Abstract
Background
Hemifacial spasm (HFS) is a neuromuscular disorder characterized by unilateral facial muscle spasms, negatively impacts quality of life due to social embarrassment. Botulinum Neurotoxin (BoNT) injections have emerged as a viable therapeutic approach. This systematic review evaluated the efficacy and safety of BoNT injections for HFS management, along with effects on patients’ quality of life and mental health.
Materials and methods
A systematic search for studies on BoNT treatment for HFS published between January 1, 2000, and May 1, 2024, was performed across major databases. Study quality was evaluated using Cochrane and Joanna Briggs Institute (JBI) tools, with data management handled by EndNote X9 and statistical analyses conducted via Review Manager (RevMan 5.4) and STATA 14.0.
Results
Thirty-five studies met the inclusion criteria: 2 RCTs comprising 83 HFS patients compared the efficacy of perioral injections of botulinum toxin and placebo, while 33 single-arm studies reported outcomes for 2786 patients post-BoNT injection. The selection of 17 single-arm studies focused on the effectiveness rate as the key outcome metric. Pooled estimate signified a remarkably high effectiveness (ES: 0.882, 95% CI: 0.830, 0.926, P < 0.001). Analysis of depression scale (SMD: -0.85, 95% CI: -1.34, -0.35, P < 0.001), anxiety scale (SMD: -1.50, 95% CI: -2.19, -0.80, P < 0.001) and total scale of quality of life (SMD: -0.64, 95% CI: -0.87, -0.41, P = 0.766) showed that BoNT therapy worked well especially in improving mental state and quality of life. Ptosis was considered as the most common adverse reaction during BoNT injections (OR: 0.30, 95% CI: 0.11, 0.81, P = 0.843).
Conclusion
BoNT injection showed validity and clinical safety in the treatment of HFS, particular for depression relief. Injections around the mouth were only effective for HFS cases with severe symptoms. A standardized strategy for BoNT injections in managing HFS, detailing parameters such as injection sites, doses, and frequencies, remained elusive. Additional RCTs are necessary to further elucidate the interplay between efficacy and these components.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-024-03883-x.
Keywords: Hemifacial spasm, Botulinum neurotoxin, Depression, Ptosis, Meta-analysis
Background
Hemifacial spasm (HFS) is a hyperkinetic movement disorder characterized by short or persistent, intermittent synchronous twitching of the muscles innervated by the facial nerve [1]. The progression of the condition typically initiates around the eyes before advancing inferiorly towards the cheek, mouth, and neck [2]. Arterial tortuosity of the posterior circulation compressing a facial nerve is the most common cause, which induces the ephaptic axono-axonal cross-talk [3]. According to the pathogenesis, HFS can be categorized into two main categories: primary and secondary. Primary HFS is mainly caused by the compression of peripheral blood vessels (anterior inferior cerebellar, posterior inferior cerebellar and vertebral motor, etc.) on the facial nerve at the brain stem. Secondary HFS often occurs after facial paralysis, tumor and trauma [4]. Primary HFS is more common than secondary one, while it is difficult to distinguish them clinically [5]. In this meta-analysis, most included studies were performed on primary type.
The primary method for diagnosing HFS is clinical recognition [6]. Electromyogram (EMG), magnetic resonance imaging (MRI) and computerized tomography (CT) are additional ways [3, 7, 8]. These diagnostic techniques aid in distinguishing HFS from other craniofacial dyskinesias such as blepharospasm, tic disorders, myokymia, and synkinesis [9]. The prevalence of HFS ranges from 5 to 13 per 100,000 individuals with an average age of approximately 40 years old, more common in women with a ratio of 1.5: 1 [4, 9, 10]. Notably, up to 90% of patients complained that HFS severely interfered their social lives. Patients with HFS experienced a poor quality of life due to social embarrassment, which can lead to social isolation, depression, even functional blindness [2].
Medications, botulinum toxin injections, neurosurgical procedures, and, most recently, doxorubicin chemomyectomy, have been employed to mitigate muscular twitches in HFS [11]. Among these, microvascular decompression (MVD) surgery is regarded as the standard treatment due to its superior efficacy in alleviating symptoms. This surgical technique involves relieving pressure on the facial nerve caused by adjacent blood vessels. Despite its benefits, MVD presents significant challenges. Achieving complete decompression without damaging the intricate web of nerves and blood vessels in the brainstem is technically demanding. Moreover, the invasive nature of the surgery itself carries inherent risks, including potential injury to the brainstem [12, 13]. In contrast, Botulinum Neurotoxin (BoNT) therapy offers a less invasive alternative that has been widely adopted since the early 1980s [11]. BoNT injections effectively manage facial spasticity associated with HFS, circumventing the risks associated with surgery. Supported by Level 1 evidence, BoNT is also indicated for the treatment of cervical dystonia, blepharospasm, chronic migraine, spasmodic dysphonia, temporomandibular joint disorders, and other conditions [14]. This potent exotoxin, produced by the bacterium Clostridium botulinum, selectively targets the neuromuscular junction, where it inhibits the release of acetylcholine, a neurotransmitter essential for muscle contraction [15].
To date, seven immunologically distinct serotypes of BoNT have been identified, labelled from A through G. Of these, BoNT type A is the most prevalently used form in clinical practice, with several commercial preparations available for use. The precise clinical outcomes and safety profile of BoNT in the context of HFS remain subjects of ongoing investigation. This ambiguity underscores the critical need for a thorough systematic review coupled with a meta-analysis to synthesize the existing research on BoNT’s application for HFS.
Method
Search strategy
This study has been submitted at PROSPERO (CRD42024568920). The article was performed according to the PRISMA guideline [16] (Additional file 1). We searched PubMed, Web of Science, Embase and Cochrane for articles published between January 1, 2000 and May 1, 2024. Searching terms are the subject headings “Hemifacial Spasm” and “Botulinum Toxins”, as well as their Medical Subject Headings (MeSH) in National Center for Biotechnology Information (NCBI) website. Two subject terms are connected by AND, and between subject terms and free terms by OR. The complete search strategy was included in Additional file 2.
Inclusion criteria
The authors extracted key characteristics of the included articles following the “PICOS principle”. Two reviewers performed a purposive screening based on the title, abstract, and full text of the article according to the inclusion criteria. The retrieved records from all databases were imported into Endnote software (version X9; Thomson Scientific), where duplicate entries were eliminated. We additionally acquired the full tests of the target literatures. Discussions and summaries were conducted in a timely manner during the screening process to ensure consistency of results. In case of disagreement between the two reviewers, it was resolved by communicating with the third reviewer.
Studies
Randomized controlled trials (RCTs) and single-arm studies of BoNT for HFS.
Patients
Patients diagnosed with HFS were included (The diagnosis of HFS was established based on clinical evidence of involuntary clonic and/or tonic contractions in the muscles innervated by the facial nerve, which were unilateral in nature) [17].
Interventions
The experimental cohort comprised HFS patients who underwent BoNT therapy, specifically targeting the periorbital and perioral regions. In the context of RCTs, the control group included HFS patients who were administered a placebo injection in analogous sites around the mouth. And for the single-arm studies, the control group was inherently designed within each participant. Individuals acted as their own controls by meticulously comparing their clinical condition pre-treatment with that post-treatment.
Outcomes
Efficacy outcomes
The primary outcomes included severity scores, global clinical improvement (GCI), visual analog scale (VAS), percentage of improvement and duration of improvement. In addition, the evaluation indicators of patients’ quality of life included the Beck Depression Inventory (BDI), Jankovic Rating Scale (JRS), behavioural and psychological symptoms assessment, etc.
Additional outcomes
Age and sex of the patient, duration of facial spasms, type and dose of botulinum toxin, postoperative adverse reaction.
Exclusion criteria
We excluded studies for the following reasons: (1) Treatment of diseases as facial syndrome, Meige’s Syndrome or blepharospasm (BS); (2) Reviews, case studies, response letters, notes and conference proceedings; (3) Full texts were not available for retrieval; (4) Articles contained incomplete data or serious errors; (5) Languages were not English.
Data extraction
Two reviewers independently completed the data extraction of the included literatures. The extracted data included basic information (authors, year of publication, type of study, intervention, etc.), patient information (age, gender, mean duration of disease, dose of injected medicine, etc.) and outcomes (duration of effect, patients’ preference, frequency of side effects, etc.). The acquired data were summarized in Microsoft Excel (version 2019) tables for subsequent statistical analysis.
Quality assessment
To assess the risk of bias of RCTs, Cochrane Collaboration Risk of Bias Tool (RevMan version 5.4; the Cochrane Collaboration) was used [18]. The Critical Appraisal tools developed in Joanna Briggs Institute (JBI) was used to evaluate the quality of the one-arm experiment [19]. Each item consists of one question, with a total of ten questions. The answers to each item were “yes”, “no”, “unclear” or “not applicable”. A score of more than 7 is considered to be of high quality and low risk.
Data analysis
Review Manager software (RevMan 5.4) and STATA software (version 14.0; Stata Corporation) were used to conduct statistical and quantitative analysis of the data of binary and continuous variables of the included literature. The subgroup analysis was conducted based on various clinical characteristics of patients or different interventions.
The odds value (OR), and 95%CI were used as the bicategorical variables, and weighted mean difference (WMD), standardized mean difference (SMD) and 95%CI were used as the continuity variables. I2 statistics were employed to assess the heterogeneity of study outcomes [20]. When I2 ≤ 25% and P ≥ 0.10, it was considered that there was no heterogeneity, and a fixed effect model was selected for analysis; If I2 > 25% and P < 0.10, there was heterogeneity, and the random effects model was selected for analysis. To determine the response rate of BoNT treatment, we used STATA 14.0 command ‘metaprop’ to generate a combined estimate along with an exact binomial and fraction-test-based confidence interval (CI) [formulas: metaprop case n,random ftt cimethod(excat) lcols(Study) xlab(0.1,0.2,0.3,0.4,0.5,0.6,0.7,0.8) dp(3)]. The variance was stabilized by employing the Freeman-Tukey double arcsine transform, which effectively grouped rates close to the margin together [21, 22]. Subgroup analysis and meta-regression were used to explore the sources of heterogeneity. Sensitivity analysis was employed to evaluate the robustness of the findings and identify potential impacts. The Egger’s linear regression test was employed to evaluate the presence of publication bias in the included trials, with statistical significance defined as P < 0.05.
Results
Study selection and subject characteristics
A total of 2306 articles were retrieved from the initial 4 databases screen and 1238 remained after removal of duplicates. With the selection function of Endnote software (version X9; Thomson Scientific), 227 reviews, 210 conference proceedings, 75 case reports, letters or notes, and 88 non-English literatures were excluded. The remaining 638 records were further screened by reading the titles and abstracts, 62 full-text articles were assessed for eligibility. Finally, 35 records [10, 17, 23–55] were included in this meta-analysis, including 2 RCTs [23, 24] and 33 single-arm studies [10, 17, 25–55] (Fig. 1). After then, we summarized the characteristics and clinical features of the study population (Tables 1, 2 and Additional file 3). The 33 single-arm studies comprised of 29 retrospective investigations and 4 prospective analyses. Of particular interest, 7 studies specifically focused on the effects of BoNT injections on patients’ quality of life (Table 2).
Fig. 1.
PRISMA flowchart of the literature search and study selection
Table 1.
Characteristics of the RCTs (mean ± SD)
| Study | Participants (M/F) | Age (years) | Disease duration (years) | Intervention | Type of BoNT | Dosage (U/site) | Duration of improvement (weeks) | Follow-up | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Colakoglu et al., 2011 [23] | 23 (9/14) | 61.95 ± 11.73 | 9.26 ± 4.91 |
Intervention group: BoNT injection into both orbicularis oculi and perioral muscles; Control group: BoNT into orbicularis oculi and placebo into perioral muscles Then crossed over |
Botulinum toxin Type A (BOTOX®) |
Depending on the severity Perioral: 16.86 ± 3.89; Orbicularis oculi: 6 ± 1.68 |
NR | NR | Severity scores, VAS |
| Jitpimolmard et al., 2022 [24] |
A: 30 (11/19) B: 30 (11/19) |
A: 49.7 ± 13.2 B: 51.3 ± 11.7 |
A: 5.0 ± 5.7 B: 3.1 ± 3.1 |
Intervention group: BoNT injection into both orbicularis oculi and perioral muscles; Control group: BoNT into orbicularis oculi and placebo into perioral muscles |
Botulinum toxin A (Botox, Allergan, Irvine, CA, USA) | 15 |
A:22.97(18.85) B:17.53(14.9) |
18 months | Frequency of spasms/5 min, VAS, percentage of improvement; duration of improvement |
M male, F female, U unit, NR not report, VAS visual analog scale (0 = no spasm, 5 = moderate spasm, 10 = severe spas)
Table 2.
Characteristics of the single-arm studies (mean ± SD)
| Study | Study design | Region | Participant (M/F) | Age (years) | Disease duration (years) | Injection site | Type of BoNT | Dose (U) | Dosing interval (months) / Dose frequency | Follow-up duration |
|---|---|---|---|---|---|---|---|---|---|---|
| Rollnik et al., 2000 [25] | R | Germany | 21 (7/14) | 59.0 ± 12.9 | NR | Orbicularis oculi muscle | ② | (24 ± 6.2) *10–3 | 5 injections | 17 months |
| Defazio et al., 2002 [26] | R | Italian | 65 (19/46) | 50.0 ± 11.3 | NR | Orbicularis oculi muscle | ③ | 17.3 ± 7.9 | When symptoms reappear (3–4 injections) | 1 years |
| Hsiung et al., 2002 [27] | R | Canada | 70 (40/30) | 57 | NR | NR | ① | 29.4 | 3 | 2 years |
| Meena Gupta et al., 2003 [28] | R | India | 62 (29/33) | 46.5 ± 7.12 | 6.2 ± 1.2 | Orbicularis oculi, mentalis, platysma | ② | 142.5 ± 6.2 | 3 | 3.7 years |
| Au et al., 2004 [29] | R | Asian countries | 137 (49/91) | NR | 5 | NR | NR | NR | NR | 1 month |
| Poonyathalang et al., 2005 [30] | R | Thailand | 26 | 56.7 | 3 | 0rbicularis oculi, Perioral muscles | ①④ | 7.5—12.5 | 3—4 | 13.6 months |
| Ortisi et al., 2006 [31] | R | UK | 60 | NR | NR | Orbicularis oculi muscle | NR | 20 | 3—4 | 49 months |
| Mohammadi et al., 2009 [32] | R | Germany | 19 (5/9) | 69 ± 12 | NR | Orbicularis oculi, Perioral muscles | ②⑤ | 25 ± 12 | NR | NR |
| Barbosa et al., 2010 [33] | R | Brazil | 54 (13/41) | 48.3 ± 10.8 | NR | The muscles around the eye, mouth and chin | ⑥ | 34.47—37.61 | 3 | 5.88 years |
| Rudzinska et al., 2010 [17] | R | Poland | 56 (21/35) | 60 ± 11 | 10.8 ± 5.8 | Orbicularis oculi, lower part and the upper part of the face | ②⑥ | 120 U of Dysport or 25 U of Botox | 3 injections | 2 weeks and 14 weeks |
| Kollewe et al., 2010 [34] | R | Germany | 97 | Range: 37—98 | NR | Orbicularis oculi of the upper, lower eyelid, the zygomaticus major, buccinator, corrugator or frontalis muscles | ②⑥ | (22 ± 10) *10–3 | 3 injections | NR |
| A. Çoban et al., 2012 [35] | R | Turkey | 92 (42/50) | 60 ± 12 | 8 ± 4 | NR | ②⑥ | 25—135 | NR | 4 years |
| Wang et al., 2014 [36] | R | China | 665 (238/427) | 46.6 ± 11.5 | 7.1 ± 6.2 | Orbicularis oculi, zygomaticus major, nasalis, and mentalis, orbicularis oris, rizorius and platysma | ① | 10—100 | NR | NR |
| Ababneh et al., 2014 [37] | R | Turkey | 11 | 59.7 ± 12.5 | 5.6 ± 7.2 | NR | ⑥ | 28.1 ± 8.7 | 3.5 injections | 14 ± 3.1 |
| Sorgun et al., 2015 [38] | R | Turkey, Germany | 68 (31/37) | 63.1 | NR | Orbicularis oculi or Perioral muscles | ②⑥ | 34.5 | ≥ 2 injections | NR |
| Choe et al., 2016 [39] | R | Korea | 26 (21/5) | 61.8 ± 14.6 | NR | The frontalis, procerus, corrugator, perioral area of the orbicularis oris, levator labii superioris, depressor anguli oris, and periocular | ②⑥ | 28.6 ± 4.9 | NR | NR |
| Koyuncuoğlu et al., 2016 [40] | R | USA | 70 (26/44) | 61.6 ± 13.2 | NR | NR | ⑥ | 15.7 ± 4.80 | ≥ 2 injections | 3.2 years |
| Batisti et al., 2017 [41] | R | Paraguay | 100 (28/72) | 63.1 ± 12.4 | NR | Orbicularis oculi, procerus, corrugator supercilii, risorius, orbicularis oris, and platysma muscles | ② | 28 ± 8.6 | 5.8 months | 2.6 years |
| Pandey et al., 2018 [42] | R | India | 34 (17/17) | 43.7 ± 12.2 | 4.8 ± 3.3 | NR | ① | 21 | 1–5 injections | 2 years |
| Gutierrez et al., 2021 [43] | R | Philippines | 162 | 47.7 ± 10.7 | 12.7 ± 5.8 | NR | ②⑥ | 17.9 ± 4.52 or 60.9 ± 9.13 | 6 ± 5 months | 2.96 years |
| Herrero-Infante et al., 2021 [10] | R | Spain | 125 (33/92) | 58.63 ± 15.4 | 6.7 ± 4.3 | NR | ⑥ | 21.53 ± 11.74 | > 3 injections | 10.45 years |
| Yahalom et al., 2022 [44] | R | Israel | 12 (5/7) | 56.6 ± 16.3 | 6.8 ± 11.6 | NR | ⑦ | 18.9 ± 12.8 | When symptoms reappear | NR |
| Tian, S et al., 2024 [45] | R | China | 118 (45/73) | 54.2 ± 11.95 | 0.08—20 | Orbicularis oculi, frontalis, orbicularis oris, and platysma | ① | 1–5 | NR | 2–4 weeks |
| Suputtitada et al., 2004 [46] | P | Thailand | 112 (41/71) | 45 ± 11.1 | 3.4 + 3.1 | Orbicularis occuli, orbicularis oris, zygomaticus, nasalis, mentalis, platysma, frontalis, corrugator | ⑥ | 25 ± 0.6 | 3 months | 4 weeks |
| Tunç et al., 2008 [47] | P | Turkey | 46 (20/26) | 54.4 ± 12.4 | 35.4 ± 23.6 | NR | ⑥ | NR | NR | NR |
| Cillino et al., 2010 [48] | P | Italy, France | 58 (21/37) | 71.7 ± 11.4 | 13.3 ± 8.8 | NR | ⑥ | 18.7 ± 9.4 | NR | 10 years |
| Rudzińska et al., 2012 [49] | R | Poland | 85 (27/58) | 60.8 ± 10.3 | 7.1 ± 5.1 | the five standard locations within the face | ⑥ | NR | NR | 2 / 12 weeks |
| Gürsoy et al., 2013 [50] | R | Turkey | 53 (13/40) | 58.0 ± 11.9 | 8.1 ± 5.3 | 3 or 5 sites in the pretarsal regions of the orbicularis oculi muscle | ⑥ | 21.10 ± 6.66 | 5.82 injections | 3 months / 4 weeks |
| Weiss et al., 2017 [51] | R | Germany | 73 (32/41) | 65.2 ± 10.4 | 9.0 ± 6.3 | NR | ②⑥⑧ | 26.3 ± 10.4 | NR | NR |
| Dong et al., 2019 [52] | R | China | 90 (36/54) | 52.3 ± 9.5 | NR | frontalis, orbicularis oculi, zygomaticus, temporalis, buccinator, masseter muscle, levator labii superioris, and mentalis | ⑨ | 33 – 66 | 1 – 2 weeks | 2 months |
| Yuksel et al., 2019 [53] | P | Turkey | 40 (19/21) | NR | NR | NR | NR | NR | NR | 4 weeks |
| Wei et al., 2022 [54] | R | China | 65 (27/38) | 54.7 ± 11.8 | NR | Orbicularis oculi muscle, zygomatic muscle, orbicularis oris muscle, and mental muscle of the affected side | ⑨ | NR | NR | 2 months |
| Trashin, A. V et al., 2023 [55] | R | Russia | 14 (4/10) | 58.07 | 5.6 | NR | NR | NR | NR | NR |
Type of BoNT: ①Botulinum toxin type A; ②Botulinum toxin type A (Dysport®); ③Botulinum toxin type A (Allergan, Irvine, Calif); ④Botulinum toxin A (Botox, Allergan, Irvine, CA, USA); ⑤Botulinum toxin type A (ASBTA); ⑥Botulinum toxin Type A (BOTOX®); ⑦Incobotulinumtoxin A (Xeomin®, INCO);⑧Botulinum toxin Type A (Xeomin®); ⑨BTX-A dry powder (Hengli, Lanzhou Institute of Biological Products, Lanzhou, China)
R Retrospective single-arm study, P Prospective single-arm study, GCI global clinical improvement, NR not reported, SE subjective evaluation, VAS visual analog scale
The aggregate analysis comprised a total of 2869 participants, with a distribution of 83 individuals contributing to the RCTs though a self-comparison model, and 2786 featured in single-arm studies. The demographic profile revealed a mean age spectrum spanning from 43.7 to 71.7 years, indicative of a study population that cut across several decades of adult life. Notably, over half of the enrolled participants were female, highlighting the representation of a predominantly female cohort in the research.
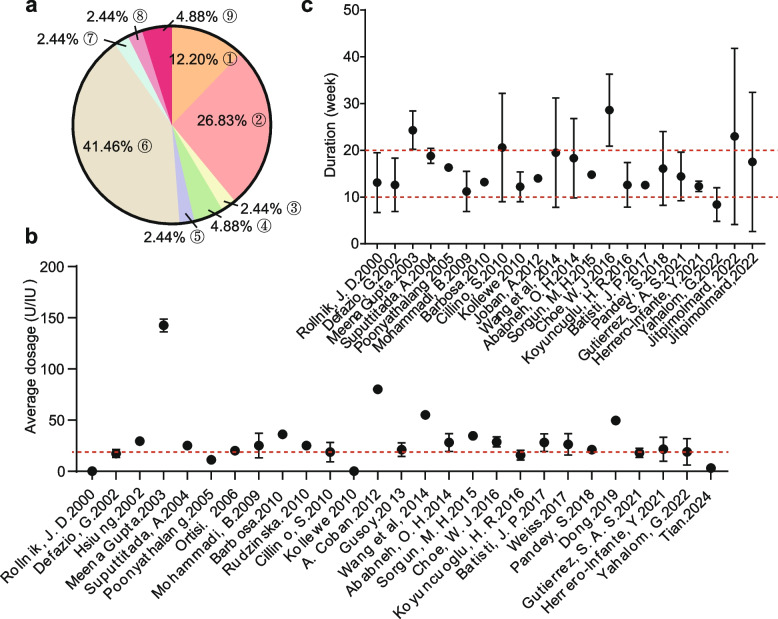
Currently, there are various commercial botulinum toxin products available. The mainstream products of concern in this study primarily include: ②Botulinum toxin type A (Dysport®) and ④Botulinum toxin type A (Botox®, manufactured by Allergan, located in Irvine, CA, USA) (Fig. 2a). Despite the use of different commercially available brands of BoNT, the targeted injection location consistently focused on the periorbital region, in both RCTs and single-arm trials (Tables 1 and 2). The administered dose was typically at around 20 units (U) (Fig. 2b). The medicine begun functioning within a week and lasted approximately 20 weeks, after which regular injections were required (Fig. 2c).
Fig. 2.
Visualization of BoNT injection modalities. a Commercial Type of BoNT, the numeric codes correspond to those in the footnote of Table 2. b Average dosage injection per site. The red dashed line indicated the location of 20 U. c The duration of effect, which corresponded to the interval between repeat injections. The red dashed lines indicated the 10-week and 20-week marks
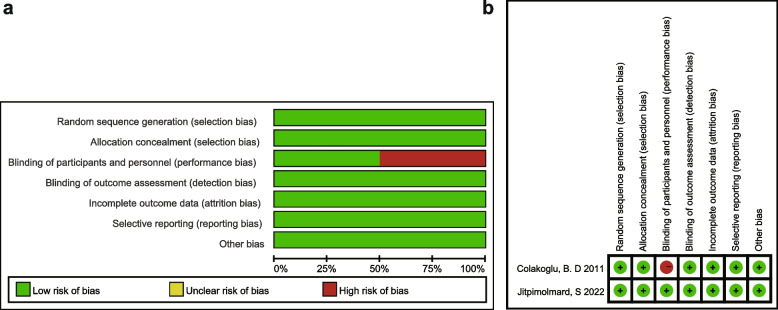
Quality assessment
The risk of bias for the RCTs [23, 24] was assessed by two reviewers (Fig. 3). Only Jitpimolmard, S. et al. [24] addressed the double-blind approach. The remaining six evaluation indicators in both trials were considered low risk. Besides that, all 33 single-arm studies [10, 17, 25–55] had JBI scores of more than 7 points (Additional file 4), indicating that they were of high quality. Finally, all 35 literatures were included in the following meta-analysis.
Fig. 3.
Quality evaluation of RCTs. a Risk of bias graph: authors’ judgements about each risk of bias item presented as percentages across all included studies. b Risk of bias summary and overall quality
Efficiency of BoNT was affected by injection site, indicated in RCTs
Two RCTs [23, 24] were included according to the criteria described above. The objective of both studies was to assess the relationship between therapeutic effect and injection site. The outcomes of single injection around eye and simultaneous injection around eye and mouth were compared. Unfortunately, despite contacting the corresponding author, we were unable to access the raw data available for further analysis. Due to lack of comparable outcomes for the forest plot, a qualitative overview was provided.
Colakoglu, B. D. et al. conducted a randomized, single-blind, crossover, clinical trial [23]. In their study, severity scores by physicians and VAS scores by patients were used as effect indicators (Table 1). All the patients benefited from BoNT treatment regardless of the methods. For orbicularis oculi muscle, there was no significant difference in Δ scores of both method (severity scores: P = 0.48; VAS scores: P = 0.49). When evaluating the effect on perioral muscles, the Δscore of BoNT method was significantly higher than that of the placebo method (severity scores: P = 0.001; VAS scores: P = 0.006). The post hoc analysis indicated that the significance was mainly from severe subgroups. And when the patients were asked to indicate their preference for a particular method, most of the severe HFS cases preferred the BoNT method (85.7%). Thus, the study suggested that BoNT application to lower facial muscles might not be necessary in patients with mild lower facial involvement.
In another RCT [24], efficacy of BoNT for HFS treatment was assessed 6 weeks after the first intervention. Among all the outcomes evaluation (Table 1), only percentage of mouth improvement in two groups was obvious (95% CI: 4.5, 26.2, P = 0.006). Given that facial contractions start around the eye and spread down to the mouth in most patients with HFS, the study supported that the mouth BoNT injection for HFS was not necessary.
High effective rates of BoNT injection were observed in single-arm studies
Thirty-three single-arm trials encompassing a total of 2786 participants were included (Table 2 and Additional file 3), with the 7 studies [49–55] at the end focusing the influence of BoNT on the quality of life in HFS patients. The injection dosage, injected muscles, and rating scales were variable, thus leading to challenges in finding the source of heterogeneity. The statistical and qualitative analysis of the included single-arm trials were performed based on the difference of the outcome effect size.
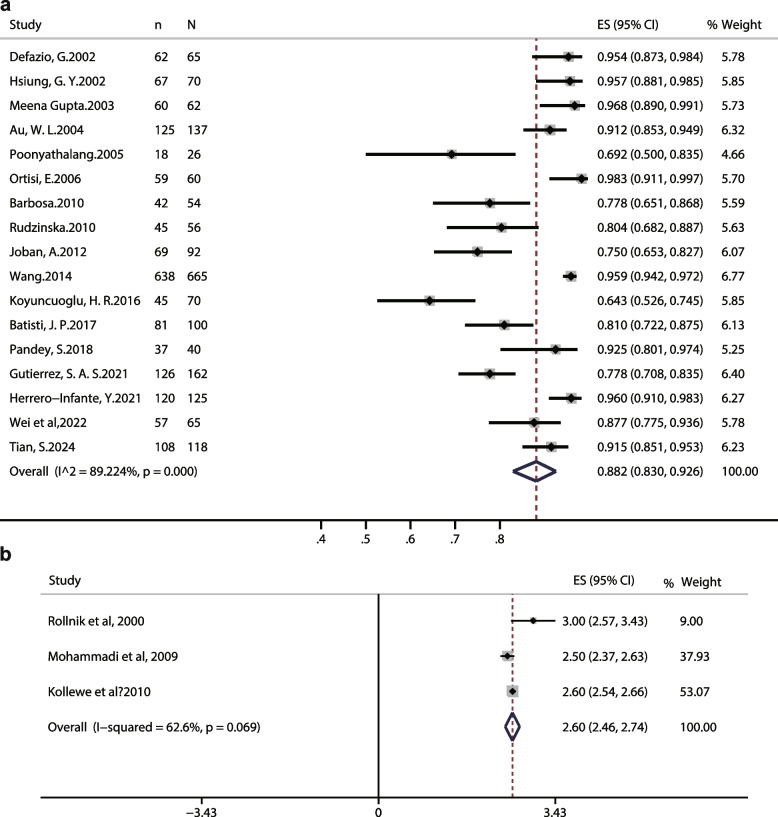
Among these retrospective studies, the effective rate (%) was used as the effect size in 17 trials, which is a subjective factor. The random effects model was employed for analysis. After applying double arcsine transformation and converting back to the original proportion, the effective rate of HFS patients included was estimated to be 0.882 (95% CI: 0.83, 0.926, P < 0.001), and the percentage of variation across studies (I2) was 89.224%, which is interpreted as considerable heterogeneity (Fig. 4a).
Fig. 4.
Forest plots of the effectiveness of BoNT in single-arm trials. a The favorable response of HFS patients with BoNT injection. b GCI index of HFS patients with BoNT injection
Among the 17 included studies, the majority of patients with HFS were middle-aged and elderly women, except for Husing 2002 [27]. Subgroup analysis was performed in terms of geographic regions, mean dose per injection site, and follow-up durations. Results showed that these factors did not account for the heterogeneity of the effective rate (Additional file 5). After then, we conducted a meta-regression analysis to investigate potential covariates influencing the effective rate of BoNT treatment for HFS patients and contributing to observed heterogeneity (Additional file 6). In the univariate meta-regression, factor of geographic regions was slightly associated with the heterogeneity observed (P = 0.088). Average dosage (P = 0.483) and follow-up durations (P = 0.273) were irrelevant. In the multivariate regression analysis, the effective rate of BoNT treatment was not substantially altered by these factors (P = 0.236, 0.430 and 0.662). The comprehensive results indicated that the 3 study-level factors could not be considered as the source of heterogeneity in effective rate.
The sensitivity analysis demonstrated that the results of the effective rate of BoNT for HFS patients remained consistent and robust, regardless of the exclusion of any individual study, thus affirming the stability and reliability of these findings (Additional file 7a). Egger’s test was performed to assess the presence of publication bias in the obtained effective rate (Additional file 7b), and the results revealed a significant indication of publication bias (P = 0.000).
Various outcomes were employed to assess the efficacy in single-arm trials
Three single-arm studies [25, 32, 34] included 137 participants used global clinical improvement (GCI) scores as effect indicators (Fig. 4b). Patients rated the GCI on a 0–3 scale at each interaction (0 = no effect, 1 = slight improvement, 2 = moderate improvement, 3 = marked improvement). Based on the heterogeneity (I2 = 62.6%, P = 0.069), random effects model was used. The forest plot showed that the patient’s symptoms indicated by GCI scores were improved significantly after BoNT injection (CGI: 2.60, 95% CI: 2.46, 2.74) (Fig. 4b).
VAS was another outcome in the retrospective study [38], which revealed that patients expressed a 73.7% (range: 0–100) improvement. In the prospective studies, severity and consciousness scores were evaluated by Tunç, T [47], that demonstrated a significant improvement (Severity scores: pre 3.32 ± 0.73, post 0.89 ± 0.75; Awareness scores: pre 3.69 ± 0.51, post 1.02 ± 0.95). The remission rate was regarded as main indicator in other 2 studies [46, 48], which showed the efficacy of BoNT treatment was more than 96%.
BoNT administration showed significant improvement in the quality of life and mental state of HFS patients
Facial symptoms of HFS would deteriorate health-related quality of life via multiple processes, including involuntary eye closure or facial expressions, social embarrassment, and mental health issues [54]. In addition to relieving face symptoms, the effects of BoNT on health-related quality of life during HFS treatment required further exploration practitioners in a real-life therapeutic environment.
Various questionnaires were used to evaluate the changes in quality of life in HFS patients after BoNT treatment (Table 2 and Additional file 3), such as the HFS-36 questionnaire, BDI and CMI self-assessment questionnaire. Seven single-arm studies involving 406 participants were analyzed for changes in quality-of-life indicators before and after treatment. The indicators were classified into 7 categories: general life satisfaction, satisfaction with health, physical health, metal health, depression scale, anxiety scale and total scale. The relative index SMD was chosen to provide an intuitive display of the summary results, allowing the same indications to be normalized across multiple scales.
Two one-arm experiments with 126 participants measured the general life satisfaction before and after BoNT injection [50, 51] with 126 participants. The effect sizes revealed no significant difference (SMD: 0.66, 95%CI: -0.13, 1.45, P = 0.002) (Fig. 5a). The total score of the questionnaire was used in two trials with 150 participants [49, 54]. The combined effect size (SMD: -0.64, 95%CI: -0.87, -0.41, P = 0.766) showed a significant difference in overall quality of life in HFS patients after BoNT refraction compared with before injection, indicating the improvement in patient’s life problems (Fig. 5b).
Fig. 5.
Forest plot of BoNT’s effect on HFS-related quality of life. The forest plot for the effect of BoNT on (a) general life satisfaction, b total scale, and c anxiety scale
Depression and anxiety, as major comorbidities of HFS, are often described in conjunction. Anxiety scale was evaluated in 3 reports with 208 participants. According to the respective effect sizes there were significant improvement in anxiety scale after BoNT injection compared with before (SMD: -1.50, 95%CI: -2.19, -0.80, P < 0.001) (Fig. 5c). Depression scale was evaluated in 6 reports with 406 participants [49, 51–54]. According to the respective effect sizes there was significant improvement in depression scale after BoNT injection (SMD: -0.85, 95%CI: -1.34, -0.35, P < 0.001), but with great heterogeneity (I2 = 91%) (Fig. 6a). Subgroup analysis considering region and gender was conducted (Fig. 6b). It was suggested that the proportion of women might have an impact on heterogeneity. The sensitivity analysis which employed for evaluating the robustness of the outcomes showed that the exclusion of any single study did not affect the estimate of the overall effect (Fig. 6c).
Fig. 6.
Analysis of the depression scores in HFS patients treated with BoNT. a The forest plot for the efficacy, b subgroup analysis and c sensitivity analysis of BoNT on depression scale
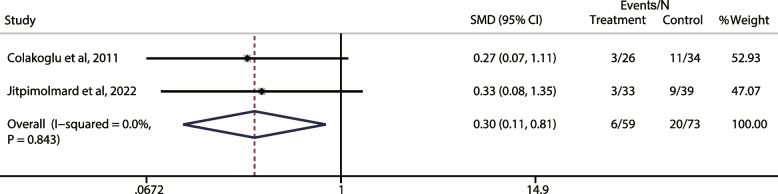
Adverse reaction after BoNT injection in HFS treatment
Fixed effect model was used to combine 2 RCTs (I2 = 0%). In terms of the common side effect “mouth drooping”, there was significant difference between the lower facial BoNT group and the placebo group (OR:0.3, 95%CI: 0.11, 0.81, P = 0.843) (Fig. 7).
Fig. 7.
Meta-analysis of the association of common side effects- mouth drooping
The adverse events following BoNT intervention in one-arm studies occurred more frequently was ptosis (14 out of the 25 reports) (Additional file 3), which was probably caused by toxin diffusion to the levator palpebrae superioris muscle [38]. Other side effects were also listed, including facial weakness, diplopia, facial asymmetry, etc. These effects are reversible, mild and transient. No systemic side effects have been recorded.
Discussion
BoNT has become the preferred therapeutic option for HFS, owing to its efficacy and safety characteristics [56]. BoNT treatment could mitigate symptoms in a vast majority (85%—95%) of HFS patients [11]. Through a comprehensive review, our analysis integrated qualitative and quantitative data to evaluate symptom alleviation following BoNT injections. Despite the scarcity of RCTs for a definitive efficacy assessment, a meta-analysis of 17 single-arm studies (ES: 0.882, 95% CI: 0.83, 0.926, P < 0.001) confirmed previous findings, consolidating BoNT’s reputation as an effective therapy strategy for HFS. The heterogeneity among these studies was large (I2 = 89.2%, P < 0.001). Meta-regression and subgroup analyses were performed to explore the source of this heterogeneity. Across all studies, female participants constituted a majority, exceeding 50%, mirroring established epidemiological patterns regarding the gender and age distribution of HFS incidence [10]. Given this concordance, age and gender were excluded as potential moderators in the subgroup analysis. Instead, the investigation focused on geographic variability, the mean dose administered per injection site, and the duration of follow-up as potential sources of heterogeneity. Regrettably, these factors did not correlate with observed heterogeneity. We posit that the heterogeneity could emanate from intrinsic features of single-arm trial designs and the paucity of comprehensive literature in this domain.
In our meta-analysis of single-arm trials, we observed a potential publication bias (Additional file 7b). In the context of single-arm trials, the impact of publication bias is particularly pronounced. This might result from that such studies often lack a control group, and are primarily used to evaluate the safety and preliminary efficacy of new therapies or interventions. The sensitivity analysis indicated that the study’s findings are likely robust (Additional file 7a), meaning that publication bias, even if present, is unlikely to significantly alter the conclusions.
HFS imposes not only physical discomfort but also severe social burdens on patients. Neuroscience research showed correlation between HFS and alterations in the basal ganglio-thalamocortical motor circuit [53], along with bilateral thalamic glucose hypermetabolism, implicating regions associated with anxiety and depression [57]. Individuals with HFS exhibit higher prevalence rates of depression and anxiety [12, 56]. While the emergence of such comorbidities may result from neurobiological mechanisms, definitive therapeutic targets for alleviating depressive and anxious symptoms in HFS patients remained elusive.
When evaluating modifications in quality of life (QoL), it is recommended to employ the HFS-36 questionnaire, CMI self-assessment questionnaire, WHOQOL-BREF TR, and other validated instruments. Anxiety and depression symptom scores were assessed using BDI, HDRS, HAS, SAS, and SDS criteria. The findings of our study indicated that the administration of BoNT injections resulted in significant improvements in both the physical and mental well-being of patients, which is consistent with previous research studies [12, 51]. We included 6 single-arm studies which summarized changes in depression in HFS patients before and after BoNT injection and showed significant improvements (SMD: -0.85, 95%CI: -1.34, -0.35, P < 0.001), but with great heterogeneity (I2 = 91%). There was great heterogeneity among the reports, and subgroup analysis suggested that the proportion of females might be related. Significant variability was evident among the reports, with subgroup analyses hinting at a potential correlation with the female predominance. It is noteworthy that women afflicted with HFS tend to be postmenopausal, thus susceptible to hormonal influences, and demonstrate heightened disease-related concerns [52].
Inevitably, BoNT injections’ effectiveness declined with time, necessitating re-injection at regular intervals. The collation and analysis of the data revealed that the intervals between injections clustered around 20 weeks (Fig. 2c). Necessity for repeated administrations might be explained by the neuroscience mechanism underlying BoNT’s activity. BoNT operates as a highly effective endotoxin, selectively blocking the acetylcholine release at the neuromuscular junction, which induces a diminution in motor unit contraction [58]. The dynamic turnover of neuromuscular junctions is expedited in the aftermath of BoNT exposure, precipitating a gradual return of muscular function, which typically commences around 3 months post-treatment and reaches completion by 6 months. This recovery is underpinned by collateral sprouting at the nerve terminal, enabling the resumption of acetylcholine release and consequent muscle activity [59]. Notably, it was reported that an increase in BoNT resistance was observed in the treatment of craniocervical muscle spasms or blepharospasm, especially when patients received frequent, repetitive, high-dose or lifetime injections [60]. Regarding the treatment of HFS, there have been no case reports published that could serve as an indicator for future attention.
In addition to the intervals between injections, factors such as the injection sites, follow-up durations, onset times of effects, and evaluation metrics varied considerably across different studies. Comprehensive summary of the included studies revealed that the orbicularis oculi muscle is the predominant target for BoNT injections in HFS management (Tables 1 and 2). Occasionally, this is complemented by injections into the orbicularis oris, frontalis, and mentalis muscles; however, these adjunctive sites are less common due to the risk of inducing lower facial muscle weakness [26]. Especially, the two RCTs included confirmed the necessity of orbicularis oculi muscle injection, and also pointed out that BoNT perioral therapy is still necessary for patients with severe facial spasm.
Aside from accurate targeting, judicious dosing is essential to achieve optimal outcomes while minimizing adverse effects in HFS patients. Here, the average total injection dose was mostly around 20 U (Fig. 2b), with clinical benefits enduring for approximately 20 weeks (Fig. 2c). Recent study has presented a novel BoNT injection paradigm based on conventional treatments [39]. By taking into account factors such as the etiology, duration, electrophysiological features and patient’s age, the strategy incorporated combined injections into multiple muscular regions to enhance therapeutic outcomes. Concurrently, personalized dose adjustments are implemented to prevent adverse reactions that may result from excessive medication use. Employing this innovative method resulted in a protracted duration of therapeutic effect, with an average sustained benefit of 28.6 weeks [39]. Such studies highlight the significance of personalized precision medicine in the context of BoNT treatment for HFS.
Based on a comprehensive review of the included literatures, the brands Dysport® and BOTOX® are recommended for the treatment of HFS. The average total injection dose typically ranged around 20 U, with the injection interval determined by the specific circumstances of each patient, primarily when symptoms recur. The dose variation of BoNT was mentioned in two studies [27, 46]. It was reported that the doses of BoNT used in the second and fifth years increased compared to the first year, though these increases were not significant. Over a period of two years, patients with HFS showed the highest response rate of sustained benefit [27]. Another report indicated a decrement in the mean dose for each subsequent injection following the initial treatment. During the ten years of treatment, the average injection dose was reduced by approximately 0.5 U annually [46]. This aspect was not addressed in the other included literature, thus insufficient evidence existed to support us in formulating reasonable recommendations.
While the therapeutic benefits of BoNT treatment in HFS are widely acknowledged, it is pertinent to address the spectrum of adverse reactions reported in the literature. In our included reports, the main side effect was ptosis and facial weakness (Additional file 3, Fig. 7). The incidence of these adverse effects did not vary with the duration of treatment, but might be related to the type of disease [57]. Pandey, S. et al. suggested that patients with secondary type of HFS suffered more side effects than primary ones, even under lower BoNT dosage [42]. Furthermore, BoNT might inadvertently spread due to various factors, affecting adjacent muscles and eliciting undesirable side effects. Influences on toxin dispersion include injection sites, dilution volume, product-specific attributes, dosage, and application technique [61]. Here, no case of BoNT resistance or migration has been reported in HFS treatment. And the reported side effects were short-lived and reversible.
The main limitation of this review is the variability between the included studies. Most included studies differed between injection methods and often no verified objective scoring system was used in determining the change in HFS after therapy. Because of this, we were not able to objectively compare results or draw clear conclusions regarding the optimal treatment using BoNT. More clinical studies, especially RCTs, are needed to further investigate the optimal treatment timing, dose, formulation and injection site of BoNT to improve the treatment of HFS. Moreover, both the evaluation indicators and scales for HFS symptom relief and quality of life are mostly subjective ideas of patients and medical staff, and there is a lack of objective evaluation indicators.
Conclusion
Our analysis supported that BoNT treatment demonstrated notable efficacy in alleviating symptoms of HFS, enhancing quality of life, and improving mental state, with a particularly significant effect on alleviating depressive moods. Gender might be a primary factor influencing the effectiveness of BoNT in mitigating depression. The effects of BoNT did not last indefinitely, requiring re-injection at intervals. No significant adverse reactions were reported following multiple injections. The most common adverse reaction was ptosis, or drooping of the eyelids, but this is transient. Although BoNT injections have shown effectiveness and clinical safety as a treatment for HFS, there was currently no standardized injection protocol, and evaluations of treatment outcomes also varied widely. We have summarized factors affecting treatment and injection techniques across different studies, such as dosage, site, frequency, and follow-up periods. This information could aid in the development of standard guidelines, but more RCTs are needed to substantiate these findings.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- BDI
Beck Depression Inventory
- BS
Blepharospasm
- CI
Confidence interval
- EQ-VAS
EuroQol-visual analogue scale
- HAS
Hamilton Anxiety Scale
- HDRS
Hamilton Depression Rating Scale
- JBI
Joanna Briggs Institute
- JRS
Jankovic Rating Scale
- OR
Odds value
- QoL
Quality of life
- SAS
Self-rating anxiety scale
- SDS
Self-rating depression scale
- SMD
Standardized mean difference
- VAS
Visual analog scale
- WHOQOL-BREF TR
The World Health Organization Quality of Life Measurement Instrument Short Form: Turkish Version
- WMD
Weighted mean difference
Authors’ contributions
All authors contributed to the study conception and design. Screening, data extraction and curation: Bingqian Wang, Min Hu and Jun Ma; Meta-analysis: Bingqian Wang; Writing - original draft preparation: Bingqian Wang; Editing the manuscript critically: Bingqian Wang, Min Hu, Jun Ma, Xiaoxi Wei, Huichuan Qi and Xingfu Bao.
Funding
This study was funded by Natural Science Foundation of Jilin Province, China (YDZJ202301ZYTS468) and Jilin University Bethune Program Project (2022B45).
Availability of data and materials
The datasets generated during the current study are available in the repository or from the corresponding author on reasonable request.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors ensured our conform to the authorship policies and consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yaltho TC, Jankovic J. The many faces of hemifacial spasm: differential diagnosis of unilateral facial spasms. Mov Disord. 2011;26(9):1582–92. 10.1002/mds.23692. [DOI] [PubMed] [Google Scholar]
- 2.Heuser K, Kerty E, Eide PK, Cvancarova M, Dietrichs E. Microvascular decompression for hemifacial spasm: postoperative neurologic follow-up and evaluation of life quality. Eur J Neurol. 2007;14(3):335–40. 10.1111/j.1468-1331.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- 3.Perren F, Magistris MR. Is hemifacial spasm accompanied by hemodynamic changes detectable by ultrasound? Article. Acta Neurochirurgica. 2014;156(8):1557–60. 10.1007/s00701-014-2132-7. [DOI] [PubMed] [Google Scholar]
- 4.Lu AY, Yeung JT, Gerrard JL, Michaelides EM, Sekula RF, Bulsara KR. Hemifacial spasm and neurovascular compression. TheScientificWorldJOURNAL. 2014;2014(2014):349319. 10.1155/2014/349319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee P, Alam MS, Koka K, Pherwani R, Noronha OV, Mukherjee B. Role of neuroimaging in cases of primary and secondary hemifacial spasm. Indian J Ophthalmol. 2021;69(2):253–6. 10.4103/ijo.IJO_415_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlowski M, Gess B, Evers S. The Babinski-2 sign in hemifacial spasm. Mov Disord. 2013;28(9):1298–300. 10.1002/mds.25472. [DOI] [PubMed] [Google Scholar]
- 7.Lefaucheur JP, Ben Daamer N, Sangla S, Le Guerinel C. Diagnosis of primary hemifacial spasm. Neurochirurgie. 2018;64(2):82–6. 10.1016/j.neuchi.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Hermier M. Imaging of hemifacial spasm. Neurochirurgie. 2018;64(2):117–23. 10.1016/j.neuchi.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Ozzello DJ, Giacometti JN. Botulinum toxins for treating essential blepharospasm and hemifacial spasm. Int Ophthalmol Clin Winter. 2018;58(1):49–61. 10.1097/iio.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 10.Herrero-Infante Y, Rodríguez-Sanz A, Máñez-Miró J, Vivancos-Matellano F. Hemifacial spasm through the last three decades: from etiology to efficacy and safety of long-term botulinum toxin treatment. Clin Neurol Neurosurg. 2021;203:106555. 10.1016/j.clineuro.2021.106555. [DOI] [PubMed] [Google Scholar]
- 11.Frei K, Truong DD, Dressler D. Botulinum toxin therapy of hemifacial spasm: comparing different therapeutic preparations. Conference Paper. Eur J Neurol. 2006;13(SUPPL. 1):30–5. 10.1111/j.1468-1331.2006.01442.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Joo KM, Park K. Challenging microvascular decompression surgery for hemifacial spasm. World Neurosurg. 2021;151:e94–9. 10.1016/j.wneu.2021.03.133. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Lyu L, Chen C, Yin S, Jiang S, Zhou P. The outcome of microvascular decompression for hemifacial spasm: a systematic review and meta-analysis. Neurosurg Rev. 2022;45(3):2201–10. 10.1007/s10143-022-01739-x. [DOI] [PubMed] [Google Scholar]
- 14.Persaud R, Garas G, Silva S, Stamatoglou C, Chatrath P, Patel K. An evidence-based review of botulinum toxin (Botox) applications in non-cosmetic head and neck conditions. JRSM Short Rep. 2013;4(2):10. 10.1177/2042533312472115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhry N, Srivastava A, Joshi L. Hemifacial spasm: the past, present and future. J Neurol Sci. 2015;356(1–2):27–31. 10.1016/j.jns.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–89. 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Rudzińska M, Wójcik M, Szczudlik A. Hemifacial spasm non-motor and motor-related symptoms and their response to botulinum toxin therapy. J Neural Transm (Vienna). 2010;117(6):765–72. 10.1007/s00702-010-0416-5. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Zhang L, Gu S, et al. Comparative effectiveness of extracorporeal shock wave, ultrasound, low-level laser therapy, noninvasive interactive neurostimulation, and pulsed radiofrequency treatment for treating plantar fasciitis: a systematic review and network meta-analysis. Medicine (Baltimore). 2018;97(43):e12819. 10.1097/md.0000000000012819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papalia GF, Petrucci G, Russo F, et al. COVID-19 pandemic increases the impact of low back pain: a systematic review and metanalysis. Int J Environ Res Public Health. 2022;19(8):4599. 10.3390/ijerph19084599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong MCS, Huang J, Wang J, et al. Global, regional and time-trend prevalence of central obesity: a systematic review and meta-analysis of 13.2 million subjects. Eur J Epidemiol. 2020;35(7):673–83. 10.1007/s10654-020-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colakoglu BD, Cakmur R, Uzunel F. Is it always necessary to apply botulinum toxin into the lower facial muscles in hemifacial spasm?: a randomized, single-blind, crossover trial. Eur Neurol. 2011;65(5):286–90. 10.1159/000327534. [DOI] [PubMed] [Google Scholar]
- 24.Jitpimolmard S, Thinkhamrop B, Tiamkao S, et al. A double-blind, placebo-controlled study of appropriate site of botulinum toxin therapy in hemifacial spasm. Adv Ther. 2022;39(5):2025–34. 10.1007/s12325-022-02077-6. [DOI] [PubMed] [Google Scholar]
- 25.Rollnik JD, Matzke M, Wohlfarth K, Dengler R, Bigalke H. Low-dose treatment of cervical dystonia, blepharospasm and facial hemispasm with albumin-diluted botulinum toxin type A under EMG guidance. An open label study. Eur Neurol. 2000;43(1):9–12. 10.1159/000008121. [DOI] [PubMed] [Google Scholar]
- 26.Defazio G, Abbruzzese G, Girlanda P, et al. Botulinum toxin A treatment for primary hemifacial spasm: a 10-year multicenter study. Arch Neurol. 2002;59(3):418–20. 10.1001/archneur.59.3.418. [DOI] [PubMed] [Google Scholar]
- 27.Hsiung GY, Das SK, Ranawaya R, Lafontaine AL, Suchowersky O. Long-term efficacy of botulinum toxin A in treatment of various movement disorders over a 10-year period. Mov Disord. 2002;17(6):1288–93. 10.1002/mds.10252. [DOI] [PubMed] [Google Scholar]
- 28.Gupta M, Singh G, Khwaja G. Botulinum toxin in the treatment of dystonias–a hospital based study. J Assoc Physicians India. 2003;51:447–53. [PubMed] [Google Scholar]
- 29.Au WL, Tan LC, Tan AK. Hemifacial spasm in Singapore: clinical characteristics and patients’ perceptions. Ann Acad Med Singap. 2004;33(3):324–8. [PubMed] [Google Scholar]
- 30.Poonyathalang A, Preechawat P, Jamnansiri U. Low-dose botulinum toxin a for treatment of blepharospasm and hemifacial spasm. Jpn J Ophthalmol Jul-Aug. 2005;49(4):327–8. 10.1007/s10384-005-0200-5. [DOI] [PubMed] [Google Scholar]
- 31.Ortisi E, Henderson HW, Bunce C, Xing W, Collin JR. Blepharospasm and hemifacial spasm: a protocol for titration of botulinum toxin dose to the individual patient and for the management of refractory cases. Eye (Lond). 2006;20(8):916–22. 10.1038/sj.eye.6702054. [DOI] [PubMed] [Google Scholar]
- 32.Mohammadi B, Kollewe K, Wegener M, Bigalke H, Dengler R. Experience with long-term treatment with albumin-supplemented botulinum toxin type A. J Neural Transm (Vienna). 2009;116(4):437–41. 10.1007/s00702-009-0200-6. [DOI] [PubMed] [Google Scholar]
- 33.Barbosa ER, Takada LT, Gonçalves LR, Costa RM, Silveira-Moriyama L, Chien HF. Botulinum toxin type A in the treatment of hemifacial spasm: an 11-year experience. Arq Neuropsiquiatr. 2010;68(4):502–5. 10.1590/s0004-282x2010000400006. [DOI] [PubMed] [Google Scholar]
- 34.Kollewe K, Mohammadi B, Dengler R, Dressler D. Hemifacial spasm and reinnervation synkinesias: long-term treatment with either Botox or Dysport. J Neural Transm (Vienna). 2010;117(6):759–63. 10.1007/s00702-010-0409-4. [DOI] [PubMed] [Google Scholar]
- 35.Çoban A, Matur Z, Hanaǧasi HA, Parman Y. Efficacy of botulinum toxin injections in the treatment of various types of facial region disorders. Article Turk Noroloji Dergisi. 2012;18(4):133–61. 10.4274/Tnd.26097. [Google Scholar]
- 36.Wang L, Hu X, Dong H, et al. Clinical features and treatment status of hemifacial spasm in China. Chin Med J (Engl). 2014;127(5):845–9. [PubMed] [Google Scholar]
- 37.Ababneh OH, Cetinkaya A, Kulwin DR. Long-term efficacy and safety of botulinum toxin A injections to treat blepharospasm and hemifacial spasm. Clin Exp Ophthalmol. 2014;42(3):254–61. 10.1111/ceo.12165. [DOI] [PubMed] [Google Scholar]
- 38.Sorgun MH, Yilmaz R, Akin YA, Mercan FN, Akbostanci MC. Botulinum toxin injections for the treatment of hemifacial spasm over 16 years. J Clin Neurosci. 2015;22(8):1319–25. 10.1016/j.jocn.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 39.Choe WJ, Kim J. Increasing the area and varying the dosage of Botulinum toxin a injections for effective treatment of hemifacial spasm. Acta Otolaryngol. 2016;136(9):952–5. 10.3109/00016489.2016.1165864. [DOI] [PubMed] [Google Scholar]
- 40.Koyuncuoğlu HR, Demirci S. Applications of botulinum toxin at a neurology clinic: an eleven-year experience. Article. Turk Noroloji Dergisi. 2016;22(1):8–12. 10.4274/tnd.04809. [Google Scholar]
- 41.Batisti JP, Kleinfelder AD, Galli NB, Moro A, Munhoz RP, Teive HA. Treatment of hemifacial spasm with botulinum toxin type a: effective, long lasting and well tolerated. Arq Neuropsiquiatr. 2017;75(2):87–91. 10.1590/0004-282x20160191. [DOI] [PubMed] [Google Scholar]
- 42.Pandey S, Jain S. Clinical features and response to botulinum toxin in primary and secondary hemifacial spasm. Neurol India. 2018;66(4):1036–42. 10.4103/0028-3886.236959. [DOI] [PubMed] [Google Scholar]
- 43.Gutierrez SAS, Yu JRT, Yalung PM, Jamora RDG. Real-world experience with botulinum toxin A for the treatment of hemifacial spasm: a study of 1138 injections. Clin Neurol Neurosurg. 2021;205:106632. 10.1016/j.clineuro.2021.106632. [DOI] [PubMed] [Google Scholar]
- 44.Yahalom G, Janah A, Rajz G, Eichel R. Therapeutic approach to botulinum injections for hemifacial spasm, synkinesis and blepharospasm. Toxins (Basel). 2022;14(5):362. 10.3390/toxins14050362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian S, Zheng H, Wu L, Wu W. Factors influencing short-term prognosis after botulinum toxin type A treatment for hemifacial spasm: a retrospective study. Heliyon. 2024;10(2):e24898. 10.1016/j.heliyon.2024.e24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suputtitada A, Phanthumchinda K, Locharernkul C, Suwanwela NC. Hemifacial spasm: results of treatment with low dose botulinum toxin injection. J Med Assoc Thai. 2004;87(10):1205–11. [PubMed] [Google Scholar]
- 47.Tunç T, Cavdar L, Karadağ YS, Okuyucu E, Coşkun O, Inan LE. Differences in improvement between patients with idiopathic versus neurovascular hemifacial spasm after botulinum toxin treatment. J Clin Neurosci. 2008;15(3):253–6. 10.1016/j.jocn.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Cillino S, Raimondi G, Guépratte N, et al. Long-term efficacy of botulinum toxin A for treatment of blepharospasm, hemifacial spasm, and spastic entropion: a multicentre study using two drug-dose escalation indexes. Eye (Lond). 2010;24(4):600–7. 10.1038/eye.2009.192. [DOI] [PubMed] [Google Scholar]
- 49.Rudzińska M, Wójcik M, Malec M, et al. Factors affecting the quality of life in hemifacial spasm patients. Article. Neurologia i Neurochirurgia Polska. 2012;46(2):121–9. 10.5114/ninp.2012.28254. [DOI] [PubMed] [Google Scholar]
- 50.Gürsoy AE, Ugurad I, Babacan-Yildiz G, Kolukisa M, Çelebi A. Effects of botulinum toxin type A on quality of life assessed with the WHOQOL-BREF in hemifacial spasm and blepharospasm. Article. Neurol Psychiatry Brain Res. 2013;19(1):12–8. [Google Scholar]
- 51.Weiss D, Sturm J, Hieber L, et al. Health-related quality of life outcomes from botulinum toxin treatment in hemifacial spasm. Ther Adv Neurol Disord. 2017;10(4):211–6. 10.1177/1756285616682676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong H, Fan S, Luo Y, Peng B. Botulinum toxin relieves anxiety and depression in patients with hemifacial spasm and blepharospasm. Neuropsychiatr Dis Treat. 2019;15:33–6. 10.2147/ndt.S181820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuksel B, Genc F, Yaman A, Goksu EO, Ak PD, Gomceli YB. Evaluation of stigmatization in hemifacial spasm and quality of life before and after botulinum toxin treatment. Acta Neurol Belg. 2019;119(1):55–60. 10.1007/s13760-018-1018-5. [DOI] [PubMed] [Google Scholar]
- 54.Wei JS, Hu X, Xia L, Shang J, Han Q, Zhang DY. Evaluation of the effect of botulinum toxin A on the physical and mental health of patients with hemifacial spasm. Article in Press. Neurologia. 2022. 10.1016/j.nrl.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Trashin AV, Shulev YA, Bogdanova EM. Quality of life in patients with hemifacial spasm after microvascular decompression and botulinum toxin therapy. Zh Vopr Neirokhir Im N N Burdenko. 2023;87(1):64–9. 10.17116/neiro20238701164. Otsenka kachestva zhizni patsientov s gemifatsial’nym spazmom posle mikrovaskulyarnoi dekompressii i botulinoterapii. [DOI] [PubMed] [Google Scholar]
- 56.Kongsaengdao S, Maneeton N, Maneeton B. The five-year prospective study of quality of life in hemifacial spasm treated with abo-botulinum toxin A. Toxins (Basel). 2021;13(3):215. 10.3390/toxins13030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bao F, Wang Y, Liu J, et al. Structural changes in the CNS of patients with hemifacial spasm. Neuroscience. 2015;289:56–62. 10.1016/j.neuroscience.2014.12.070. [DOI] [PubMed] [Google Scholar]
- 58.Dobryansky M, Korsh J, Shen AE, Aliano K. Botulinum toxin type A and B primary resistance. Aesthet Surg J. 2015;35(2):Np28-30. 10.1093/asj/sju027. [DOI] [PubMed] [Google Scholar]
- 59.Wenzel RG. Pharmacology of botulinum neurotoxin serotype A. Am J Health Syst Pharm. 2004;61(22 Suppl 6):S5-10. 10.1093/ajhp/61.suppl_6.S5. [DOI] [PubMed] [Google Scholar]
- 60.de Jongh FW, Schaeffers A, Kooreman ZE, et al. Botulinum toxin A treatment in facial palsy synkinesis: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;280(4):1581–92. 10.1007/s00405-022-07796-8. [DOI] [PubMed] [Google Scholar]
- 61.Borba A, Matayoshi S, Rodrigues M. Avoiding complications on the upper face treatment with botulinum toxin: a practical guide. Aesthetic Plast Surg. 2022;46(1):385–94. 10.1007/s00266-021-02483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available in the repository or from the corresponding author on reasonable request.
No datasets were generated or analysed during the current study.