Abstract
Recent efforts in the study of vector-borne parasitic diseases (VBPDs) have emphasized an increased consideration for preventing drug resistance and promoting the environmental safety of drugs, from the beginning of the drug discovery pipeline. The intensive use of the few available antileishmanial drugs has led to the spreading of hyper-resistant Leishmania infantum strains, resulting in a chronic burden of the disease. In the present work, we have investigated the biochemical mechanisms of resistance to antimonials, paromomycin, and miltefosine in three drug-resistant parasitic strains from human clinical isolates, using a whole-cell mass spectrometry proteomics approach. We identified 14 differentially expressed proteins that were validated with their transcripts. Next, we employed functional association networks to identify parasite-specific proteins as potential targets for novel drug discovery studies. We used SeqAPASS analysis to predict susceptibility based on the evolutionary conservation of protein drug targets across species. MATH-domain-containing protein, adenosine triphosphate (ATP)-binding cassette B2, histone H4, calpain-like cysteine peptidase, and trypanothione reductase emerged as top candidates. Overall, this work identifies new biological targets for designing drugs to prevent the development of Leishmania drug resistance, while aligning with One Health principles that emphasize the interconnected health of people, animals, and ecosystems.
Keywords: ecotoxicology, Leishmania infantum, drug resistance, one health, proteomics, mass spectrometry
Leishmaniasis is a prevalent vector-borne parasitic disease (VBPD) and represents the second most widespread category of neglected tropical diseases (NTDs).1,2 These diseases, sustained by parasites of the Leishmania genus (Trypanosomatidae family), necessitate two hosts to complete their life cycle: a vertebrate host (mammals) acting as a disease reservoir and an invertebrate host (sand flies) serving as the vector.3 The nonflagellated promastigote form of the parasite infects the immune cells of vertebrates.4 Following the establishment of a parasitic guest–host crosstalk with infected macrophages or monocytes, the promastigote transforms into an amastigote, multiplies, and ultimately causes cell lysis and systemic infection.5 Therapeutic options vary depending on the Leishmania strain and endemic region, and include liposomal amphotericin B (Visceral Leishmaniases, VL), miltefosine (VL and cutaneous infection), and drug combinations like paromomycin administered with antimonials (African Leishmania strains).6,7 The mechanisms of action of these drugs remain poorly understood and are believed to target a pool of proteins, rather than individual hits.8 Antimonials, for instance, act by binding to thiol groups of mitochondrial proteins and inhibiting the formation of adenosine triphosphate (ATP) molecules and their precursors.9,10 Paromomycin inhibits parasite metabolism and mitochondrial respiration,10,11 while miltefosine, by mimicking membrane phosphocholine, weakens the integrity of the cell and mitochondrial membrane and causes parasite osmotic lysis.12,13
The unsupervised, excessive use of a limited array of available drugs has led to the selection of specific hyper-resistant strains, resulting in the rapid onset of drug resistance.14 Moreover, the drugs themselves or their metabolites can disperse into the environment, which contributes to this issue.15,16 These instances collectively lead to the limited therapeutic success of currently marketed drugs.17,18 Investigating the mechanism of action through modulation of the genome and proteome of parasites makes the omics field a suitable platform to investigate drug resistance.18
To date, only a few genes and proteins have been validated as contributing to Leishmania drug resistance, including ABC membrane transporter proteins, which mediate drug influx and efflux across the plasma membrane. Overexpression of the MRPA gene may be associated with intracellular thiol accumulation in the antimony resistance phenotype, along with trypanothione reductase.19 These proteins belong to the ATPases family and are the primary contributors to Sb(III), Sb(V), and paromomycin resistance, due to the sequestration of metal–thiol adducts into vesicular membranes.20,21 Other active transporters, like aquaporins (AQP), have been associated with a reduced cytosolic concentration of chemotherapeutics.22 Another resistance mechanism involves the up-regulation of cytosolic oxidoreductases from the trypanothione synthesis pathways (e.g., tryparedoxin peroxidase and peroxyredoxin), inducing metal ion chelation through free reduced thiols.23 Additionally, it has been demonstrated that chronic parasite exposure to metals like arsenic, often found in low, but biologically meaningful, levels in aquatic and terrestrial matrices (10–100 ppb), can induce cross-resistance to antimony, as they belong to the same chemical group.24 The close relationship between the environment and the spread of drug resistance highlights the need for implementing a One Health approach.25 Such an approach acknowledges the interdependence between healthy people, healthy animals, and healthy environments,26 and is increasingly recognized as a critical strategy toward more sustainable drug development.27 Until now, most studies on parasite resistance mechanisms have focused on promastigotes, but characterizing the amastigote proteome within actual immune cells would more directly reveal the guest–host crosstalk.21 To overcome and prevent drug-resistance development, a preventive strategy known as guest–host-directed therapy has been implemented.28 Examples of this approach include various natural compounds with immunomodulating activity that upregulate nitric oxide synthesis and IL-12 expression while downregulating IL-10 in macrophages infected with Leishmania spp.29 In addition, the use of specific PI3K inhibitors halts the reorganization of the actin cytoskeleton induced by parasite infection, thereby reducing the occurrence of resistance in murine models.30 In these instances, understanding the biochemistry of resistance mechanisms has supported therapeutic design strategies in which certain up-regulated proteins involved in drug-resistance mechanisms are targeted and down-regulated to prevent resistance development. Balancing the development of new therapeutics with reduced drug resistance susceptibility and decreased environmental impacts,31 requires the implementation of the principles of sustainable molecular design. This approach will guide the design of pharmaceuticals and other substances with desired functional characteristics while lowering environmental side effects.32,33
Over the past decade, the availability of genetic information across a wide array of species and the development and application of in silico (computational) tools have grown substantially. These advancements help increase our understanding of cross-species susceptibility to chemical exposure and support the extrapolation of molecular initiation events linked to adverse outcomes from environmental contaminants.33 Furthermore, these tools can help with identifying alternative, less hazardous chemicals that could substitute existing products and/or designing de novo chemicals that are less hazardous. This approach aligns with key principles of green chemistry and aims to reduce the environmental impacts of chemicals.32−36
In a prior study, we identified a set of differentially expressed proteins (DEPs) in THP-1 macrophages that were altered by drug-resistant L. infantum parasites.37 These strains originated from human clinical isolates taken from HIV-immunocompromised patients, resulting in disease recrudescence due to drug resistance against antimony, paromomycin, and miltefosine.38 Using a mass spectrometry (MS) bottom-up proteomic approach, we mapped the primary biochemical networks involved in drug resistance. Transferrin receptor C (TFRC) and nucleoside diphosphate kinase 3 (NDK3) were notably overexpressed in miltefosine and paromomycin samples, respectively.37 These findings were confirmed using both protein immunoblot and transcriptomics, thereby proposing TFRC and NDK3 as drug targets for a dual guest–host medicinal chemistry program.37 To apply the guest–host approach, it is necessary to identify specific/overexpressed proteins in the Leishmania parasite hosted by THP-1 human cells. In the present study, using whole-cell proteomics MS analysis, of various drug-resistant Leishmania infantum strains upon in vitro infection of THP-1 cells, we identify DEPs and highlight the metabolic pathways that are significantly up/down-regulated compared to nonresistant strains. Then, we compared L. infantum and their infected THP-1 to identify and exclude proteins belonging to the same metabolic pathways. This selection process enables the identification of proteins uniquely present in the Leishmania parasites and the THP-1 host. We further examine the evolutionary conservation of these putative protein targets across species to gauge the potential environmental impacts of these new antiparasitic agents. Specifically, we use SeqAPASS, an online FDA-approved screening tool to examine protein sequence/structural similarity among taxonomic groups and to identify species with heightened sensitivity to specific chemicals.35,39 The liquid chromatography–mass spectrometry (LC–MS)/MS characterization of new mechanisms of parasitic drug resistance stands as a useful effort in disease control, paving the way for the development of new target-based medicinal chemistry programs.
Results and Discussion
Design of the Experiment
In a previous study, we conducted a mass spectrometry (MS) proteomic analysis on THP-1 cells infected with L. infantum parasites. The samples were obtained from HIV-immunocompromised patients affected by recrudescent visceral leishmaniasis (VL).40 These samples are designated as Sb-RES (LEM3323, antimony resistant), PAR-RES (LEM2126, paromomycin resistant), and MIL-RES (LEM5159, miltefosine resistant) and were obtained as clinical isolates. We have used the same biological samples, in technical replicates for all the experiments, i.e., each biological specimen was analyzed in MS proteomics (human and L. infantum proteins detected) and in transcriptomics. The MS proteomic analysis identified human proteins that were up- or down-regulated in THP-1 cells due to the presence of drug-resistant parasites. In the present work, the same MS method is adopted to recognize proteins and pathways that are important for L. infantum to survive within THP-1 cells and to resist treatment with miltefosine, paromomycin, or antimonial cytotoxicity.37 The differentially expressed proteins (DEPs) in L. infantum strains were subsequently subjected to STRING enrichment analysis to better understand the cellular metabolic networks.41,42 The generation of enriched networks using dedicated bioinformatic tools (Gene Ontologies and REACTOME43,44) facilitated the identification of the most significant pathways expected to be involved in the resistance mechanisms. Subsequently, the pathways from both parasite and human networks enrichment from THP-1 analysis were compared to identify mutual (shared between human and Leishmania cells) and nonmutual (i.e., biochemically independent) pathways. In parallel, results from transcriptomics analysis from the same samples were used to confirm the differentially expressed proteins. Proteins whose biochemical pathways do not overlap between human and parasite origin, i.e., proteins belonging to nonmutual pathways, were investigated for their presence in non-target organisms, relevant from an ecotoxicological perspective, using the SeqAPASS tool, level 1 and 2 analyses. The highest-scored proteins serve as a starting point for investigating potential pharmaceutical targets in a drug discovery program. The SeqAPASS tool analysis was performed to verify whether the proteins might have homologues in other organisms in the environment because inhibiting them (off-targets) could potentially lead to environmental hazards. The experimental workflow is presented in Figure 1.
Figure 1.
Workflow of the process of sample analysis leading to the identification of selected proteins through an MS/ecotoxicology integrated approach.
Label-Free Analysis of L. infantum Drug-Resistant Strain Proteome
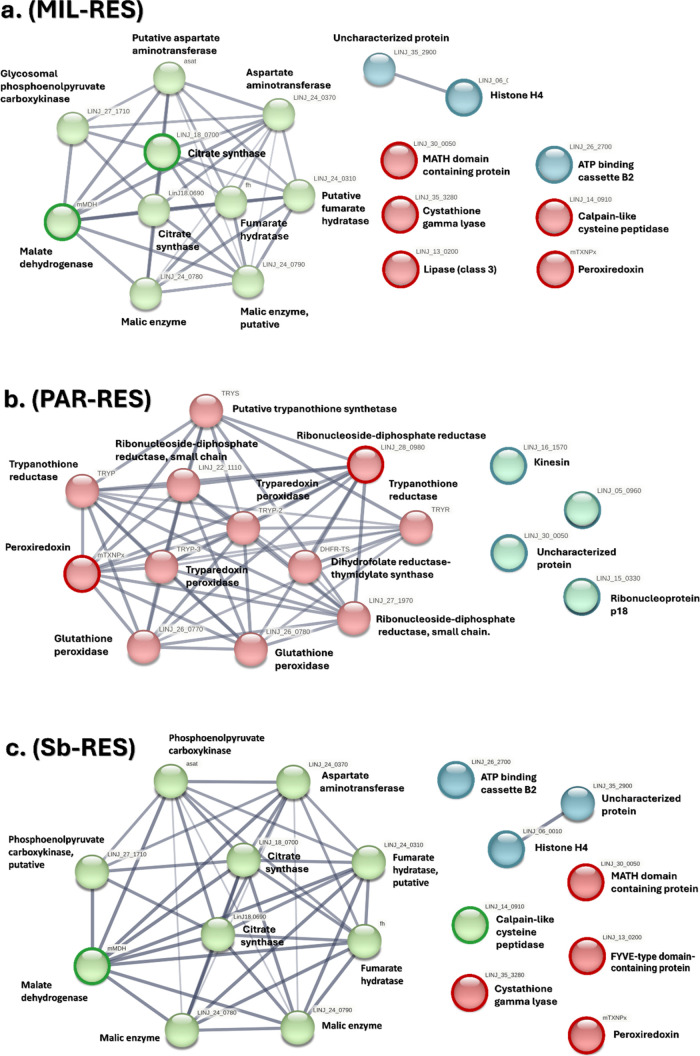
The L. infantum drug-resistant samples were processed following LC–MS/MS, and the results were analyzed using Mascot and Progenesis software for peptide identification and quantification. The MS proteome lists from each parasite drug-resistant strain were compared with those obtained from the nonresistant strains. In total, 155 differentially expressed proteins emerged from Sb-RES, 186 from PAR-RES, and 166 from MIL-RES. A t-test was used to discriminate DEPs (p < 0.05) as shown in the volcano plots in Figure 2 and the heatmap in Figure 3a. Eight DEPs emerged from sample Sb-RES, and six from samples PAR-RES and MIL-RES, two of which were mutual between Sb-RES and MIL-RES (malate dehydrogenase and ATP-binding cassette, ABC protein, subfamily B), one between Sb-RES and PAR-RES (MATH-domain-containing protein), and one mutual and overexpressed among the three samples versus control (peroxiredoxin), as in Figure 3b. The complete list of the DEPs for each strain, including their UniProt and STRING accession codes, their fold changes (FC)/p-values, and a brief description of their functions are included in Tables S1–S3.
Figure 2.
Volcano plots representing the DEPs identified from the analysis between Sb-RES parasites and nonresistant controls (a), PAR-RES parasites and nonresistant controls (b), and MIL-RES parasites and nonresistant controls (c). On the X-axis, FC (fold change) is represented as the log(2) of the ratios, and the Y-axis represents the −log(10) of the p-value. Red dots represent the up-regulated proteins (p-value <0.05, FC ≥ 1), blue dots represent the down-regulated proteins (p-value < 0.05, FC ≤ −1), gray dots represent nondifferentially expressed proteins (either with a p-value >0.05 and/or −1 < FC < 1). (d) Workflow employed for protein identification, quantification, and DEP obtainment.
Figure 3.
(a) Heatmap representing the DEPs of the three strains of THP1 cells infected with L. infantum with their respective fold changes in a color scale. Green indicates significantly down-regulated proteins, and red indicates up-regulated proteins. All the proteins with an FC < −1 and >1 and/or an associated p-value ≤0.05 were reported as not significant. (b) Venn’s diagram representing the mutual DEPs between the three resistant strains.
Mutual DEPs among Leishmania Drug-Resistant Strains
The proteins identified as significantly differentially up- or down-regulated in the comparison among at least two different drug-resistant Leishmania strains are described below. As shown in Figure 3a,b, peroxiredoxin is shared among all three strains, while malate dehydrogenase, ATP-binding cassette protein subfamily B member 2, and malate dehydrogenase belong to Sb- and MIL-RES strains. The MATH-domain-containing protein and peroxiredoxin are shared between PAR- and MIL-RES. Specifically, peroxiredoxin (UniProt gene: mTXNPx-tryparedoxin peroxidase, 226 aa. Abbreviation: TXNPx) belongs to a family of highly conserved enzymes found across the tree of life, from bacteria to humans, including Leishmania parasites.45 These enzymes are characterized by a redox-active cysteine (peroxidative cysteine), which oxidizes to sulfenic acid.46 Hosts are known to overexpress these enzymes to detoxify their cytoplasmatic environment from reactive oxygen species, such as H2O2 and alkyl hydroperoxides.47 In particular, the pair tryparedoxin/tryparedoxin peroxidase uses the electrons from trypanothione to reduce the hydrogen peroxide and alkyl hydroperoxide to water and alcohol, respectively.48 Tryparedoxin peroxidases are known to be overexpressed by Leishmania parasites infecting macrophages to neutralize the host’s free radicals and succeed in the infection.49 Interestingly, TXNPs have previously been linked to drug resistance to antimony in L. braziliensis and L. infantum lines, which exhibited increased tolerance to mild hydrogen peroxide administration in vitro. Earlier studies suggest that TXNPx overexpression may be attributed to more stable mRNA stability induced by acquired resistance to antimony.50,51 Additionally, the authors proposed that TXNPx overexpression itself could be the cause of drug resistance by directly sequestering SbIII along with the enzyme trypanothione S-transferase.47 These considerations suggest that L. infantum cytosolic tryparedoxin peroxidase could be investigated as a potential candidate for drug targeting in SbIII-sensitive lines. ATP-binding cassette (ABC) transporters represent an important family of membrane proteins, primarily located in the parasite plasma membrane and involved in transporting phosphatidylcholine analogues to the lipid layer.20,52 In our experiments, both Sb-RES and MIL-RES samples exhibited overexpression of subfamily B, member 2 of the ABC transporter, with FC values of 2.03 and 1.28, respectively. The overexpression of ABCB2 (also referred to as TAP1) was initially reported by Campos-Salinas et al.52 as one of the ATP-binding cassettes conferring resistance to miltefosine and other alkyl glycerophospholipids by reducing their cytosolic bioaccumulation.53,54 The up-regulation of its protein was confirmed through MS analysis.37 Additionally, excessive metal uptake in Leishmania ssp induces multiple ABCs overexpression and this confers resistance to antimonials.55,56 For this reason, a proposed strategy to mitigate drug resistance to SbIII involves minimizing parasite thiol levels in both the macrophage and the parasite through a dual guest–host strategy.57
During DEP′s analysis, a few proteins were associated with drug resistance (Table 1). Malate dehydrogenase (EC:1.1.1.37, mMDH) showed significant downregulation in both Sb-RES and MIL-RES. This mitochondrial constitutive enzyme catalyzes the oxidation of malate to oxalacetate by reducing NAD+ to NADH, and is a key enzyme of the tricarboxylic acid cycle.58 The MATH domain is a shared motif between two seemingly functionally unrelated proteins, TRAFs (TNF receptor-associated factors) and meprins.59 TRAF6, the most abundant TRAF isoform, positively regulates the inflammasome complex by recruiting the NLRP3 inflammasome in macrophages. Upon NFkB (nuclear factor kappa-light-chain-enhancer of activated B cells) and DAMPs (Damage-associated molecular patterns) activation, the inflammasome complex is formed, leading to the activation of caspase-1, release of IL-1β and IL-18, and pyroptosis.60Leishmania infection triggers the activation of the NLRP3 inflammasome in macrophages,61 although recent studies involving Leishmania amazonensis and Leishmania donovani, have demonstrated downregulation of components of the inflammasome in infected macrophages.62 The role and downregulation of MATH-domain-containing proteins in Leishmania-resistant lines remain to be clarified. A brief description of the DEPs found in individual drug-resistant strains is provided in Table 1.
Table 1. Description of the Proteins That Have Been Found as DEPs in Individual Leishmania Strains.
| protein name (common) | resistant strain | description |
|---|---|---|
| histone H4 | Sb-RES | histone H4 of L. infantum has a high degree of identity with the histone H4 gene of Leishmania tarentolae and a very low degree of identity with the corresponding region of histone H4 genes from other organisms. H4 synthesis in Leishmania is tightly coupled to DNA replication by a mechanism operating at the translational level.63 Despite not being directly related to drug resistance, H4 along with H3, H2A, and H2B, are studied as possible immunization agents responsible for DNA-based Leishmania immunity in vaccine development.64 |
| calpain-like cysteine peptidase | Sb-RES | calpain-like proteins constitute a recently discovered family of calcium-dependent cysteine peptidases.65 They have an important role as virulence factors that modulate the mammalian host’s immune response to Leishmania ssp.54 They are not reported to be related to drug-resistance phenomena, but their key role in Trypanosomatid infection makes them candidates for drug discovery.66 |
| Lipase—Class 3 | Sb-RES | lipases are esterases able to hydrolyze long-chain acyl-triglycerides into di- and monoglycerides, glycerol, and free fatty acids at a water/lipid interface for the survival and infection of pathogens.67 This enzyme was not correlated yet to drug resistance but gained interest in drug discovery of new Leishmania ssp. chemotherapeutic agents.68 |
| cystathione γ lyase | Sb-RES | cystathionine γ-lyase (or γ-cystathionine, EC:4.4.1.1) breaks down cystathionine into cysteine, α-ketobutyrate, and ammonia by using pyridoxal phosphate as prosthetic group. It is employed by the parasite to obtain cysteine from the host’s blood homocysteine.69 |
| acetyl-coenzyme A synthetase (AMP forming) | MIL-RES | adenosine monophosphate-forming acetyl CoA synthetase (AceCS, EC:6.2.1.1) is the key enzyme involved in the conversion of acetate to acetyl CoA. |
| dimethylargininase | MIL-RES | also known as dimethylarginine dimethylaminohydrolase (DDAH), it converts the hosts’ circulating Asymmetric dimethylarginine into dimethylamine and citrulline. Little information is reported in literature apart from its amino acidic sequences and encoding genes. |
| citrate synthase | MIL-RES | citrate synthase (E.C. 2.3.3.1) is in the mitochondrial matrix of eukaryotes and plays a crucial role in the Kreb’s Cycle. Indeed, it catalyzes the condensation of acetyl coenzyme A with oxaloacetate to regenerate citric acid.70 Along with glyoxylate cycle enzymes, it provides the parasite with ATP needed to replicate by breaking down the host’s glucose.70 |
| kinesin(s) | PAR-RES | kinesins are a large family of proteins that constitute part of the complex cytoskeletal structure of the parasite flagellum attachment zone.71 They are highly conserved (KIF superfamily) and are characterized by an ATPasic site and a microtubule-binding site.72 |
| ribonucleoprotein p18, mitochondrial precursor | PAR-RES | ribonucleoprotein p18 (Rab18) stands out among a limited set of conserved Rab proteins that can be traced back to the most recent common ancestor of eukaryotes. Even though its sequence is known, its precise role in organisms still must be uncovered.73 |
| trypanothione reductase | PAR-RES | trypanothione reductase (ThyR) is a key enzyme that prevents oxidation of the cytosolic trypanothione (N1–N8-bis(glutathionyl)-spermidine). It is employed by Leishmania to transfer electrons to the tryparedoxin-peroxidase couple, which reduces the reactive oxygen species produced by the host’s macrophages.74 Authors agree to consider it one of the most promising targets for Leishmania-specific drug design along with trypanothione synthetase (TryS), and it is also confirmed to be one of the many targets of Sb(III) complexes in Trypanosoma ssp.35,75 |
| dipeptidyl peptidase 3 | PAR-RES | dipeptidyl peptidase 3 is a member of the M49 peptidase family and is a zinc-dependent metallopeptidase able to cleave dipeptides from its substrates. It is a key enzyme for the parasite survival in the vector and host environments, as it allows to degrade exogenous protein fragments and obtain amino acids for energy production.76 Its levels were not related to drug resistance, but due to its detection suitability, its gene can be a reliable DNA marker to investigate the taxonomy of Leishmania and help disease surveillance.77 |
Functional and Physical Protein Associations of the L. infantum Strain in the Enrichment Analysis
After identifying the DEPs in the Leishmania parasite to show the interacting proteins and define the metabolic pathways, the DEPs of Sb-RES, PAR-RES, and MIL-RES, were analyzed with, STRINGprotein enrichment tool.42 This process facilitates the identification of protein pathways, providing insights into deeper interactions among proteins not identified directly in the above-described MS experiment but potentially important for understanding the functional metabolic roles of the proteins. The identified pathways are reported for each strain in Figure 4. Protein resulting from Sb-RES enrichment process are biologically connected as a group, suggested by the clustering coefficient of 0.636 (a measure of the degree to which proteins cluster together) and a PPI (protein–protein interaction) enrichment p-value of 0.0325 (threshold p < 0.05) (Figure 4a). The network enrichment of the initial eight parasitic DEPs (FDR 1 × 10–16, confidence level 0.400) for Sb-RES identified 42 enriched significant GO-terms (p < 0.05) across eight categories. For PAR-RES, the analysis of the four DEPs resulted in 16 proteins (knots) with 66 connections (edges). The clustering coefficient from the enrichment analysis was 0.756, and the PPI enrichment p-value indicated high significance (p < 1.0 × 10–16) (Figure 4b). For MIL-RES, the analysis found 16 nodes and 56 edges (Figure 3c). The overall outcome of this analysis suggests that paromomycin and antimony resistances are characterized by an over-representation of the tricarboxylic acid (TCA) energetic metabolism, along with malate dehydrogenase enzyme activity. On the other hand, miltefosine resistance lacks this up-regulated pathways and is instead characterized by an over-representation of parasite redox activity (Figure 4c). This observation suggests that paromomycin and antimonials share similar patterns associated with the parasite’s resistance mechanisms, while miltefosine induces a stress-like response in the cell.
Figure 4.
Functional and physical association networks of MIL-RES (a), PAR-RES (b), and Sb-RES (c). Different line colors represent the type of interaction and association (thin, light gray lines represent confidence >0.400 and <0.700, medium size lines represent confidence >0.700 and <0.900, and thick, darker lines represent confidence >0.900). Association parameters include i. experiments, ii. databases, iii. co-occurrence, and iv. coexpression). Nodes are colored by GO Biological Process enriched proteins. For these networks, the DEPs do not show significant conserved neighborhood (transferred by homology in other species) or significant coexpression (as gene place) with their predictive partners, but their putative homologues are neighbors or coexpressed in other organisms. The main GOs (Molecular Functions) for Sb-RES are citrate synthase activity (GO:0036440, TCA), and malate dehydrogenase (decarboxylating) (GO:0004473, TCA). The main GO′s (Molecular Functions) for PAR-RES are citrate synthase activity (GO:0036440, TCA), and malate dehydrogenase (decarboxylating) (GO:0004473, TCA). The main GO′s (Molecular Functions) for MIL-RES are peroxiredoxin activity (GO:0051920) and ribonucleoside-diphosphate reductase activity (GO:0004748). The outcome and parameters of STRING-enriched networks are reported in Table S4.
Comparison between Differentially Expressed Proteins and Genes (DEGs) in L. infantum Amastigotes
To cross-validate the biological significance of the DEPs identified in the proteomics experiments, the corresponding DEGs from previously performed transcriptomics on the same biological replicates were considered. Previously conducted transcriptomics experiments investigated mRNA levels in THP-1 human cells infected with the same three drug-resistant L. infantum strains considered in the current study.78 The authors identified >50 significantly up- or down-regulated transcripts per sample, that were compared with the present DEPs. However, a match between proteins and associated transcripts was not achieved, therefore our approach was focused on the identification of shared pathways for both transcripts and proteins using STRING and the corresponding GOs methods. The following transcript clusters: transport, uptake, and efflux across cellular membranes, energy and redox metabolism, and detoxification were compared with all the GOs identified for the proteins of the present study. The matching items (DEGs/DEPs) for these clusters are reported in Table 2, and the GOs entry lists for transcripts and proteins are in Tables S5 and S6.
Table 2. Differentially Expressed Genes (DEGs, from Transcriptomics) and Proteins (DEPs, from Proteomics), which Share the Same Functional Pathway.
| DEGs | DEPs | STRAIN | GOs |
|---|---|---|---|
| ATP-binding cassette protein subfamily B, member 2, putative | Sb-RES | transport, uptake, efflux across cellular membranes (GO:0090662, GO:0090484, GO:0043191) | |
| MIL-RES | |||
| ATP-binding cassette protein subfamily C, member 1, putative | MIL-RES | ||
| ATP-binding cassette protein subfamily C, member 2, putative | MIL-RES | ||
| acetyl-coenzyme A synthetase (AMP forming) | MIL-RES | energy metabolism and biosynthetic processes (GO:0016615, GO:0030060, GO:0004148, GO:0036440, GO:0004775, GO:0004739, GO:0004591, GO:0004473, GO:0004471) | |
| fatty-acyl-CoA synthetase 2 | Sb-RES | ||
| MIL-RES | |||
| dimethylargininase | MIL-RES | ||
| argininosuccinate synthase | MIL-RES | ||
| PAR-RES | |||
| trypanothione reductase | PAR-RES | redox metabolism and cell detoxification (GO:0016624, GO:0016903, GO:0016668, GO:0016491, GO:0004602, GO:0004601, GO:0016616) | |
| tryparedoxin peroxidase | PAR-RES | ||
| dipeptidyl peptidase 3 | PAR-RES | ||
| metallopeptidase, clan MF, family M17 | Sb-RES |
Interestingly, the transcriptomics experiments successfully identified genes coding for subfamily A and C of the ATP-binding cassette, while our proteomics experiment identified isoform B. Notably, both data sets revealed significant overexpression of the ABC transporter for the MIL-RES sample. Additionally, there was a distinct up-regulation in amino acid biosynthetic processes, particularly supported by the overexpression of Dimethylargininase (DEP) and Arginosuccinate synthase (DEG). These two enzymes play an important role in arginine metabolism and degradation, as parasites encounter arginine deprivation during host infection, which must be taken up by the monocytes.79,80 The up-regulation of acetyl-coenzyme A synthetase (AMP forming) in the proteome is corroborated by the overexpression of fatty-acyl-CoA synthetase 2 (FAS-2). The former, also known as Acetate CoA ligase (EC 6.2.1.1) is the exclusive cellular source of acetyl-CoA, while the latter (FAS-2) consumes an acetyl-CoA and seven malonyl-CoA molecules to produce palmitoyl-CoA, thus belonging to the same metabolic pathway (biosynthesis of fatty acids).81 Lastly, the up-regulation of tryparedoxin peroxidase (DEG in the PAR-RES line) aligns with the overexpression of the trypanothione reductase protein in the same strain. Exploiting cytosolic NADP(H), they cooperatively reduce free oxygen radicals and hydroperoxides to alcohols in a cascade of redox reactions.50 Trypanothione reductase is strictly related to tryparedoxin peroxidase, since the first reduces trypanothione used by the latter to neutralize hydrogen peroxide molecules during Leishmania infection.91 This has already been related to a physiological oxidative stress response of the parasite to maintain its homeostasis under the administration of toxic xenobiotics and antiparasitic agents. The identified DEPs emerged as belonging to the same pathways discovered in the transcriptomic analysis. Therefore, we proceeded with host-target protein analysis.
Host–Guest Crosstalk for Homo sapiens THP-1 and L. infantum
With the aim to identify pathways specific to the parasite and not shared with the human host, a bioinformatic analysis was developed on the DEPs previously selected from the MS analysis of the L. infantum samples (Table 1). The biological pathways from the human host and from the Leishmania parasite are compared by using GOs and REACTOME methods. Those pathways belonging to both systems are recognized and excluded from the final list of host–guest crosstalk analyzed in the subsequent chapter for the SeqAPASS study. Consequently, only pathways that are quantitatively and selectively regulated in the THP-1 host and L. infantum parasite are involved in the selective host–guest crosstalk. The approach considers pathways rather than individual proteins, providing a more global view of biological interactions. For this, the 14 identified DEPs (Table 1) were elaborated with the STRING tool, and five main metabolic pathways from the REACTOME database were identified as significant (p < 0.05). The pathways include glyoxylate and dicarboxylate metabolism, carbon metabolism, citrate cycle (TCA cycle), glutathione metabolism, and pyruvate metabolism (p < 0.05). The results highlight a strong involvement of cellular sugar/lipid metabolism and redox metabolism. The nonenriched STRING network and its associated REACTOME terms are presented in Figure S1. After network enrichment, the cellular metabolism emerges as the main pathway for L. infantum-resistant strains (Figure S2). The corresponding GOs and their REACTOME pathways (p < 0.05) are detailed in Tables S7 and S8. The same process was applied to the THP-1 DEPs (Figure S3, Tables S9 and S10). The comparison between parasitic and THP-1 GO pathways revealed the sharing of three mutual Biological Process (BP), namely: Metabolic Processes (MP) (GO:0044237), Cellular Metabolic Processes (CMP) (GO:0008152), and Cellular Processes (CP) (GO:0009987) (Figure 5a). Similarly, the comparison between L. infantum and THP-1 REACTOME Pathways identified four mutual pathways: metabolism of amino acid and derivatives (HSA-71291/MAP-71291), pyruvate metabolism (HAS-00620/MAP-70268), cell metabolism (MAP-1430728/HSA-1430728) and citric acid cycle (TCA cycle) (MAP-71403/HSA-1428517) (Figure 5b). The final proteins selection workflow for SeqAPASS analysis is reported in Figure 5c. The complete pathway lists are detailed in Tables S11 and S12.
Figure 5.
Comparison of the L. infantum and THP-1 pathways in GO/REACTOME. (a) Venn diagram representing the comparison between parasitic and THP-1 GO pathways revealing the sharing of metabolic processes (MP) (GO:0044237), cellular metabolic processes (CMP) (GO:0008152), and cellular processes (CP) (GO:0009987). (b) Venn diagram representing the comparison between L. infantum and THP-1 REACTOME pathways revealing the sharing of pyruvate metabolism (HAS-00620/MAP-70268), metabolism of amino acid and derivatives (HSA-71291/MAP-71291), cell metabolism (MAP-1430728/HSA-1430728), and citric acid cycle (TCA cycle) (MAP-71403/HSA-1428517). (c) Flow of the analysis leading to the Venn diagram (a) and (b) and final protein selection for the SeqAPASS analysis.
Then, a network enrichment of the nonmutual parasitic DEPs from the initial MS analysis was performed (histone H4, ATP-binding cassette protein subfamily B -member 2- putative, calpain-like cysteine peptidase and trypanothione reductase) after performing the exclusion process reported in Figure 5. The STRING enrichment of the four selected DEPs (p < 1.0 × 10–16) generated four independent clusters. The most significant cluster contains proteins involved in transcription and translation, generated around histone H4. A cluster represents part of the folate metabolism and redox system; a two-protein system covers part of the membrane transport system, as shown in Figure 6.
Figure 6.
Enriched STRING Network of the four parasite DEPs. The red cluster represents the RNA and DNA mediated transcription cluster (GO:0006351 p = 9.10 × 10–9 and GO:0006383 p = 0.011), the green cluster represents oxidoreductase activity (GO:0016624, p = 0.018), and the blue cluster represents the ABC transporters (CL:8031, p = 0.0068). Network parameters: number of nodes: 24. number of edges: 51. average node degree: 5.37. avg. local clustering coefficient: 0.744. expected number of edges: 18. PPI enrichment p-value: < 6.6 × 10–11.
SeqAPASS Analysis
To understand whether the identified proteins can be considered for further drug discovery studies, it is important to consider the evolutionary conservation of targets across species and the implications for the protection of biodiversity. In the current study, the seven proteins identified by proteomic analysis were subjected to a level one SeqAPASS analysis, which compared the total protein structure of each query protein at a sequence level. Peroxiredoxin (A0A6L0XFC6_LEIIN) and malate dehydrogenase (A4I9I5_LEIIN) were identified to have a large number of orthologs (1857 and 1065, respectively) above a susceptibility threshold (34.49%, 25.85%; Figure 7a,b, Table S14). This observation indicates that protein targets are highly conserved across taxa, including species in the Leishmania genus, which are intended to be targeted by a designed compound in a drug discovery program, along with species in diverse taxonomic classes such as Insecta, Actinopteri, Aves, Mammalia, Reptilia, and Amphibia. However, three target proteins—MATH-domain-containing protein, A0A6L0XK78_LEIIN; ATP-binding cassette protein subfamily B member 2, A0A6L0XH07_LEIIN; MATH-domain-containing protein, A0A6L0XK78_LEIIN; and trypanothione reductase, A0A6L0WLV7_LEIIN—only resulted in 1 ortholog. Additionally, two query proteins, histone H4, A0A6L0WIT6_LEIIN, and calpain-like cysteine peptidase, A0A6L0WUS2_LEIIN, resulted in two ortholog candidates. It is important to note that Kinetoplastea was the only taxonomic class with a predicted susceptibility for these five candidate proteins (Figure 7c–g), highlighting a distinct level of conservation or functional specificity of the new antiparasitic agents against L. infantum.
Figure 7.
SeqAPASS Level 1 analysis demonstrating the similarity of orthologs for each respective protein in L. infantum (a–g) and Strigomonas culicis (h). Parenthetical numbers indicate the number of individual species analyzed. Red horizontal lines indicate susceptibility cutoffs: (a) 34.49%, (b) 25.85%, (c) 100%, (d) 100%. (e) 100%, (f) 100%, (g) 100%, and (h) 66.34%. (* Putative Protein Sequence). (i) Amino acid alignment of calpain-like cysteine peptidase orthologs from L. infantum (A0A6L0WUS2_LEIIN) and S. culicis (S9UQJ9_9TRYP). Text in red indicates total alignment (strong conservation), whereas cyan indicates conservation between groups of strongly similar properties as below, roughly equivalent to scoring >0.5 in the Gonnet PAM 250 matrix. The panel was generated using BioEdit Sequence Alignment Editor software.
Furthermore, calpain-like cysteine peptidase has no orthologues outside of Kinetoplastea, indicating that it is an excellent candidate for developing targeted pharmaceutical interventions. While calpain is endogenous to humans, the structure of this protein did not align with our query protein, meaning it is structurally dissimilar and not expected to interact with a drug targeting calpain-like cysteine peptidase in L. infantum (Figure 7i). Given the specific conservation of these five proteins in L. infantum, the current analysis implies a crucial role or a unique function for these proteins in Kinetoplastea (Figure 7c–g), but not other taxonomic classes. This suggests that other species are not likely to be susceptible to the new antiparasitic medicine because they have unique structures and therefore can be potentially targeted by compounds that are rationally designed to interact with the protein. However, note that SeqAPASS is limited by current data availability. The susceptibility cutoff for these query proteins was 100% (fewer species) with three target protein sequences identified as putative (ATP-binding cassette protein subfamily B member 2, NCBI: CAC9497987.1, MATH-domain-containing protein, NCBI: CAC9511396.1, and calpain-like cysteine peroxidase, NCBI: CAC9469271.1). Depending on putative sequence information, for which sequence information is hypothesized to be accurate, the ability of SeqAPASS to accurately identify potential orthologues via the initial BLASTp analysis may be restricted. However, SeqAPASS analysis of calpain-like cysteine peptidase from S. culicis, the most similar, nonhypothetical protein sequence resulted in similar results, with a lower susceptibility cutoff (66.34%, Figure 7h). The confirmation of results using an established primary structure (S9UQJ9_9TRYP) strengthens the opportunity for targeting calpain-like cysteine peptidase in L. infantum. Additionally, a level two SeqAPASS analysis, which compares sequence information within a specific domain rather than the total protein sequence, revealed that there are no susceptible species outside of Kinetoplastea for the Domain of Unknown Function found on the calpain-like protein highlighted in this study (Figure S8). Despite these limitations, knowledge of SeqAPASS could be expanded as sequencing data is broadened to include additional species. Moreover, the application of SeqAPASS facilitates a comprehensive consideration of evolutionary conservation targets across species, which is essential for balancing novel drug development to mitigate potential ecological impacts. Additionally, as sequence information for nonmodel species becomes more available and accurate, the capabilities of SeqAPASS will parallel these advancements. To further our understanding of potential ecological risks, additional information must be considered, including internal disposition within and among species, which influences delivery to target sites, as demonstrated by ivermectin, a common antiparasitic drug that does not cross the blood–brain barrier in mammals. Future studies are needed to advance comparative pharmacokinetics across non-target species of ecological importance.82
Conclusions
By exploiting an LC–MS/MS proteomics pipeline, we identified 14 differentially expressed proteins (DEPs) from three different drug-resistant L. infantum strains from clinical isolates. Samples resistant to antimonials, paromomycin, and miltefosine demonstrated a peculiar biochemical pattern compared to nonresistant L. infantum samples. Peroxiredoxin is the only significant protein overexpressed in all three strains, while other proteins are up- or down-regulated in more than one sample vs. control. These proteins or similar ones were found during the RTqPCR transcriptomics experiments performed previously, suggesting an important role in drug-resistance mechanisms. Using bioinformatics tools such as REACTOME, GO’s of the biological networks linked to these proteins were identified. On these bases it is suggested that resistance to the above-mentioned drugs may lead to an imbalance of the Kreb’s cycle for antimony and paromomycin resistance and of trypanothione redox metabolic pathway in the case of miltefosine resistance. A further comparison performed between the parasites’ transitory host proteome, from H. sapiens THP-1 monocytes, and the parasite, allowed the identification of the biological networks involved in a crosstalk mechanism that on one side is exploited by the parasite to root the infection, and on the other is induced onto the host to endorse drug-resistance processes (e.g., the up-regulation of the iron uptake machinery). Five relevant proteins were identified including calpain-like cysteine peptidase, trypanothione reductase, histone H4, MATH-domain-containing protein, and the ATP-binding cassette B2. From the perspective of integrating One Health principles into the drug discovery pipeline considering the health of patients alongside that of animals and the natural environment, the differentially expressed proteins from L. infantum strains were submitted to an ecotoxicological screening to predict their species-specificity and guide the preliminary target identification process by considering the homology level in a comparative analysis with species of interest. The SeqAPASS analysis has selected calpain-like cysteine peptidase, trypanothione reductase, histone H4, MATH-domain-containing protein, and the ATP-binding cassette B2 as the best hits in terms of sequence homology, ranking the calpain-like cysteine peptidase as the best one, presenting <25% of homology with other organisms. In higher organisms, the calpain-like cysteine peptidase is a peptidase known also as a target for antiparasitic drugs. It shows a high molecular weight and a catalytic peptidase active site. In the Leishmania species studied in the present work, the identified short-length calpain-like cysteine peptidase protein does not show any enzymatic activity, no catalytic site is present, and no crystal structure was solved. The short-length calpain-like cysteine peptidase shares 47% homology sequence, including the flagellum binding motif, with the parasitic small myristoilated proteins (SMPs) from Leishmania major. The SMP structure was solved using NMR studies.83,84 The calpain-like peptidases are associated with structural functions, mainly as flagellum and microtubule-stabilizing agents with crucial roles in trypanosome morphology transitions, as well as immunogenicity and macrophage recognition66,85 Indeed, this protein family has been considered for epitope-based vaccine design, against L. major infection in vivo.86,87 By targeting the parasitic calpain-like function and the host’s TFRC or NDK3 previously related to the drug-resistance mechanism, it is expected to lower the drug resistance development in the Leishmania strains. A representation of the localization of the studied proteins within the amastigote L. infantum infecting the THP-1 cells is shown in Figure 8.
Figure 8.
Representation of L. infantum amastigote (pale brown) infecting a human THP-1 monocyte (green). The macrophage vacuole is darker green, and the most significant proteins that emerged from this study for the proposed dual guest–host targeting strategy are represented. The crystal structure of the 6 proteins are taken from the PDB. The protein represented in the cartoons are the DEPs from L. infantum (peroxiredoxin, calpain-like protein, ATP-binding cassette domain, histone H4) and THP-1 monocytes (nucleoside diphosphate kinase 3, and transferrin receptor C) and are the best MS proteins identified, not shared between the parasite and the human proteome. Referring to host protein targeting, the green arrow indicates the inhibition of human NDK3 leading to a reduction in nucleotide synthesis in the amastigote; the green arrow indicates the inhibition of the human transferrin receptor C (THP-1 cell membrane) leads to a reduction of bioavailable F2II levels. Both inhibition processes are reported with the red “no entry” sign. The cartoon was generated with the BioRender Suite.
The guest–host therapeutic concept has recently been consolidated for the treatment of different infectious diseases, for instance hepatitis, ebolavirus, tuberculosis, and pneumonia. New drug discovery programs based on X-ray crystals of the investigated proteins, and knockout models of L. infantum amastigotes to demonstrate first the importance of calpains in the context of parasite survival should be developed.
Materials and Methods
Experimental Design and Workflow
Based on the premise that drug-resistance mechanisms involve complex remodulation of genes and functional protein expressions, the primary objective of this study is to identify the differentially expressed proteins (DEPs) synthesized by three distinct clinical isolates of Leishmania when undergoing phagocytosis by THP-1 myelomonocytic cells in vitro. The investigated strains are, respectively, Sb-RES (antimony resistant), PAR-RES (paromomycin resistant), and MIL-RES (miltefosine resistant). The nonresistant strain Hi-LJPC served as a negative control. Results were obtained by ultra-high performance liquid chromatography-high-resolution mass spectrometry (UHPLC-HRMS) analysis, coupled with metadata integration, biochemical-based functional network enrichment, and other bioinformatic tools. Then, a comparative analysis was conducted between the identified DEPs and the differentially expressed genes obtained from the same samples, as reported by García-Hernández et al., to corroborate the identification of target proteins.87 The DEPs from the three parasitic strains were further investigated and their associated GO′s and REACTOME pathways were compared to the GOs and REACTOME pathways from THP-1 hosts.37 This analysis resulted in the identification of mutually shared and nonmutual biochemical pathways. The proteins associated with the former were then scrutinized from an ecotoxicological point of view, while those in the latter category were studied for medicinal chemistry purposes and putative new drug targets.
L. infantum Strain Description and Cell Preparation
Human THP-1 myelomonocytic cells were grown at 37 °C and 5% CO 2 in RPMI-1640 medium supplemented with 10% hiFBS, 2 mM glutamate, 100 U/mL penicillin, and 100 mg/mL streptomycin, as described in Perea-Martínez et al.88 Briefly, THP-1 cells (30 × 106 cells in 175 cm 2 flasks) underwent differentiation into macrophages through a 48 h treatment with 20 ng/mL of PMA, followed by 24 h of incubation in fresh medium. All L. infantum lines were cultivated as described in Raquel García-Hernández et al. prior to the infection process.87L. infantum stationary phase promastigotes were incubated for 72 h in acid medium plus 10% heat-inactivated fetal bovine serum (hiFBS) at a macrophage/parasite ratio of 1:10. Infected macrophages were then incubated in RPMI-1640 medium plus 10% hiFBS at 37 °C and 5% CO2 for 96 h. Thus, the infections of the different L. infantum lines were very similar. To determine the percentage of infection and the average of amastigotes by cell, macrophages were infected with the L. infantum lines treated in parallel under the same conditions as those employed for RNA extraction and visualized by microscopy. The following L. infantum lines were used for cell infections: i. Hi-L3323, an antimony-resistant line isolated from an immunocompromised patient with visceral leishmaniasis (VL), with a Resistance Index (RI) vs Hi-LJPC line in amastigotes >1.6-fold; ii. Hi-L2126, a paromomycin-resistant line isolated from another immunocompromised patient with VL, with a RI vs Hi-LJPC in amastigotes >3.9- fold; L. infantum Hi-L5159, a miltefosine resistant, with a measured RI vs Hi-LJPC in amastigotes higher than 13.7-fold. The Hi-LJPC nonresistant line served as the negative control. The drug resistance phenotype of the L. infantum lines was maintained throughout the time they were in in vitro culture.
Sample Treatment and LC–MS/MS Analysis
Infected THP-1 cells were detached and resuspended in lysis buffer containing 7 M urea, 2 M thiourea, 40 mM Tris, and 4% CHAPS (Sigma-Aldrich, Saint Louis, MO), supplemented with a Complete Mini EDTA-free Protease Inhibitor cocktail 2x (Roche, Basel, Switzerland). Following three freeze–thaw cycles, 150 μL of rehydration buffer (7 M urea, 2 M thiourea, 1% DTT) was added. The lysates were homogenized for 30 min and centrifuged at 20.000g at 4 °C for 45 min to remove debris. A filter-aided sample preparation (FASP) protocol was implemented for tryptic digestion before LC–MS analysis. Briefly, 200 μg of protein lysate (supernatant) was loaded onto 30 kDa MWCO filters (Millipore, Burlington, Massachusetts), denatured, reduced, alkylated, and digested with trypsin overnight.42 The recovered peptides were desalted with C-18 SPE 7 mm/3 mL extraction columns (Empore, CDS analytical, Oxford, PA) and dried in a SpeedVac evaporator (Eppendorf Corporation, Hamburg, Germany). Samples were reconstituted in mobile phase with 2% FA at a final concentration of 1 mg/mL and analyzed on an UltiMate 3000 UHPLC coupled to an Orbitrap Q-Exactive Ultra High Resolution Mass Spectrometer in a 3hour run in MS/MS data-dependent acquisition, as previously described.37 The assays were performed using two biological replicates on the same samples used for transcriptomic analysis.
Preliminary Protein Identification
Raw files were analyzed with Mascot (Matrix Science, U.K.) for peptide identification,89 and with Progenesis QI for Proteomics suite (Nonlinear, Waters Corp) for protein quantitation. Peptides were searched against a FASTA database built with annotations from SwissProt and UniProt/TrEMBL,90,91 with the L. infantum proteome (generated on 6th October 2022), 27318 annotations from SwissProt exclusively belonging to H. sapiens, and 115 entries from the Common Repository of Adventitious Proteins database (cRAP) for contaminants identification.92 A p = 0.05 was used for peptide matching on Mascot, including decoy-database research (percolator, <1% FDR). Significant peptide-to-spectrum maps were imported into the Progenesis suite for alignment and normalization against L. infantum proteins between runs. Protein abundances were quantified based on their nonconflicting peptides, each having at least one unique peptide. The t-test was used between the JPC control and Sb-RES, PAR-RES, and Sb-RES lines to assess the degree of expression and its associated p-value per each protein. Proteins with a FC ≥ 1 (ratio of 2-fold expression vs control)/FC ≤ 1 (ratio of 0.5-fold expression vs control), and p-value < 0.05 were considered as differentially expressed (DEPs).
STRING Analysis, Enrichment, and GO′s Clustering
We conducted a Functional and Physical Protein association analysis on STRING (v11.5) using the DEPs from each strain.42 For each strain, we submitted its DEPs to STRING using their sequence under the Multiple sequences query. The network settings included edges indicating both functional and physical protein associations, with the line color indicating the type of interaction evidence. The minimum required interaction score was set to an average confidence of 0.400, and the maximum number of interactors shown was set to no more than 10 in the first shell and no more than 10 in the second shell. Furthermore, the network was clustered into a specified number of clusters (three) using k-mean clustering, with the line thickness representing the strength of the data support. Finally, we identified the common enriched terms between Sb-RES, PAR-RES, and Sb-RES to gain insights into which pathways could be associated with drug resistance.
Comparison of DEPs with Their Corresponding Transcripts
A comparative analysis between the DEPs identified in this experiment and the transcripts from Garcia-Hernandez et al.48 was initially conducted on the same biological replicates by aligning the gene list to the UniProt entries representing the proteins. A subsequent analysis was performed using STRING and AmiGO on the corresponding GOs from García-Hernández et al.87 to assess the shared biochemical networks. The GOs from the cluster reported by García-Hernández et al.87 were compared with those emerging from the DEPs analysis and the proteins concurrently belonging to the same GOs were listed in Table 2.
Comparison of the Biochemical Pathways Common to H. sapiens and L. infantum
The DEPs identified in the three resistant strains were submitted to STRING enrichment, resulting in a total of 35 proteins. The GOs (Biological Process, FDR < 0.05) that emerged from this enrichment were compared to the GOs obtained after STRING enrichment of the corresponding human proteins from Tagliazucchi et al.37 A Venn diagram was constructed to illustrate mutual GO′s. The same procedure was repeated for the Reactome Pathways, maintaining the same level of significance (<5% FRD).93 The results of each analysis were compared between H. sapiens and L. infantum to distinguish mutual and nonmutual pathways. The Excel function employed for the comparison of Human/parasitic GOs and Reactome Pathways is as follows: = COUNTIF (F2:F94;B2) = 1 and IF(ISERROR(MATCHF2;$B$2:$B$535;0));″″;F2). These formulas identify duplicate values in the two columns when applied.
SeqAPASS Analysis
We used SeqAPASS (Version 7.0) to examine potential protein targets of this new antiparasitic medicine in L. infantum and other species.35 A level one SeqAPASS analysis was completed to determine whether orthologues of differentially expressed proteins are susceptible to this medication. Briefly, the National Centre for Biotechnology Information accession number for each of the seven potential target proteins was used individually as the query protein in level one analysis. An initial BLASTp analysis identified all potential alignments, regardless of taxa, length, or function. Alignments were then collated and plotted on a density plot based on similarity. SeqAPASS then identified local minimums and found the next ortholog candidate, which it set as potential susceptibility cutoffs, defined as a percent similarity. For the current analysis, the default susceptibility cutoff was employed, which identified the first local minimum and found the next ortholog candidate.39 Additionally, we observed results from BLASTp on calpain-like cysteine peptidase (A0A6L0WUS2_LEIIN), which was designated as a putative sequence, and identified calpain-like cysteine peptidase from S. culicis (S9UQJ9_9TRYP) as the most similar, nonputative protein. This accession was selected for additional SeqAPASS analysis (Supporting Information S2). A level two SeqAPASS analysis, which functions identically to a level one analysis but on a domain-specific level, was also conducted on domains from the four proteins that displayed a 100% susceptibility cutoff in level 1 analysis. Specifically, we examined TIGR01423 (trypanothione-disulfide reductase) from Trypanothione Reductase, COG1132 (MdlB, ABC-type multidrug transport system, ATPase and permease components) from ATP-Binding Cassette Protein Subfamily B Member 2, COG1196 (Smc, Chromosome segregation ATPases), and cd99121 (meprin and TRAF-C homology domain) from MATH-domain-containing Protein, and pfam09149 (domain of unknown function) from Calpain-like Cystein Peptidase. (Supporting Information S5–S8).
Statistics
DEPs were identified through a t-test comparing replicates from the three distinct drug-resistant lines with the nonresistant line. Significance parameters included a p-value <0.05 and a fold change (FC) ≥ 1 for the up-regulated proteins and FC ≤ −1 for the down-regulated proteins, corresponding to a 2-fold expression ratio. STRING significance for network generations was set to an FDR 0.400 (medium confidence), whereas STRING clustering employed a k-mean model with an inflation parameter set to 3. All the GOs and KEGGs reported in this study held a significance of p < 0.05.
Acknowledgments
We thank Dr. F. Javier Moreno from the WHO Collaborating Centre for Leishmaniasis, Instituto de Salud Carlos III (ISCIII), for providing L. infantum lines LLM2070, LLM2165, LLM2255, and LLM2221, isolated from HIV-positive patients with visceral leishmaniasis and TF, and the paromomycin-resistant L. infantum line LEM2126 (L2126) used in this study. Also, we thank Dr. Laurence Lachaud from the Centre National de Référence des Leishmanioses, Université Montpellier (Montpellier, France), for providing the drug-resistant L. infantum lines used in this work: LEM3323 (L3323) and LEM5159 (L5159), which are SbIII- and MIL-resistant lines, respectively. The authors acknowledge Dr. Sotiris Ouzounis for critically reading the manuscript and providing suggestions for the statistics part. The authors also thank Dr. Stefania Ferrari for annotating L. infantum database for protein search, which was obtained from SwissProt (L. infantum entry, exported in FASTA format, updated January 2023, updates are ongoing). The authors acknowledge the “Fondazione Cassa di Risparmio di Modena” for funding the UHPLC-ESI-HRMS Q-Exactive system at the Centro Interdipartimentale Grandi Strumenti (CIGS) of the University of Modena and Reggio Emilia.
Data Availability Statement
Raw MS data, including the values of the areas of the proteins and their associated p-values, are available on the public repository Fairdom at https://fairdomhub.org/projects/378.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.4c00185.
DEPs identified in LINF 3323 (antimony resistant) vs control differential analysis (Table S1); DEPs identified in LINF 2126 (paromomycin resistant) vs control differential analysis) (Table S2); DEPs identified in LINF 5159 (miltefosine resistant) vs control differential analysis (Table S3); parameters associated with the three STRING networks generated with all the DEPs from the three resistant lines (Table S4); L. infantum DEGs from García-Hernández et al., 2022 (Table S5); GO′s obtained from the DEPs analysis for proteins-transcripts comparison (Table S6); STRING network generated with all the DEPs from the three resistant strains and their corresponding KEGGS pathways with the FDR value before network enrichment (Figure S1); Leishmania STRING network generated with all the DEPs from the three resistant strains and their corresponding GOs (Biological Process) and REACTOME pathways with the FDR value and the associated FDR after network enrichment (Figure S2); STRING network generated with all the DEPs from the three THP-1 Human proteomes infected with the resistant strains [Tagliazucchi et al.] (Figure S3); GOs (Biological Process) associated with the enriched STRING network in Figure 4, main text, with their corresponding statistics and significance (Table S7); REACTOME Pathways associated with the enriched STRING network in Figure 4, main text, with their corresponding statistics and significance) (Table S8); GOs (Biological Processes) emerged from the network represented in Figure 3, Supporting Information, with their associated statistical parameters (Table S9); REACTOME Pathways emerged from the network represented in Figure 3, Supporting Information, with their associated statistical parameters (Table S10); GOs terms emerged from Venn’s diagram generated by the intersection between GOs from Human and Parasitic biochemical pathways (Table S11); REACTOME Pathways terms emerged from the Venn diagram generated by intersection between GOs from Human and Parasitic biochemical pathways (Table S12); terms of the enriched STRING network generated with the DEPs that do not share the same GOs as the DEPs belonging to the THP-1 cells (Table S13); results from Level 1 SeqAPASS analysis for a novel antiparasitic drug (Table S14); boxplot describing SeqAPASS data illustrating the percent similarity of (A) all protein sequences across species compared to the primary amino acid sequences and (B) functional domain of L. infantum trypanothione Reductase (Figure S5); boxplot describing SeqAPASS data illustrating the percent similarity of (A) all protein sequences across species compared to the primary amino acid sequences and (B) functional domain of L. infantum ATP-Binding cassette protein (Figure S6); boxplot describing SeqAPASS data illustrating the percent similarity of (A) all protein sequences across species compared to the primary amino acid sequences, (B) functional domain of COG1196 Smc and (C) cd99121 MATH domain of L. infantum MATH-domain-containing protein (putative) (Figure S7); boxplot describing SeqAPASS data illustrating the percent similarity of (A) all protein sequence across species compared to the primary amino acid sequences and (B) functional domain of L. infantum Calpain-like cysteine peptidase (Figure S8) (PDF)
Sequence alignment to predict across species susceptibility (SeqAPASS) raw data (XLSX)
This work was inspired and developed within the COST Action “OneHealthdrugs” CA21111. Thanks are due to Grant RTI2018-097210-B-100 funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A Way of Making Europe” awarded to F. Gamarro.
The authors declare no competing financial interest.
Special Issue
Published as part of ACS Infectious Diseases virtual special issue “One Health and Vector Borne Parasitic Diseases”.
Supplementary Material
References
- Akuffo R.; Wilson M.; Sarfo B.; Attram N.; Mosore M.-T.; Yeboah C.; Cruz I.; Ruiz-Postigo J.-A.; Boakye D.; Moreno J.; Anto F. Prevalence of Leishmania Infection in Three Communities of Oti Region, Ghana. PLoS Neglected Trop. Dis. 2021, 15 (5), e0009413 10.1371/journal.pntd.0009413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P.; Scott P. Leishmaniasis: Complexity at the Host–Pathogen Interface. Nat. Rev. Microbiol. 2011, 9 (8), 604–615. 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- Bates P. A. Revising Leishmania’s Life Cycle. Nat. Microbiol. 2018, 3 (5), 529–530. 10.1038/s41564-018-0154-2. [DOI] [PubMed] [Google Scholar]
- Navea-Pérez H. M.; Díaz-Sáez V.; Corpas-López V.; Merino-Espinosa G.; Morillas-Márquez F.; Martín-Sánchez J. Leishmania infantum in Wild Rodents: Reservoirs or Just Irrelevant Incidental Hosts?. Parasitol. Res. 2015, 114 (6), 2363–2370. 10.1007/s00436-015-4434-y. [DOI] [PubMed] [Google Scholar]
- Wheeler R. J.; Gluenz E.; Gull K. The Cell Cycle of Leishmania: Morphogenetic Events and Their Implications for Parasite Biology. Mol. Microbiol. 2011, 79 (3), 647–662. 10.1111/j.1365-2958.2010.07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrillo L.; Castro A.; Matía B.; Molina L.; García-Martínez J.; Jaqueti J.; García-Arata I.; Carrillo E.; Moreno J.; Ruiz-Giardin J. M.; San Martín J. Clinical Aspects of Visceral Leishmaniasis Caused by L. infantum in Adults. Ten Years of Experience of the Largest Outbreak in Europe: What Have We Learned?. Parasites Vectors 2019, 12 (1), 359. 10.1186/s13071-019-3628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafim T. D.; Iniguez E.; Oliveira F. Leishmania infantum. Trends Parasitol. 2020, 36 (1), 80–81. 10.1016/j.pt.2019.10.006. [DOI] [PubMed] [Google Scholar]
- Ponte-Sucre A.; Diaz E.; Padrón-Nieves M.. The Concept of Fitness and Drug Resistance in Leishmania. In Drug Resistance in Leishmania Parasites; Springer: Vienna, 2012; pp 431–449. [Google Scholar]
- Baiocco P.; Franceschini S.; Ilari A.; Colotti G. Trypanothione Reductase from Leishmania infantum: Cloning, Expression, Purification, Crystallization and Preliminary X-Ray Data Analysis. Protein Pept. Lett. 2009, 16 (2), 196–200. 10.2174/092986609787316306. [DOI] [PubMed] [Google Scholar]
- Bhandari V.; Sundar S.; Dujardin J. C.; Salotra P. Elucidation of Cellular Mechanisms Involved in Experimental Paromomycin Resistance in Leishmania Donovani. Antimicrob. Agents Chemother. 2014, 58 (5), 2580–2585. 10.1128/AAC.01574-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci M.; Luciani R.; Gianquinto E.; Pozzi C.; Pisa di.; Iacono F.; Dello L.; Landi G.; Tagliazucchi L.; Mangani S.; Spyrakis F.; Costi M. P. Repurposing the Trypanosomatidic GSK Kinetobox for the Inhibition of Parasitic Pteridine and Dihydrofolate Reductases. Pharmaceuticals 2021, 14 (12), 1246. 10.3390/ph14121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perezvictoria F.; Sanchezcanete M.; Seifert K.; Croft S.; Sundar S.; Castanys S.; Gamarro F. Mechanisms of Experimental Resistance of Leishmania to Miltefosine: Implications for Clinical Use. Drug Resistance Updates 2006, 9 (1–2), 26–39. 10.1016/j.drup.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Monge-Maillo B.; Lopez-Velez R. Miltefosine for Visceral and Cutaneous Leishmaniasis: Drug Characteristics and Evidence-Based Treatment Recommendations. Clin. Infect. Dis. 2015, e35671 10.1093/cid/civ004. [DOI] [PubMed] [Google Scholar]
- Croft S. L.; Sundar S.; Fairlamb A. H. Drug Resistance in Leishmaniasis. Clin. Microbiol. Rev. 2006, 19 (1), 111–126. 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno D.; Maia C.; Aït-Oudhia K. Antimony Resistance and Environment: Elusive Links to Explore during Leishmania Life Cycle. Int. J. Parasitol.: Drugs Drug Resist. 2012, 2, 200–203. 10.1016/j.ijpddr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal G.; Wyllie S.; Singh N.; Sundar S.; Fairlamb A. H.; Chatterie M. Increased Levels of Thiols Protect Antimony Unresponsive Leishmania Donovani Field Isolates against Reactive Oxygen Species Generated by Trivalent Antimony. Parasitology 2007, 134 (12), 1679–1687. 10.1017/S0031182007003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretti-Mira A. C.; de Oliveira-Neto M. P.; Da-Cruz A. M.; de Oliveira M. P.; Craft N.; Pirmez C. Therapeutic Failure in American Cutaneous Leishmaniasis Is Associated with Gelatinase Activity and Cytokine Expression. Clin. Exp. Immunol. 2011, 163 (2), 207–214. 10.1111/j.1365-2249.2010.04285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte-Sucre A.; Gamarro F.; Dujardin J.-C.; Barrett M. P.; López-Vélez R.; García-Hernández R.; Pountain A. W.; Mwenechanya R.; Papadopoulou B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Neglected Trop. Dis. 2017, 11 (12), e0006052 10.1371/journal.pntd.0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M. K.; Rai S.; Ashutosh; Ravinder; Gupta S.; Sundar S.; Goyal N. Characterization of Natural Antimony Resistance in Leishmania Donovani Isolates. Am. J. Trop. Med. Hyg. 2007, 76 (4), 681–688. 10.4269/ajtmh.2007.76.681. [DOI] [PubMed] [Google Scholar]
- Perea A.; Manzano J. I.; Castanys S.; Gamarro F. The LABCG2 Transporter from the Protozoan Parasite Leishmania Is Involved in Antimony Resistance. Antimicrob. Agents Chemother. 2016, 60 (6), 3489–3496. 10.1128/AAC.02813-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanys-Muñoz E.; Alder-Baerens N.; Pomorski T.; Gamarro F.; Castanys S. A Novel ATP-Binding Cassette Transporter from Leishmania Is Involved in Transport of Phosphatidylcholine Analogues and Resistance to Alkyl-Phospholipids. Mol. Microbiol. 2007, 64 (5), 1141–1153. 10.1111/j.1365-2958.2007.05653.x. [DOI] [PubMed] [Google Scholar]
- Mandal G.; Wyllie S.; Singh N.; Sundar S.; Fairlamb A. H.; Chatterjee M. Increased Levels of Thiols Protect Antimony Unresponsive Leishmania Donovani Field Isolates against Reactive Oxygen Species Generated by Trivalent Antimony. Parasitology 2007, 134 (12), 1679–1687. 10.1017/S0031182007003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S.; Maharjan M.; Singh S.; Chatterjee M.; Madhubala R. Assessing Aquaglyceroporin Gene Status and Expression Profile in Antimony-Susceptible and -Resistant Clinical Isolates of Leishmania Donovani from India. J. Antimicrob. Chemother. 2010, 65 (3), 496–507. 10.1093/jac/dkp468. [DOI] [PubMed] [Google Scholar]
- Singh G.; Jayanarayan K. G.; Dey C. S.. Arsenite Resistance in Leishmania and Possible Drug Targets. In Drug Targets in Kinetoplastid Parasites; Springer: New York; pp 1–8. [DOI] [PubMed] [Google Scholar]
- Venturelli A.; Tagliazucchi L.; Lima C.; Venuti F.; Malpezzi G.; Magoulas G. E.; Santarem N.; Calogeropoulou T.; Cordeiro-da-Silva A.; Costi M. P. Current Treatments to Control African Trypanosomiasis and One Health Perspective. Microorganisms 2022, 10 (7), 1298. 10.3390/microorganisms10071298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram M. G.; Costi M. P.; Thoré E. S. J.; Sabo-Attwood T.; Brooks B. W. One Health. Curr. Biol. 2024, 34 (11), R517–R519. 10.1016/j.cub.2024.04.025. [DOI] [PubMed] [Google Scholar]
- Ilbeigi K.; Barata C.; Barbosa J.; Bertram M. G.; Caljon G.; Costi M. P.; Kroll A.; Margiotta-Casaluci L.; Thoré E. S. J.; Bundschuh M. Assessing Environmental Risks during the Drug Development Process for Parasitic Vector-Borne Diseases: A Critical Reflection. ACS Infect. Dis. 2024, 10 (4), 1026–1033. 10.1021/acsinfecdis.4c00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B.; Varikuti S.; Halsey G.; Volpedo G.; Hamza O. M.; Satoskar A. R. Host-Directed Therapies for Parasitic Diseases. Future Med. Chem. 2019, 11 (15), 1999–2018. 10.4155/fmc-2018-0439. [DOI] [PubMed] [Google Scholar]
- Saha P.; Bhattacharjee S.; Sarkar A.; Manna A.; Majumder S.; Chatterjee M. Berberine Chloride Mediates Its Anti-Leishmanial Activity via Differential Regulation of the Mitogen Activated Protein Kinase Pathway in Macrophages. PLoS One 2011, 6 (4), e18467 10.1371/journal.pone.0018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings H. E.; Barbi J.; Reville P.; Oghumu S.; Zorko N.; Sarkar A.; Keiser T. L.; Lu B.; Rückle T.; Varikuti S.; Lezama-Davila C.; Wewers M. D.; Whitacre C.; Radzioch D.; Rommel C.; Seveau S.; Satoskar A. R. Critical Role for Phosphoinositide 3-Kinase Gamma in Parasite Invasion and Disease Progression of Cutaneous Leishmaniasis. Proc. Natl. Acad. Sci. U.S.A. 2012, 109 (4), 1251–1256. 10.1073/pnas.1110339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orive G.; Lertxundi U.; Brodin T.; Manning P. Greening the Pharmacy. Science 2022, 377 (6603), 259–260. 10.1126/science.abp9554. [DOI] [PubMed] [Google Scholar]
- Coish P.; Brooks B. W.; Gallagher E. P.; Kavanagh T. J.; Voutchkova-Kostal A.; Zimmerman J. B.; Anastas P. T. Current Status and Future Challenges in Molecular Design for Reduced Hazard. ACS Sustainable Chem. Eng. 2016, 4 (11), 5900–5906. 10.1021/acssuschemeng.6b02089. [DOI] [Google Scholar]
- Brooks B. W. Greening Chemistry and Ecotoxicology towards Sustainable Environmental Quality. Green Chem. 2019, 21 (10), 2575–2582. 10.1039/C8GC03893G. [DOI] [Google Scholar]
- Brooks B. W.; van den Berg S.; Dreier D. A.; LaLone C. A.; Owen S. F.; Raimondo S.; Zhang X. Towards Precision Ecotoxicology: Leveraging Evolutionary Conservation of Pharmaceutical and Personal Care Product Targets to Understand Adverse Outcomes Across Species and Life Stages. Environ. Toxicol. Chem. 2024, 43, 526. 10.1002/etc.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLone C. A.; Villeneuve D. L.; Lyons D.; Helgen H. W.; Robinson S. L.; Swintek J. A.; Saari T. W.; Ankley G. T. Editor’s Highlight: Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS): A Web-Based Tool for Addressing the Challenges of Cross-Species Extrapolation of Chemical Toxicity. Toxicol. Sci. 2016, 153 (2), 228–245. 10.1093/toxsci/kfw119. [DOI] [PubMed] [Google Scholar]
- Brooks B. W.; Sabo-Attwood T.; Choi K.; Kim S.; Kostal J.; LaLone C. A.; Langan L. M.; Margiotta-Casaluci L.; You J.; Zhang X. Toxicology Advances for 21(St) Century Chemical Pollution. One Earth 2020, 2 (4), 312–316. 10.1016/j.oneear.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi L.; Perea-Martinez A.; Fiorini G.; Manzano J. I.; Genovese F.; García-Hernández R.; Pinetti D.; Gamarro F.; Costi M. P. Label-Free Mass Spectrometry Proteomics Reveals Different Pathways Modulated in THP-1 Cells Infected with Therapeutic Failure and Drug Resistance Leishmania infantum Clinical Isolates. ACS Infect. Dis. 2023, 9 (3), 470–485. 10.1021/acsinfecdis.2c00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Martínez A.; Garc\′{\i}a-Hernández R.; Manzano J. I.; Gamarro F. Transcriptomic Analysis in Human Macrophages Infected with Therapeutic Failure Clinical Isolates of Leishmania infantum. ACS Infect. Dis. 2022, 8 (4), 800–810. 10.1021/acsinfecdis.1c00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. A.; Blatz D. J.; LaLone C. A. Defining the Biologically Plausible Taxonomic Domain of Applicability of an Adverse Outcome Pathway: A Case Study Linking Nicotinic Acetylcholine Receptor Activation to Colony Death. Environ. Toxicol. Chem. 2023, 42 (1), 71–87. 10.1002/etc.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Hernández R.; Manzano J. I.; Perea-Martínez A.; Gamarro F. New Insights on Drug-Resistant Clinical Isolates of Leishmania infantum-Infected Human Macrophages as Determined by Comparative Transcriptome Analyses. OMICS 2022, 26 (3), 165–177. 10.1089/omi.2021.0185. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D.; Gable A. L.; Nastou K. C.; Lyon D.; Kirsch R.; Pyysalo S.; Doncheva N. T.; Legeay M.; Fang T.; Bork P.; Jensen L. J.; von Mering C. The STRING Database in 2021: Customizable Protein-Protein Networks, and Functional Characterization of User-Uploaded Gene/Measurement Sets. Nucleic Acids Res. 2021, 49 (D1), D605–D612. 10.1093/nar/gkaa1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D.; Gable A. L.; Lyon D.; Junge A.; Wyder S.; Huerta-Cepas J.; Simonovic M.; Doncheva N. T.; Morris J. H.; Bork P.; Jensen L. J.; Mering C. von. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47 (D1), D607–D613. 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M.; Ball C. A.; Blake J. A.; Botstein D.; Butler H.; Cherry J. M.; Davis A. P.; Dolinski K.; Dwight S. S.; Eppig J. T.; Harris M. A.; Hill D. P.; Issel-Tarver L.; Kasarskis A.; Lewis S.; Matese J. C.; Richardson J. E.; Ringwald M.; Rubin G. M.; Sherlock G. Gene Ontology: Tool for the Unification of Biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25 (1), 25–29. 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassal B.; Matthews L.; Viteri G.; Gong C.; Lorente P.; Fabregat A.; Sidiropoulos K.; Cook J.; Gillespie M.; Haw R.; Loney F.; May B.; Milacic M.; Rothfels K.; Sevilla C.; Shamovsky V.; Shorser S.; Varusai T.; Weiser J.; Wu G.; Stein L.; Hermjakob H.; D′Eustachio P. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2019, D596 10.1093/nar/gkz1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B.; Dubey V. In Silico Studies on Tryparedoxin Peroxidase of Leishmania infantum: Structural Aspects. Curr. Pharm. Biotechnol. 2009, 10 (6), 626–630. 10.2174/138920109789069305. [DOI] [PubMed] [Google Scholar]
- Rhee S. G.; Kil I. S. Multiple Functions and Regulation of Mammalian Peroxiredoxins. Annu. Rev. Biochem. 2017, 86 (1), 749–775. 10.1146/annurev-biochem-060815-014431. [DOI] [PubMed] [Google Scholar]
- Wyllie S.; Vickers T. J.; Fairlamb A. H. Roles of Trypanothione-Transferase and Tryparedoxin Peroxidase in Resistance to Antimonials. Antimicrob. Agents Chemother. 2008, 52 (4), 1359–1365. 10.1128/AAC.01563-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo A.; Colotti G.; Boffi A.; Baiocco P.; Ilari A. The Crystal Structures of the Tryparedoxin-Tryparedoxin Peroxidase Couple Unveil the Structural Determinants of Leishmania Detoxification Pathway. PLoS Neglected Trop. Dis. 2012, 6 (8), e1781 10.1371/journal.pntd.0001781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar R.; Tsuji N.; Morales T. H.; Carmody A. B.; Ozols V. O.; Welton J.; Tang L. Removal of Hydrogen Peroxide by a 1-Cysteine Peroxiredoxin Enzyme of the Filarial Parasite Dirofilaria Immitis. Parasitol. Res. 2000, 86 (3), 200–206. 10.1007/s004360050032. [DOI] [PubMed] [Google Scholar]
- Andrade J. M.; Murta S. M. F. Functional Analysis of Cytosolic Tryparedoxin Peroxidase in Antimony-Resistant and Susceptible Leishmania Braziliensis and Leishmania infantum Lines. Parasites Vectors 2014, 7 (1), 406. 10.1186/1756-3305-7-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrangolo F. S. V.; Liarte D. B.; Andrade L. C.; de Melo M. F.; Andrade J. M.; Ferreira R. F.; Santiago A. S.; Pirovani C. P.; Silva-Pereira R. A.; Murta S. M. F. Comparative Proteomic Analysis of Antimony-Resistant and -Susceptible Leishmania Braziliensis and Leishmania infantum Chagasi Lines. Mol. Biochem. Parasitol. 2013, 190 (2), 63–75. 10.1016/j.molbiopara.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Campos-Salinas J.; Cabello-Donayre M.; García-Hernández R.; Pérez-Victoria I.; Castanys S.; Gamarro F.; Pérez-Victoria J. M. A New ATP-Binding Cassette Protein Is Involved in Intracellular Haem Trafficking in Leishmania. Mol. Microbiol. 2011, 79 (6), 1430–1444. 10.1111/j.1365-2958.2010.07531.x. [DOI] [PubMed] [Google Scholar]
- Castanys-Muñoz E.; Alder-Baerens N.; Pomorski T.; Gamarro F.; Castanys S. A Novel ATP-binding Cassette Transporter from Leishmania Is Involved in Transport of Phosphatidylcholine Analogues and Resistance to Alkyl-phospholipids. Mol. Microbiol. 2007, 64 (5), 1141–1153. 10.1111/j.1365-2958.2007.05653.x. [DOI] [PubMed] [Google Scholar]
- Branquinha M. H.; Marinho F. A.; Sangenito L. S.; Oliveira S. S. C.; Goncalves K. C.; Ennes-Vidal V.; d′Avila-Levy C. M.; Santos A. L. S. Calpains: Potential Targets for Alternative Chemotherapeutic Intervention against Human Pathogenic Trypanosomatids. Curr. Med. Chem. 2013, 20 (25), 3174–3185. 10.2174/0929867311320250010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero I.; Téllez J.; Romanha A. J.; Steindel M.; Grisard E. C. Upregulation of Cysteine Synthase and Cystathionine β-Synthase Contributes to Leishmania Braziliensis Survival under Oxidative Stress. Antimicrob. Agents Chemother. 2015, 59 (8), 4770–4781. 10.1128/AAC.04880-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan H. L.; Roberts W. L.; Rainey P. M.; Beverley S. M. The PGPA Gene of Leishmania major Mediates Antimony (SbIII) Resistance by Decreasing Influx and Not by Increasing Efflux. Mol. Biochem. Parasitol. 1994, 68 (1), 145–149. 10.1016/0166-6851(94)00154-5. [DOI] [PubMed] [Google Scholar]
- Carter K. C.; Sundar S.; Spickett C.; Pereira O. C.; Mullen A. B. The In Vivo Susceptibility of Leishmania Donovani to Sodium Stibogluconate Is Drug Specific and Can Be Reversed by Inhibiting Glutathione Biosynthesis. Antimicrob. Agents Chemother. 2003, 47 (5), 1529–1535. 10.1128/AAC.47.5.1529-1535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram J. C.; Coombs G. H. Purification of Particulate Malate Dehydrogenase and Phosphoenolpyruvate Carboxykinase from Leishmania Mexicana Mexicana. Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol. 1985, 827 (3), 310–319. 10.1016/0167-4838(85)90216-X. [DOI] [PubMed] [Google Scholar]
- Uren A. G.; Vaux D. L. TRAF Protiens and Meprins Share a Conserved Domain. Trends Biochem. Sci. 1996, 21 (7), 244–245. 10.1016/S0968-0004(96)30022-4. [DOI] [PubMed] [Google Scholar]
- Lopez-Castejon G. Control of the Inflammasome by the Ubiquitin System. FEBS J. 2020, 287 (1), 11–26. 10.1111/febs.15118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Junior D. S.; Costa D. L.; Carregaro V.; Cunha L. D.; Silva A. L. N.; Mineo T. W. P.; Gutierrez F. R. S.; Bellio M.; Bortoluci K. R.; Flavell R. A.; Bozza M. T.; Silva J. S.; Zamboni D. S. Inflammasome-Derived IL-1β Production Induces Nitric Oxide-Mediated Resistance to Leishmania. Nat. Med. 2013, 19 (7), 909–915. 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- Giraud E.; Rouault E.; Fiette L.; Colle J.-H.; Smirlis D.; Melanitou E. Osteopontin in the Host Response to Leishmania Amazonensis. BMC Microbiol. 2019, 19 (1), 32. 10.1186/s12866-019-1404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M.; Quijada L.; Alonso C.; Requena J. M. Histone Synthesis in Leishmania infantum Is Tightly Linked to DNA Replication by a Translational Control. Biochem. J. 2000, 346, 99–105. 10.1042/bj3460099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión J.; Folgueira C.; Alonso C. Transitory or Long-Lasting Immunity to Leishmania major Infection: The Result of Immunogenicity and Multicomponent Properties of Histone DNA Vaccines. Vaccine 2008, 26 (9), 1155–1165. 10.1016/j.vaccine.2007.12.051. [DOI] [PubMed] [Google Scholar]
- Goll D. E.; Thompson V. F.; Li H.; Wei W.; Cong J. The Calpain System. Physiol. Rev. 2003, 83 (3), 731–801. 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Ennes-Vidal V.; Branquinha M. H.; Dos Santos A. L. S.; d′Avila-Levy C. M. The Diverse Calpain Family in Trypanosomatidae: Functional Proteins Devoid of Proteolytic Activity?. Cells 2021, 10 (2), 299 10.3390/cells10020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ayed S.; Ali M. B.; Bali A.; Gargouri Y.; Laouini D.; Ben Ali Y. Secretory Lipase from the Human Pathogen Leishmania major: Heterologous Expression in the Yeast Pichia Pastoris and Biochemical Characterization. Biochimie 2018, 146, 119–126. 10.1016/j.biochi.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Parameswaran S.; Saudagar P.; Dubey V. K.; Patra S. Discovery of Novel Anti-Leishmanial Agents Targeting LdLip3 Lipase. J. Mol. Graphics Modell. 2014, 49, 68–79. 10.1016/j.jmgm.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Giordana L.; Mantilla B. S.; Santana M.; Silber A. M.; Nowicki C. Cystathionine γ-Lyase, an Enzyme Related to the Reverse Transsulfuration Pathway, Is Functional in Leishmania Spp. J. Eukaryotic Microbiol. 2014, 61 (2), 204–213. 10.1111/jeu.12100. [DOI] [PubMed] [Google Scholar]
- Martin E.; Simon M. W.; Schaefer F. W.; Mukkada A. J. Enzymes of Carbohydrate Metabolism in Four Human Species of Leishmania: A Comparative Survey. J. Protozool. 1976, 23 (4), 600–607. 10.1111/j.1550-7408.1976.tb03850.x. [DOI] [PubMed] [Google Scholar]
- Dhom-Lemos L.; Viana A. G.; Cunha J. L. R.; Cardoso M. S.; Mendes T. A. O.; Pinheiro G. R. G.; Siqueira W. F.; Lobo F. P.; Teles L. F.; Bueno L. L.; Guimarães-Carvalho S. F.; Bartholomeu D. C.; Fujiwara R. T. Leishmania infantum Recombinant Kinesin Degenerated Derived Repeat (RKDDR): A Novel Potential Antigen for Serodiagnosis of Visceral Leishmaniasis. PLoS One 2019, 14 (1), e0211719 10.1371/journal.pone.0211719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales R. M.; Vaselek S.; Neish R.; Berry L.; Brunet C. D.; Crobu L.; Kuk N.; Mateos-Langerak J.; Robinson D. R.; Volf P.; Mottram J. C.; Sterkers Y.; Bastien P. The Kinesin of the Flagellum Attachment Zone in Leishmania Is Required for Cell Morphogenesis, Cell Division and Virulence in the Mammalian Host. PLOS Pathog. 2021, 17 (6), e1009666 10.1371/journal.ppat.1009666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejgaard S. Y.; Presley J. F. Rab18: New Insights into the Function of an Essential Protein. Cell. Mol. Life Sci. 2019, 76 (10), 1935–1945. 10.1007/s00018-019-03050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiulli G.; Lantella A.; Forte E.; Angelucci F.; Colotti G.; Ilari A.; Malatesta F. Leishmania infantum Trypanothione Reductase Is a Promiscuous Enzyme Carrying an NADPH:O2 Oxidoreductase Activity Shared by Glutathione Reductase. Biochim. Biophys. Acta, Gen. Subj. 2015, 1850 (9), 1891–1897. 10.1016/j.bbagen.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Saha G.; Khamar B. M.; Singh O. P.; Sundar S.; Dubey V. K. Leishmania Donovani Evades Caspase 1 Dependent Host Defense Mechanism during Infection. Int. J. Biol. Macromol. 2019, 126, 392–401. 10.1016/j.ijbiomac.2018.12.185. [DOI] [PubMed] [Google Scholar]
- Diaz J. R.; Ramírez C. A.; Nocua P. A.; Guzman F.; Requena J. M.; Puerta C. J. Dipeptidyl Peptidase 3, a Novel Protease from Leishmania Braziliensis. PLoS One 2018, 13 (1), e0190618 10.1371/journal.pone.0190618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bel Hadj Ali I.; Chouaieb H.; Aoun Y. S. B.; Harigua-Souiai E.; Souguir H.; Yaacoub A.; Dbouni O.; El; Harrat Z.; Mukhtar M. M.; Said M. B.; Haddad N.; Fathallah-Mili A.; Guizani I. Dipeptidyl Peptidase III as a DNA Marker to Investigate Epidemiology and Taxonomy of Old World Leishmania Species. PLoS Neglected Trop. Dis. 2021, 15 (7), e0009530 10.1371/journal.pntd.0009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Hernández R.; Perea-Martínez A.; Manzano J. I.; Terrón-Camero L. C.; Andrés-León E.; Gamarro F. Transcriptome Analysis of Intracellular Amastigotes of Clinical Leishmania infantum Lines from Therapeutic Failure Patients after Infection of Human Macrophages. Microorganisms 2022, 10 (7), 1304. 10.3390/microorganisms10071304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Pinkovich A.; Kannan S.; Nitzan-Koren R.; Puri M.; Pawar H.; Bar-Avraham Y.; McDonald J.; Sur A.; Zhang W.-W.; Matlashewski G.; Madhubala R.; Michaeli S.; Myler P. J.; Zilberstein D. Sensing Host Arginine Is Essential for Leishmania Parasites′ Intracellular Development. mBio 2020, 11 (5), e02023-20 10.1128/mBio.02023-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Pinkovich A.; Balno C.; Strasser R.; Zeituni-Molad M.; Bendelak K.; Rentsch D.; Ephros M.; Wiese M.; Jardim A.; Myler P. J.; Zilberstein D. An Arginine Deprivation Response Pathway Is Induced in Leishmania during Macrophage Invasion. PLoS Pathog. 2016, 12 (4), e1005494 10.1371/journal.ppat.1005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya R.; Dhembla C.; Makde R. D.; Sundd M.; Kundu S. An Overview of the Fatty Acid Biosynthesis in the Protozoan Parasite Leishmania and Its Relevance as a Drug Target against Leishmaniasis. Mol. Biochem. Parasitol. 2021, 246, 111416 10.1016/j.molbiopara.2021.111416. [DOI] [PubMed] [Google Scholar]
- Ponte-Sucre A.; Gamarro F.; Dujardin J.-C.; Barrett M. P.; López-Vélez R.; García-Hernández R.; Pountain A. W.; Mwenechanya R.; Papadopoulou B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Neglected Trop. Dis. 2017, 11 (12), e0006052 10.1371/journal.pntd.0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng J.; Saunders E. C.; Gooley P. R.; McConville M. J.; Naderer T.; Tull D. Membrane Targeting of the Small Myristoylated Protein 2 (SMP-2) in Leishmania Major. Mol. Biochem. Parasitol. 2013, 190 (1), 1–5. 10.1016/j.molbiopara.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Tull D.; Naderer T.; Spurck T.; Mertens H. D. T.; Heng J.; McFadden G. I.; Gooley P. R.; McConville M. J. Membrane Protein SMP-1 Is Required for Normal Flagellum Function in Leishmania. J. Cell Sci. 2010, 123 (4), 544–554. 10.1242/jcs.059097. [DOI] [PubMed] [Google Scholar]
- Oliveira M.; Martins V.; Santos T.; Lage D.; Ramos F. M.; Salles B.; Costa L.; Dias D.; Ribeiro P.; Schneider M.; Machado-de-Ávila R.; Teixeira A.; Coelho E.; Chávez-Fumagalli M. Small Myristoylated Protein-3, Identified as a Potential Virulence Factor in Leishmania Amazonensis, Proves to Be a Protective Antigen against Visceral Leishmaniasis. Int. J. Mol. Sci. 2018, 19 (1), 129. 10.3390/ijms19010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangenito L. S.; Ennes-vidal V.; Marinho F. A.; Mota F. F. D. A.; Santos A. L. S.; D′avila-levy C. M.; Branquinha M. H. Arrested Growth of Trypanosoma Cruzi by the Calpain Inhibitor MDL28170 and Detection of Calpain Homologues in Epimastigote Forms. Parasitology 2009, 136 (4), 433–441. 10.1017/S0031182009005629. [DOI] [PubMed] [Google Scholar]
- García-Hernández R.; Manzano J. I.; Perea-Martínez A.; Gamarro F. New Insights on Drug-Resistant Clinical Isolates of Leishmania infantum Infected Human Macrophages as Determined by Comparative Transcriptome Analyses. OMICS: J. Integr. Biol. 2022, 26 (3), 165–177. 10.1089/omi.2021.0185. [DOI] [PubMed] [Google Scholar]
- Perea-Martínez A.; García-Hernández R.; Manzano J. I.; Gamarro F. Transcriptomic Analysis in Human Macrophages Infected with Therapeutic Failure Clinical Isolates of Leishmania infantum. ACS Infect. Dis. 2022, 8 (4), 800–810. 10.1021/acsinfecdis.1c00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. N.; Pappin D. J.; Creasy D. M.; Cottrell J. S. Probability-Based Protein Identification by Searching Sequence Databases Using Mass Spectrometry Data. Electrophoresis 1999, 20 (18), 3551–3567. . [DOI] [PubMed] [Google Scholar]
- Boeckmann B. The SWISS-PROT Protein Knowledgebase and Its Supplement TrEMBL in 2003. Nucleic Acids Res. 2003, 31 (1), 365–370. 10.1093/nar/gkg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A.; Martin M.-J.; Orchard S.; Magrane M.; Agivetova R.; Ahmad S.; Alpi E.; Bowler-Barnett E. H.; Britto R.; Bursteinas B.; Bye-A-Jee H.; Coetzee R.; Cukura A.; Da Silva A.; Denny P.; Dogan T.; Ebenezer T.; Fan J.; Castro L. G.; Garmiri P.; Georghiou G.; Gonzales L.; Hatton-Ellis E.; Hussein A.; Ignatchenko A.; Insana G.; Ishtiaq R.; Jokinen P.; Joshi V.; Jyothi D.; Lock A.; Lopez R.; Luciani A.; Luo J.; Lussi Y.; MacDougall A.; Madeira F.; Mahmoudy M.; Menchi M.; Mishra A.; Moulang K.; Nightingale A.; Oliveira C. S.; Pundir S.; Qi G.; Raj S.; Rice D.; Lopez M. R.; Saidi R.; Sampson J.; Sawford T.; Speretta E.; Turner E.; Tyagi N.; Vasudev P.; Volynkin V.; Warner K.; Watkins X.; Zaru R.; Zellner H.; Bridge A.; Poux S.; Redaschi N.; Aimo L.; Argoud-Puy G.; Auchincloss A.; Axelsen K.; Bansal P.; Baratin D.; Blatter M.-C.; Bolleman J.; Boutet E.; Breuza L.; Casals-Casas C.; de Castro E.; Echioukh K. C.; Coudert E.; Cuche B.; Doche M.; Dornevil D.; Estreicher A.; Famiglietti M. L.; Feuermann M.; Gasteiger E.; Gehant S.; Gerritsen V.; Gos A.; Gruaz-Gumowski N.; Hinz U.; Hulo C.; Hyka-Nouspikel N.; Jungo F.; Keller G.; Kerhornou A.; Lara V.; Le Mercier P.; Lieberherr D.; Lombardot T.; Martin X.; Masson P.; Morgat A.; Neto T. B.; Paesano S.; Pedruzzi I.; Pilbout S.; Pourcel L.; Pozzato M.; Pruess M.; Rivoire C.; Sigrist C.; Sonesson K.; Stutz A.; Sundaram S.; Tognolli M.; Verbregue L.; Wu C. H.; Arighi C. N.; Arminski L.; Chen C.; Chen Y.; Garavelli J. S.; Huang H.; Laiho K.; McGarvey P.; Natale D. A.; Ross K.; Vinayaka C. R.; Wang Q.; Wang Y.; Yeh L.-S.; Zhang J.; Ruch P.; Teodoro D. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49 (D1), D480–D489. 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellacheruvu D.; Wright Z.; Couzens A. L.; Lambert J.-P.; St-Denis N. A.; Li T.; Miteva Y. V.; Hauri S.; Sardiu M. E.; Low T. Y.; Halim V. A.; Bagshaw R. D.; Hubner N. C.; Al-Hakim A.; Bouchard A.; Faubert D.; Fermin D.; Dunham W. H.; Goudreault M.; Lin Z.-Y.; Badillo B. G.; Pawson T.; Durocher D.; Coulombe B.; Aebersold R.; Superti-Furga G.; Colinge J.; Heck A. J. R.; Choi H.; Gstaiger M.; Mohammed S.; Cristea I. M.; Bennett K. L.; Washburn M. P.; Raught B.; Ewing R. M.; Gingras A.-C.; Nesvizhskii A. I. The CRAPome: A Contaminant Repository for Affinity Purification-Mass Spectrometry Data. Nat. Methods 2013, 10 (8), 730–736. 10.1038/nmeth.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassal B.; Matthews L.; Viteri G.; Gong C.; Lorente P.; Fabregat A.; Sidiropoulos K.; Cook J.; Gillespie M.; Haw R.; Loney F.; May B.; Milacic M.; Rothfels K.; Sevilla C.; Shamovsky V.; Shorser S.; Varusai T.; Weiser J.; Wu G.; Stein L.; Hermjakob H.; D′Eustachio P. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2020, 48 (D1), D498–D503. 10.1093/nar/gkz1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw MS data, including the values of the areas of the proteins and their associated p-values, are available on the public repository Fairdom at https://fairdomhub.org/projects/378.