Abstract
Zfp318, a mouse gene with a Cys2/His2 zinc finger motif, is mainly expressed in germ cells in the testis. It encodes two alternative transcripts, which regulate androgen receptor‐mediated transcriptional activation or repression by overexpression of them. However, the role of Zfp318 is still obscure in vivo, especially in spermatogenesis. To elucidate the role of Zfp318 during gamete production, we established a knockout mouse line. Zfp318‐null male mice exhibited infertility, whereas Zfp318‐null female mice displayed normal fertility. ZFP318 was expressed during multiple stages of spermatogenesis, from spermatocytes to round spermatids. The nuclei of secondary spermatocytes showed high levels of expression. Histological analysis and quantitative analysis of DNA content showed decreased numbers of both spermatids in the seminiferous tubules and mature spermatozoa in the epididymides of Zfp318‐null mice. These results suggest that Zfp318 is expressed as a functional protein in testicular germ cells and plays an important role in meiosis during spermatogenesis.
Keywords: male infertility, sperm count, spermatogenesis, testis, zinc finger
Zfp318‐null male mice exhibited infertility, decreased numbers of both spermatids in the seminiferous tubules and mature spermatozoa in the epididymides.Zfp318 is expressed as a functional protein in testicular germ cells and plays an important role in meiosis during spermatogenesis.
Introduction
Spermatogenesis is a complicated, well‐coordinated process, with a gene expression program that uses both transcriptional and translational mechanisms (Sassone‐Corsi 1997; Eddy 2002). Despite the expression of a large number of different transcription factors in male germ cells, few have been reported to have functional significance in spermatogenesis (Eddy 2002). According to more recent reports with gene knockout technology, Zbtb16/PLZF (Buaas et al. 2004; Costoya et al. 2004), Sox3 (Weiss et al. 2003; Raverot et al. 2005), and Sohlh1 (Ballow et al. 2006) are essential for spermatogonial stem cell self‐renewal and mitosis during spermatogenesis. In post‐meiotic germ cell development, CREM‐τ acts as a switch that regulates the expression of genes required for haploid germ cell development (Blendy et al. 1996; Nantel et al. 1996). TATA box‐binding protein‐like 1 (TBPL1) is also required for spermiogenesis, and inactivation of TBPL1 results in complete arrest of spermiogenesis at step 7 in stage VII seminiferous tubules (Martianov et al. 2001).
Testicular zinc finger protein, TZF, is a polypeptide comprising 924 amino acid residues. It contains a Cys2/His2 zinc finger motif at the C‐terminal end (Inoue et al. 2000), and its transcript is expressed during spermatogenesis (Ishizuka et al. 2003). We designated the gene as TZF, which is herein referred to as Zfp318 according to the Mouse Genome Informatics nomenclature. Several Cys2/His2 zinc finger proteins are also expressed at specific stages during mouse spermatogenesis. For example, deficiency of ZFP105 in pachytene spermatocytes resulted in undifferentiated spermatogenic cells and decreased male fertility (Zhou et al. 2010). Ovol2/MOVO may play an important role in the XY body during spermatogenesis, possibly in XY body formation and meiotic sex chromosome inactivation (Chizaki et al. 2012). Gtsf1/Cue110 is required for spermatogenesis and is involved in retrotransposon suppression in male germ cells (Yoshimura et al. 2009). Zmynd15 was recently identified as a transcriptional repressor that was essential for spermiogenesis (Yan et al. 2010). We previously showed that short‐form ZFP318 homodimers act as transcriptional repressors in the regulation of androgen receptor (AR)‐mediated gene transcriptional activation (Ishizuka et al. 2005; Tao et al. 2006a,b). Interestingly, long‐form ZFP318, an alternative splice variant of Zfp318, can form both homodimers and heterodimers with short‐form ZFP318 and acts as a transcriptional activator of AR‐mediated gene transcription. It contains 2025 amino acid residues, and its 902°N‐terminal amino acids are identical to those of short‐form ZFP318. The mRNAs of both splice variants of Zfp318 are highly expressed in mouse testes, especially in germ cells (Ishizuka et al. 2003). If both proteins colocalize in spermatocytes, transcriptional activation and repression may occur depending on the expression of other proteins. Our previous study showed that short‐form ZFP318 recruits endogenous histone deacetylase 2 (HDAC2) to suppress AR transcriptional activation, while the long‐form ZFP318 homodimer or heterodimer recruits a coactivator complex to activate AR‐mediated transcription (Tao et al. 2006b). These results suggest that Zfp318 might function in mouse spermatogenesis via AR‐mediated gene transcriptional activation. However, the study of cell‐specific AR knockout showed that deletion of the AR gene in mouse germ cells does not affect spermatogenesis and male fertility (Wang et al. 2009). Therefore, there is no direct evidence that Zfp318 has any crucial role in germ cells in spermatogenesis.
To elucidate the function of Zfp318 in spermatogenesis, we here first described the detailed expression pattern of Zfp318 in mouse testes and established a Zfp318 knockout mouse line. Zfp318‐null mice exhibited male‐specific sterility because of a defect in spermatogenesis. The loss of Zfp318 appeared to affect meiosis specifically, resulting in a reduced number of haploid sperm cells. Our current findings support that Zfp318 is important for mouse spermatogenesis.
Material and methods
Generation of mutant mice lacking Zfp318
The targeting strategy is shown in supplementary data (Fig. S1A). 129SvJ ES cell clones carrying the targeted mutation were injected into C57BL/6 blastocysts, and chimeric male mice were crossed with C57BL/6 females to establish heterozygous (Zfp318 +/−) mutant lines. We established the congenic strains of 129SvJ and C57BL/6, which lacked Zfp318, by backcrossing at least seven generations. Northern and Southern blot analyses confirmed Zfp318 deletion (Fig. S1B,C). For breeding studies, wild‐type (+/+), heterozygous (+/−), and homozygous (–/–) male mice (8–10 weeks old each; n = 10) were caged for 2 weeks with four females (8 weeks old, ddY mice) each, and pregnancies/deliveries were recorded. All animal experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee of Toin University of Yokohama.
Western blot analyses
Mouse testes from the congenic strain 129SvJ were homogenized by a POLYTRON Homogenizer (KINEMATICA AG, Lucerne, Switzerland) in TBS supplemented with cOmplete Protease Inhibitor Cocktail (EDTA‐free) (Roche, Mannheim, Germany) and treated with Benzonase Nuclease (Novagen, Billerica, MA, USA). Proteins were separated by a NuPAGE SDS–PAGE Gel System using 3–8% Tris‐Acetate Gels (Novex, Carlsbad, CA, USA) and transferred to PVDF membranes. The anti‐ZFP318 antibody (human ZNF318 antibody; HPA027031, Sigma‐Aldrich, Saint Louis, MO, USA), horse radish peroxidase (HRP)‐conjugated anti‐rabbit IgG (GE, Fairfield, CT, USA), and ECL Prime (GE) were used for the detection of ZFP318.
In situ hybridization
The digoxigenin (DIG) antisense and sense Zfp318 riboprobes for in situ hybridization were synthesized using a DIG RNA Labeling Kit (Roche). The sequence positions which are specific to short and long transcripts of Zfp318 were 2825–3122 and 5665–6157, respectively. After being perfused, congenic 129SvJ wild‐type mouse testes were dissected, fixed with Tissue Fixative (Genostaff, Tokyo, Japan), and embedded in paraffin according to standard procedures. Paraffin blocks were sectioned at 8‐μm thickness. After being treated with proteinase K (10 μg/mL) for 30 min at 37°C, the sections were post‐fixed in 4% paraformaldehyde for 10 min, washed with phosphate‐buffered saline (PBS), and placed in 0.2 mol/L HCl for 10 min. Then, they were washed with PBS and acetylated by incubation in 0.1 mol/L triethanolamine and 0.25% acetic anhydride for 10 min. Hybridization was performed with probes at concentrations of 100 ng/mL in Probe Diluent (Genostaff) at 60°C for 16 h. After treatment with blocking reagent, the sections were incubated with alkaline phosphatase (AP)‐conjugated anti‐DIG antibodies (Roche) diluted 1:1000 with TBST for 2 h. The sections were visualized with BM Purple AP Substrate (Roche) overnight, and counterstained with Kernechtrot stain solution (Mutoh, Tokyo, Japan).
Immunohistochemical and histological analysis
The testes from congenic 129SvJ mice were dissected and fixed in Bouin's fixative for 2 h at room temperature (RT). The tissues were embedded in paraffin and then cut into 4‐μm‐thick sections. For immunohistochemical analysis, antigens were retrieved by heating in citrate buffer (pH 6.0), and sections were incubated with 10% goat serum (Nichirei, Tokyo, Japan) as blocking solution. The anti‐ZFP318 antibody was diluted to 6 μg/mL in 10% goat serum. The sections were incubated with the antibody, washed, and incubated with secondary antibody (Simple Stain Mouse MAX PO, Nichirei). The AEC (3‐amino‐ 9‐ethylcarbazole) Substrate Kit (Nichirei) was applied until a red color developed. For histological analysis, spermatogenesis was examined in sections stained with hematoxylin and eosin (H&E) or PAS and hematoxylin. Photomicrographs were taken with the All‐in‐One Fluorescence Microscope system (BZ‐8100 and BZ‐Analyzer, Keyence). The seminiferous tubules were staged according to a reference (Russell et al. 1990).
Preparation of testicular and epididymal cells
Testicular and epididymal cells were prepared as previously described (Krishnamurthy et al. 2000). Briefly, the tissues were minced in PBS and gently aspirated to disperse the cells. The cell suspension was filtered through a 35‐μm nylon mesh, and cells were washed in PBS. After centrifugation and aspiration, the cells were resuspended in PBS, fixed in 70% chilled ethanol, and stored at 4°C until further analysis. Cells from the cauda epididymis were prepared and stored in the same manner.
Quantification of testicular and epididymal cells by DNA flow cytometry
Mouse testicular and epididymal cells were stained essentially as described previously (Krishnamurthy et al. 1998). Briefly, an aliquot of 1 × 106 to 2 × 106 ethanol‐fixed testicular and epididymal cells were washed with PBS and treated with 0.25% pepsin solution for 10 min at 37°C. After centrifugation, cells were stained with propidium iodide (PI) staining solution (25 μg/mL PI, 40 μg/mL RNase, and 0.3% Tween 20 in PBS) at RT for 20 min, filtered through a 35‐μm nylon mesh, and diluted with 0.01% ethylenediaminetetraacetic acid (EDTA) in PBS. The PI‐stained cells were analyzed by flow cytometry with the FACSVantage (Becton Dickinson, Franklin Jakes, NJ, USA). The fluorescence signals (absorbance, 620 nm) of the PI‐stained cells were recorded and analyzed using CellQuest software (Becton Dickinson).
Results
Localization of Zfp318 mRNAs and proteins in mouse testis
The localization of Zfp318 mRNAs in mouse testes was determined by in situ hybridization. The hybridization signal was associated with the seminiferous tubules or limited to certain populations of germ cells for a probe of short‐form (Fig. 1A) and long‐form (Fig. 1B) Zfp318, respectively. These signals were approximately equal between a probe of short‐form and long‐form. The long‐form Zfp318 signals were stronger than the short‐form. Detailed examination of positive signals for long‐form Zfp318 revealed that Zfp318 was localized to primary spermatocytes and in the initial steps of round spermatid formation in the seminiferous tubules (Fig. 1C) No signal was detected in spermatogonia, somatic cells, which include Sertoli cells, peritubular myoid cells, and interstitial cells (Fig. 1A, B). No hybridization signal was observed when each sense probe was used as a control. These splice variants encode two proteins, respectively, comprising 902 and 2025 amino acids (12) and containing a Cys2/His2 zinc finger motif (11). Amino acid sequence alignment and analysis revealed that ZFP318 is highly conserved in mice and humans. To determine ZFP318 localization, we performed immunohistochemistry on testis sections using the anti‐human ZNF318 antibody, which recognizes homologous amino acid sequences of both mouse ZFP318 splice variants and human ZNF318 (Fig. S2, underlined, 88% homologous). Results of Western blotting analysis revealed that this anti‐human ZNF318 antibody recognized both the mouse ZFP318 variants, and no non‐specific reaction was observed in the extract from the testis of Zfp318 −/− mice (Fig. 2A). Moreover, the main variant of ZFP318 expressed in mouse testis was the long‐form. Using the antibody, ZFP318 expressed inside of seminiferous tubules depending on the stages of spermatogenesis (Fig. 2B). Nucleus‐restricted localization was observed in the germ cells (Figs 2B and 3). The staining intensity differed depending on the differentiation of the cells in spermatogenesis. Moderate staining was observed in primary spermatocytes from leptotene to diplotene stages, with increased intensity in the early pachytene stage (Fig. 3B, D). Interestingly, the nuclei of secondary spermatocytes, a stage between meiosis I and II and specifically stage XII in the epithelium, showed strong staining (Fig. 3F). Newly formed round spermatids, after completion of meiosis II, displayed faint nuclear staining, which was not apparent in round spermatids later in spermiogenesis, likely after step 4. Somatic cells, which include Sertoli cells, peritubular myoid cells, and interstitial cells, did not show obvious staining. Strong staining was occasionally observed in the nuclei of spermatogonia, but it was declared nonspecific since staining was also observed in the testes from Zfp318 −/− mice.
Figure 1.
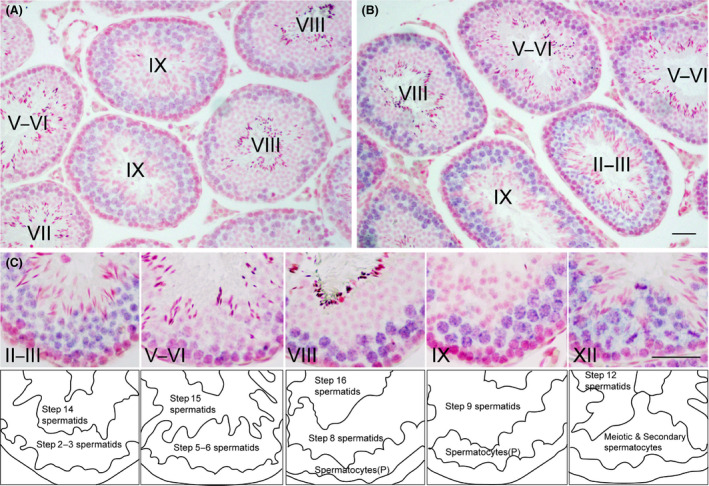
Localization of Zfp318 mRNA in testis. (A, B) In situ hybridization analysis of Zfp318 mRNA localization in the testes of adult mice for a short transcript (A) and a long transcript (B). The hybridization signals (blue) are confined to the luminal compartments. (C) Higher magnification reveals that the long transcript is localized in the nuclei of pachytene spermatocytes through round spermatids. Roman numerals indicate the stage of the cycle of the mouse seminiferous epithelium. Bars: 50 μm.
Figure 2.
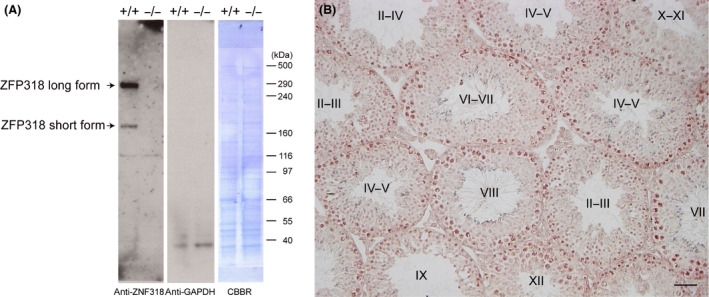
Immunohistochemistry of ZFP318. (A) This antibody recognizes both forms of ZFP318 in extract from the normal adult testis. The long‐form ZFP318 is the major splice variant. There is no signals in extract from the Zfp318 −/− testis. Anti‐GAPDH‐HRP detection and CBBR (Coomassie Brilliant Blue R‐250) staining of the same membrane show as a loading control. (B) Normal adult testis immunostained with the antibody and with hemotoxylin. Roman numerals indicate the stage of the cycle of the mouse seminiferous epithelium. Bar: 50 μm.
Figure 3.
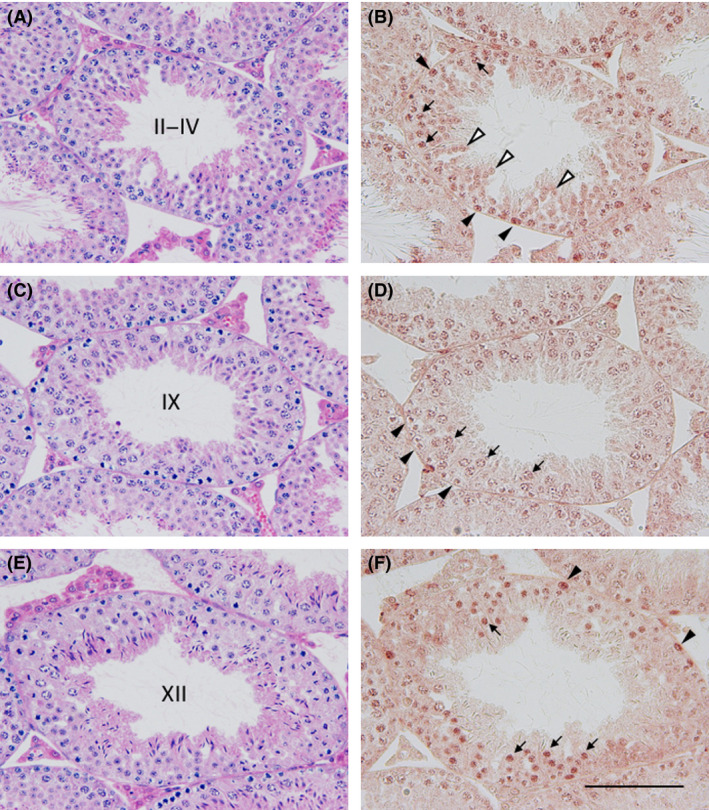
Sections of the normal adult testis stained with HE (A, C and E) and the neighboring sections immunostained using an antibody against ZFP318 (B, D and F). Roman numerals reveal stage of the cycle of the mouse seminiferous epithelium. Positive signals are observed in the nuclei of spermatogonia (B and F, arrowheads), of pachytene spermatocytes (B and D, arrows) and of the secondary spermatocytes (F, arrows). Nuclei of round spermatids (B, white arrowheads) and of leptotene spermatocytes (D, arrowheads) also show weak reactions. Bar: 100 μm.
Characteristics of Zfp318‐null mice
To elucidate the role of Zfp318 in spermatogenesis, we produced Zfp318‐null using homologous recombination in ES cells (Fig. S1). Southern blot analysis of genomic DNA confirmed insertion of the targeting vector into the mutant mice (Fig. S1B). Analysis of testicular RNA and protein from Zfp318 −/− mice revealed the absence of short‐ and long‐form mRNA (Figs S1C, 2A). Zfp318 −/− male mice exhibited normal health and behavior, but 129SvJ males had reduced body, testes, and epididymal weights. The testes and epididymides of Zfp318 −/− mice at 10 weeks of age were significantly reduced, weighing 67.6 ± 6.9 mg (n = 10; 22.3% reduction) and 22.0 ± 5.2 mg (n = 6; 30.6% reduction), respectively, when compared with those of wild‐type controls, which weighted 87.0 ± 5.4 mg (n = 10) and 31.7 ± 2.4 mg (n = 5), respectively. Even when the significantly lower body weights are adjusted for (n = 10; ~10% reduction, Fig. 4A), the testes and epididymides weights of Zfp318 −/− male mice remained significantly decreased (Fig. 4B, C). Furthermore, Zfp318 −/− males were infertile, with complete infertility observed in 129SvJ congenics and subfertility in C57BL/6 congenics (Table 1). Therefore, 129SvJ congenic mice were used for the detailed examinations in this study.
Figure 4.
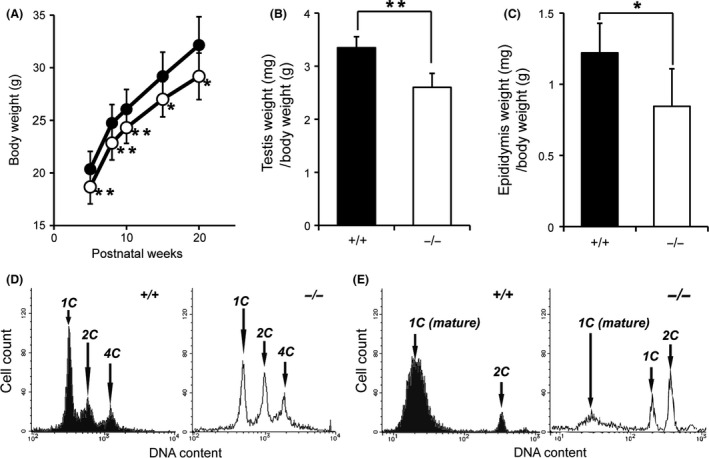
Zfp318 −/− male mice (129SvJ) display reduced body, testes, and epididymides weights and less mature spermatozoa. (A) Zfp318 −/− mice (open circle, n = 10) display approximately 10% reduction in body weight compared to Zfp318 +/+ mice (closed circle, n = 10). (B) Zfp318 −/− (10 weeks old) testes display ~22.3% reduction in weight (n = 10). (C) Zfp318 −/− (10 weeks old) epididymides display approximately 30.8% reduction in weight (n = 6). A t‐test was used for the evaluation of statistical significance. *P < 0.05, **P < 0.001. (D, E) Flow cytometric analysis of the DNA content of the whole testis (D) and epididymis (E). (D) 1C, haploid spermatids; 2C, diploid cells at the G1 phase of the cell cycle (mainly Sertoli cells and spermatogonia); 4C, cells at the G2 phase of the cell cycle (mainly primary spermatocytes). Each panel is representative of four or six animals, with the data averages stated in Results. (E) 1C (mature), mature haploid spermatids with condensed nuclear DNA; 1C, haploid spermatids; 2C, diploid cells at the G1 phase of the cell cycle (mainly epithelial cells of the epididymis and spermatogonia). Each panel is representative of three or four animals, with the data averages stated in Results.
Table 1.
Fertility assessment of Zfp318‐null mice
| Genetic background | 129SvJ | C57BL/6 | ||||
|---|---|---|---|---|---|---|
| Genotype | +/+ | +/− | −/− | +/+ | +/− | −/− |
| Number of animals | 10 | 10 | 11 | 10 | 10 | 11 |
| Pregnancies/mated females | 28/40 | 32/40 | 0/44 | 35/40 | 34/40 | 13/44 |
| Average number of progeny/pregnancy | 10.3 | 11.1 | 0 | 11.2 | 11.3 | 6.0 |
Ratio of sperm cells in the testes and epididymides in Zfp318‐null and wild‐type mice
As the classical histological examination did not reveal quantitative changes between the germ cells of Zfp318 +/+ and Zfp318 −/− mice, we performed a more rapid and sensitive analysis of germ cells by quantitative DNA flow cytometry. Figure 4D depicts representative histograms of PI‐stained testicular cells. Based on the DNA content, three quantifiable populations were discernible: spermatids (1C), secondary spermatocytes and spermatogonia, testicular somatic cells (2C), primary spermatocytes, and G2 spermatogonia (4C). Analysis of testicular cells revealed that Zfp318 −/− mice exhibited a significant decrease in 1C cells (15.4 ± 2.2% versus 19.9 ± 2.7%, P < 0.05) and significant increases in 2C (12.3 ± 1.7% vs 6.0 ± 2.0%, P < 0.05) and 4C (7.7 ± 1.0% vs 3.4 ± 0.8%, P < 0.001) cells when compared with Zfp318 +/+ mice. Figure 4E contains representative histograms of PI‐stained epididymal cells. The histograms elucidated the detection of an additional population with a lower DNA content: elongated and mature spermatids (1C (mature)). Analysis of epididymal cells revealed that Zfp318 −/− mice exhibited a significant decrease in 1C (mature) cells (15.7 ± 7.1% versus 37.3 ± 10.0%, P < 0.05) when compared with Zfp318 +/+ mice. A new population, which was absent from Zfp318 +/+ mice, called 1C (4.5 ± 1.6%), was seen in Zfp318 −/− mice.
Impaired spermatogenesis in Zfp318‐null mice
Histological examination of the testis revealed no obvious abnormality in the spermatogenesis of Zfp318 +/− mice (data not shown). Zfp318 −/− mice, however, exhibited defects in spermatogenesis (Fig. 5B, D). As spermiogenesis proceeded, fewer elongated spermatids with malformed nuclei were observed in the seminiferous epithelium. Vacuoles were sporadically observed in the epithelium, indicating degeneration and dissolution of germ cells (Fig. 5B). Additionally, irregular sized nuclei with similar morphology to those of the round spermatids were detected in the layer of the spermatids in the epithelium (Fig. 5a–d). The nuclear diameter of the cells was comparable to those of pachytene spermatocytes that co‐existed in the epithelium. The abnormal cells with larger nuclei, evaluated mainly by the co‐existing spermatogonia and spermatocytes, were detected in the epithelium at stages I to V of the cycle, but not in the later stages of the epithelium. The impaired spermatogenesis of the mutant mice was evidenced with little accumulation of the sperm containing sloughed germ cells in the lumen of the epididymal duct (Fig. 5F).
Figure 5.
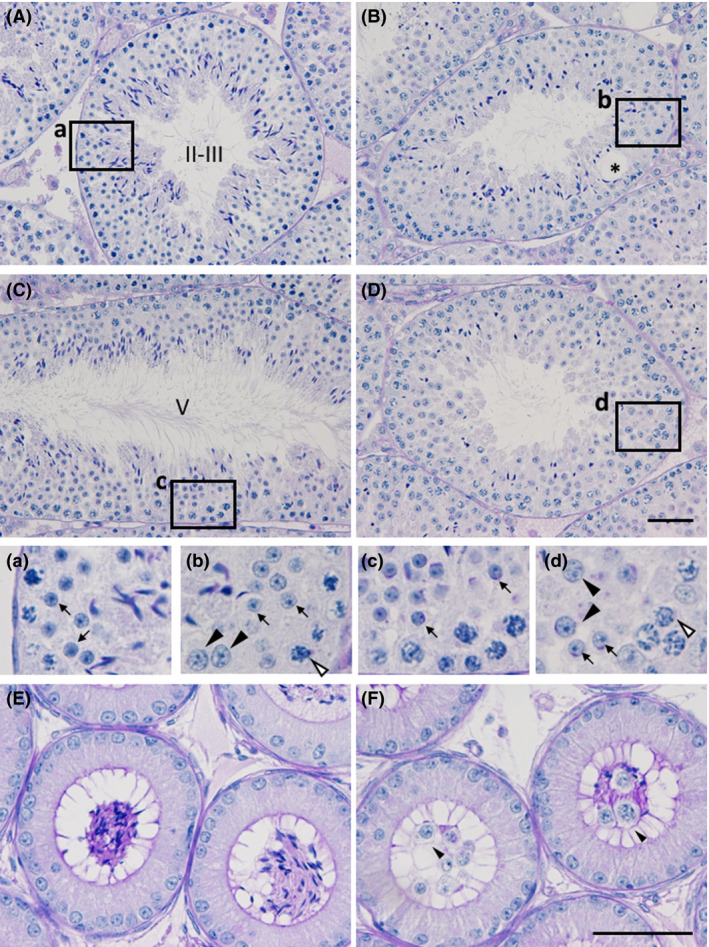
Histological analysis of testes and epididymides of the Zfp318 −/− mouse. PAS‐hematoxylin‐stained sections of testes (A‐D) and caput epididymides (E and F). Depicted areas in A–D are enlarged in a–d, respectively. Roman numerals in A and C show the stage of the cycle of the mouse seminiferous epithelium. Seminiferous tubules in B and D were regarded as the corresponding stage of A and C, respectively. The wild‐type male shows normal testicular morphology (A and C), whereas the mutant testis shows affected spermatogenesis including vacuole formation (B, asterisk) and sparsely located elongated spermatids (D). Note the cells with the large nuclei (b and d, arrowheads) among the normal round spermatids (arrows), comparable to those of pachytene spermatocytes (b and d, white arrowheads). Accumulation of the sperm is seen in the lumen of the wild‐type epididymal duct (E). Sloughed germ cells with few sperm are observed in the mutant (F). Bars: 50 μm.
Discussion
We generated and analyzed the male reproductive phenotype of Zfp318‐null mice, and the results indicate that the gene is indispensable for proper spermatogenesis, and hence, male fertility. Spermatogenesis proceeded normally up to the meiosis prophase just before the spermatozoa morphogenesis steps. Round to elongated spermatids exhibited nuclear malformation from the first step onwards, and completed spermatozoa were decreased in number. We have previously demonstrated that Zfp318 germ cell‐specific transcripts first appear at post‐natal day 16, as spermatocytes enter meiotic prophase (Ishizuka et al. 2003). In this study, the expression of these transcripts was observed from pachytene spermatocytes at meiotic prophase to the initial steps of round spermatids in seminiferous tubules, suggesting that Zfp318 is involved primarily in meiosis, and the protein expression had the same distribution. The nuclei of secondary spermatocytes showed strong staining, which was diminished in the later stages of round spermatids and elongated spermatids. Therefore, ZFP318 was not a component of spermatozoa but may act as a regulatory factor of meiosis or a transcriptional factor of genes required in spermiogenesis. Because no developmental difference was observed until that of secondary spermatocytes between wild‐type and Zfp318‐null testes, the essential role of ZFP318 in spermatogenesis appears to involve meiosis. Quantitative DNA flow cytometry was conducted to further investigate the role of ZFP318 in the regulation of spermatogenesis. The quantities of primary (4C) and secondary (2C) spermatocytes increased, while spermatids (1C) decreased. Histological examination also revealed the morphological changes related to this phenomenon, including irregularly sized nuclei of the round spermatids and malformation of the nuclei of spermatids. These results also suggest a meiosis‐promoting function of the gene.
The two splice variants of Zfp318 are nuclear proteins that alternatively function as coactivator and corepressor of AR (Tao et al. 2006a). However, the deletion of the AR gene in mouse germ cells does not affect spermatogenesis and male fertility (Wang et al. 2009). AR‐mediated transcriptional activation in germ cells is not essential for spermatogenesis. Western blotting showed that both the splice variants of Zfp318 were expressed in testicular germ cells, with the main variant being the long‐form. These results suggest that long‐form homodimers of ZFP318 could be part of a coactivator complex for transcriptional activation of nuclear receptors other than AR. ZFP318 has one (short‐form) or two (long‐form) U‐1 like zinc finger domains which can directly bind to DNA without AR. Moreover, once considered to function exclusively as sequence‐specific DNA‐binding motifs, zinc‐fingers are now known to also recognize RNA and other proteins (Gamsjaeger et al. 2007). Recently, Zfp318 was identified by genetic screen in mice as a transacting factor responsible for expression of IgD, the alternatively spliced Igh product made by mature B lymphocytes (Enders et al. 2014; Pioli et al. 2014). Indeed, our Zfp318‐null mice extinguished IgD expression on mature B cells and increased IgM (Enders et al. 2014). ZFP318 also has a conserved domain concerning chromosome segregation protein (PRK03918) or tumor suppressor, trichoplein or mitostatin, was first defined as a meiosis‐specific nuclear structural protein (pfam13868) (Vecchione et al. 2009), which has a crucial role in MT‐anchoring activity at the centrosome in proliferating cells (Ibi et al. 2011). Although ZFP318 seems to play an important role progressing meiosis in spermatogenesis, further study will be need to show if ZFP318 functions as a meiosis‐specific nuclear structural protein.
Supporting information
Fig. S1. Zfp318 knockout scheme and validation.
Fig. S2. Amino acid sequences of human ZNF318 (NP_055160), murine ZFP318 isoform 1 (long‐form) (NP_997554), and murine ZFP318 isoform 2 (short‐form) (NP_067321).
Acknowledgments
We would like to thank H. Nishimura, K. Takada, S. Kasagi, T. Yokoyama, H. Yumiyama, K. Sasaki, S. Kamakura, K. Yamasaki, T. Inoue, and A. Kurotobi for their help in the maintenance of mice and K. Nunoichi and Y. Maruyama for their technical assistance in producing the Zfp318 −/− mice. This work was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan and by grants from the Promotion and Mutual Aid Corporation for Private Schools of Japan.
References
- Ballow, D. , Meistrich, M. L. , Matzuk, M. & Rajkovic, A. 2006. Sohlh1 is essential for spermatogonial differentiation. Dev. Biol. 294, 161–167. [DOI] [PubMed] [Google Scholar]
- Blendy, J. A. , Kaestner, K. H. , Weinbauer, G. F. , Nieschlag, E. & Schutz, G. 1996. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature 380, 162–165. [DOI] [PubMed] [Google Scholar]
- Buaas, F. W. , Kirsh, A. L. , Sharma, M. , Mclean, D. J. , Morris, J. L. , Griswold, M. D. , De Rooij, D. G. & Braun, R. E. 2004. Plzf is required in adult male germ cells for stem cell self‐renewal. Nat. Genet. 36, 647–652. [DOI] [PubMed] [Google Scholar]
- Chizaki, R. , Yao, I. , Katano, T. , Matsuda, T. & Ito, S. 2012. Restricted expression of Ovol2/MOVO in XY body of mouse spermatocytes at the pachytene stage. J. Androl. 33, 277–286. [DOI] [PubMed] [Google Scholar]
- Costoya, J. A. , Hobbs, R. M. , Barna, M. , Cattoretti, G. , Manova, K. , Sukhwani, M. , Orwig, K. E. , Wolgemuth, D. J. & Pandolfi, P. P. 2004. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 36, 653–659. [DOI] [PubMed] [Google Scholar]
- Eddy, E. M. 2002. Male germ cell gene expression. Recent Prog. Horm. Res. 57, 103–128. [DOI] [PubMed] [Google Scholar]
- Enders, A. , Short, A. , Miosge, L. A. , Bergmann, H. , Sontani, Y. , Bertram, E. M. , Whittle, B. , Balakishnan, B. , Yoshida, K. , Sjollema, G. , Field, M. A. , Andrews, T. D. , Hagiwara, H. & Goodnow, C. C. 2014. Zinc‐finger protein ZFP318 is essential for expression of IgD, the alternatively spliced Igh product made by mature B lymphocytes. Proc. Natl. Acad. Sci. U S A. 111, 4513–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamsjaeger, R. , Liew, C. K. , Loughlin, F. E. , Crossley, M. & Mackay, J. P. 2007. Sticky fingers: zinc‐fingers as protein‐recognition motifs. Trends Biochem. Sci. 32, 63–70. [DOI] [PubMed] [Google Scholar]
- Ibi, M. , Zou, P. , Inoko, A. , Shiromizu, T. , Matsuyama, M. , Hayashi, Y. , Enomoto, M. , Mori, D. , Hirotsune, S. , Kiyono, T. , Tsukita, S. , Goto, H. & Inagaki, M. 2011. Trichoplein controls microtubule anchoring at the centrosome by binding to Odf2 and ninein. J. Cell Sci. 124, 857–864. [DOI] [PubMed] [Google Scholar]
- Inoue, A. , Ishiji, A. , Kasagi, S. , Ishizuka, M. , Hirose, S. , Baba, T. & Hagiwara, H. 2000. The transcript for a novel protein with a zinc finger motif is expressed at specific stages of mouse spermatogenesis. Biochem. Biophys. Res. Commun. 273, 398–403. [DOI] [PubMed] [Google Scholar]
- Ishizuka, M. , Ohshima, H. , Tamura, N. , Nakada, T. , Inoue, A. , Hirose, S. & Hagiwara, H. 2003. Molecular cloning and characteristics of a novel zinc finger protein and its splice variant whose transcripts are expressed during spermatogenesis. Biochem. Biophys. Res. Commun. 301, 1079–1085. [DOI] [PubMed] [Google Scholar]
- Ishizuka, M. , Kawate, H. , Takayanagi, R. , Ohshima, H. , Tao, R. H. & Hagiwara, H. 2005. A zinc finger protein TZF is a novel corepressor of androgen receptor. Biochem. Biophys. Res. Commun. 331, 1025–1031. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy, H. , Weinbauer, G. F. , Aslam, H. , Yeung, C. H. & Nieschlag, E. 1998. Quantification of apoptotic testicular germ cells in normal and methoxyacetic acid‐treated mice as determined by flow cytometry. J. Androl. 19, 710–717. [PubMed] [Google Scholar]
- Krishnamurthy, H. , Danilovich, N. , Morales, C. R. & Sairam, M. R. 2000. Qualitative and quantitative decline in spermatogenesis of the follicle‐stimulating hormone receptor knockout (FORKO) mouse. Biol. Reprod. 62, 1146–1159. [DOI] [PubMed] [Google Scholar]
- Martianov, I. , Fimia, G. M. , Dierich, A. , Parvinen, M. , Sassone‐Corsi, P. & Davidson, I. 2001. Late arrest of spermiogenesis and germ cell apoptosis in mice lacking the TBP‐like TLF/TRF2 gene. Mol. Cell 7, 509–515. [DOI] [PubMed] [Google Scholar]
- Nantel, F. , Monaco, L. , Foulkes, N. S. , Masquilier, D. , Lemeur, M. , Henriksen, K. , Dierich, A. , Parvinen, M. & Sassone‐Corsi, P. 1996. Spermiogenesis deficiency and germ‐cell apoptosis in CREM‐mutant mice. Nature 380, 159–162. [DOI] [PubMed] [Google Scholar]
- Pioli, P. D. , Debnath, I. , Weis, J. J. & Weis, J. H. 2014. Zfp318 regulates IgD expression by abrogating transcription termination within the Ighm/Ighd locus. J. Immunol. 193, 2546–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raverot, G. , Weiss, J. , Park, S. Y. , Hurley, L. & Jameson, J. L. 2005. Sox3 expression in undifferentiated spermatogonia is required for the progression of spermatogenesis. Dev. Biol. 283, 215–225. [DOI] [PubMed] [Google Scholar]
- Russell, L. D. , Ettlin, R. A. , Sinha Hikim, A. P. & Clegg, E. D. 1990. Histological and Histopathological Evaluation of the Testis. Clearwater, Fl, USA, Cache River Press. [Google Scholar]
- Sassone‐Corsi, P. 1997. Transcriptional checkpoints determining the fate of male germ cells. Cell 88, 163–166. [DOI] [PubMed] [Google Scholar]
- Tao, R. H. , Kawate, H. , Ohnaka, K. , Ishizuka, M. , Hagiwara, H. & Takayanagi, R. 2006a. Opposite effects of alternative TZF spliced variants on androgen receptor. Biochem. Biophys. Res. Commun. 341, 515–521. [DOI] [PubMed] [Google Scholar]
- Tao, R. H. , Kawate, H. , Wu, Y. , Ohnaka, K. , Ishizuka, M. , Inoue, A. , Hagiwara, H. & Takayanagi, R. 2006b. Testicular zinc finger protein recruits histone deacetylase 2 and suppresses the transactivation function and intranuclear foci formation of agonist‐bound androgen receptor competitively with TIF2. Mol. Cell. Endocrinol. 247, 150–165. [DOI] [PubMed] [Google Scholar]
- Vecchione, A. , Fassan, M. , Anesti, V. , Morrione, A. , Goldoni, S. , Baldassarre, G. , Byrne, D. , D'arca, D. , Palazzo, J. P. , Lloyd, J. , Scorrano, L. , Gomella, L. G. , Iozzo, R. V. & Baffa, R. 2009. MITOSTATIN, a putative tumor suppressor on chromosome 12q24.1, is downregulated in human bladder and breast cancer. Oncogene 28, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. S. , Yeh, S. , Tzeng, C. R. & Chang, C. 2009. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell‐specific androgen receptor knockout mice. Endocr. Rev. 30, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, J. , Meeks, J. J. , Hurley, L. , Raverot, G. , Frassetto, A. & Jameson, J. L. 2003. Sox3 is required for gonadal function, but not sex determination, in males and females. Mol. Cell. Biol. 23, 8084–8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, W. , Si, Y. , Slaymaker, S. , Li, J. , Zheng, H. , Young, D. L. , Aslanian, A. , Saunders, L. , Verdin, E. & Charo, I. F. 2010. Zmynd15 encodes a histone deacetylase‐dependent transcriptional repressor essential for spermiogenesis and male fertility. J. Biol. Chem. 285, 31418–31426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura, T. , Toyoda, S. , Kuramochi‐Miyagawa, S. , Miyazaki, T. , Miyazaki, S. , Tashiro, F. , Yamato, E. , Nakano, T. & Miyazaki, J. 2009. Gtsf1/Cue110, a gene encoding a protein with two copies of a CHHC Zn‐finger motif, is involved in spermatogenesis and retrotransposon suppression in murine testes. Dev. Biol. 335, 216–227. [DOI] [PubMed] [Google Scholar]
- Zhou, H. , Liu, L. H. , Zhang, H. , Lei, Z. & Lan, Z. J. 2010. Expression of zinc finger protein 105 in the testis and its role in male fertility. Mol. Reprod. Dev. 77, 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Zfp318 knockout scheme and validation.
Fig. S2. Amino acid sequences of human ZNF318 (NP_055160), murine ZFP318 isoform 1 (long‐form) (NP_997554), and murine ZFP318 isoform 2 (short‐form) (NP_067321).


