Abstract
Rationale:
Podocyte infolding glomerulopathy (PIG) is a rare glomerular disease, its diagnosis mainly depends on pathological manifestations of the kidney. Few clinical cases of PIG have been reported, but it is sometimes associated with connective tissue diseases. Here we describe a case of systemic lupus erythematosus (SLE) with PIG and undertake a review of the literature.
Patient concerns:
A 34-year-old female patient was admitted to our hospital in August 2019 with repeated facial erythema and proteinuria for more than 10 years. The patient was previously diagnosed with SLE.
Diagnosis:
Systemic lupus erythematosus.
Interventions:
Renal biopsy was performed to investigate ongoing proteinuria and the results were consistent with PIG. Treatment with methylprednisolone, hydroxychloroquine sulfate, mycophenolate mofetil, and candesartan ester.
Outcomes:
Improved the patient’s condition and resolved the proteinuria.
Lessons:
This study reported a case of PIG and SLE. The patient was diagnosed according to biopsy, and the disease remain stable after immunosuppressive therapy. It is recommended to carefully study renal biopsies from patients with proteinuria and underlying autoimmune diseases to identify additional cases.
Keywords: case report, diagnostic criteria, pathogenesis, pathological characteristics, podocyte infolding glomerulopathy
1. Introduction
Podocyte infolding glomerulopathy (PIG), a pathological condition characterized by unique ultrastructural findings, was discovered in 1992 when Sato et al[1] reported on 5 renal biopsy specimens using electron microscopy (EM). These findings include the inversion and folding of podocytes into the glomerular basement membrane (GBM), which appear as microspheres and microtubules under EM. Initially, PIG was considered to be a variant of membranous nephropathy (MN), which is characterized by annular subepithelial deposition. However, in 2008, PIG was recognized as a possible distinct disease entity.[2]
Relatively few cases of PIG have been reported in the literature, some of which are associated with connective tissue diseases, including systemic lupus erythematosus (SLE). However, it is not clear whether PIG is a distinct disease in these patients or if it relates to co-existing diagnoses.[3] Therefore, this study aims to report a case of a 34-year-old woman with SLE and PIG, and review relevant literature reporting clinical cases of PIG.
2. Case presentation
A 34-year-old Chinese woman was admitted to our hospital in August 2019 with recurrent facial erythema and proteinuria persisting for more than 10 years. On physical examination, the patient exhibited flaky light erythema on both cheeks without flaking or itching. Laboratory examination revealed no abnormalities in blood routine and virus infection. Immunoassay testing yielded the following results: antinuclear antibody (ANA) > 500 AU/mL (normal range: 0–32), anti-double-stranded DNA antibody 36.1 IU/ml (normal range: 0–24), anti-SSA/Ro52 antibody positive (+), anti-SSA/Ro60 antibody positive (+), anti-U1-snRNP antibody positive (+). Urinalysis showed protein (2+) and microalbumin > 0.15 g/L. Urinary protein excretion was 446 mg/d and urine protein creatinine ratio was 0.05.
The patient had previously been diagnosed with SLE for 10 years and had been undergoing long-term medication, including prednisone, hydroxychloroquine sulfate, methotrexate, and azathioprine. Nine months ago, she was hospitalized in the rheumatology department of our hospital, and the treatment regimen was changed to methylprednisolone 20 mg once daily, hydroxychloroquine sulfate 0.2 g twice daily, and merti-mescaline dispersible tablets 0.75 g. Since then, the patient’s condition has stabilized and she was discharged from the hospital. Regular outpatient reviews showed persistent urinary protein levels ranging from 1+ to 3+ levels. The patient denied any family history.
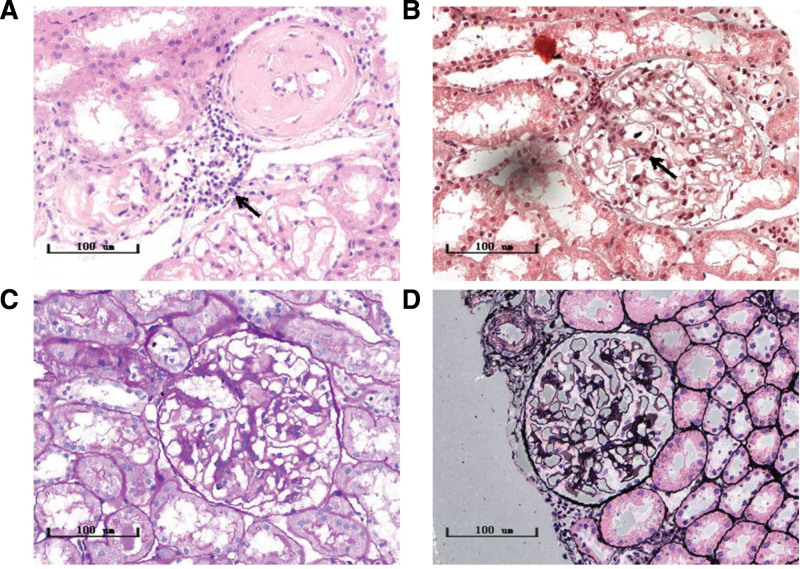
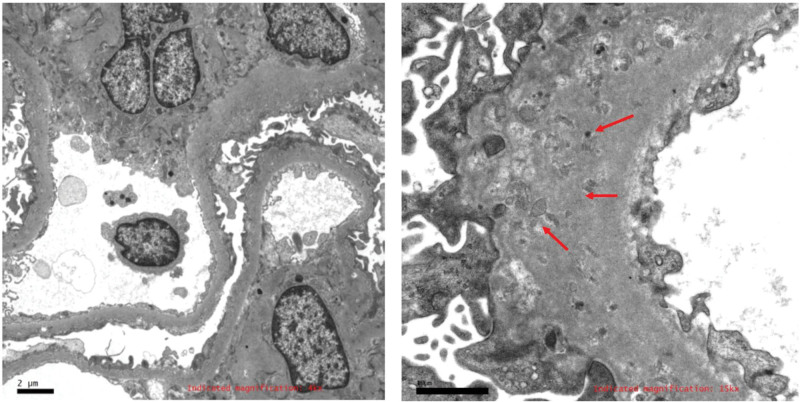
As the previous treatment regimen for SLE was not effective it was suggested that the case undergo a kidney biopsy. The results of the kidney biopsy were as follows: The volume of the glomeruli increased slightly, with slight proliferation of mesangial cells. The capillary loops were open, but the basement membrane appeared thickened and relatively rigid, with no obvious spike-like structure. There were no fuchsinophilic protein deposits in the mesangial area and subendothelial area, but deposits were found under the epithelium. No mesangial insertion or double track formation was observed. Proliferation of parietal epithelial cells and crescents was observed. Renal tubular epithelial cells exhibited granular and vacuolar degeneration, with small focal atrophy (approximately 5% of the area). There was small focal inflammatory cell infiltration in the renal interstitium, with no obvious fibrosis. The arterial wall showed thickening of the intima and narrowing of the lumen (Fig. 1). No significant regularity in immunoglobulin staining was observed. The EM specimen was stained with toluidine blue, revealing 2 glomeruli. Observed under the ultrathin section electron microscope, the capillary loops were open, parietal cells did not proliferate, and the basement membrane was slightly irregularly thickened, exceeding 800 nm in thickness. Fusion of most foot processes was observed, along with the presence of microspheres or microcapsules near the epithelial side of the basement membrane. Mesangial cells and matrix showed slight proliferation, with no exact electron-dense deposits found under the epithelium, mesangial area, or endothelium. Renal tubular epithelial cells exhibited vacuolar degeneration, while the renal interstitium showed no special lesions (Fig. 2). These characteristics led to the diagnosis of PIG in this case.
Figure 1.
Histological analysis of the kidney biopsy. The light microscope specimens were stained with (A) Hematoxylin and Eosin (HE); (B) Masson; (C) Periodic acid–Schiff (PAS); (D) Periodic Schiff–Methenamine Silver (PASM).
Figure 2.
The electron microscope specimen was stained with toluidine blue, and 2 glomeruli were seen. More microspheres or microcysts are seen nearby, the capillary collaterals are open, the wall cells are not proliferating, the basement membrane is irregularly thickened, and the peduncles are mostly united.
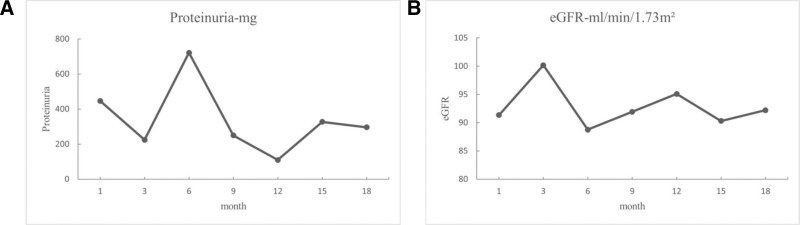
The patient received methylprednisolone 12 mg once daily, hydroxychloroquine sulfate 0.2 g twice daily, mycophenolate mofetil dispersible tablets 0.75 g twice daily, and candesartan ester 2 mg once daily as treatment, which was maintained until March 2020. Starting March, 2020, the corticosteroid dosage was reduced to 8 mg/d, and mycophenolate mofetil dispersible tablets were changed to 0.5 g. The patient was followed up regularly for over 1 year. Her condition improved, with Creatinine at 49.8 (normal range: 41–73 μmol/L), cystatin at 0.67 (normal range: 0.54–1.15 mg/L), urea at 3.3 (normal range: 2.6–7.5 mmol/L), and no abnormalities in blood routine. Blood pressure and renal function were within normal ranges (Fig. 3A), and urinary protein was <500 mg/d (Fig. 3B).
Figure 3.
Follow-up monitoring of renal function and urinary protein. (A) Changes in eGFR over time (months); (B) changes in proteinuria with time (months).
Written consent for publication of this case report was obtained from the patient.
3. Discussion
This study reported a case of PIG in a female patient with SLE. The patient had a history of SLE with facial erythema and proteinuria for over ten years, but the treatment regimens with prednisone and hydroxychloroquine sulfate had been unsuccessful in resolving all symptoms. The patient underwent a kidney biopsy, revealing characteristics of PIG. She was treated with methylprednisolone, hydroxychloroquine sulfate, mycophenolate mofetil, and candesartan ester, which improved the patient’s condition and resolved the proteinuria.
The diagnosis of PIG in this case was based on the diagnostic criteria for PIG that were put forward by the Japanese Society of Nephrology (JSN), which established an expert group on PIG and analyzed data of 25 cases of PIG reported in Japan.[2] These criteria include the presence of non-silvered vacuoles similar to those found in MN in the GBM under light microscopy and the presence of microsphere or microtubule structures of 50 to 150 nm within the GBM visible under EM.[2] However, since the diagnosis of PIG relies solely on the morphologic manifestations of EM, it is still unclear whether PIG should be regarded as a distinct disease entity.
It has been observed that the majority of cases with PIG have underlying lesions, while some exhibit only mild lesions with few specific changes seen on light microscopy. The light microscopy appearance of PIG is similar to that of MN, and is characterized by a stiff, thickened GBM with non-silver-loving vacuoles. However, it can be differentiated from MN by fluorescence and EM. Masuda et al[4] retrospectively analyzed 126 renal biopsies of primary MN and suggested that it is necessary to exclude cellular debris and virus-like particles to differentiate them from MN before diagnosing PIG, especially when microstructures are present in the GBM.
A literature review was conducted to comprehend the pathological characteristics and underlying causes of PIG. PubMed was searched using the keywords “podocyte infolding glomerulopathy.” Studies that did not report on treatment or prognosis were excluded. From the available literature, it was found that all 32 diagnosed cases of PIG so far originated from Asia, with a higher prevalence among (Table 1). The most frequently associated condition reported was SLE (16/32, 50.0%), while other diseases included tumors, chronic thyroiditis, hydronephrosis, hepatitis B virus infection, and dry syndrome. EM consistently revealed GBM thickening, fusion or loss of peduncles, and the formation of microspheres or microtubules in most cases (Table 2). Immunofluorescence staining was positive for G and C3 in the majority of patients, with only 9 cases being completely negative. These 9 cases included 5 cases of SLE, 2 cases of thyroid disease, 1 case of primary Sjögren’s syndrome (pSS), and 1 case of multiple myeloma. No significant regularity in immunoglobulin staining was observed. Our patient in this case also had a fully negative result, possibly due to prior immunotherapy.
Table 1.
Clinical profiles of the PIG cases reported in the literature.
| Case No | Sex | Age | Comorbidity | Cr (µmol/L) | Proteinuria | Therapy | Prognosis |
|---|---|---|---|---|---|---|---|
| 12 | M | 31 | SLE | 168 | 0.5 g/d | PSL | CR |
| 22 | F | 37 | SLE | 106.1 | 1 g/d | PSL, MMF | CR |
| 32 | F | 40 | SLE | 44.2 | 1.5 g/d | PSL | NR |
| 42 | F | 30 | SLE | 44.2 | 1.6 g/d | PSL | CR |
| 52 | F | 61 | SLE, Takayasu arteritis | 79.6 | 1.7 g/d | PSL, CsA | PR |
| 62 | F | 29 | SLE, hydronephrosis | 61.9 | 1.6 g/d | PSL | PR |
| 72 | F | 46 | SLE, hydronephrosis | 44.2 | 0.6 g/d | PSL | PR |
| 82 | F | 27 | SLE | 35.4 | 2.7 g/d | PSL, MMF | CR |
| 92 | M | 53 | SLE, bilateral urethral stone | 79.6 | 3.1 g/d | PSL | PR |
| 102 | F | 23 | SLE | 44.2 | 1.8 g/d | PSL | CR |
| 112 | F | 31 | SLE | 79.6 | 0.5 g/d | PSL | CR |
| 122 | F | 24 | SLE, SS | 53 | 6.0 g/d | PSL | CR |
| 132 | M | 49 | PBC, SS, cystitis, SLE | 97.2 | 2.2 g/d | PSL | CR |
| 142 | F | 20 | SLE? | 123.8 | 1.4 g/d | PSL | CR |
| 152 | F | 47 | RA, pSS | 53 | 1.3 g/d | PSL | PR |
| 162 | F | 51 | pSS | 53 | 3.7 g/d | PSL | CR |
| 172 | F | 30 | MCTD | 79.6 | 0.3 g/d | PSL | NR |
| 182 | F | 54 | Basedow disease | 221 | 6.0 g/d | PR | |
| 192 | F | 57 | Hypothyroidism, chronic thyroiditis | 53 | 0.3 g/d | CR | |
| 202 | M | 45 | Absent | 61.9 | 2.6 g/d | PSL | PR |
| 212 | F | 42 | Ovarian mature teratoma | 68.1 | 7.5 g/d | ARB | PR |
| 222 | F | 69 | Absent | 79.6 | 1.6 g/d | PR | |
| 232 | M | 46 | HBV infection | 106.1 | 4.0 g/d | Diuretics | PR |
| 242 | M | 59 | Tumor lysis syndrome | 450.8 | 0.6 g/d | PSL | NR |
| 252 | F | 45 | Absent | 70.7 | 1.5 g/d | PSL | CR |
| 2613 | F | 14 | Absent | 48.6 | 2.4 g/d | PSL | CR |
| 2714 | M | 79 | Multiple myeloma | 113.2 | 1.4 g/d | PSL | Absent |
| 2815 | F | 44 | Absent | 39.8 | 0.3 g/d | PSL, Kanarb | CR |
| 2916 | F | 45 | UCTD | 145.9 | 5.8 g/d | PSL, MMF, RTX | PR |
| 3017 | F | 27 | pSS | 168 | 0.6 g/d | PSL, HCQ | Lost |
| 3117 | F | 23 | SLE | 47 | 16.8 g/d | PSL, HCQ, MMF | CR |
| 32 | F | 34 | SLE | 48.3 | 0.4 g/d | MePr, HCQ, MMF | CR |
ARB = angiotensin receptor blocker, CR = complete response, Cr = creatinine, CsA = cyclosporin, F = female, HBV = hepatitis B virus, HCQ = hydroxychloroquine, M = male, MCTD = mixed connective tissue disease, MMF = mycophenolate mofetil, NR = not reported, PBC = primary biliary cirrhosis, PIG = podocyte infolding glomerulopathy, PR = partial response, PSL = prednisolone, pSS = primary Sjogren syndrome, RA = rheumatoid arthritis, RTX = rituximab, SLE = systemic lupus erythematosus, SS = Sjogren syndrome, UCTD = undifferentiated connective tissue disease.
Table 2.
Pathological characteristics of PIG cases reported in the literature.
| Case No. | Sex | Age | Pathology | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LM diagnosis | Mesangial deposit | GBM thickening | FPE | Microspheres | Microtubules | IF staining | |||
| 12 | M | 31 | MGA | Mild | Present | Present | Present | Absent | All negative |
| 22 | F | 37 | LN class II | Mild | Present | Present | Present | Present | G, A, C3, C1q |
| 32 | F | 40 | LN class II | Mild | Present | Present | Present | Present | G, A, C3, C1q |
| 42 | F | 30 | LN class II | Mild | Present | Present | Present | Absent | G, A, C3, C1q, C5b-9 |
| 52 | F | 61 | LN class II | Mild | Present | Present | Present | Absent | G, M, C1q |
| 62 | F | 29 | MN | Absent | Present | Present | Present | Absent | All negative |
| 72 | F | 46 | MN | Absent | Present | Present | Present | Absent | All negative |
| 82 | F | 27 | LN class V | Absent | Present | Present | Present | Present | G, A, M, C3, C1q, C5b-9 |
| 92 | M | 53 | MN | Absent | Present | Present | Present | Present | All negative |
| 102 | F | 23 | LN class V | Mild | Present | Present | Present | Present | G |
| 112 | F | 31 | LN class V | Absent | Present | Present | Present | Present | G |
| 122 | F | 24 | LN class V | Mild | Present | Present | Present | Present | G, M, C1q |
| 132 | M | 49 | MPGN (type 3) | Present | Present | Present | Absent | Present | G, A |
| 142 | F | 20 | MGA | Absent | Present | NR | Present | Absent | G |
| 152 | F | 47 | MGA | Absent | Present | NR | Present | Absent | G, A, M |
| 162 | F | 51 | MGA | Absent | Present | Present | Present | Absent | All negative |
| 172 | F | 30 | MGA | Absent | Present | Present | Present | Absent | G |
| 182 | F | 54 | FSGS | Present | Present | Present | Present | Absent | All negative |
| 192 | F | 57 | FSGS | Absent | Present | Present | Present | Absent | All negative |
| 202 | M | 45 | FSGS | Present | Present | Present | Present | Present | G,A,C3 |
| 212 | F | 42 | FSGS + MN | Absent | Present | Present | Present | Absent | G |
| 222 | F | 69 | MN | Absent | Present | Present | Present | Absent | G,A,M,C3 |
| 232 | M | 46 | MN | Absent | Present | Present | Present | Present | G |
| 242 | M | 59 | MN | Absent | Present | Present | Present | Absent | M |
| 252 | F | 45 | MN | Absent | Present | Present | Present | Present | G,A,C3 |
| 2613 | F | 14 | FSGS | Absent | Absent | Present | Present | Absent | M,C3, C1q |
| 2714 | M | 79 | NR | Absent | Present | Present | Present | Absent | All negative |
| 2815 | F | 44 | NR | Mild | Present | Present | Present | Present | M |
| 2916 | F | 45 | MN | Absent | Present | Present | Present | Absent | G, C3 |
| 3017 | F | 27 | FSGS | Absent | Present | Present | Present | Absent | M |
| 3117 | F | 23 | LN class II | Absent | Present | Present | Present | Absent | M |
| 32 | F | 34 | LN class II | Absent | Present | Present | Present | Present | All negative |
A = IgA, F = female, FPE = foot process effacement, FSGS = focal segmental glomerular sclerosis, G = IgG, GBM = glomerular basement membrane, IF = immunofluorescent, LM = light microscopy, LN = lupus nephritis, M = male; M (in IF staining column), IgM, MGA = mild glomerular abnormality, MN = membranous nephropathy, MPGN = membranous proliferative glomerulonephritis, NR = not reported, PIG = podocyte infolding glomerulopathy.
Currently, the pathogenesis of PIG remains unclear. Nakajima et al[5] analyzed extracellular structures, including microspheres and fibrous structures, in various glomerular diseases using immunoelectron microscopy, and found complement or complement fragments, distributed on their membranes. Hinglais et al[6] found that circular granules and striated membrane-like structures expressing the membrane-attacking complex C5b-9 in GBM by immunohistochemistry. This suggests a potential association between PIG and the activation of a specific complement system on podocytes, possibly associated with podocyte injury disrupting matrix biosynthesis and degradation balance, and GBM thickening with increased permeability.[7,8] Feng et al[3] identified mutations in the INF2 gene, known for inherited focal segmental glomerulosclerosis (FSGS).[9–11] Additionally, a case report by Xiong et al[12] showed a novel SMARCAL1 mutation. These suggest that genetic mutations may be involved in the PIG process.
Generally, the clinical outcome for PIG is favorable. The majority of cases (27/32, 84.4%) received immunosuppressive therapy, and achieved at least partial remission. Only a very small number of patients showed disease progression. From this perspective, PIG may be considered a relatively benign disease.
In conclusion, this study reported a case of PIG and SLE. The patient was diagnosed through biopsy, and the disease remained stable after immunosuppressive therapy. The literature review indicates that the relationship between PIG and autoimmune disorders is unclear, and further investigation is needed to understand the specific pathophysiological mechanisms involved. EM remains the gold standard for diagnosing PIG, and it is recommended to thoroughly examine renal biopsies from patients with proteinuria and underlying autoimmune diseases to identify additional cases and comprehend the clinicopathologic features of PIG.
Acknowledgments
The authors thank Guangzhou University of Chinese Medicine, Dongguan hospital, for its technical assistance for this study.
Author contributions
Conceptualization: Huiqing Zhang.
Data curation: Huiqing Zhang, Jie Lin, Hanqi Lu, Yunliang Zhong, Bin Kuang.
Formal analysis: Lie Deng, Qiang Li.
Methodology: Huiqing Zhang, Jie Lin, Hanqi Lu, Yunliang Zhong, Lie Deng, Bin Kuang.
Supervision: Bin Kuang, Qiang Li.
Writing – original draft: Huiqing Zhang, Jie Lin.
Writing – review & editing: Huiqing Zhang, Jie Lin, Hanqi Lu, Yunliang Zhong, Lie Deng, Bin Kuang, Qiang Li.
Abbreviations:
- ANA
- antinuclear antibody
- EM
- electron microscopy
- GBM
- glomerular basement membrane
- MN
- membranous nephropathy
- PIG
- podocyte infolding glomerulopathy
- SLE
- systemic lupus erythematosus
This research was funded by Guangdong Provincial Basic and Applied Basic Research Funding Committee, Regional Joint Fund-Regional Cultivation Program, grant number 2022A1515140140; Dongguan Science and Technology Bureau, Dongguan Social Science and Technology Development Project (High-level Hospital Construction Special Project), grant number 20231800917372; Dongguan Science and Technology Bureau, Dongguan Social Science and Technology Development Key Project, grant numbers 20221800906132 and 20211800904412.
Consent for publication is not applicable.
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. Written consent for publication of this case report was obtained from the patient.
The authors declare that they have no competing interests.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Zhang H, Lin J, Lu H, Zhong Y, Deng L, Kuang B, Li Q. Systemic lupus erythematosus with podocyte infolding glomerulopathy: A case report and literature review. Medicine 2024;103:43(e39809).
Contributor Information
Huiqing Zhang, Email: 1360981240@qq.com.
Jie Lin, Email: 331614068@qq.com.
Hanqi Lu, Email: 393398106@qq.com.
Yunliang Zhong, Email: 37294218@qq.com.
Lie Deng, Email: 872714876@qq.com.
Bin Kuang, Email: kb20041001@126.com.
References
- [1].Sato H, Saito T, Yoshinaga K. Intramembranous fine deposit disease associated with collagen disorders: a new morphological entity? Virchows Arch A Pathol Anat Histopathol. 1992;420:447–51. [DOI] [PubMed] [Google Scholar]
- [2].Joh K, Taguchi T, Shigematsu H, et al. Proposal of podocytic infolding glomerulopathy as a new disease entity: a review of 25 cases from nationwide research in Japan. Clin Exp Nephrol. 2008;12:421–31. [DOI] [PubMed] [Google Scholar]
- [3].Feng Y, Wang W, Zou Y, et al. Podocyte infolding glomerulopathy: a case series report and literature review. J Clin Med. 2023;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Masuda Y, Mii A, Shimizu A, et al. Invagination and infolding of podocytes in glomerular basement membrane in the cases of primary membranous nephropathy. Clin Exp Nephrol. 2008;12:440–9. [DOI] [PubMed] [Google Scholar]
- [5].Nakajima M, Hewitson TD, Mathews DC, Kincaid-Smith P. Localisation of complement components in association with glomerular extracellular particles in various renal diseases. Virchows Arch A Pathol Anat Histopathol. 1991;419:267–72. [DOI] [PubMed] [Google Scholar]
- [6].Hinglais N, Kazatchkine MD, Bhakdi S, et al. Immunohistochemical study of the C5b-9 complex of complement in human kidneys. Kidney Int. 1986;30:399–410. [DOI] [PubMed] [Google Scholar]
- [7].Asanuma K, Shirato I, Ishidoh K, Kominami E, Tomino Y. Selective modulation of the secretion of proteinases and their inhibitors by growth factors in cultured differentiated podocytes. Kidney Int. 2002;62:822–31. [DOI] [PubMed] [Google Scholar]
- [8].Lee HS. Mechanisms and consequences of TGF-ß overexpression by podocytes in progressive podocyte disease. Cell Tissue Res. 2012;347:129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Iguchi A, Sohma A, Yamazaki H, et al. A case of podocytic infolding glomerulopathy with focal segmental glomerulosclerosis. Case Rep Nephrol Urol. 2013;3:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harada M, Kamijo Y, Ehara T, Shimojo H, Shigematsu H, Higuchi M. A case of podocytic infolding glomerulopathy with multiple myeloma. BMC Nephrol. 2014;15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao Y, Zhang H, Wang H, Ye M, Jin X. Role of formin INF2 in human diseases. Mol Biol Rep. 2022;49:735–46. [DOI] [PubMed] [Google Scholar]
- [12].Kwon KW, Jeong HJ, Lee JH. Podocytic infolding glomerulopathy: a case report. Ultrastruct Pathol. 2016;40:374–7. [DOI] [PubMed] [Google Scholar]