Abstract
The developmental transition from juvenile to adult is often accompanied by many systemic changes in morphology, metabolism, and reproduction. Curiously, both mammalian puberty and insect metamorphosis are triggered by a pulse of steroid hormones, which can harmonize gene expression profiles in the body and thus orchestrate drastic biological changes. However, understanding of how the timing of steroid hormone biosynthesis is regulated at the molecular level is poor. The principal insect steroid hormone, ecdysteroid, is biosynthesized from dietary cholesterol in the specialized endocrine organ called the prothoracic gland. The periodic pulses of ecdysteroid titers determine the timing of molting and metamorphosis. To date, at least nine families of ecdysteroidogenic enzyme genes have been identified. Expression levels of these genes correlate well with ecdysteroid titers, indicating that the transcriptional regulatory network plays a critical role in regulating the ecdysteroid biosynthesis pathway. In this article, we summarize the transcriptional regulation of ecdysteroid biosynthesis. We first describe the development of prothoracic gland cells during Drosophila embryogenesis, and then provide an overview of the transcription factors that act in ecdysteroid biosynthesis and signaling. We also discuss the external signaling pathways that target these transcriptional regulators. Furthermore, we describe conserved and/or diverse aspects of steroid hormone biosynthesis in insect species as well as vertebrates.
Keywords: ecdysteroid, insect, metamorphosis, prothoracic gland, transcription
The developmental transition from juvenile to adult is often accompanied by many systemic changes in morphology, metabolism, and reproduction. Curiously, both mammalian puberty and insect metamorphosis are triggered by a pulse of steroid hormones, which can harmonize gene expression profiles in the body and thus orchestrate drastic biological changes. However, understanding of how the timing of steroid hormone biosynthesis is regulated at the molecular level is poor. The principal insect steroid hormone, ecdysteroid, is biosynthesized from dietary cholesterol in the specialized endocrine organ called the prothoracic gland. The periodic pulses of ecdysteroid titers determine the timing of molting and metamorphosis. To date, at least nine families of ecdysteroidogenic enzyme genes have been identified. Expression levels of these genes correlate well with ecdysteroid titers, indicating that the transcriptional regulatory network plays a critical role in regulating the ecdysteroid biosynthesis pathway. In this article, we summarize the transcriptional regulation of ecdysteroid biosynthesis. We first describe the development of prothoracic gland cells during Drosophila embryogenesis, then provide an overview of the transcription factors that act in ecdysteroid biosynthesis and signaling. We also discuss the external signaling pathways that target these transcriptional regulators. Furthermore, we describe conserved and/or diverse aspects of steroid hormone biosynthesis in insect species as well as vertebrates.
Introduction
Temporal coordination of organismal development, simply called developmental timing, is one of the fundamental aspects in developmental biology (Ambros 2000; Thummel 2001; Banerjee & Slack 2002; Rougvie 2005). In various multicellular organisms, appropriate regulation of developmental timing allows organisms to be sexually mature adults from the juvenile stage. For example, in mammals including humans, puberty is one of the major temporal changes during development, which initiates a series of drastic morphological and physiological changes that make organisms reproductive. Such a drastic developmental transition to transform sexually immature individuals to fecund adults, known as metamorphosis, is also found in evolutionarily distant animals such as insects.
Both mammalian puberty and insect metamorphosis are triggered by steroid hormones, which are small fat‐soluble bioactive molecules that can pass through the cell membrane into the cytoplasm (Miller & Auchus 2011; Yamanaka et al. 2013a; Niwa & Niwa 2014b). Steroid hormones can systemically harmonize changes of gene expression in the body and thus orchestrate the drastic biological changes. One of the crucial keys to determine the timing of both puberty and metamorphosis is temporal regulation of steroid hormone biosynthesis in vivo, which generates temporally specific peaks of hemolymph steroid hormone titers to trigger developmental transitions (Hariharan 2012). Therefore, understanding the mechanisms that modulate the timing of biosynthesis is important to comprehend developmental timing at the molecular level.
An initial step towards the elucidation of steroid hormone biosynthesis is identification and characterization of steroidogenic enzymes responsible for converting precursor sterols to active steroid hormones. Studies on mammalian steroidogenic enzymes have been conducted since the 1980s, and a number of essential steroidogenic enzymes have been identified (Miller 1988; Hanukoglu 1992). By contrast, insect steroidogenic enzymes have been reported only since 2000. The previous 15 years, however, have been a fruitful period in terms of the elucidation of a number of insect steroidogenic enzymes (Niwa & Niwa 2014a). The principal insect steroid hormones are ecdysteroids, including ecdysone and its active derivative 20‐hydroxyecdysone (20E) that trigger metamorphosis as well as molting. Molecular genetics and biochemical studies using the fruit fly Drosophila melanogaster and the silkworm Bombyx mori have revealed that for ecdysteroid biosynthesis in the ecdysone‐producing organ, called the prothoracic gland (PG, Fig. 1), at least nine families of enzymes are required: noppera‐bo (nobo) (Enya et al. 2014, 2015), neverland (nvd) (Yoshiyama et al. 2006; Yoshiyama‐Yanagawa et al. 2011), non‐molting glossy/shroud (sro) (Niwa et al. 2010), Cyp307a1/spook (spo) (Niwa et al. 2005; Ono et al. 2006), Cyp307a2/spookier (spok) (Ono et al. 2006), Cyp6t3 (Ou et al. 2011), Cyp306a1/phantom (phm) (Niwa et al. 2004; Warren et al. 2004), Cyp302a1/disembodied (dib) (Chávez et al. 2000; Warren et al. 2002), and Cyp315a1/shadow (sad) (Warren et al. 2002). After release from the PG to the hemolymph, ecdysone is converted to 20E by another enzyme Cyp314a1/shade (shd) in the peripheral tissues (Petryk et al. 2003). All of these enzymes (except nvd, spok, and Cyp6t3) are collectively referred to as the Halloween genes (Rewitz et al. 2007; Niwa & Niwa 2014a).
Figure 1.
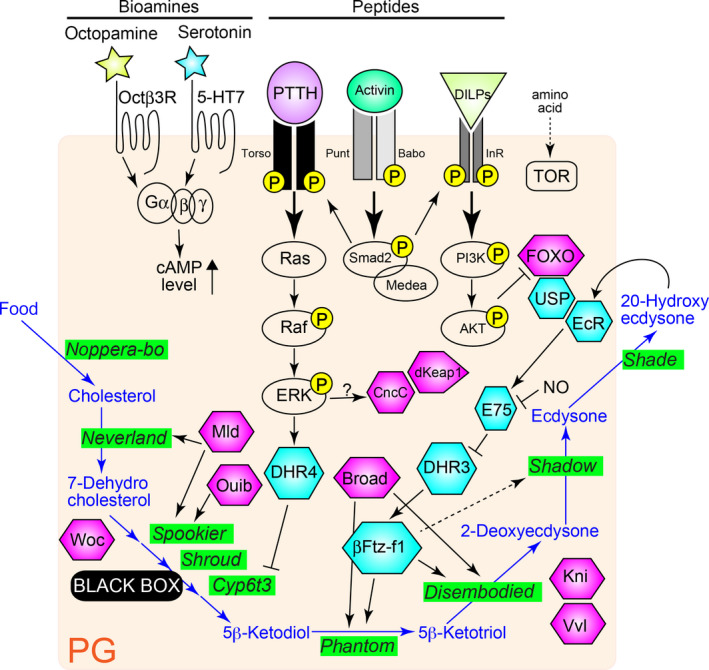
Overview of the ecdysteroid biosynthesis pathway and the regulatory mechanisms through transcriptional network in the prothoracic gland (PG) of Drosophila melanogaster. Several distinct signaling pathways regulate ecdysteroid biosynthesis and some pathways potentially target transcription factors (TFs) to regulate ecdysteroidogenic enzyme gene expressions. The ecdysteroid biosynthesis pathway is colored in blue. It starts with dietary cholesterol. Ecdysteroid biosynthesis enzymes are highlighted in green. Hexagons represent ecdysteroidogenic TFs that are listed in Table 1. Among them, nuclear receptors are colored in sky blue. In each signaling pathway, only the key components are depicted. Yellow “P” means phosphorylation. DILPS, Drosophila insulin‐like peptides; ERK, Extracellular signal‐regulated kinase; InR, Insulin receptor; NO, nitric oxide; PI3K, Phosphoinositide 3‐kinase; PG, prothoracic gland; PTTH, Prothoracicotropic hormone; TOR, Target of rapamycin.
After the discovery of the ecdysteroidogenic enzymes, researchers promptly realized that the expression levels of these biosynthesis genes in the PG correlate very well with the levels of hemolymph ecdysteroid titers (Warren et al. 2002, 2006; Niwa et al. 2005; Parvy et al. 2005). This is reminiscent of mammalian steroidogenic gene expression, which well reflects steroid hormone production (Mizutani et al. 2015). Therefore, it turns out that the timing of steroid hormone biosynthesis depends on transcriptional regulation of the steroidogenic enzyme genes. Indeed, transcriptional regulation of mammalian steroidogenic genes has been an important research issue for a long time, and the key steroidogenic transcriptional factors (TFs) have been identified. The nuclear receptor NR5A1, also known as Ad4BP/Steroidogenic Factor 1 (SF‐1), is recognized as a master regulator for steroidogenic gene expression, as this TF controls the transcription of almost all steroidogenic genes in steroidogenic tissues (Parker et al. 2002; Morohashi et al. 2013). By contrast, elucidation of ecdysteroidogenic TFs and their regulatory roles in insects has lagged far behind that of mammalian steroidogenic transcriptional regulation.
In this review, we will examine the recent progress of the spatio‐temporal transcriptional regulatory mechanisms underlying ecdysteroid biosynthesis in the PG. Based on studies published in the previous 15 years, we will illustrate that multiple transcription factors are cooperatively working in a network to achieve the differentiation and morphogenesis of the PG cells, and the appropriate control of ecdysteroid biosynthesis during larval development. In addition, we will also discuss how TFs are regulated by extracellular stimuli, affecting ecdysteroid biosynthesis by controlling ecdysteroidogenic gene expressions.
Transcription factors specify the origin of the prothoracic gland cells
In mammals, Ad4BP/SF‐1 serves as a master TF to induce differentiation of the cell into a steroidogenic cell lineage (Parker et al. 2002; Morohashi et al. 2013; Mizutani et al. 2015). In insects, much less is known about molecular mechanisms to regulate the development of the PG itself during embryogenesis. Very recently, Sánchez‐Higueras et al. (2014) reported that differentiation and morphogenesis of the PG requires a proper combination of TFs during Drosophila embryogenesis (Fig. 2). The study demonstrates that the PG has a homologous origin with the respiratory tracheal system in Drosophila. The PG and the corpora allata (CA), which biosynthesizes juvenile hormone, are derived from identical primordia in successive segments of the head and trunk of the embryo: the PG arises in the labial (Lb) segment, the CA arises in the maxillary (Mx) segment, and the trachea arises in thoracic T2 to abdominal A8 segments. In each segment, the identity of the PG, CA, and tracheas is initially specified by a unique Homeotic TF with the Signal Transducers and Activator of Transcription (STAT) via inducing expression of the POU‐domain TF gene ventral veins lacking (also known as ventral veinless; vvl). In detail, Deformed (Dfd) and Sex comb reduced (Scr) control vvl expressions in the Mx and Lb patches, res‐pectively. Subsequently, a subgroup of vvl‐expressing cells activates the Zn‐finger gene snail, a key regulator of the epithelial‐mesenchymal transition. The snail‐expressing cells then migrate dorsally and merge into the corpora cardiaca (CC), which originates from anterior mesodermal cells. In cyclorrhaphous Diptera, including Drosophila, the PG, CA, and CC form a composite endocrine organ “the ring gland (RG)”. The differentiated PG and CA cells eventually express the specific marker TF genes, spalt and seven‐up, respectively (Sánchez‐Higueras et al. 2014). In summary, the specification of the embryonic PG primordia requires a specific combination of TFs, at least including Scr, STAT, Vvl, and Snail (Fig. 2). These TF codes might be essential for establishing the cellular status of the PG cells expressing the special set of ecdysteroidogenic genes.
Figure 2.
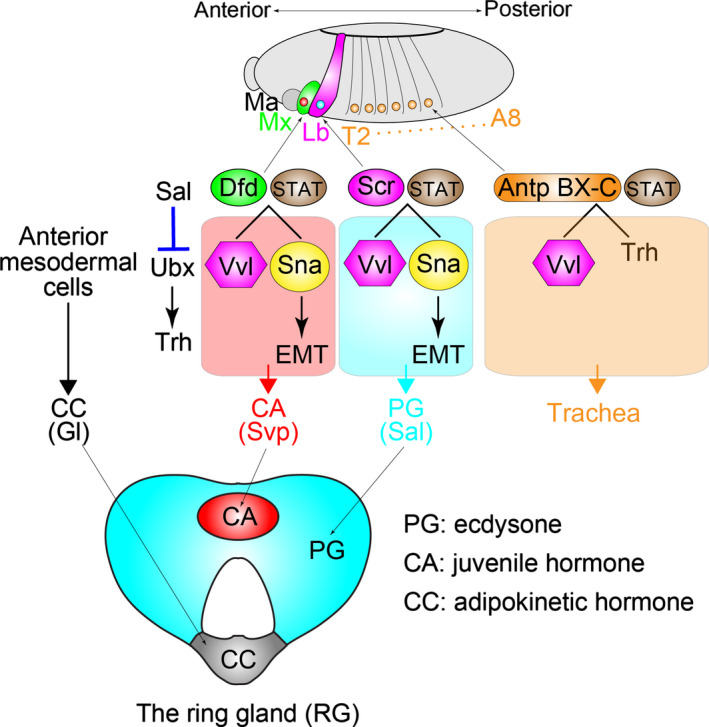
Schematic of ectodermal endocrine and respiratory primordia in embryos and their specification gene regulatory network. A part of this cartoon is adapted from figure 4 in Sánchez‐Higueras et al. (2014). In each segment, Homeotic genes and Signal Transducers and Activators of Transcription (STAT) induce expression of the early transcription factors. Deformed (Dfd)‐STAT in the maxillary primordium (Mx) and Sex combs reduced (Scr)‐STAT in the labial primordium (Lb) induce ventral veins lacking (vvl) and snail (sna) expression. Spalt (Sal) represses trunk Homeotic gene expression in these primordia, preventing trachealess (trh) expression. The sna‐expressing cells undergo the epithelial‐mesenchymal transition (EMT) and migrate to form the corpora allata (CA) and the prothoracic gland (PG) cells. The corpora cardiaca (CC) cells are separately derived from anterior mesodermal cells. Representative lineage markers are Glass (Gl) in the CC, Seven‐up (Svp) in the CA, and Sal in the PG. These cells make a composite endocrine organ called the ring gland (RG).
Transcription factors are required for expression of ecdysteroidogenic genes in the prothoracic gland
After the PG is specified, a number of ecdysteroidogenic enzyme genes and ecdysteroidogenic regulators begin to be expressed, which is an important characteristic of this endocrine organ. Such gene expressions are induced and/or maintained by a number of TFs, hereinafter referred to as “ecdysteroidogenic TFs”. Some but not all ecdysteroidogenic TFs directly bind to the promoters of ecdysteroidogenic genes, confirmed by an electrophoresis mobility shift assay or chromatin immunoprecipitation assay (Xiang et al. 2010; Deng & Kerppola 2013; Danielsen et al. 2014; Meng et al. 2015). The known validated and putative ecdysteroidogenic TFs required in the PG are listed in Table 1.
Table 1.
A list of ecdysteroidogenic transcription factors
| TF names | Protein family | Reported ecdysteroidogenic genes whose expression are affected† | Organisms analyzed in published studies | References |
|---|---|---|---|---|
| Antp | homeotic | phm | Bombyx mori | Meng et al. (2015) |
| Br | C2H2 zinc finger | phm , dib , sad, npc1 | Drosophila melanogaster | Xiang et al. (2010); Moeller et al. (2013) |
| CncC | basic leucine zipper | nvd , spok , dib , sad | D. melanogaster | Deng & Kerppola (2013) |
| DHR3 | nuclear receptor | phm, dib, sad | D. melanogaster | Parvy et al. (2014) |
| DHR4 | nuclear receptor | Cyp6t3 | D. melanogaster | Ou et al. (2011) |
| dKeap1 | BTB | nvd , spok , phm , dib , sad | D. melanogaster | Deng & Kerppola (2013) |
| E75 | nuclear receptor | phm | D. melanogaster | Bialecki et al. (2002); Cáceres et al. (2011); Parvy et al. (2014) |
| EcR | nuclear receptor | phm, dib, sad | D. melanogaster | Moeller et al. (2013); Parvy et al. (2014) |
| FOXO | Forkhead | phm, dib | D. melanogaster | Koyama et al. (2014) |
| βFtz‐f1 | nuclear receptor | phm, dib, sad | D. melanogaster | Parvy et al. (2005, 2014) |
| Kni | C2C2 zinc finger | phm , dib, sad | D. melanogaster | Danielsen et al. (2014) |
| Mld‡ | C2H2 zinc finger | nvd, spok, sro | D. melanogaster | Ono et al. (2006); Danielsen et al. (2014) |
| Ouib‡ | C2H2 zinc finger | spok | D. melanogaster | Komura‐Kawa et al. (2015). |
| USP | C2C2 zinc finger, ligand binding | phm, dib | D. melanogaster | Koyama et al. (2014) |
| Vvl/POU‐M2 | POU | spok, sro, phm , dib, sad, torso |
D. melanogaster Tribolium castaneum B. mori |
Cheng et al. (2014); Danielsen et al. (2014); Meng et al. (2015) |
†Underlines indicate genes whose promoter sequences can be physically associated with TFs, which are shown by electrophoresis mobility shift assay and/or chromatin immunoprecipitation analyses. ‡It must be noted that these TF genes are found only in genomes of Drosophilidae species. Abbreviations: Antp, Antennapedia; Br, Broad; CncC, Cap'n'collar; DHR3, Drosophila Hormone Receptor 3; DHR4, Drosophila Hormone Receptor 4; E75, Ecdysone‐induced protein 75; EcR, Ecdysone receptor; FOXO, Forkhead box, sub‐group O; βFtz‐F1, β‐fushi tarazu transcription factor 1; Kni, Knirps; Mld, Molting defective; Ouib, Ouija board; USP, Ultraspiracle; Vvl, Ventral veins lacking
The first identified TF that influences the expression levels of validated ecdysteroidogenic genes was βFtz‐f1, a critical regulator of insect metamorphosis in many tissues (Parvy et al. 2005). βFtz‐f1 is a homologue of Ad4BP/SF‐1, suggesting a conserved role of steroidogenic TFs. Studies on Drosophila clearly show that the protein levels of Phm and Dib are significantly reduced with the loss of βFtz‐f1 function in PG cells (Parvy et al. 2005).
Most of the ecdysteroidogenic TFs, listed in Table 1, have originally been characterized as TFs for their non‐steroidogenic functions: βFtz‐f1, Ultraspiracle (USP) (Koyama et al. 2014), Broad (Br) (Xiang et al. 2010; Moeller et al. 2013), DHR3 (Parvy et al. 2014), and DHR4 (Ou et al. 2011) are encoded by the well‐known ecdysteroid‐inducible genes that are expressed in many types of cells (Thummel 2001; Ou & King‐Jones 2013). Other TFs are known for their roles in spatial pattern formation. For example, Vvl described above is involved in cellular differentiation of several types of cells including the PG (Cheng et al. 2014; Danielsen et al. 2014; Sánchez‐Higueras et al. 2014). Knirps is quite well known as a gap gene during embryogenesis (Danielsen et al. 2014). Moreover, TFs involved in metabolic responses have also been identified, such as the Cap'n'collar (CncC)‐dKeap1 complex and Forkhead box, sub‐group O (FOXO) that are mediators of the xenobiotic metabolism signaling pathway and of the insulin/insulin‐like peptide signaling pathway, respectively (Deng & Kerppola 2013; Koyama et al. 2014).
In addition to typical TFs, chromatin remodeling factors influence ecdysteroid biosynthesis by affecting expression of many but not all ecdysteroidogenic genes in the PG, as evidenced by genetic analyses on the dATAC histone acetylase complex (Pankotai et al. 2010; Borsos et al. 2015) and the insulator protein CTCF (Fresán et al. 2015). It should be noted that these chromatin remodeling factors are also known as crucial proteins for many biological processes other than ecdysteroid biosynthesis, indicating that these ‘generalist’‐type transcription regulators act on a certain group of ecdysteroidogenic genes in the PG.
The evolutionarily conserved function of steroidogenic TFs
Many of the ecdysteroidogenic TFs are evolutionarily conserved across a wide variety of animal species from insects to mammals. The most epitomized example is βFtz‐f1, an insect orthologue of vertebrate Ad4BP/SF1. As described above, Ad4BP/SF1 is the key regulator of vertebrate steroidogenic organ specifications and steroidogenic gene expressions (Parker et al. 2002; Morohashi et al. 2013; Mizutani et al. 2015). It is also interesting to note that a chromosomal deletion of a human homologue of vvl, known as POU3F2, is associated with hypogonadotropic hypogonadism and adrenal insufficiency (Bonaglia et al. 2008; Izumi et al. 2013). Insect orthologues of vvl are also involved in ecdysteroid biosynthesis not only in D. melanogaster but also in other insects such as the red flour beetle Tribolium castaneum (Cheng et al. 2014) and B. mori (referred as POU‐M2) (Meng et al. 2015). Therefore, the βFtz‐f1/SF‐1 and Vvl/POU3F2 families emphasize the conserved regulatory mechanisms of steroidogenesis between insects and vertebrates.
On the other hand, the ecdysteroidogenic C2H2 zinc finger TF Molting defective (Mld) is very unique because its orthologues are found only in genomes of Drosophilidae species (Neubueser et al. 2005; Ono et al. 2006). The less conservative nature of Mld does not mean a lesser importance of the TF; Mld plays an essential role in ecdysteroid biosynthesis via inducing nvd and spok (Ono et al. 2006; Danielsen et al. 2014). It is also noteworthy that the classical temperature‐sensitive dominant mutant lethal(3)dts3, which shows ecdysteroid deficiency (Walker et al. 1987), has been recognized and used as an allele of mld (Simon et al. 2003; Ishimoto et al. 2013). Besides mld, we have also recently identified another novel ecdysteroidogenic C2H2 zinc finger TF gene designated ouija board (ouib), which is required for expression of spok but whose orthologues are found only in Drosophilidae genomes (Komura‐Kawa et al. 2015). These data imply that some essential ecdysteroidogenic TFs might have rapidly evolved only in very small insect clade(s), and thus future studies using a variety of insects would be valuable to unravel ecdysteroidogenic TFs that are not well conserved in Drosophilidae.
PTTH signaling regulates transcription of ecdysteroidogenic genes in the prothoracic glands
In general, expression levels of ecdysteroidogenic genes correlate well with temporal fluctuation of the ecdysteroid titer during development. Thus, regarding the issue of “Time in development”, an important question to be addressed is how the ecdysteroidogenic TFs described above contribute to timing of ecdysteroid biosynthesis in the PG.
Previous studies have demonstrated that timing of ecdysteroid biosynthesis in the PG is influenced by multiple extracellular stimuli (Niwa & Niwa 2014b). The most important and classical humoral factor is the neuropeptide Prothoracicotropic hormone (PTTH), which stimulates the biosynthesis and secretion of ecdysteroids in the PG (Tanaka 2011). The temporal coordination of PTTH secretion regulates timing of molting and metamorphosis in many insects (Mizoguchi et al. 2001, 2015; Halme et al. 2010; Yamanaka et al. 2013b). While PTTH has been extensively studied for its short‐term prothoracicotropic activity, which requires the de novo translation of proteins (Gilbert et al. 2002), PTTH also influences transcription in the PG, which might have a long‐term effect on ecdysteroid biosynthesis (Ou & King‐Jones 2013). For example, in vitro experimental assays have demonstrated that a recombinant PTTH protein stimulates transcription of spo, dib, and phm in the PG of the silkworm B. mori (Namiki et al. 2005; Niwa et al. 2005; Yamanaka et al. 2007). Conversely, PTTH neuron‐ablated animals or loss‐of‐function animals of torso, which encodes a PTTH receptor (Rewitz et al. 2009b) exhibit drastic reduction of many ecdysteroidogenic enzyme genes in D. melanogaster (McBrayer et al. 2007; Niwa et al. 2010; Enya et al. 2014). Therefore, the PTTH signaling pathway in the PG, which consists of Torso, Ras small GTPase, Raf kinase, and Extracellular signal‐related kinase (ERK) (Rewitz et al. 2009b), should control the activities of some ecdysteroidogenic TFs (Fig. 1).
Although ecdysteroidogenic TFs acting downstream of the Torso‐Ras‐ERK pathway are not fully understood, one striking example is the nuclear receptor DHR4 (Ou et al. 2011). The activity of DHR4 is regulated by its subcellular localization between the nucleus and cytoplasm of the PG cells. Furthermore, DHR4 protein accumulates in the PG nuclei at developmental times when the ecdysteroid titer is high. A crucial function of DHR4 is to negatively regulate expression of the ecdysteroidogenic P450 gene Cyp6t3, which functions in the ‘Black Box’ in the biosynthesis pathway but whose substrate is still unknown (Ou et al. 2011).
Currently, DHR4 is the only good example of ecdysteroidogenic TFs acting downstream of PTTH signaling. Nevertheless, DHR4 is not extensively involved in regulating expression of any other characterized ecdysteroidogenic enzyme genes (Ou et al. 2011). Therefore, PTTH signaling must target other ecdysteroidogenic TFs in the PG to control ecdysteroidogenic gene expressions. Recently, it was shown that loss of cncC function suppresses a developmental acceleration phenotype of the activated Ras overexpression (Deng & Kerppola 2013), suggesting that the CncC‐dKeap1 complex would be a candidate acting downstream of the PTTH signaling pathway.
Regulation of ecdysteroidogenic gene expression in the prothoracic gland by 20‐hydroxyecdysone and the ecdysteroid‐signaling cascade
In addition to PTTH, other humoral factors that influence ecdysteroidogenic gene expressions in the PG are ecdysteroids per se, particularly 20E, the most biologically active form of ecdysteroids, and its downstream signaling. 20E has a large impact on ecdysteroid biosynthesis in the PG (Gilbert et al. 2002). For example, in cultured B. mori PGs, both ecdysteroid biosynthesis activity and responsiveness to PTTH are inhibited by 20E administration (Takaki & Sakurai 2003). Curiously, the inhibitory effect of 20E on the PG is dependent on the larval developmental stage of B. mori, implying that 20E seems to have a feedback effect on the PG to generate a peak of ecdysteroid level from juvenile to adult transition.
An in vivo biological significance of the feedback effect of 20E has recently been demonstrated by genetics of D. melanogaster (Fig. 3). During the larval stages, reduced ecdysteroid‐EcR signaling in the PG decreases ecdysteroidogenic gene expressions, leading to a delay in the larva‐to‐pupa transition (Moeller et al. 2013). This indicates that ecdysteroid has a positive‐feedback effect on the PG, rapidly amplifying its own synthesis to trigger pupariation. Indeed, the isoforms of the ecdysteroid‐regulated factor Br, Br‐Z1, and Br‐Z4 bind directly to the phm and dib promoters/enhancers to regulate their transcriptions. By contrast, after pupariation, reduced ecdysteroid‐EcR signaling increases ecdysteroidogenic gene expressions, leading to incomplete metamorphosis. This means that a negative‐feedback signal ensures the decline in ecdysteroid levels in the prepupa‐to‐pupa transition. Notably, these opposing signals depend on the different responses of Br isoforms to ecdysteroid levels: low levels of ecdysteroid should quickly induce Br‐Z4, whereas high levels of ecdysteroid should induce Br‐Z1 that transcriptionally silences the ecdysteroidogenic genes (Moeller et al. 2013). The inhibitory effect of Br is reminiscent of the fact that overexpression of Br in the PG blocks molting, and this larval arrest phenotype can be rescued by feeding 20E (Zhou et al. 2004).
Figure 3.
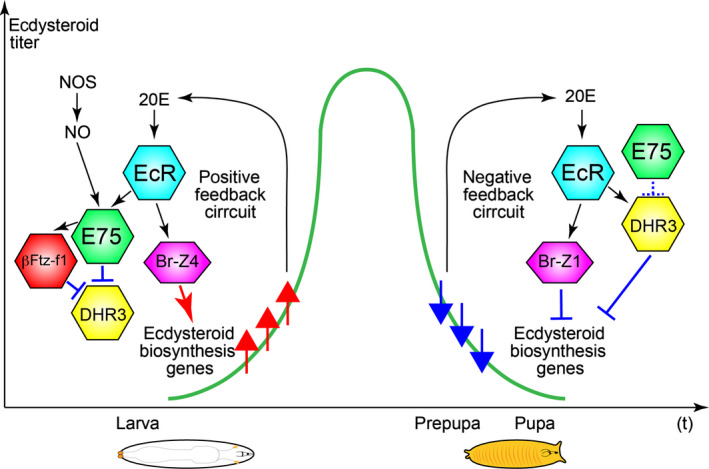
Feedback control of ecdysteroids affects ecdysteroidogenic gene expressions in the larva‐to‐pupa transition in Drosophila. The names of TFs are listed in Table 1. In the late 3rd instar stage, ecdysteroid biosynthesis is amplified by the positive feedback of 20‐hydroxyecdysone (20E)‐EcR signaling and nitric oxide (NO)‐E75 signaling to trigger pupariation. Br‐B4 upregulates the expressions of ecdysteroidogenic enzyme genes such as phm and dib. After pupariation, 20E‐EcR signaling negatively regulates ecdysteroid biosynthesis by Br‐Z1, which suppresses the expressions of ecdysteroidogenic enzyme genes. Moreover, DHR3 also represses ecdysteroidogenic enzyme gene expressions. The positive and negative feedback circuits accomplish the temporal peak of ecdysteroid titer in the developmental transition. NOS, nitric oxide synthase.
Other ecdysteroid‐response genes modulate timing of ecdysteroid biosynthesis in the PG. Similar to the repressive function of Br‐Z1 described above, the ecdysteroid‐inducible nuclear receptor DHR3 represses ecdysteroidogenic enzyme genes through EcR function during the prepupal‐to‐pupal transition (Parvy et al. 2014). Conversely, before the transition, the other ecdysteroid‐inducible nuclear receptor E75 positively regulates ecdysteroid biosynthesis (Bialecki et al. 2002). Indeed, E75 and βFtz‐f1 counteracts DHR3 to avoid premature repression of ecdysteroid biosynthesis (Cáceres et al. 2011; Parvy et al. 2014). These data demonstrate that the metamorphic 20E peak relies on ecdysteroid‐mediated feedback control of PG activity through transcriptional regulatory networks.
E75 contains a heme moiety and thus can bind to nitric oxide (NO), an important secondary messenger acting as a short‐range signaling molecule in a vast array of important physiological processes (Reinking et al. 2005). NO is produced by NO synthase (NOS) in the PG and blocks the function of E75 (Wildemann & Bicker 1999; Cáceres et al. 2011). While it is unclear whether and how NO synthesis is temporally regulated in the PG, the NO signaling pathway possibly modulates ecdysteroid biosynthesis in the PG (Jaszczak et al. 2015).
Other extracellular signals that influence ecdysteroidogenic gene expression
Recent studies have accumulated evidence that ecdysteroidogenic gene expression in the PGs is influenced not only by PTTH and 20E, but also by other extracellular signals and their signaling pathways (Fig. 1). These pathways include the insulin like peptides‐TOR pathway (Colombani et al. 2005; Koyama et al. 2013), the TGFβ/Activin‐Smad pathway (Gibbens et al. 2011), serotonin and its receptor 5HT‐7 (Shimada‐Niwa & Niwa 2014), octopamine and its receptor Octβ3R (Ohhara et al. 2012, 2015), and Bommo‐FMRFamide that is a prothoracicostatic neuropeptide (Yamanaka et al. 2007), all of which are required for determining the proper timing of ecdysteroid biosynthesis in the PG during development (Niwa & Niwa 2014b). It will be interesting to determine which ecdysteroidogenic TFs act downstream of any of these signaling cascades, and whether any ecdysteroidogenic TFs are regulated by those multiple signaling inputs.
Some ecdysteroidogenic TFs, however, control expression of genes encoding essential components of these signaling pathways. For example, Vvl and Kni are required for expression of torso, Insulin receptor, akt, 4E‐BP, and S6 kinase (Danielsen et al. 2014), indicating that some ecdysteroidogenic TFs directly and indirectly regulate ecdysteroid biosynthesis by transcriptional control. Related to this point, ecdysteroidogenic TFs also regulate transcription of genes that are involved in uptake and transport of extracellular cholesterol or plant sterols, which are the precursors of ecdysteroids. For example, Br plays an indispensable role in controlling expression of not only ecdysteroidogenic genes (Danielsen et al. 2014) but also the Niemann‐Pick type C1 gene, which encodes the evolutionarily conserved cholesterol transporter (Xiang et al. 2010). βFtz‐f1 is also required for ecdysteroid biosynthesis in the PG via regulating expression of a scavenger receptor gene Snmp1, which appears to be involved in lipid uptake in the PG cells (Talamillo et al. 2008, 2013).
It must be noted that regulatory mechanisms to control ecdysteroidogenic gene expression might be diversified among insect species. For example, several studies have reported that juvenile hormones (JHs) have significant effects on some ecdysteroidogenic enzyme genes and torso in the PG of B. mori (Yamanaka et al. 2007; Young et al. 2012; Ogihara et al. 2015). This is less likely the case with the PG in D. melanogaster, considering that JHs appear not to have the typical “status quo” effect on larval development in D. melanogaster (Niwa et al. 2008; Liu et al. 2009; Riddiford et al. 2010; Ono 2014; Wen et al. 2015). Furthermore, some prothoracicotropic factors might primarily control translation, but not transcription, in the PG. A recent example is Pigment dispersing factor (PDF), as PDF stimulates ecdysteroid biosynthesis in the PG of B. mori but does not influence any known ecdysteroidogenic enzyme genes (Iga et al. 2014).
Protein modifications and protein–protein interactions to modulate the activity of ecdysteroidogenic TFs
Downstream of the signaling pathways, the activity of ecdysteroidogenic TFs must be modulated at the protein level. The striking example is the protein SUMOylation. A recent study has demonstrated that the activity of βFtz‐f1 in ecdysteroidogenic cells, including PG and ovarian follicle cells, is modulated by its SUMOylation (Talamillo et al. 2008, 2013). Curiously, mammalian Ad4BP/SF‐1 is also SUMOylated. Moreover, the disruption of Ad4BP/SF‐1 SUMOylation in mice exhibits the inappropriate activation of target genes, leading to abnormalities of endocrine development (Lee et al. 2011), suggesting that a part of post‐translational modification of ecdysteroidogenic TFs is evolutionarily conserved between insects and vertebrates. βFtz‐f1 also appears to be controlled by its acetylation at least in D. melanogaster cultured cells, resulting in its protein stabilization (Borsos et al. 2015). In addition, phosphorylation might be one of the essential post‐translational regulations in the PG, as PTTH signaling involves the Ras‐ERK pathway (Rewitz et al. 2009b). However, currently, there are no reports demonstrating the phosphorylation of any validated ecdysteroidogenic TFs including DHR4 (Rewitz et al. 2009a).
Physical interactions between ecdysteroidogenic TFs and other regulator proteins have also been recognized as another layer of mechanisms regulating ecdysteroid biosynthesis in the PG, as shown by the interaction between USP and FOXO (Koyama et al. 2014) and among CDK8, CyclinC, EcR, and USP (Xie et al. 2015). Further protein interactome analyses would be important in the future.
Outlook
In the previous 15 years, significant progress has been made that allows for a better understanding of transcriptional regulation of ecdysteroid biosynthesis, particularly in the fruit fly D. melanogaster. It is now obvious that ecdysteroidogenic genes are regulated by multiple numbers of TFs in the PG. However, the current list of ecdysteroidogenic TFs is incomplete, as candidate ecdysteroidogenic TFs are still present as previously discussed (Ou & King‐Jones 2013). For example, mutant animals of without children (woc), encoding a C2H2‐type zinc finger protein, is a larval lethal with ecdysteroid deficiency (Wismar et al. 2000). Because the mutant can be partially rescued by feeding 7‐dehydrocholesterol (Warren et al. 2001), Woc has been hypothesized to activate transcription of a gene involved in the cholesterol 7,8‐dehydrogenastion to produce 7dC. However, it is still unclear whether Woc regulates the expression of nvd, encoding cholesterol 7,8‐dehydrogenase (Yoshiyama et al. 2006), or any other downstream targets. Another example is a basic‐Helix‐Loop‐Helix TF gene HLH54F that is predominantly expressed in the PG of both D. melanogaster and B. mori (Namiki et al. 2009). No validated targets of HLH54F in the PG have been reported. More interestingly, the central circadian clock genes period and timeless, which do not encode actual TFs but regulatory proteins modulating transcription, are rhythmically expressed in the pupal PG and play a role in controlling eclosion rhythms (Myers et al. 2003; Morioka et al. 2012). Therefore, gene expression levels of a certain set of genes in the PG might be transcriptionally oscillated under the control of the central clock network. Identification and characterization of the oscillatory genes may elucidate a connection between clock and metamorphosis.
Undoubtedly, the current studies on ecdysteroidogenic TFs are almost only focusing on their functions in the PG cells, while ecdysteroid biosynthesis occurs in other types of cells during embryonic and adult stages. In the embryo, ecdysteroid biosynthesis is activated during mid‐embryogenesis before the development of PG primordial cells. At this stage, a subset of epidermal cells and the amnioserosa cells appears to be responsible for ecdysteroid biosynthesis, as the Halloween genes are strongly expressed in these cells (Chávez et al. 2000; Warren et al. 2002, 2004; Petryk et al. 2003; Niwa et al. 2004, 2010; Namiki et al. 2005; Ono et al. 2006; Yoshiyama et al. 2006; Enya et al. 2014). Importantly, the temporal fluctuation of the Halloween gene expressions during mid‐embryogenesis correlates very well with that of the embryonic ecdysteroid titer (Niwa et al. 2010; Enya et al. 2014). In the case of the adults, female ovarian follicle cells are the classically famous sites of ecdysteroid biosynthesis and indeed require ecdysteroidogenic enzymes for biosynthesis (Ono et al. 2006; Domanitskaya et al. 2014; Sieber & Spradling 2015) (T. Ameku and R.N., unpublished data). However, it is unclear which TFs regulate such embryonic and ovarian ecdysteroidogenic gene expressions, except for βFtz‐f1 that appears to regulate dib expression in the follicle cells (Talamillo et al. 2013).
We must now unravel the higher regulatory mechanisms that control ecdysteroid biosynthesis through transcription. As described above, the most important issue to draw a signaling network for controlling ecdysteroid biosynthesis is to understand which ecdysteroidogenic TFs act downstream of which extracellular stimulus‐triggered signaling pathway. As PTTH signaling promotes activated ERK phosphorylating nuclear target proteins such as TFs, it is feasible to hypothesize that PTTH signaling regulates the activity of several ecdysteroidogenic TFs including DHR4 (Ou et al. 2011). A proteomic approach would be helpful to identify PTTH‐stimulated phosphorylated proteins in the PG in future, while a previous trial using the tobacco hornworm Manduca sexta did not identify any known ecdysteroidogenic TFs described above (Rewitz et al. 2009a).
>All of the evidence clearly demonstrates the necessity of ecdysteroidogenic TFs to control ecdysteroidogenic gene expression in the PG. By contrast, there is no reported study examining whether any ecdysteroidogenic TFs are sufficient to induce ecdysteroidogenic gene expression in non‐steroidogenic cells. In the case of mammals, Ad4BP/SF‐1 is both necessary and sufficient for the induction and maintenance of steroidogenic genes. For example, overexpression of Ad4BP/SF‐1 differentiates cultured stem cells into steroidogenic cell lineages with the expression of various steroidogenesis‐related genes (Miyamoto et al. 2011). Moreover, transgenic expression of Ad4BP/SF‐1 in mice leads to ectopic adrenal formation (Zubair et al. 2009). By contrast, it is unlikely that βFtz‐f1, the insect homologue of Ad4BP/SF‐1, acts as a master regulator to induce ecdysteroidogenic gene expression in the PG because βFtz‐f1 plays a crucial role in ecdysteroid‐dependent transcriptional cascades not only in the PG, but also in many other tissues (Thummel 2001). Besides βFtz‐f1, other ecdysteroidogenic TFs identified to date are also highly expressed in non‐ecdysteroidogenic cells and have important functions other than ecdysteroid biosynthesis. Therefore, it is an interesting open question to examine whether and how forced expression of one or more TFs can differentiate the PG cells and/or induce ecdysteroidogenic gene expression. This point might be important to comprehensively understand the evolutionary commonality of steroidogenic TF function during animal evolution.
Finally, we would like to point out that the current published studies on ecdysteroidogenic TFs have focused on their roles in regulating just a handful of identified ecdysteroidogenic enzyme genes and other known regulatory protein genes, but no studies have examined the entire transcriptome in the PG. It is possible that the set of known genes is just the tip of the iceberg of ecdysteroidogenic TF‐regulating genes. Interestingly, a recent study using mice reveals that Ad4BP/SF‐1 governs the coordinated regulation of not only typical steroidogenic genes, but also essential genes within a glycolytic pathway (Baba et al. 2014). In the future, next‐generation sequencing approaches could help to employ RNA‐sequencing to comprehensively understand the ecdysteroidogenic TF‐dependent regulation of gene expression profiles at the transcriptome system level.
Acknowledgments
We thank Tomotsune Ameku for valuable comments on the manuscript. This work was supported by grants to R.N. from JST/PRESTO and Japan Society for the Promotion of Science KAKENHI Grant Number 25712010.
References
- Ambros, V. 2000. Control of developmental timing in Caenorhabditis elegans . Curr. Opin. Genet. Dev. 10, 428–433. [DOI] [PubMed] [Google Scholar]
- Baba, T. , Otake, H. , Sato, T. , Miyabayashi, K. , Shishido, Y. , Wang, C.‐Y. , Shima, Y. , Kimura, H. , Yagi, M. , Ishihara, Y. , Hino, S. , Ogawa, H. , Nakao, M. , Yamazaki, T. , Kang, D. , Ohkawa, Y. , Chung, B.‐C. & Morohashi, K. 2014. Glycolytic genes are targets of the nuclear receptor Ad4BP/SF‐1. Nat. Commun. 5, 3634. [DOI] [PubMed] [Google Scholar]
- Banerjee, D. & Slack, F. 2002. Control of developmental timing by small temporal RNAs: A paradigm for rna‐mediated regulation of gene expression. BioEssays 24, 119–129. [DOI] [PubMed] [Google Scholar]
- Bialecki, M. , Shilton, A. , Fichtenberg, C. , Segraves, W. A. & Thummel, C. S. 2002. Loss of the ecdysteroid‐inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila . Dev. Cell 3, 209–220. [DOI] [PubMed] [Google Scholar]
- Bonaglia, M. C. , Ciccone, R. , Gimelli, G. , Gimelli, S. , Marelli, S. , Verheij, J. , Giorda, R. , Grasso, R. , Borgatti, R. , Pagone, F. , Rodrìguez, L. , Martinez‐Frias, M.‐L. , van Ravenswaaij, C. & Zuffardi, O. 2008. Detailed phenotype–genotype study in five patients with chromosome 6q16 deletion: narrowing the critical region for Prader‐Willi‐like phenotype. Eur. J. Hum. Genet. 16, 1443–1449. [DOI] [PubMed] [Google Scholar]
- Borsos, B. N. , Pankotai, T. , Kovács, D. , Popescu, C. , Páhi, Z. & Boros, I. M. 2015. Acetylations of Ftz‐F1 and histone H4K5 are required for the fine‐tuning of ecdysone biosynthesis during Drosophila metamorphosis. Dev. Biol. 404, 80–87. [DOI] [PubMed] [Google Scholar]
- Cáceres, L. , Necakov, A. S. , Schwartz, C. , Kimber, S. , Roberts, I. J. & Krause, H. M. 2011. Nitric oxide coordinates metabolism, growth, and development via the nuclear receptor E75. Genes Dev. 25, 1476–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez, V. M. , Marqués, G. , Delbecque, J. P. , Kobayashi, K. , Hollingsworth, M. , Burr, J. , Natzle, J. E. & O'Connor, M. B. 2000. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development 127, 4115–4126. [DOI] [PubMed] [Google Scholar]
- Cheng, C. , Ko, A. , Chaieb, L. , Koyama, T. , Sarwar, P. , Mirth, C. K. , Smith, W. A. & Suzuki, Y. 2014. The POU factor ventral veins lacking/Drifter directs the timing of metamorphosis through ecdysteroid and juvenile hormone signaling. PLoS Genet. 10, e1004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani, J. , Bianchini, L. , Layalle, S. , Pondeville, E. , Dauphin‐Villemant, C. , Antoniewski, C. , Carré, C. , Noselli, S. & Léopold, P. 2005. Antagonistic actions of ecdysone and insulins determine final size in Drosophila . Science 310, 667–670. [DOI] [PubMed] [Google Scholar]
- Danielsen, T. E. , Moeller, M. E. , Dorry, E. , Komura‐Kawa, T. , Fujimoto, Y. , Troelsen, J. T. , Herder, R. , O'Connor, M. B. , Niwa, R. & Rewitz, K. F. 2014. Transcriptional control of steroid biosynthesis genes in the Drosophila prothoracic gland by Ventral veins lacking and Knirps. PLoS Genet. 10, e1004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, H. & Kerppola, T. K. 2013. Regulation of Drosophila metamorphosis by xenobiotic response regulators. PLoS Genet. 9, e1003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanitskaya, E. , Anllo, L. & Schüpbach, T. 2014. Phantom, a cytochrome P450 enzyme essential for ecdysone biosynthesis, plays a critical role in the control of border cell migration in Drosophila . Dev. Biol. 386, 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enya, S. , Ameku, T. , Igarashi, F. , Iga, M. , Kataoka, H. , Shinoda, T. & Niwa, R. 2014. A Halloween gene noppera‐bo encodes a glutathione S‐transferase essential for ecdysteroid biosynthesis via regulating the behaviour of cholesterol in Drosophila . Sci. Rep. 4, 6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enya, S. , Daimon, T. , Igarashi, F. , Kataoka, H. , Uchibori, M. , Sezutsu, H. , Shinoda, T. & Niwa, R. 2015. The silkworm glutathione S‐transferase gene noppera‐bo is required for ecdysteroid biosynthesis and larval development. Insect Biochem. Mol. Biol. 61, 1–7. [DOI] [PubMed] [Google Scholar]
- Fresán, U. , Cuartero, S. , O'Connor, M. B. & Espinàs, M. L. 2015. The insulator protein CTCF regulates Drosophila steroidogenesis. Biol. Open 4, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbens, Y. Y. , Warren, J. T. , Gilbert, L. I. & O'Connor, M. B. 2011. Neuroendocrine regulation of Drosophila metamorphosis requires TGFβ/Activin signaling. Development 138, 2693–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, L. I. , Rybczynski, R. & Warren, J. T. 2002. Control and biochemical nature of the ecdysteroidogenic pathway. Annu. Rev. Entomol. 47, 883–916. [DOI] [PubMed] [Google Scholar]
- Halme, A. , Cheng, M. & Hariharan, I. K. 2010. Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila . Curr. Biol. 20, 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu, I. 1992. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J. Steroid Biochem. Mol. Biol. 43, 779–804. [DOI] [PubMed] [Google Scholar]
- Hariharan, I. K. 2012. How growth abnormalities delay “puberty” in Drosophila . Sci. Signal. 5, pe27. [DOI] [PubMed] [Google Scholar]
- Iga, M. , Nakaoka, T. , Suzuki, Y. & Kataoka, H. 2014. Pigment dispersing factor regulates ecdysone biosynthesis via Bombyx neuropeptide G protein coupled receptor‐B2 in the prothoracic glands of Bombyx mori. PLoS ONE 9, e103239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto, H. , Wang, Z. , Rao, Y. , Wu, C.‐F. & Kitamoto, T. 2013. A novel role for ecdysone in Drosophila conditioned behavior: Linking GPCR‐mediated non‐canonical steroid action to cAMP signaling in the adult brain. PLoS Genet. 9, e1003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi, K. , Housam, R. , Kapadia, C. , Stallings, V. A. , Medne, L. , Shaikh, T. H. , Kublaoui, B. M. , Zackai, E. H. & Grimberg, A. 2013. Endocrine phenotype of 6q16.1‐q21 deletion involving SIM1 and Prader‐Willi syndrome‐like features. Am. J. Med. Genet. A 161, 3137–3143. [DOI] [PubMed] [Google Scholar]
- Jaszczak, J. S. , Wolpe, J. B. , Dao, A. Q. & Halme, A. 2015. Nitric oxide synthase regulates growth coordination during Drosophila melanogaster imaginal disc regeneration. Genetics 200, 1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura‐Kawa, T. , Hirota, K. , Shimada‐Niwa, Y. , Yamauchi, R. , Shimell, M. , Shinoda, T. , Fukamizu, A. , O'Connor, M. B. & Niwa, R. 2015. The Drosophila zinc finger transcription factor Ouija board controls ecdysteroid biosynthesis through specific regulation of spookier . PLOS Genet. DOI: 10.1371/journal.pgen.1005712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama, T. , Mendes, C. C. & Mirth, C. K. 2013. Mechanisms regulating nutrition‐dependent developmental plasticity through organ‐specific effects in insects. Front. Physiol. 4, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama, T. , Rodrigues, M. A. , Athanasiadis, A. , Shingleton, A. W. & Mirth, C. K. 2014. Nutritional control of body size through FoxO‐Ultraspiracle mediated ecdysone biosynthesis. Elife 3, e03091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, F. Y. , Faivre, E. J. , Suzawa, M. , Lontok, E. , Ebert, D. , Cai, F. , Belsham, D. D. & Ingraham, H. A. 2011. Eliminating SF‐1 (NR5A1) Sumoylation in vivo results in ectopic Hedgehog signaling and disruption of endocrine development. Dev. Cell 21, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Sheng, Z. , Liu, H. , Wen, D. , He, Q. , Wang, S. , Shao, W. , Jiang, R.‐J. , An, S. , Sun, Y. , Bendena, W. G. , Wang, J. , Gilbert, L. I. , Wilson, T. G. , Song, Q. & Li, S. 2009. Juvenile hormone counteracts the bHLH‐PAS transcription factors MET and GCE to prevent caspase‐dependent programmed cell death in Drosophila . Development 136, 2015–2025. [DOI] [PubMed] [Google Scholar]
- McBrayer, Z. , Ono, H. , Shimell, M. , Parvy, J.‐P. , Beckstead, R. B. , Warren, J. T. , Thummel, C. S. , Dauphin‐Villemant, C. , Gilbert, L. I. & O'Connor, M. B. 2007. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila . Dev. Cell 13, 857–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, M. , Cheng, D. , Peng, J. , Qian, W. , Li, J. , Dai, D. , Zhang, T. & Xia, Q. 2015. The homeodomain transcription factors Antennapedia and POU‐M2 regulate the transcription of the steroidogenic enzyme gene Phantom in the silkworm. J. Biol. Chem. 290, 24438–24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, W. L. 1988. Molecular biology of steroid hormone synthesis. Endocr. Rev. 9, 295–318. [DOI] [PubMed] [Google Scholar]
- Miller, W. L. & Auchus, R. J. 2011. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, K. , Yazawa, T. , Mizutani, T. , Imamichi, Y. , Kawabe, S. , Kanno, M. , Matsumura, T. , Ju, Y. & Umezawa, A. 2011. Stem cell differentiation into steroidogenic cell lineages by NR5A family. Mol. Cell. Endocrinol. 336, 123–126. [DOI] [PubMed] [Google Scholar]
- Mizoguchi, A. , Ohashi, Y. , Hosoda, K. , Ishibashi, J. & Kataoka, H. 2001. Developmental profile of the changes in the prothoracicotropic hormone titer in hemolymph of the silkworm Bombyx mori: Correlation with ecdysteroid secretion. Insect Biochem. Mol. Biol. 31, 349–358. [DOI] [PubMed] [Google Scholar]
- Mizoguchi, A. , Kamimura, M. , Kiuchi, M. & Kataoka, H. 2015. Positive feedback regulation of prothoracicotropic hormone secretion by ecdysteroid–a mechanism that determines the timing of metamorphosis. Insect Biochem. Mol. Biol. 58, 39–45. [DOI] [PubMed] [Google Scholar]
- Mizutani, T. , Ishikane, S. , Kawabe, S. , Umezawa, A. & Miyamoto, K. 2015. Transcriptional regulation of genes related to progesterone production. Endocr. J. 62, 757–763. [DOI] [PubMed] [Google Scholar]
- Moeller, M. E. , Danielsen, E. T. , Herder, R. , O'Connor, M. B. & Rewitz, K. F. 2013. Dynamic feedback circuits function as a switch for shaping a maturation‐inducing steroid pulse in Drosophila . Development 140, 4730–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka, E. , Matsumoto, A. & Ikeda, M. 2012. Neuronal influence on peripheral circadian oscillators in pupal Drosophila prothoracic glands. Nat. Commun. 3, 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi, K. , Baba, T. & Tanaka, M. 2013. Steroid hormones and the development of reproductive organs. Sex Dev. 7, 61–79. [DOI] [PubMed] [Google Scholar]
- Myers, E. M. , Yu, J. & Sehgal, A. 2003. Circadian control of eclosion: Interaction between a central and peripheral clock in Drosophila melanogaster . Curr. Biol. 13, 526–533. [DOI] [PubMed] [Google Scholar]
- Namiki, T. , Niwa, R. , Sakudoh, T. , Shirai, K.‐I. , Takeuchi, H. & Kataoka, H. 2005. Cytochrome P450 CYP307A1/Spook: a regulator for ecdysone synthesis in insects. Biochem. Biophys. Res. Commun. 337, 367–374. [DOI] [PubMed] [Google Scholar]
- Namiki, T. , Niwa, R. , Higuchi, A. , Yoshiyama, T. , Mita, K. & Kataoka, H. 2009. A basic‐HLH transcription factor, HLH54F, is highly expressed in the prothoracic gland in the silkworm Bombyx mori and the fruit fly Drosophila melanogaster . Biosci. Biotechnol. Biochem. 73, 762–765. [DOI] [PubMed] [Google Scholar]
- Neubueser, D. , Warren, J. T. , Gilbert, L. I. & Cohen, S. M. 2005. molting defective is required for ecdysone biosynthesis. Dev. Biol. 280, 362–372. [DOI] [PubMed] [Google Scholar]
- Niwa, R. & Niwa, Y. S. 2014a. Enzymes for ecdysteroid biosynthesis: their biological functions in insects and beyond. Biosci. Biotechnol. Biochem. 78, 1283–1292. [DOI] [PubMed] [Google Scholar]
- Niwa, Y. S. & Niwa, R. 2014b. Neural control of steroid hormone biosynthesis during development in the fruit fly Drosophila melanogaster . Genes Genet. Syst. 89, 27–34. [DOI] [PubMed] [Google Scholar]
- Niwa, R. , Matsuda, T. , Yoshiyama, T. , Namiki, T. , Mita, K. , Fujimoto, Y. & Kataoka, H. 2004. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila . J. Biol. Chem. 279, 35942–35949. [DOI] [PubMed] [Google Scholar]
- Niwa, R. , Sakudoh, T. , Namiki, T. , Saida, K. , Fujimoto, Y. & Kataoka, H. 2005. The ecdysteroidogenic P450 Cyp302a1/disembodied from the silkworm, Bombyx mori, is transcriptionally regulated by prothoracicotropic hormone. Insect Mol. Biol. 14, 563–571. [DOI] [PubMed] [Google Scholar]
- Niwa, R. , Niimi, T. , Honda, N. , Yoshiyama, M. , Itoyama, K. , Kataoka, H. & Shinoda, T. 2008. Juvenile hormone acid O‐methyltransferase in Drosophila melanogaster . Insect Biochem. Mol. Biol. 38, 714–720. [DOI] [PubMed] [Google Scholar]
- Niwa, R. , Namiki, T. , Ito, K. , Shimada‐Niwa, Y. , Kiuchi, M. , Kawaoka, S. , Kayukawa, T. , Banno, Y. , Fujimoto, Y. , Shigenobu, S. , Kobayashi, S. , Shimada, T. , Katsuma, S. & Shinoda, T. 2010. Non‐molting glossy/shroud encodes a short‐chain dehydrogenase/reductase that functions in the “Black Box” of the ecdysteroid biosynthesis pathway. Development 137, 1991–1999. [DOI] [PubMed] [Google Scholar]
- Ogihara, M. H. , Hikiba, J. , Iga, M. & Kataoka, H. 2015. Negative regulation of juvenile hormone analog for ecdysteroidogenic enzymes. J. Insect Physiol. 80, 42–47. [DOI] [PubMed] [Google Scholar]
- Ohhara, Y. , Kayashima, Y. , Hayashi, Y. , Kobayashi, S. & Yamakawa‐Kobayashi, K. 2012. Expression of β‐adrenergic‐like octopamine receptors during Drosophila development. Zoolog. Sci. 29, 83–89. [DOI] [PubMed] [Google Scholar]
- Ohhara, Y. , Shimada‐Niwa, Y. , Niwa, R. , Kayashima, Y. , Hayashi, Y. , Akagi, K. , Ueda, H. , Yamakawa‐Kobayashi, K. & Kobayashi, S. 2015. Autocrine regulation of ecdysone synthesis by β3‐octopamine receptor in the prothoracic gland is essential for Drosophila metamorphosis. Proc. Natl Acad. Sci. USA 112, 1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, H. 2014. Ecdysone differentially regulates metamorphic timing relative to 20‐hydroxyecdysone by antagonizing juvenile hormone in Drosophila melanogaster . Dev. Biol. 391, 32–42. [DOI] [PubMed] [Google Scholar]
- Ono, H. , Rewitz, K. F. , Shinoda, T. , Itoyama, K. , Petryk, A. , Rybczynski, R. , Jarcho, M. , Warren, J. T. , Marqués, G. , Shimell, M. J. , Gilbert, L. I. & O'Connor, M. B. 2006. Spook and Spookier code for stage‐specific components of the ecdysone biosynthetic pathway in Diptera. Dev. Biol. 298, 555–570. [DOI] [PubMed] [Google Scholar]
- Ou, Q. & King‐Jones, K. 2013. What goes up must come down. Transcription factors have their say in making ecdysone pulses. Curr. Top. Dev. Biol. 103, 35–71. [DOI] [PubMed] [Google Scholar]
- Ou, Q. , Magico, A. & King‐Jones, K. 2011. Nuclear receptor DHR4 controls the timing of steroid hormone pulses during Drosophila development. PLoS Biol. 9, e1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankotai, T. , Popescu, C. , Martín, D. , Grau, B. , Zsindely, N. , Bodai, L. , Tora, L. , Ferrús, A. & Boros, I. 2010. Genes of the ecdysone biosynthesis pathway are regulated by the dATAC histone acetyltransferase complex in Drosophila . Mol. Cell. Biol. 30, 4254–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, K. L. , Rice, D. A. , Lala, D. S. , Ikeda, Y. , Luo, X. , Wong, M. , Bakke, M. , Zhao, L. , Frigeri, C. , Hanley, N. A. , Stallings, N. & Schimmer, B. P. 2002. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog. Horm. Res. 57, 19–36. [DOI] [PubMed] [Google Scholar]
- Parvy, J. P. , Blais, C. , Bernard, F. , Warren, J. T. , Petryk, A. , Gilbert, L. I. , O'Connor, M. B. & Dauphin‐Villemant, C. 2005. A role for βFTZ‐F1 in regulating ecdysteroid titers during post‐embryonic development in Drosophila melanogaster . Dev. Biol. 282, 84–94. [DOI] [PubMed] [Google Scholar]
- Parvy, J.‐P. , Wang, P. , Garrido, D. , Maria, A. , Blais, C. , Poidevin, M. & Montagne, J. 2014. Forward and feedback regulation of cyclic steroid production in Drosophila melanogaster . Development 141, 3955–3965. [DOI] [PubMed] [Google Scholar]
- Petryk, A. , Warren, J. T. , Marqués, G. , Jarcho, M. P. , Gilbert, L. I. , Kahler, J. , Parvy, J.‐P. , Li, Y. , Dauphin‐Villemant, C. & O'Connor, M. B. 2003. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20‐hydroxyecdysone. Proc. Natl Acad. Sci. USA 100, 13773–13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinking, J. , Lam, M. M. S. , Pardee, K. , Sampson, H. M. , Liu, S. , Yang, P. , Williams, S. , White, W. , Lajoie, G. , Edwards, A. & Krause, H. M. 2005. The Drosophila nuclear receptor E75 contains heme and is gas responsive. Cell 122, 195–207. [DOI] [PubMed] [Google Scholar]
- Rewitz, K. F. , O'Connor, M. B. & Gilbert, L. I. 2007. Molecular evolution of the insect Halloween family of cytochrome P450s: Phylogeny, gene organization and functional conservation. Insect Biochem. Mol. Biol. 37, 741–753. [DOI] [PubMed] [Google Scholar]
- Rewitz, K. F. , Larsen, M. R. , Lobner‐Olesen, A. , Rybczynski, R. , O'Connor, M. B. & Gilbert, L. I. 2009a. A phosphoproteomics approach to elucidate neuropeptide signal transduction controlling insect metamorphosis. Insect Biochem. Mol. Biol. 39, 475–483. [DOI] [PubMed] [Google Scholar]
- Rewitz, K. F. , Yamanaka, N. , Gilbert, L. I. & O'Connor, M. B. 2009b. The insect neuropeptide PTTH activates receptor tyrosine kinase Torso to initiate metamorphosis. Science 326, 1403–1405. [DOI] [PubMed] [Google Scholar]
- Riddiford, L. M. , Truman, J. W. , Mirth, C. K. & Shen, Y.‐C. 2010. A role for juvenile hormone in the prepupal development of Drosophila melanogaster . Development 137, 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougvie, A. E. 2005. Intrinsic and extrinsic regulators of developmental timing: from miRNAs to nutritional cues. Development 132, 3787–3798. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Higueras, C. , Sotillos, S. & Castelli‐Gair Hombría, J. 2014. Common origin of insect trachea and endocrine organs from a segmentally repeated precursor. Curr. Biol. 24, 76–81. [DOI] [PubMed] [Google Scholar]
- Shimada‐Niwa, Y. & Niwa, R. 2014. Serotonergic neurons respond to nutrients and regulate the timing of steroid hormone biosynthesis in Drosophila . Nat. Commun. 5, 5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber, M. H. & Spradling, A. C. 2015. Steroid signaling establishes a female metabolic state and regulates SREBP to control oocyte lipid accumulation. Curr. Biol. 25, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, A. F. , Shih, C. , Mack, A. & Benzer, S. 2003. Steroid control of longevity in Drosophila melanogaster . Science 299, 1407–1410. [DOI] [PubMed] [Google Scholar]
- Takaki, K. & Sakurai, S. 2003. Regulation of prothoracic gland ecdysteroidogenic activity leading to pupal metamorphosis. Insect Biochem. Mol. Biol. 33, 1189–1199. [DOI] [PubMed] [Google Scholar]
- Talamillo, A. , Sánchez, J. , Cantera, R. , Pérez, C. , Martín, D. , Caminero, E. & Barrio, R. 2008. Smt3 is required for Drosophila melanogaster metamorphosis. Development 135, 1659–1668. [DOI] [PubMed] [Google Scholar]
- Talamillo, A. , Herboso, L. , Pirone, L. , Pérez, C. , González, M. , Sánchez, J. , Mayor, U. , Lopitz‐Otsoa, F. , Rodriguez, M. S. , Sutherland, J. D. & Barrio, R. 2013. Scavenger receptors mediate the role of SUMO and Ftz‐f1 in Drosophila steroidogenesis. PLoS Genet. 9, e1003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, Y. 2011. Recent topics on the regulatory mechanism of ecdysteroidogenesis by the prothoracic glands in insects. Front. Endocrinol. 2, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel, C. S. 2001. Molecular mechanisms of developmental timing in C. elegans and Drosophila . Dev. Cell 1, 453–465. [DOI] [PubMed] [Google Scholar]
- Walker, V. K. , Watson, K. L. , Holden, J. J. & Steel, C. G. H. 1987. Vitellogenesis and fertility in Drosophila females with low ecdysteroid titres; the l(3)3DTS mutation. J. Insect Physiol. 33, 137–142. [Google Scholar]
- Warren, J. T. , Wismar, J. , Subrahmanyam, B. & Gilbert, L. I. 2001. Woc (without children) gene control of ecdysone biosynthesis in Drosophila melanogaster . Mol. Cell. Endocrinol. 181, 1–14. [DOI] [PubMed] [Google Scholar]
- Warren, J. T. , Petryk, A. , Marque, G. , Jarcho, M. , Parvy, J.‐P. , Dauphin‐villemant, C. , O'Connor, M. B. & Gilbert, L. I. 2002. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster . Proc. Natl Acad. Sci. USA 99, 11043–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, J. T. , Petryk, A. , Marqués, G. , Parvy, J.‐P. , Shinoda, T. , Itoyama, K. , Kobayashi, J. , Jarcho, M. , Li, Y. , O'Connor, M. B. , Dauphin‐Villemant, C. & Gilbert, L. I. 2004. Phantom encodes the 25‐hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem. Mol. Biol. 34, 991–1010. [DOI] [PubMed] [Google Scholar]
- Warren, J. T. , Yerushalmi, Y. , Shimell, M. J. , O'Connor, M. B. , Restifo, L. L. & Gilbert, L. I. 2006. Discrete pulses of molting hormone, 20‐hydroxyecdysone, during late larval development of Drosophila melanogaster: Correlations with changes in gene activity. Dev. Dyn. 235, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, D. , Rivera‐Perez, C. , Abdou, M. , Jia, Q. , He, Q. , Liu, X. , Zyaan, O. , Xu, J. , Bendena, W. G. , Tobe, S. S. , Noriega, F. G. , Palli, S. R. , Wang, J. & Li, S. 2015. Methyl farnesoate plays a dual role in regulating Drosophila metamorphosis. PLoS Genet. 11, e1005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildemann, B. & Bicker, G. 1999. Developmental expression of nitric oxide/cyclic GMP synthesizing cells in the nervous system of Drosophila melanogaster . J. Neurobiol. 38, 1–15. [DOI] [PubMed] [Google Scholar]
- Wismar, J. , Habtemichael, N. , Warren, J. T. , Dai, J. D. , Gilbert, L. I. & Gateff, E. 2000. The mutation without children rgl causes ecdysteroid deficiency in third‐instar larvae of Drosophila melanogaster . Dev. Biol. 226, 1–17. [DOI] [PubMed] [Google Scholar]
- Xiang, Y. , Liu, Z. & Huang, X. 2010. br regulates the expression of the ecdysone biosynthesis gene npc1 . Dev. Biol. 344, 800–808. [DOI] [PubMed] [Google Scholar]
- Xie, X.‐J. , Hsu, F.‐N. , Gao, X. , Xu, W. , Ni, J.‐Q. , Xing, Y. , Huang, L. , Hsiao, H.‐C. , Zheng, H. , Wang, C. , Zheng, Y. , Xiaoli, A. M. , Yang, F. , Bondos, S. E. & Ji, J.‐Y. 2015. CDK8‐Cyclin C mediates nutritional regulation of developmental transitions through the ecdysone receptor in Drosophila . PLoS Biol. 13, e1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka, N. , Honda, N. , Osato, N. , Niwa, R. , Mizoguchi, A. & Kataoka, H. 2007. Differential regulation of ecdysteroidogenic P450 gene expression in the silkworm, Bombyx mori . Biosci. Biotechnol. Biochem. 71, 2808–2814. [DOI] [PubMed] [Google Scholar]
- Yamanaka, N. , Rewitz, K. F. & O'Connor, M. B. 2013a. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu. Rev. Entomol. 58, 497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka, N. , Romero, N. M. , Martin, F. A. , Rewitz, K. F. , Sun, M. , O'Connor, M. B. & Léopold, P. 2013b. Neuroendocrine control of Drosophila larval light preference. Science 341, 1113–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama, T. , Namiki, T. , Mita, K. , Kataoka, H. & Niwa, R. 2006. Neverland is an evolutionally conserved Rieske‐domain protein that is essential for ecdysone synthesis and insect growth. Development 133, 2565–2574. [DOI] [PubMed] [Google Scholar]
- Yoshiyama‐Yanagawa, T. , Enya, S. , Shimada‐Niwa, Y. , Yaguchi, S. , Haramoto, Y. , Matsuya, T. , Shiomi, K. , Sasakura, Y. , Takahashi, S. , Asashima, M. , Kataoka, H. & Niwa, R. 2011. The conserved Rieske oxygenase DAF‐36/Neverland is a novel cholesterol‐metabolizing enzyme. J. Biol. Chem. 286, 25756–25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, S. C. , Yeh, W. L. & Gu, S. H. 2012. Transcriptional regulation of the PTTH receptor in prothoracic glands of the silkworm, Bombyx mori . J. Insect Physiol. 58, 102–109. [DOI] [PubMed] [Google Scholar]
- Zhou, X. , Zhou, B. , Truman, J. W. & Riddiford, L. M. 2004. Overexpression of broad: a new insight into its role in the Drosophila prothoracic gland cells. J. Exp. Biol. 207, 1151–1161. [DOI] [PubMed] [Google Scholar]
- Zubair, M. , Oka, S. , Parker, K. & Morohashi, K. 2009. Transgenic expression of Ad4BP/SF‐1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol. Endocrinol. 23, 1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]


