Abstract
This study aimed to evaluate the ability of selected microRNAs as biomarkers of atrial fibrillation (AF) in ischemic stroke patients in comparison with other established biochemical biomarkers. A prospective case–control study of consecutive ischemic stroke patients with AF admitted to a comprehensive stroke center was conducted. The control group consisted of patients with ischemic stroke with no AF detected on prolonged (at least 3 weeks) Holter ECG monitoring. As potential biomarkers of AF, we analyzed the plasma levels of microRNAs (miR-21, miR-29b, miR-133b, miR-142-5p, miR-150, miR-499, and miR-223-3p) and 13 biochemical biomarkers at admission. The predictive accuracy of biomarkers was assessed by calculating the area under the receiver operating characteristic curve. The data of 117 patients were analyzed (61 with AF, 56 with no AF, 46% men, median age 73 years, median National Institutes of Health Stroke Scale 6). Biochemical biomarkers (N-terminal pro-B-type natriuretic peptide [NT-proBNP], high-sensitivity cardiac troponin I, fibrinogen, C-reactive protein, eGFR, and total triglycerides) were significantly associated with AF. NT-proBNP had the best diagnostic performance for AF with area under the receiver operating characteristic curve 0.92 (95%, CI 0.86–0.98); a cutoff value of >528 ng/L had a sensitivity of 79% and a specificity of 97%. None of the other biomarkers, including microRNAs, was associated with AF. Conventional biochemical biomarkers (NT-proBNP, high-sensitivity cardiac troponin I, fibrinogen, C-reactive protein, eGFR, and triglycerides), but not microRNAs (miR-21, miR-29b, miR-133b, miR-142-5p, miR-150, miR-499, and miR-223-3p) were significantly associated with AF in our ischemic stroke cohort.
Keywords: atrial fibrillation, biomarker, microRNA, stroke
1. Introduction
Atrial fibrillation (AF) is the most frequent cardiac arrhythmia, with a prevalence of 1% to 2% in the European population, and is one of the most important causes of ischemic stroke.[1] Ischemic stroke in patients with AF is more severe and recurrent, causing higher mortality and disability than ischemic stroke in patients without AF.[2] About 20% to 30% of ischemic stroke cases are caused by diagnosed permanent AF.[3] However, paroxysmal and undetected AF is estimated to be causal to a substantial part of cryptogenic strokes and minor parts of other etiological stroke subgroups.[4] Detection of paroxysmal AF after stroke is crucial because it means a change in secondary stroke prevention treatment to oral anticoagulation, which is more effective than antiplatelet therapy in these patients, reducing the risk of stroke recurrence by up to two-thirds.[5] The failure of the NAVIGATE ESUS[6] and RE-SPECT ESUS[7] trials to show a reduction in recurrent strokes in patients with embolic stroke of undetermined source treated empirically with non-vitamin K antagonist oral anticoagulants compared with aspirin leads to the conclusion that ECG monitoring in search for AF after ischemic stroke is still essential. Extensive prospective studies (Embrace, CRYSTAL AF) showed that using prolonged ECG monitoring (telemetry, long-term Holter monitoring, loop recorder, implantable cardiac monitor), significantly more cases of paroxysmal AF were detected in ischemic stroke patients than using classical monitoring in the ICU.[8,9] Because prolonged ECG monitoring has a limited capacity to allow all patients to be monitored after stroke without a time delay, a tool to select patients with the highest probability of AF detection may allow the most at-risk patients to be prioritized in the indication for long-term ECG monitoring.
In addition to clinical scoring scales (CHA2-DS2-VASc, ATRIA, and HATCH), biomarkers can be used to predict AF in cryptogenic stroke patients. Biomarkers are generally defined as measures that can be assessed as indicators of biological and etiopathogenic processes or predictors of therapeutic intervention.[10] The biomarker predictors of paroxysmal AF can be divided into electrophysiological, morphological, and molecular categories.
Electrophysiological biomarkers encompass different 12-lead ECG features underlying the etiopathogenesis of AF, such as variability of P waves in different leads or their prolongation, indicating conduction pathology in the atria.[11] Alterations in ion channel expression in the myocardium may result in prolonged QTc interval in paroxysmal AF. Premature atrial contraction, that is, supraventricular extrasystole, is also a significant predictor.[12] Morphological biomarkers of AF could be based on echocardiographic measurements of the size and function of the left atrium, where structural changes may occur owing to arrhythmia.[13] The morphological and topographic characteristics of ischemic lesions on brain computed tomography or magnetic resonance imaging can correspond to AF-caused cardioembolic etiology if acute ischemia is found in more areas of the arterial blood supply.
Molecular biomarkers of AF can be divided into several groups: cardiac, inflammatory, metabolic, renal, prothrombotic, and genetic. Cardiac biomarkers of AF are well-known heart failure markers, such as N-terminal pro-B-type natriuretic peptide (NT-proBNP). In patients with cryptogenic stroke and detected AF, NT-proBNP levels were significantly higher than those in the cryptogenic stroke group without AF.[14] A significant reduction in 2-year stroke recurrence and mortality in the warfarin group in the Warfarin–Aspirin recurrent stroke study of cryptogenic stroke patients without arterial hypertension was found in a subgroup of patients with NT-proBNP levels > 750 pg/mL.[15] Another biomarker for predicting AF is high-sensitivity cardiac troponin I (hs-cTnI).[16] The value of inflammatory biomarkers in AF remains unclear. Some studies showed a significantly higher level of C-reactive protein (CRP) in patients with AF than in controls,[17] whereas others showed no such relationship.[18] Among metabolic biomarkers, glycated hemoglobin has been reported to have both positive and negative correlations with AF.[19] A negative correlation was found between AF and LDL and cholesterol levels.[20] In addition, AF is more prevalent in patients with severe renal insufficiency and a decreased glomerular filtration rate.[21] Biomarkers associated with thrombogenesis are also applicable to predicting AF. A meta-analysis of 59 studies found an association between AF and several coagulation activation parameters (D-dimer, fibrinogen, thrombin–antithrombin complex, and antithrombin III).[22]
Small noncoding RNA molecules, microRNAs (miRNAs), have been hypothesized to serve as clinically relevant biomarkers owing to their relative stability in the bloodstream.[23] miRNAs play a role in posttranscriptional processes and regulate gene expression primarily by blocking mRNA translation.[24] miRNAs could contribute to etiopathological processes in AF, such as ion channel expression, fibrotic myocardial remodeling, and cardiomyocyte apoptosis. Both upregulation and downregulation of different miRNAs have been described in patients with AF and ischemic stroke.[25] A series of miRNA molecules have been investigated for their association with AF and ischemic stroke. miR-150 is a promising biomarker, as its levels have been reported to be significantly reduced in patients with AF compared with control groups.[26] miR-150 is involved in the inflammatory system and inhibits cellular proliferation and extracellular matrix production, processes that can lead to myocardial fibrosis and AF.[26] Reduced plasma levels in patients with AF have also been described in the case of miR-21[27] and miR-29,[28] which play a role in fibrotic myocardium remodeling. miR-29 directly regulates the levels of proteins necessary for collagen formation,[28] and miR-21 increases the survival of fibroblasts.[29] The association between AF and both miR-21 and miR-150 levels has also been confirmed by the fact that they returned to normal values after catheter ablation.[27] miR-499 regulates the potassium channel KCNN3 and its expression is increased in the atrium of patients with AF.[30] A notable increase in plasma levels of miR-133b (involved in apoptosis and fibrosis) in patients with acute new-onset AF has been described.[27] Lower expression of miR-142-5p (involved in apoptosis of cardiomyocytes) and higher expression of miR-223-3p (involved in promoting myocardial hypertrophy through targeting apoptosis repressor) in patients with AF compared to patients with sinus rhythm has been found.[31]
1.1. Aim of the study
This study aimed to evaluate the ability of selected miRNAs as biomarkers of AF in patients with ischemic stroke compared to other established biochemical biomarkers by determining the association of their plasma levels with the occurrence of AF in patients with ischemic stroke.
2. Methods
2.1. Study population and examinations
Consecutive patients with acute ischemic stroke and AF admitted to a comprehensive stroke center between 2017 and 2019 were prospectively included in this study. AF was diagnosed based on medical history or detected upon admission. The control group consisted of patients with acute ischemic stroke, no history of AF, and no AF detection within the first day of hospitalization. The exclusion criteria were acute infection, immunological disorder, immunosuppressive therapy, active cancer, renal or hepatic failure, gravidity, history of stroke, and myocardial infarction within the previous month. All patients in the control group underwent ECG monitoring in the ICU and standard ward and underwent a 3 to 4 weeks ambulatory Holter ECG monitoring after the hospital discharge. Patients were excluded from the control group if AF was detected using any monitoring modality. Given the observational exploratory nature of this study, the sample size was not calculated. Recruitment was aimed at 80 patients in the AF group and 100 patients in the control group (expecting a possible loss of patients due to later AF detection). No matching criteria for patient characteristics other than recent acute stroke were provided, as the recruitment of consecutive patients in the study aimed to respect real-life clinical settings and minimize sampling bias. Several other limitations typical of case–control studies (recall bias, observation bias) did not need to be addressed, as the study did not aim to prove the causal relationship between biomarkers and AF, relied on exact laboratory measurements, and was conducted prospectively.
Based on the results of standard auxiliary examination methods (MRI of the brain, CTA of supra-aortic arteries, transthoracic or transesophageal echocardiography), the etiology of stroke was identified using the TOAST classification (large vessel disease, cardioembolism, small vessel disease, other determined etiology, undetermined etiology, and embolic stroke of undetermined source),[32] and selected echocardiographic (left atrial diameter, left ventricular ejection fraction, mitral valve disease) and brain imaging parameters (presence of acute brain ischemia in more areas of arterial blood supply and presence of old brain ischemic lesions) were recorded. Important clinical data (age, sex, and BMI) and medical history (smoking, heavy alcohol drinking [defined as more than 1 drink per day], arterial hypertension, diabetes mellitus, previous stroke or TIA, and ischemic heart disease) of the patients were collected for the study. The CHA2DS2-VASc score was then calculated. Stroke severity was quantified using the National Institutes of Health Stroke Scale (NIHSS) score at admission and the modified Rankin scale. AF was defined as an arrhythmia with irregular R-R intervals, absence of P waves, and frequency of fibrillation waves > 300/min. Deaths were detected during the hospitalization. The cause of death was obtained from the death certificate. Levels of biochemical biomarkers and miRNAs were compared between the deceased and the survivors. No longitudinal follow-up of the clinical events was conducted in this study.
2.2. Biochemical biomarkers and microRNAs
Blood samples were collected from the peripheral venous blood draw within 24 hours of hospital admission to analyze the levels of biochemical biomarkers and miRNAs. As potential biomarkers of AF, we analyzed plasma levels of hemostatic markers of prothrombotic state (D-dimer, fibrinogen, antithrombin III), cardiac biomarkers (NT-proBNP, hs-cTnI), a marker of inflammation (CRP), parameters of renal function (creatinine, estimated glomerular filtration rate [eGFR]), glycated hemoglobin, and lipid metabolism parameters (total cholesterol, total triglycerides, high-density lipoprotein, and low-density lipoprotein). All biochemical biomarkers were assessed using standard procedures in the hospital biochemistry laboratory.
miRNA quantification was performed in the laboratory for molecular diagnostics. Blood samples were collected in TEMPUS Blood RNA Tubes (Thermo Fisher Scientific, Waltham, MA) and stored at ‐70 °C. Isolation of the total RNA on the principle of guanidium isocyanate isolation was performed using Preserved Blood RNA Purification Kit I (Norgen Biotek, Thorold, Ontario, Canada) in concordance with the standard instructions in the kit manual. The selection of miRNAs (miR-21, miR-29b, miR-133b, miR-142-5p, miR-150, miR-499, and miR-223-3p) analyzed in our study was based on their reported association with AF and ischemic stroke.[26–31] Particular microRNA molecules were quantified by real-time polymerase chain reaction using a Rotor-Gene Q cycler (Qiagen, Hilden, Germany) and Cohesion miRNA RT-PCR Quantification kits (hsa-mir-X Real-time RT-PCR Detection kit; Cohesion biosciences, London, UK) working on the principle of two-step real-time quantitative assay (stem-loop reverse transcription and real-time quantitative polymerase chain reaction using specific primers). The concentrations (copies) of particular miRNA molecules and their corresponding cycle thresholds (ΔCT) were recorded.
2.3. Ethics, data processing, and statistical analysis
All subjects included in this study provided written informed consent. The study was approved by the Ethics Committee of the Second Faculty of Medicine, Charles University and Motol University Hospital. Anonymized data were stored in the REDCAp electronic data capture system (Vanderbilt University, Nashville, TN). The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request. Given the prospective nature of this study, no missing data needed to be addressed. Baseline characteristics were compared between the groups (AF vs non-AF and deaths vs survivors) using the chi-square test for categorical variables and the Mann–Whitney U test or Student t test for continuous variables. The analyzed continuous variables were presented as median with interquartile range (IQR) value or mean with standard deviation (SD). The IQR was defined as the difference between the 75th (Q3) and 25th (Q1) percentiles of the data. Discriminatory accuracy was assessed using the area under the receiver operating characteristic curve (AUC). P values <.05 were considered to indicate statistical significance. Statistical analysis was performed using the SPSS Statistics 29 software (IBM, Armonk, NY).
3. Results
3.1. Patient characteristics
In total, 248 patients were screened for this study. Of these, 173 patients who did not meet the exclusion criteria and signed an informed consent form were included in the study. Eleven patients were later excluded because they were identified as stroke mimics, and 21 patients were excluded because of deterioration of blood samples caused by lab freezer failure. In the control group, 24 patients were excluded because of AF detection (in 20 patients, AF was detected by ECG monitoring during hospitalization and in 4 patients on prolonged 3-week Holter monitoring after hospital discharge). Finally, 117 patients (61 in the AF group and 56 in the non-AF group) were included in this study (Table 1). Men constituted 46% of all the patients, median age was 73 years (IQR 24), median BMI was 26 (IQR 6.2), 31% were smokers, 6.8% had a history of heavy alcohol drinking, median CHA2DS2-VASc score was 4 (IQR 3), 72.6% had arterial hypertension, 26.5% had diabetes mellitus, 31.6% had hyperlipidemia, 21.3% had a previous stroke or transitory ischemic attack, 17.9% had ischemic heart disease. On admission, 23.9% of patients were using anticoagulant therapy (warfarin 19 patients, dabigatran 5, apixaban 2, low-molecular-weight heparin 2), 22.2% were using antiplatelet therapy (aspirin 21 patients, clopidogrel 3, dual antiplatelet therapy 2), and 24.8% were using antiarrhythmic therapy (beta-blockers 23 patients, amiodarone 2, propafenone 2, verapamil 1, digoxin 1). No patients had a history of catheter ablation prior to hospitalization. The median NIHSS score at admission was 6 (IQR 10), mRS at discharge was 1 (IQR 2), 70% of patients received intravenous thrombolysis (IVT), and in 29% mechanical thrombectomy was done. Based on the study design, 50.4% of strokes were cardioembolic, followed by cryptogenic strokes (31.6%), embolic strokes of undetermined source (6.8%), large vessel disease (6.8%), small vessel disease (3.4%), and other determined etiologies (0.9%). Based on the echocardiography, 33.3% of patients had mitral valve disease, the median left atrial diameter was 41 mm (IQR 11), and the mean left ventricular ejection fraction was 58.1% (SD 6.8). Information about the smoking history of 4 patients in the AF group was missing; otherwise, all variables analyzed in the study showed no missing data.
Table 1.
Patient characteristics.
| Atrial fibrillation (n = 61) | No atrial fibrillation (n = 56) | All patients (n = 117) | Statistical significance, P*, AF vs no AF | |
|---|---|---|---|---|
| Age—years, median (IQR) | 78 (13) | 68 (18) | 73 (24) | <.001* |
| Female sex—n (%) | 38 (62.3%) | 25 (44.6%) | 63 (53.8%) | .09 |
| BMI—median (IQR) | 26 (7.2) | 25.9 (5.5) | 26 (6.2) | .05 |
| Smoker—n (%) | 12 (21.1%)** | 23 (41.1%) | 35 (31%)** | .02* |
| Heavy alcohol use—n (%) | 5 (8.2%) | 3 (5.4%) | 8 (6.8%) | .54 |
| Arterial hypertension—n (%) | 52 (85.2%) | 33 (58.9%) | 85 (72.6%) | .004* |
| Diabetes mellitus—n (%) | 19 (31.1%) | 12 (21.4%) | 31 (26.5%) | .28 |
| Hyperlipidemia—n (%) | 22 (36.1%) | 15 (26.8%) | 37 (31.6%) | .34 |
| Previous stroke or TIA—n (%) | 16 (26.2%) | 9 (16%) | 25 (21.3%) | .04* |
| Ischemic heart disease—n (%) | 15 (24.6%) | 6 (10.7%) | 21 (17.9%) | .06 |
| CHA2DS2-VASc (IQR) | 4 (2) | 2 (3) | 4 (3) | <.001* |
| NIHSS on admission (IQR) | 12 (11) | 4.5 (4) | 6 (10) | <.001* |
| mRS on discharge (IQR) | 2 (3) | 1 (1.3) | 1 (2) | <.001* |
| IVT—count (%) | 34 (55.7%) | 48 (85.7%) | 82 (70%) | <.001* |
| Mechanical thrombectomy—n (%) | 24 (39.3%) | 10 (17.9%) | 34 (29%) | .02* |
| Preadmission antiarrhythmic therapy—n (%) | 22 (36.1%) | 7 (12.5%) | 29 (24.8%) | .005* |
| Preadmission anticoagulant therapy—n (%) | 27 (44.3%) | 1 (1.8%) | 28 (23.9%) | <.001* |
| Preadmission antiplatelet therapy—n (%) | 16 (26.2%) | 10 (17.9%) | 26 (22.2%) | .37 |
| ECHO - LA, mm—median (IQR) | 44 (11) | 37 (6) | 41 (11) | <.001* |
| ECHO - LVEF, %—mean (SD) | 56.7 (7.1) | 59.6 (6.2) | 58.1 (6.8) | .002* |
| ECHO—Mitral valve disease—n (%) | 31 (50.8%) | 8 (14.3%) | 39 (33.3%) | <.001* |
| Etiology of stroke—n (%) | ||||
| Large vessel disease | 1 (1.6%) | 7 (12.5%) | 8 (6.8%) | |
| Small vessel disease | 1 (1.6%) | 3 (5.4%) | 4 (3.4%) | |
| Cardioembolism | 59 (96.7%) | 0 | 59 (50.4%) | |
| Other determined etiology | 0 | 1 (1.8%) | 1 (0.9%) | |
| Undetermined etiology | 0 | 37 (66.1%) | 37 (31.6%) | |
| ESUS | 0 | 8 (14.3%) | 8 (6.8%) | |
| In-hospital mortality—n (%) | 6 (9.8%) | 0 (0%) | 6 (5.1%) | |
AF = atrial fibrillation, BMI = body mass index, ECHO = echocardiography, ESUS = embolic stroke of undetermined source, IQR = interquartile range, IVT = intravenous thrombolysis, LA = left atrium diameter, LVEF = left ventricular ejection fraction, mRS = Modified Rankin Scale, NIHSS = National Institute of Health Stroke Scale, SD = standard deviation, TIA = transient ischemic attack.
* P < 0.05.
4 values missing.
The AF and non-AF groups differed significantly in several parameters. Patients with AF were significantly older, had higher CHA2DS2-VASc scores, had more often arterial hypertension, and had significantly more severe strokes, corresponding to higher NIHSS and mRS scores as well as a higher proportion of patients receiving mechanical thrombectomy in the AF group. The use of antiarrhythmic and anticoagulant therapy was significantly more prevalent in patients with AF. In contrast, smoking was significantly more prevalent among the patients without AF (41.1% vs 21.1%). The proportion of patients receiving IVT was higher in the non-AF group (85.7% vs 55.7%). The other clinical parameters did not differ between the 2 groups.
All echocardiographic parameters were significantly different between the AF and non-AF groups. The diameter of the left atrium was significantly larger in the AF group (44 mm vs 37 mm, P < .001), with AUC 0.78 (95% CI 0.67 to 0.90), a cutoff value > 40 mm had a sensitivity of 70% and a specificity of 25%. Left ventricular ejection fraction was significantly lower in the AF group (P = .002, AUC 0.67 [95% CI 0.57 to 0.77]), with a mean value of 56.7% (SD 7.1) vs 59.6% (SD 6.2). Finally, mitral valve disease was more prevalent among patients with AF (50.8% vs 14.3%, P < .001).
Death occurred in 6 (5.1%) patients during the hospitalization. The cause of death was stroke in all cases. Patients who died were all in the AF group (mortality 9.8%) and had a significantly larger left atrial diameter (67 vs 40 mm, P = .02), higher CRP (64.9 vs 10.8 mg/L, P < .001), higher NT-proBNP (1332 vs 7083 ng/mL, P < .001), and lower ΔCT miR-29b (15.17 vs 17.34, P = .02). None of the other biomarkers were associated with mortality.
3.2. Biomarkers
Thirteen biochemical biomarkers and 3 echocardiographic parameters were compared between the AF and non-AF groups (Table 2). The levels (median) of cardiac biomarkers were significantly different between AF and non-AF groups: NT-proBNP 1578 ng/L vs 177 ng/L (P < .001), hs-cTnI 20.5 vs 5.6 ng/L (P < .001). The diagnostic performance of NT-proBNP for AF had AUC 0.92 (95% CI 0.86–0.98), a cutoff value of >528 ng/L had a sensitivity of 79% and a specificity of 97%. The diagnostic performance of hs-cTnI had AUC 0.76 (0.66–0.87). The levels of hemostatic markers of prothrombotic state were not significantly different between AF and non-AF groups (D-dimer, antithrombin III), except for the fibrinogen level, which was higher in the AF group (3.15 g/L vs 2.55 g/L, P = .04) with AUC 0.62 (0.51–0.73). CRP level was significantly higher in the AF group than in the non-AF group (11 mg/L vs 5 mg/L, P = .002) with an AUC of 0.67 (0.57–0.76). The estimated glomerular filtration rate was significantly lower in the AF group (66.6 mL/min/1.73 m2 vs 85.2 mL/min/1.73 m2, P < .001) with AUC 0.71 (0.62–0.81) but creatinine levels did not differ. Among the metabolic markers, only total triglycerides were significantly higher in patients with AF (1.11 mmol/L vs 1.10 mmol/L, P = .02) with an AUC of 0.63 (0.53–0.74), and no difference was found in the levels of glycated hemoglobin, total cholesterol, high-density lipoprotein, and low-density lipoprotein.
Table 2.
Conventional biochemical biomarkers.
| Biomarker-median (IQR) | Atrial fibrillation (n = 61) | No atrial fibrillation (n = 56) | All patients (n = 117) | Statistical significance, P*, AF vs no AF | AUC, (asymptotic 95% confidence interval) |
|---|---|---|---|---|---|
| D-dimer, µg/L | 386 (1747) | 263 (338) | 320 (650) | .23 | 0.576 (0.456–0.697) |
| Fibrinogen, g/L | 3.15 (1.36) | 2.55 (1.02) | 3.10 (1.32) | .04* | 0.619 (0.510–0.728) |
| Antithrombin III, % | 90 (17) | 90 (23) | 90 (20) | .60 | 0.468 (0.349–0.587) |
| NT-proBNP, ng/L | 1578 (2676) | 177 (205) | 475 (1385) | <.001* | 0.916 (0.857–0.975) |
| hs-cTnI, ng/L | 20.5 (80.8) | 5.6 (7.0) | 10.7 (49.8) | <.001* | 0.760 (0.655–0.866) |
| CRP, mg/L | 11.0 (23.6) | 5.0 (4.5) | 5.0 (17.4) | .002* | 0.665 (0.567–0.763) |
| eGFR, mL/min/1.73 m2 | 66.6 (30) | 85.2 (21.6) | 79.2 (30) | <.001* | 0.712 (0.619–0.805) |
| Creatinine, µmol/L | 86.0 (41.0) | 75.0 (23.0) | 79.0 (38.0) | .20 | 0.569 (0.465–0.673) |
| HbA1c, mmol/mol | 40 (13) | 37 (8) | 39 (11.3) | .07 | 0.603 (0.493–0.714) |
| LDL-c, mmol/L | 2.38 (1.18) | 2.65 (1.59) | 2.49 (1.43) | .16 | 0.577 (0.469–0.685) |
| Total cholesterol, mmol/L | 3.90 (1.50) | 4.70 (1.75) | 4.10 (1.52) | .05 | 0.606 (0.500–0.711) |
| HDL-c, mmol/L | 0.98 (0.33) | 1.32 (0.43) | 1.03 (0.56) | .88 | 0.508 (0.399–0.618) |
| TG, mmol/L | 1.11 (1.17) | 1.10 (0.82) | 1.11 (0.94) | .02* | 0.634 (0.530–0.737) |
AF = atrial fibrillation, AUC = area under the curve for the presence of AF, CRP = C-reactive protein, eGFR = estimated glomerular filtration rate, HbA1c = glycated hemoglobin, HDL = high-density lipoprotein, hs-cTnI = high-sensitive cardiac troponin I, IQR = interquartile range, LDL = low-density lipoprotein, NT-proBNP = amino-terminal pro-B-type natriuretic peptide, TG = total triglycerides.
* P < 0.05.
None of the miRNA levels (Table 3), expressed as CTs, were significantly different between the AF and non-AF groups. Only 2 miRNAs had AUC > 0.5, namely miR-150 (0.61, 95% CI 0.50–0.71) and miR-499 (0.52, 95% CI 0.40–0.60).
Table 3.
miRNA levels.
| miRNA (SD) | Atrial fibrillation (n = 61) | No atrial fibrillation (n = 56) | All patients (n = 117) | Statistical significance, P*, AF vs no AF | AUC, (asymptotic 95% confidence interval) |
|---|---|---|---|---|---|
| miR-21 | 17.19 (3.26) | 17.26 (2.28) | 17.22 (2.81) | .75 | 0.47 (0.36–0.58) |
| miR-29b | 23.33 (5.21) | 24.18 (4.56) | 23.74 (4.90) | .31 | 0.45 (0.34–0.55) |
| miR-133b | 22.93 (2.26) | 23.42 (2.22) | 23.17 (2.27) | .27 | 0.45 (0.35–0.56) |
| miR-142-5p | 21.79 (6.21) | 20.72 (5.68) | 21.29 (5.96) | .58 | 0.55 (0.43–0.66) |
| miR-150 | 18.20 (2.57) | 17.46 (2.50) | 17.85 (2.55) | .09 | 0.61 (0.50–0.71) |
| miR-223-3p | 12.41 (2.19) | 12.36 (2.20) | 12.39 (2.19) | .97 | 0.50 (0.39–0.61) |
| miR-499 | 27.25 (4.04) | 26.85 (4.39) | 27.06 (4.20) | .67 | 0.52 (0.40–0.63) |
miRNA levels are expressed as ΔCT (cycle threshold) normalized values. AUC is the area under the curve for the presence of atrial fibrillation (AF).
AUC = area under the receiver operating characteristic curve, SD = standard deviation.
* P < 0.05.
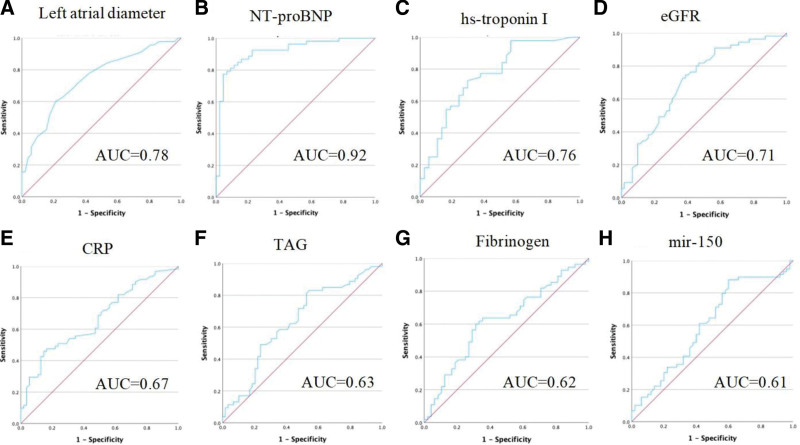
Receiver operating characteristic curves of the biomarkers with a statistically significant difference between the AF and non-AF groups are shown in Figure 1.
Figure 1.
Receiver operating characteristic (ROC) curves of biomarkers. (A) ROC curve of left atrial diameter, area under the curve (AUC) = 0.78. (B) ROC curve of NT-proBNP, AUC = 0.92. (C) ROC curve of hs-cTnI, AUC = 0.76. (D) ROC curve of eGFR, AUC = 0.71. (E) ROC curve of CRP, AUC = 0.67. (F) ROC curve of total triglycerides, AUC = 0.63. (G) ROC curve of fibrinogen, AUC = 0.62. (H) ROC curve of miR-150, AUC = 0.61. CRP = C-reactive protein, hs-cTnI = high-sensitivity cardiac troponin I.
4. Discussion
Most of the clinical characteristics of the AF group (older age, higher prevalence of arterial hypertension, higher prevalence of previous stroke or TIA, higher CHA2DS2-VASc score, higher NIHSS and mRS, higher proportion of mechanical thrombectomy treatment, higher proportion of anticoagulant, and antiarrhythmic therapy) and all echocardiographic parameters (larger left atrial diameter, lower left ventricular ejection fraction, higher prevalence of mitral valve disease) did not show a surprising result in comparison with non-AF group characteristics.[2,13] However, a difference in a few baseline characteristics should be pointed out. Smoking was more prevalent among the patients without AF, probably corresponding to more prevalent large vessel disease as an etiology of stroke in the non-AF group. Heavy alcohol drinking was almost equally distributed between the groups and overall detected in only 6.8% of patients. Especially in the AF group, the number could have been underestimated because of the inaccuracy in history taking due to older age and worse stroke impairment. The proportion of patients receiving IVT was higher in the non-AF group. There could be 2 possible explanations. First, the patients in the AF group were using anticoagulants more frequently, which contraindicated IVT. Second, patients in the AF group were older, more likely to live alone, and therefore more likely to arrive at the hospital later and surpass the time window for IVT treatment, which is shorter than the time window for the mechanical thrombectomy.
The biochemical biomarkers tested in our ischemic stroke cohort were selected based on the results of previous studies[13–22] as well as their routine clinical obtainability. Of 13 tested conventional biochemical biomarkers, 6 (sorted by the highest AUC: elevated levels of NT-proBNP, hs-cTnI, decreased eGFR, elevated levels of CRP, triglycerides, and fibrinogen) were significantly associated with AF in our ischemic stroke cohort. In concordance with the previous studies, cardiac biomarkers (NT-proBNP, hs-cTnI) had the highest AUC, reflecting the myocardial stress imposed by the arrhythmia and other associated cardiac comorbidities.[14–16] A decreased eGFR showed the strongest association with AF besides cardiac markers. In decreased renal function, the possible underlying mechanism resulting in AF could be the activation of the renin–angiotensin–aldosterone system, which leads to increased atrial pressure, promoting atrial fibrosis, and modulating ion channels, finally impacting structural and electrical atrial remodeling.[21] The low-grade elevation of CRP in the AF group (similarly as described before[17]) could be a sign of a chronic inflammatory condition that could influence cardiac and vascular remodeling, promoting AF and encouraging its persistence. Also, it has been shown before that CRP increases the risk of Ca influx in atrial myocytes.[33] Among the metabolic markers, total triglyceride level was the only one associated with AF. Higher triglyceride levels in patients with AF have also been described in a large population-based study.[34] The direct link with AF is unclear, but total triglyceride level is a component of the metabolic syndrome, which has been associated with the incidence of AF.[35] However, no association between AF and other metabolic biomarkers (glycated hemoglobin, total cholesterol, high-density lipoprotein, and low-density lipoprotein) was found in our ischemic stroke cohort. Of hemostatic markers, only fibrinogen showed higher levels in association with AF, albeit with an AUC of 0.62. This finding was also described in previous studies.[22] Fibrinogen is a positive acute-phase reactant protein. Increased fibrinogen levels were prospectively correlated with a higher risk of AF, which supports the notion that inflammation plays a role in the genesis of AF.[36] Surprisingly, our study found no association between AF and elevated D-dimer level, which is considered an established biomarker of AF.[22] This could be explained by the fact that the blood samples were mostly taken after the administration of intravenous thrombolysis, which may have skewed the D-dimer levels across both the AF and non-AF groups.
In our study, we did not observe notably altered plasma levels of the selected miRNAs in ischemic stroke patients with AF compared to non-AF controls. The selection of miRNAs tested in our study was based on their strongest reported association with AF and ischemic stroke.[26–31] However, heterogeneity in the design and methods of the studies often led to conflicting results when both upregulation and downregulation of particular miRNAs were described in connection with AF.[25] The levels of miR-150 were significantly reduced in patients with AF compared with those in the control group.[26] In contrast, our study showed nonsignificantly higher levels of miR-150 in the AF group. However, the populations differed, as the patients in the reference study were younger (median 51 years) and dominantly of Asian ethnicity. Reduced plasma levels of miR-21[27] and miR-29b[28] were observed in patients with AF, which was in concordance with our results, although the result was not statistically significant. In the reference studies, the patients were younger (median 59 years[27] and 63–68 years[29]) and were not presented with stroke. The expression of miR-499 has been reported to be elevated in the atrial tissue of patients with AF.[30] This finding was supported by our results of elevated plasma levels, although the difference was not statistically significant. In our study, patients were older, and we did not exclude patients with diabetes mellitus and uncontrolled hypertension as in the reference study. The reported increase in miR-133b plasma level in patients with AF[27] was not confirmed by our results, which showed nonsignificantly lower levels of miR-133b in the AF group. However, the patients in the reported study were younger (median 59 years) and were not presented with acute stroke. The reported lower expression of miR-142-5p and higher expression of miR-223-3p in patients with AF compared with patients with sinus rhythm[31] also conflicts with our results, as miR-142-5p was found to be nonsignificantly upregulated in the AF group and the levels of miR-223-3p were almost equal in both groups. As in most of the reference studies, the patients were younger (mean age 63 years), of Asian ethnicity, and were not presented with acute stroke. These significant differences in the target population were probably the main reason why the results of our study did not confirm the previous findings.
In-hospital mortality of 5.1% observed in our study corresponds to previously described values of ischemic stroke in-hospital mortality in highly developed stroke care systems.[37] All the deceased patients were part of the AF group, which had an in-hospital mortality of 9.8%, confirming the poorer prognosis of AF-associated ischemic stroke.[2] In patients 72 hours after ischemic stroke, lower plasma levels of miR-150-5p were associated with 90-day mortality compared to traditional demographic and vascular risk factors.[38] However, this association was not confirmed in our study, which could be caused by the short follow-up period and the fact that stroke was the cause of all deaths and miR-150-5p is supposed to reduce the risk of systemic secondary complications after stroke rather than influence the stroke lesion.[38] The only miRNA associated with mortality in our cohort was miR-29b (lower ΔCT 15.17 vs 17.34, P = .02). This result is consistent with the idea of a protective role of miR-29b in the development of the inflammatory response in the stroke lesion.[39] Of echocardiographic and biochemical biomarkers, large left atrial diameter, high CRP level, and high NT-proBNP level were significant predictors of mortality in our cohort. All of these biomarkers were associated with AF. On the contrary, other biomarkers linked to AF were not found to be predictors of mortality (ventricular ejection fraction, hs-cTnI, fibrinogen, eGFR, and total triglycerides). This dichotomy needs to be interpreted with caution as the overall number of deaths in the study is low. The higher probability of large embolus formation, as well as confined penumbra perfusion, could be the reasons for the higher mortality of stroke in patients with heart failure.
Our study has several limitations. Only a limited number of candidate miRNAs were selected because of the capacity of the laboratory, which was busy processing samples during the COVID-19 pandemic. The pandemic overload in the laboratory has also delayed the analytical processes in this study. Pre-analytical degradation of miRNAs could occur as the blood samples were stored for different times, depending on the inclusion of the subjects. However, this potential risk should be equal in both AF and non-AF groups. In addition, the possible effects of medication were not considered in our study. In particular, antithrombotic therapy can influence the expression of certain miRNAs and hamper their use as biomarkers.[40] miRNAs quantification relied on a one-time blood draw taken within 24 hours of hospital admission. It has been reported that half-lives of skeletal-muscle-enriched miRNAs were approximately 11 to 20 hours[41] so the association of miRNA and AF could have been missed because the occurrence of AF did not match the timing of blood sampling. Comparing the miRNA levels at different intervals since stroke could yield data on their temporal occurrence and half-lives, but it was not feasible for capacity reasons within the scope of this study. Another confounding issue could be that the AF and non-AF groups could not be matched in terms of individual clinical characteristics because patients with AF are usually older and present with more comorbidities. However, this reflects a real-life clinical setting in which biomarkers are supposed to be used.
The advantage of this study is the complete prospectively obtained dataset, which means that no data imputation was required. A unique strength of this study is the possibility of simultaneously comparing conventional biochemical biomarkers and experimental miRNAs in one set of patients, thus obtaining an image of their direct comparison. So far, not many studies have evaluated the association of miRNAs with AF in ischemic stroke,[42] and some of them were tested by our study for the first time in this clinical setting.
The occurrence of miRNAs, both spatial (circulating or tissue-specific) and temporal, is rather unpredictable, and the complexity of their interactions is considerable.[26] The results of our study confirmed the difficulty in choosing a suitable candidate miRNA as a biomarker. The conflicting results of biomarker studies on circulating miRNAs raise the question of how effective it is to consider a biomarker whose role in the etiology of the process is not sufficiently understood. Identifying a suitable miRNA biomarker candidate could become more feasible after elucidating its role in pathophysiological processes associated with AF. In contrast, the significant association between conventional biochemical biomarkers and AF aligns with the known concept of their involvement in pathophysiological processes in AF.
5. Conclusion
The blood levels of the selected miRNAs (miR-21, miR-29b, miR-133b, miR-142-5p, miR-150, miR-499, and miR-223-3p) were not associated with AF in our ischemic stroke cohort. Biochemical biomarkers (NT-proBNP, hs-cTnI, fibrinogen, CRP, eGFR, and total triglycerides) were significantly associated with AF in ischemic stroke patients. These conventional and clinically available biomarkers can be used, in addition to clinical characteristics and echocardiographic parameters, to select high-risk cryptogenic stroke patients for priority prolonged ECG monitoring, leading to faster AF detection and affecting the efficacy of secondary stroke prevention. The role of miRNAs as clinically usable biomarkers of AF in ischemic stroke patients remains questionable.
Author contributions
Conceptualization: Petr Janský, Vojtěch Kaplan, Tereza Šrámková, Petra Kešnerová, Jaroslava Paulasová-Schwabová, Václav Maťoška, Aleš Tomek.
Data curation: Petr Janský, Filip Kolman, Petra Kloudová, Václav Maťoška.
Formal analysis: Petr Janský, Václav Maťoška, Aleš Tomek.
Funding acquisition: Petra Kešnerová, Aleš Tomek.
Investigation: Petr Janský, Vojtěch Kaplan, Tereza Šrámková, Petra Kloudová, Kateřina Benešová, Anna Olšerová, Hana Magerová, Vlastimil Šulc, Hana Halmová, Silvia Kmetonyová, Ivana Šarbochová.
Methodology: Petr Janský, Vojtěch Kaplan, Tereza Šrámková, Jaroslava Paulasová-Schwabová, Václav Maťoška, Aleš Tomek.
Project administration: Petr Janský, Filip Kolman, Petra Kloudová, Kateřina Benešová, Anna Olšerová, Hana Magerová, Vlastimil Šulc, Hana Halmová, Silvia Kmetonyová, Ivana Šarbochová.
Resources: Aleš Tomek.
Supervision: Petra Kešnerová, Jaroslava Paulasová-Schwabová, Václav Maťoška, Aleš Tomek.
Visualization: Petr Janský, Aleš Tomek.
Writing – original draft: Petr Janský, Václav Maťoška.
Writing – review & editing: Aleš Tomek.
Abbreviations:
- AF
- atrial fibrillation
- AUC
- area under the receiver operating characteristic curve
- CRP
- C-reactive protein
- CT
- cycle thresholds
- hs-cTnI
- high-sensitivity cardiac troponin I
- IQR
- interquartile range
- IVT
- intravenous thrombolysis
- miRNA
- microRNA
- NIHSS
- National Institutes of Health Stroke Scale
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- SD
- standard deviation
The study was supported by Czech Health Research Council project number AZV NV19-08-00362. The study was supported by the Charles University Grant Agency (GA UK), project No. 618217.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Janský P, Kaplan V, Šrámková T, Kolman F, Kloudová P, Benešová K, Olšerová A, Kešnerová P, Magerová H, Šulc V, Halmová H, Kmetonyová S, Paulasová-Schwabová J, Šarbochová I, Maťoška V, Tomek A. MicroRNAs and other biomarkers of atrial fibrillation in ischemic stroke patients. Medicine 2024;103:43(e40165).
Contributor Information
Vojtěch Kaplan, Email: kaplanvojtech@gmail.com.
Tereza Šrámková, Email: terez.ruzickova@gmail.com.
Kateřina Benešová, Email: katerinabican@gmail.com.
Anna Olšerová, Email: a.olserova@gmail.com.
Petra Kešnerová, Email: kesnerova.petra@gmail.com.
Hana Magerová, Email: ha.nohejlova@gmail.com.
Vlastimil Šulc, Email: vlsulc@gmail.com.
Hana Halmová, Email: ha.nohejlova@gmail.com.
Silvia Kmetonyová, Email: silvia.kmetony@gmail.com.
Jaroslava Paulasová-Schwabová, Email: schwabova@gmail.com.
Ivana Šarbochová, Email: ivanasarb@gmail.com.
Václav Maťoška, Email: vaclav.matoska@gmail.com.
Aleš Tomek, Email: ales.tomek@gmail.com.
References
- [1].Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–4. [DOI] [PubMed] [Google Scholar]
- [3].Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- [4].Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14:377–87. [DOI] [PubMed] [Google Scholar]
- [5].Koudstaal PJ. Anticoagulants versus antiplatelet therapy for preventing stroke in patients with nonrheumatic atrial fibrillation and a history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2000;2:CD000187. [DOI] [PubMed] [Google Scholar]
- [6].Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378:2191–201. [DOI] [PubMed] [Google Scholar]
- [7].Diener HC, Sacco RL, Easton JD, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380:1906–17. [DOI] [PubMed] [Google Scholar]
- [8].Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–86. [DOI] [PubMed] [Google Scholar]
- [9].Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–77. [DOI] [PubMed] [Google Scholar]
- [10].Atkinson AC, Degruttola W, Demets V, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. [DOI] [PubMed] [Google Scholar]
- [11].Dogan U, Dogan EA, Tekinalp M, et al. P-wave dispersion for predicting paroxysmal atrial fibrillation in acute ischemic stroke. Int J Med Sci. 2012;9:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kolb C, Nürnberger S, Ndrepepa G, Zrenner B, Schömig A, Schmitt C. Modes of initiation of paroxysmal atrial fibrillation from analysis of spontaneously occurring episodes using a 12-lead Holter monitoring system. Am J Cardiol. 2001;88:853–7. [DOI] [PubMed] [Google Scholar]
- [13].Phang RS, Isserman SM, Karia D, et al. Echocardiographic evidence of left atrial abnormality in young patients with lone paroxysmal atrial fibrillation. Am J Cardiol. 2004;94:511–3. [DOI] [PubMed] [Google Scholar]
- [14].Sakai K, Shibazaki K, Kimura K, et al. Brain natriuretic peptide as a predictor of cardioembolism in acute ischemic stroke patients: brain natriuretic peptide stroke prospective study. Eur Neurol. 2013;69:246–51. [DOI] [PubMed] [Google Scholar]
- [15].Longstreth WT, Jr, Kronmal RA, Thompson JL, et al. Amino terminal pro-B-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke. 2013;44:714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Beaulieu-Boire I, Leblanc N, Berger L, Boulanger JM. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis. 2013;22:978–83. [DOI] [PubMed] [Google Scholar]
- [17].Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. [DOI] [PubMed] [Google Scholar]
- [18].Liuba I, Ahlmroth H, Jonasson L, et al. Source of inflammatory markers in patients with atrial fibrillation. Europace. 2008;10:848–53. [DOI] [PubMed] [Google Scholar]
- [19].Sandhu RK, Conen D, Tedrow UB, et al. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc. 2014;3:e000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lopez FL, Agarwal SK, Maclehose RF, et al. Blood lipid levels, lipid-lowering medications, and the incidence of atrial fibrillation: the atherosclerosis risk in communities study. Circ Arrhythm Electrophysiol. 2012;5:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Iguchi Y, Kimura K, Kobayashi K, et al. Relation of atrial fibrillation to glomerular filtration rate. Am J Cardiol. 2008;102:1056–9. [DOI] [PubMed] [Google Scholar]
- [22].Wu N, Tong S, Xiang Y, et al. Association of hemostatic markers with atrial fibrillation: a meta-analysis and meta-regression. PLoS One. 2015;10:e0124716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gilad S, Meiri E, Yogev Y, et al. Serum microRNAs are promising novel biomarkers. PLoS One. 2008;3:e3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- [25].Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu Z, Zhou C, Liu Y, et al. The expression levels of plasma micoRNAs in atrial fibrillation patients. PLoS One. 2012;7:e44906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McManus DD, Tanriverdi K, Lin H, et al. Plasma microRNAs are associated with atrial fibrillation and change after catheter ablation (the miRhythm study). Heart Rhythm. 2015;12:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dawson K, Wakili R, Ordög B, et al. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation. 2013;127:1466–75, 1475e1. [DOI] [PubMed] [Google Scholar]
- [29].Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4. [DOI] [PubMed] [Google Scholar]
- [30].Ling TY, Wang XL, Chai Q, et al. Regulation of the SK3 channel by microRNA-499—potential role in atrial fibrillation. Heart Rhythm. 2013;10:1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang S, Min J, Yu Y, et al. Differentially expressed miRNAs in circulating exosomes between atrial fibrillation and sinus rhythm. J Thorac Dis. 2019;11:4337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- [33].Ding M, Wennberg A, Gigante B, Walldius G, Hammar N, Modig K. Lipid levels in midlife and risk of atrial fibrillation over 3 decades-Experience from the Swedish AMORIS cohort: a cohort study. PLoS Med. 2022;19:e1004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chang SN, Tsai CT, Wu CK, et al. A functional variant in the promoter region regulates the C-reactive protein gene and is a potential candidate for increased risk of atrial fibrillation. J Intern Med. 2012;272:305–15. [DOI] [PubMed] [Google Scholar]
- [35].Kumar P, Gehi AK. Atrial fibrillation and metabolic syndrome: understanding the connection. J Atr Fibrillation. 2012;5:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mukamal KJ, Tolstrup JS, Friberg J, Grønbaek M, Jensen G. Fibrinogen and albumin levels and risk of atrial fibrillation in men and women (the Copenhagen City Heart Study). Am J Cardiol. 2006;98:75–81. [DOI] [PubMed] [Google Scholar]
- [37].Qureshi AI, Baskett WI, Huang W, et al. Acute ischemic stroke and COVID-19: an analysis of 27 676 patients. Stroke. 2021;52:905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Scherrer N, Fays F, Mueller B, et al. MicroRNA 150-5p improves risk classification for mortality within 90 days after acute ischemic stroke. J Stroke. 2017;19:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ma X, Yun HJ, Elkin K, Guo Y, Ding Y, Li G. MicroRNA-29b suppresses inflammation and protects blood-brain barrier integrity in ischemic stroke. Mediators Inflamm. 2022;2022:1755416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].de Boer HC, van Solingen C, Prins J, et al. Aspirin treatment hampers the use of plasma microRNA-126 as a biomarker for the progression of vascular disease. Eur Heart J. 2013;34:3451–7. [DOI] [PubMed] [Google Scholar]
- [41].Oikawa S, Yuan S, Kato Y, Akimoto T. Skeletal muscle-enriched miRNAs are highly unstable in vivo and may be regulated in a Dicer-independent manner. FEBS J. 2023;290:5692–703. [DOI] [PubMed] [Google Scholar]
- [42].Boxhammer E, Dienhart C, Rezar R, Hoppe UC, Lichtenauer M. Deciphering the role of microRNAs: unveiling clinical biomarkers and therapeutic avenues in atrial fibrillation and associated stroke-a systematic review. Int J Mol Sci. 2024;25:5568. [DOI] [PMC free article] [PubMed] [Google Scholar]