcinoma cancer. J Cancer Res Clin Oncol. 2023;149:203–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–99. [DOI] [PubMed] [Google Scholar]
- 20.Bugter J, Fenderico N, Maurice M. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer. 2021;21:5–21. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Zheng L, Shang W, Yang Z, Li T, Liu F, et al. Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ. 2022;29:2190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. [DOI] [PubMed] [Google Scholar]
- 24.Zou Y, Zheng S, Xie X, Ye F, Hu X, Tian Z, et al. N6-methyladenosine regulated FGFR4 attenuates ferroptotic cell death in recalcitrant HER2-positive breast cancer. Nat Commun. 2022;13:2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huster D, Purnat TD, Burkhead JL, Ralle M, Fiehn O, Stuckert F, et al. High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J Biol Chem. 2007;282:8343–55. [DOI] [PubMed] [Google Scholar]
- 26.Voli F, Valli E, Lerra L, Kimpton K, Saletta F, Giorgi F, et al. Intratumoral copper Modulates PD-L1 expression and influences tumor immune evasion. Cancer Res. 2020;80:4129–44. [DOI] [PubMed] [Google Scholar]
- 27.Guo J, Cheng J, Zheng N, Zhang X, Dai X, Zhang L, et al. Copper promotes tumorigenesis by activating the PDK1-AKT oncogenic pathway in a copper transporter 1 dependent manner. Adv Sci. 2021;8:e2004303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J, Liu X, Li X, Li H, Shi L, Xia X, et al. Copper regulates the host innate immune response against bacterial infection via activation of ALPK1 kinase. Proc Natl Acad Sci USA. 2024;121:e2311630121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zulkifli M, Spelbring AN, Zhang Y, Soma S, Chen S, Li L, et al. FDX1-dependent and independent mechanisms of elesclomol-mediated intracellular copper delivery. Proc Natl Acad Sci USA. 2023;120:e2216722120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Z, Brose J, Schimo S, Ackland SM, La Fontaine S, Wedd AG. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: detection probes and affinity standards. J Biol Chem. 2011;286:11047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Li N, Zhang M, Sun M, Bian J, Yang B, et al. APEX2-based proximity labeling of Atox1 identifies CRIP2 as a nuclear copper-binding protein that regulates autophagy activation. Angew Chem Int Ed. 2021;60:25346–55. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Wu VH, Allevato MM, Gilardi M, He Y, Luis Callejas-Valera J, et al. Syngeneic animal models of tobacco-associated oral cancer reveal the activity of in situ anti-CTLA-4. Nat Commun. 2019;10:5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen D, Wang C. Targeting cancer stem cells in squamous cell carcinoma. Precis Clin Med. 2019;2:152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Zhao H, Liang W, Jiang E, Zhou X, Shao Z, et al. Autophagy regulates the cancer stem cell phenotype of head and neck squamous cell carcinoma through the noncanonical FOXO3/SOX2 axis. Oncogene. 2022;41:634–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun S, Liu S, Duan SZ, Zhang L, Zhou H, Hu Y, et al. Targeting the c-Met/FZD8 signaling axis eliminates patient-derived cancer stem-like cells in head and neck squamous carcinomas. Cancer Res. 2014;74:7546–59. [DOI] [PubMed] [Google Scholar]
- 36.Fang L, Zhu Q, Neuenschwander M, Specker E, Wulf-Goldenberg A, Weis W, et al. A small-molecule antagonist of the β-catenin/TCF4 interaction blocks the self-renewal of cancer stem cells and suppresses tumorigenesis. Cancer Res. 2016;76:891–901. [DOI] [PubMed] [Google Scholar]
- 37.Guo B, Yang F, Zhang L, Zhao Q, Wang W, Yin L, et al. Cuproptosis induced by ROS responsive nanoparticles with elesclomol and copper combined with αPD-L1 for enhanced cancer immunotherapy. Adv Mater. 2023;35:e2212267. [DOI] [PubMed] [Google Scholar]
- 38.Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, Palumaa P. Affinity gradients drive copper to cellular destinations. Nature. 2010;465:645–48. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Jensen R. Understanding and overcoming immunosuppression shaped by cancer stem cells. Cancer Res. 2023;83:2096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellano M, Pollock P, Walters M, Sparrow L, Down L, Gabrielli B, et al. CDKN2A/p16 is inactivated in most melanoma cell lines. Cancer Res. 1997;57:4868–75. [PubMed] [Google Scholar]
- 41.Dok R, Kalev P, Van Limbergen E, Asbagh L, Vázquez I, Hauben E, et al. p16INK4a impairs homologous recombination-mediated DNA repair in human papillomavirus-positive head and neck tumors. Cancer Res. 2014;74:1739–51. [DOI] [PubMed] [Google Scholar]
- 42.Spranger S, Bao R, Gajewski T. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–5. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Sun Z. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11:5365–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeuchi Y, Tanegashima T, Sato E, Irie T, Sai A, Itahashi K, et al. Highly immunogenic cancer cells require activation of the WNT pathway for immunological escape. Sci Immunol. 2021;6:eabc6424. [DOI] [PubMed] [Google Scholar]
- 45.Solier S, Müller S, Cañeque T, Versini A, Mansart A, Sindikubwabo F, et al. A druggable copper-signalling pathway that drives inflammation. Nature. 2023;617:386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pezacki AT, Matier CD, Gu X, Kummelstedt E, Bond SE, Torrente L, et al. Oxidation state-specific fluorescent copper sensors reveal oncogene-driven redox changes that regulate labile copper(II) pools. Proc Natl Acad Sci USA. 2022;119:e2202736119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang D, Kroemer G, Kang R. Targeting cuproplasia and cuproptosis in cancer. Nat Rev Clin Oncol. 2024;21:370–88. [DOI] [PubMed] [Google Scholar]
- 48.Nagai M, Vo NH, Shin Ogawa L, Chimmanamada D, Inoue T, Chu J, et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radic Biol Med. 2012;52:2142–50. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Li S, Wang S, Yang Q, Wu Z, Zhang M, et al. LIMP-2 enhances cancer stem-like cell properties by promoting autophagy-induced GSK3β degradation in head and neck squamous cell carcinoma. Int J Oral Sci. 2023;15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L, Yang Q, Li Y, Yang L, Liu J, Li H, et al. Targeting CMTM6 suppresses stem cell-like properties and enhances antitumor immunity in head and neck squamous cell carcinoma. Cancer Immunol Res. 2020;8:179–91. [DOI] [PubMed] [Google Scholar]
- 51.Arnesano F, Natile G. Interference between copper transport systems and platinum drugs. Semin Cancer Biol. 2021;76:173–88. [DOI] [PubMed] [Google Scholar]
- 52.Członkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, et al. Wilson disease. Nat Rev Dis Primers. 2018;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang S, Wu Z, Zhu S, Wan S, Zhang M, Zhang B, et al. CTLA-4 blockade induces tumor pyroptosis via CD8 T cells in head and neck squamous cell carcinoma. Mol Ther. 2023;31:2154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaya-Okur H, Wu S, Codomo C, Pledger E, Bryson T, Henikoff J, et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat Commun. 2019;10:1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barbie D, Tamayo P, Boehm J, Kim S, Moody S, Dunn I, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq and CUT&Tag data have been deposited in GEO under the following accession numbers: GSE248083, GSE248084, and GSE248246.