Abstract
The objective of this study was to evaluate analgesic efficacy of tramadol (opioid-like analgesia), carprofen (NSAID) and a combination of both drugs (multimodal therapy), in a rat laparotomy model. Sprague Dawley rats (Crl: CD (SD)) were randomly assigned to 1 of 2 groups: surgery (abdominal laparotomy with visceral manipulation and anesthesia) or sham surgery (anesthesia only). All rats were treated with one of the following: tramadol (12.5 mg/kg IP), carprofen (5 mg/kg SC), a combination of tramadol (12.5 mg/kg IP) and carprofen (5 mg/kg SC), or saline (5 mg/kg SC; sham surgery rats only). Analgesia was administered 10 minutes before anesthesia and surgery, 4 hours after surgery (PM treatment) and the following morning 24 hours after surgery (AM treatment). Running wheel activity was measured daily both pre-surgery and post-surgery. Feed and water consumption and body weight were evaluated prior to and 24, 48 and 72 hours following surgery. Locomotor activity and fecal corticosterone concentration were evaluated prior to and 24 hours following surgery among all groups. Clinical observations were made at 2, 12, and 24 hours following surgery to evaluate signs of pain and distress including hunched posture, back-arching, ruffled hair coat, writhing, stretching, and lethargy. Surgery rats treated with tramadol had decreased locomotor activity following surgery, unlike surgery groups treated with carprofen or multimodal therapy. All surgery groups, regardless of analgesia, showed decreased running wheel activity on the day of surgery compared to their respective baseline levels. Running wheel activity remained decreased in the carprofen and multimodal groups on day 1 post-surgery compared to baseline but returned to baseline levels by day 2 post-surgery. Surgery groups treated with tramadol or multimodal therapy also showed decreased food consumption on the day of surgery compared to their baselines. No significant differences in body weight or fecal corticosterone concentration were present among treatment groups. Carprofen appeared more effective in controlling pain in this rat laparotomy model than tramadol alone. The use of the combination of carprofen and tramadol resulted in a slightly improved analgesic effect compared to tramadol alone.
The Animal Welfare Act (AWA) defines a painful procedure as any procedure that would reasonably be expected to cause more than slight or momentary pain or distress in a human [1]. It also states we must ensure that animal pain and distress are minimized, including adequate veterinary care with the appropriate use of anesthetic, analgesic, tranquilizing drugs or euthanasia [1]. The use of adequate anesthesia and analgesia in research animals undergoing painful procedures is an ethical, legal and scientific imperative [2]. Pain management also represents refinement as one of the 3Rs (reduce, refine, and replace) and it is each researcher’s responsibility to assure that research animals receive the proper analgesia during procedures to reduce and minimize pain.
Non-steroidal anti-inflammatory drugs (NSAIDs), opioids and local analgesics are used to control postoperative pain in rodents and other species in veterinary medicine [3]. There has been considerable research focused on the use of NSAIDs as an alternative to opioids in veterinary medicine for the treatment of acute and chronic pain in rats as they are not controlled drugs and are readily available [4], [5]. Carprofen, an NSAID, has been shown to provide sufficient analgesia to alleviate acute post-operative pain in rats that underwent laparotomies [6]. NSAIDs, however, can have adverse side effects including diarrhea, anorexia, gastrointestinal ulcers and renal effects [7]. Opioids, such as buprenorphine, have been shown to provide effective analgesia following laparotomy in rats [4]. Opioids are regulated by the Drug Enforcement Agency (DEA), which controls their possession, storage, and usage. Opioids can also cause adverse side effects including sedation, constipation and respiratory depression [8]. In rats, buprenorphine has been reported to cause pica [9]. Tramadol, an opioid-like drug, is a centrally acting analgesic agent that is used in the management of pain in human and veterinary medicine [10]. It has a dual mode of action as a mu-opioid agonist as well as a serotonin and norepinephrine reuptake inhibitor, which distinguishes it from other opioids [11], [8]. Tramadol is a drug of interest to the veterinary community because it generally has fewer side effects than more potent opioids [8, 12]. Additionally, it is not controlled drug by the DEA unlike opioid drugs yet provides the analgesic benefits of opioids. Although there are increasing numbers of reports of tramadol’s use in dogs and cats, there is limited literature reporting its clinical use in rats.
Multimodal or balanced analgesia involves the use of two or more analgesic agents to provide additive analgesic effects [13]. It may be beneficial to combine drugs from different analgesic classes as they alter more than one nociceptive pathway, producing a synergistic effect and requiring lower doses than for either drug alone [8]. Previous work by Liles and Flecknell 1994 demonstrated a single dose of buprenorphine (opioid) and carprofen (NSAID) combined was as effective as two separate doses of buprenorphine in a rat laparotomy model [4]. In addition, research conducted in dogs undergoing ovariohysterectomies demonstrated a combination of carprofen and pethidine, an opioid, demonstrated better post-surgical outcome than either drug administered alone [13].
The objective of this study was to compare the analgesia efficacy of tramadol, carprofen, or a combination of tramadol and carprofen (multimodal therapy) in a rat laparotomy model. The parameters measured to assess analgesic efficacy were open field activity, running wheel activity, feed and water consumption, body weight, clinical observations and fecal corticosterone concentration.
Materials and Methods
Animals.
Seventy 7-week-old male Sprague-Dawley rats Crl:CD (SD), weighing 175 to 220 grams, were obtained from Charles River Laboratories (Raleigh, NC). Each rat was individually housed and maintained in an Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC), International accredited facility at the National Institute of Environmental Health Sciences (NIEHS). All animal rooms were maintained under controlled environmental conditions [temperature (72 ± 2°F), relative humidity (40–60%), air exchanges (12–15/h), lighting (12/12 h light/dark cycle)]. Feed, bedding, and water were monitored for microbial pathogens and toxic chemicals, including pesticide residues. Rats received autoclaved NIH-31 diet (Zeigler Brothers, Inc., Gardner, PA) and reverse osmosis/deionized water ad libitum. Rats were housed in polycarbonate microisolator cages with autoclaved hardwood bedding (Sani-Chip®, PJ Murphy Forest Products, Corp., Montville, NJ). Feed, bedding, and water were monitored for microbial pathogens and toxic chemicals, including pesticide residues. Each rat received an autoclaved paper towel as environmental enrichment. The rats were allowed to acclimate for one week and were tattooed for identification. Each rat was handled daily and play tickling was mimicked by making rapid finger movements across the nape to acclimate them to manipulation and restraint[14]. All rats were from colonies that tested negative for the following viral agents: Sendai virus, pneumonia virus of mice, sialodacryoadenitis virus, Kilham rat virus, H1 virus, rat minute virus, reovirus, rat theilovirus, lymphocytic choriomeningitis virus, hantavirus, mouse adenovirus, rat parvovirus, and rat respiratory virus. In addition, the colonies were free of the following bacterial and fungal agents: Bordetella bronchiseptica, Clostridium piliformis, Corynebacterium kutscheri, Mycoplasma pulmonis, Salmonella spp., Streptobacillus moniliformis, Streptococcus pneumoniae, Helicobacter hepaticus, Helicobacter bilis and other Helicobacter spp., Klebsiella oxytoca, Klebsiella pneumoniae, Pasteurella multocida, Pasteurella pneumotropica, Pasteurella aeruginosa, Staphylococcus aureus, and beta-hemolytic Streptococcus spp. Animals were also free of endoparasites and ectoparasites. All procedures were reviewed and approved by the NIEHS Animal Care and Use Committee.
Experimental design.
Rats (10/group) were randomly allocated to one of the following seven treatment groups: Tramadol (12.5 mg/kg) no surgery (NSx-T), tramadol (12.5 mg/kg) surgery (Sx-T), carprofen (5 mg/kg) no surgery (NSx-C), carprofen (5 mg/kg) surgery (Sx-C), tramadol (12.5 mg/kg) + carprofen (5 mg/kg) no surgery (NSx-TC), tramadol (12.5 mg/kg) + carprofen (5 mg/kg) surgery (Sx-TC), or control saline no surgery (NSx-S). Carprofen was obtained from Pfizer Animal Health (New York, NY) and tramadol was obtained from Triangle Compounding Pharmacy (Cary, NC). All compounds were given 10 minutes prior to anesthesia, 4 hours after surgery (PM treatment) and the following morning (AM treatment). Tramadol and saline were given intraperitoneally (IP) and carprofen given subcutaneously (SC). All rats were anesthetized with 2% isoflurane in oxygen and placed in dorsal recumbency. Each rat undergoing surgery had its hair clipped and its skin aseptically prepared with betadine and alcohol for a ventral laparotomy. Ventral laparotomies were performed by 2 veterinary surgeons. Starting at the caudal sternum, a 3 cm skin incision was made using a #10 scalpel. A second incision, 2.5 cm in length, was made through the linea alba into the abdominal cavity. A sterile cotton swab tip was moistened with sterile saline, inserted into the abdomen and rotated 5 times within the abdominal viscera to mimic visceral manipulation. The surgeon then waited 30 seconds and repeated the 5 rotation visceral manipulation. The linea alba was closed using 4–0 Vicryl (Ethicon, Cincinnati, OH) with 5 sutures evenly spaced. The skin incision was closed with 5 EZ clip staples (Stoelting Co., Wood Dale, IL). The rats not receiving surgery were kept anesthetized for 5 minutes and then moved back to their cage for recovery. All rats were recovered from anesthesia in their cages on a hot water pad set at 100°F.
Clinical observation.
An observer, blinded to the analgesia treatment groups, evaluated each rat in their home cage at 2, 12, and 24 hours post-surgery. Animals were observed post-surgery for clinical signs of pain and distress including hunched posture, back-arching, ruffled hair coat, writhing, stretching, twitching, inappetance, lethargy, falling/staggering, poor gait and swelling and/or bleeding at the incision site [15], [6].
Running wheel activity.
Three days prior to surgery, each rat was transferred to a cage with free access to a running wheel. Wheel revolutions were recorded and tallied using commercially available equipment and software (VitalView, Mini Mitter, Bend, OR). The primary measure of wheel activity, amplitude, was the maximum hourly wheel rotations per day. The number of wheel revolutions each hour was recorded continuously 3 days before surgery through 2 days after surgery. Baseline wheel activity was defined as the average of the 3 pre-surgery days of activity.
Locomotor activity.
Locomotor activity was measured in an open field chamber using commercially available instrumentation and software (Opto-Max, Columbus Instruments, Columbus, OH). Each chamber was constructed of clear Plexiglas (40cm x 40cm x 20cm). Photocells were located 1.2 cm apart on the walls around the chamber. The photocells emitted infrared beams and the number of beam interruptions associated with grooming, exploring, or movement was recorded a total of 30 minutes for each session. Locomotor activity was measured 1 day before (baseline) and 1 day after surgery.
Feed and water consumption.
Feed and water consumption was recorded daily from 1 day before (baseline) surgery through 2 days after surgery.
Body weight.
Body weight was recorded 1 day before (baseline) and 1 day and 3 days after surgery.
Fecal corticosterone.
Corticosterone concentration was measured in the feces using a corticosterone enzyme immunoassay kit (Stressgen Assay Designs, Ann Arbor, MI) at the Endocrinology Laboratory at the Brookfield Zoo (Brookfield, IL). Two fecal pellets were collected daily 2 days before surgery (baseline) and daily through 2 days after surgery, except on the day of surgery. Each collection was individually analyzed using the immunoassay kit. All rats were placed in clean cages after surgery to assure the pellets collected represented post-surgery samples.
A summary timeline of data collection during the study is shown in Table 1.
Table 1.
Data Collection Timeline
| Parameter | Day -3 | Day -2 | Day -1 | Day 0 | Day 1 | Day 2 | Day 3 |
|---|---|---|---|---|---|---|---|
| (Pre-surgery) | (Pre-surgery) | (Pre-surgery) | (Surgery Day pm) | (Post- Surgery) | (Post-surgery) | (Post-surgery) | |
| Running Wheel Activity | X | X | X |
X | X | X | |
| Locomotor Activity | X | X | |||||
| Feed and Water Consumption | X | X | X | X | |||
| Body Weight | X | X | X | ||||
| Fecal Corticosterone | X | X | X | X |
Necropsy and histology
All rats were euthanized 3 days after the day of surgery with an overdose of pentobarbital intraperitoneally and tissues collected in 10% neutral buffered formalin and processed for histological examination with hematoxylin and eosin (H&E) stain. Histology was performed on skin, lungs, heart, kidneys, liver, and spleen to look for drug effect differences among treatment groups.
Data processing and statistical analysis
Body weights, locomotor activity and log corticosterone levels, which were normally distributed, were compared across treatment groups by analysis of variance (ANOVA) and two-sample t-tests; differences across days were tested using repeated measures ANOVA and paired t-tests [16]. Food and water consumption, which were not normally distributed, were compared across treatment groups using nonparametric ANOVA (Kruskal-Wallis ANOVA) and Mann-Whitney tests [17]. To assess changes between days, the Wilcoxon signed ranks test, a nonparametric analog of the paired t-test, was used.
Running wheel activity over each 24 hour cycle was modeled using a sigmoidally transformed cosine curve described by Marler et al. [18]. The amplitude or maximum hourly number of wheel rotations, as estimated by this model, was the primary running wheel endpoint. These data were not normally distributed, so Kruskal-Wallis ANOVA and Mann-Whitney tests were used to compare treatment groups and the Wilcoxon signed ranks test was used to compare days within the same treatment group. Other endpoints available from this sigmoidal model were the time at which activity increased or decreased, the rate at which activity increased or decreased, the length of time each animal was active or inactive, and the time at which the maximum activity occurred.
Results
Locomotor Activity.
Figure 1 shows the mean and standard error of total locomotor activity in an open field pre-surgery and post-surgery for each group. There were no significant differences in pre-surgery locomotor activity levels among the treatment groups. On day 1 post-surgery, the Sx-TC group had significantly more activity than the NSx-TC group. The Sx-T group had a significant (p<0.05) decrease in locomotor activity on day 1 post-surgery compared to their pre-surgery baseline, which was not seen in the other surgery groups (Sx-C and Sx-TC). Among the non-surgery groups, the NSx-C and NSx-TC groups had significant decreases in locomotor activity on day 1 post-surgery compared to their pre-surgery baseline levels.
Figure 1. Mean and standard error of locomotor activity in an open field pre-surgery and day 1 post-surgery.
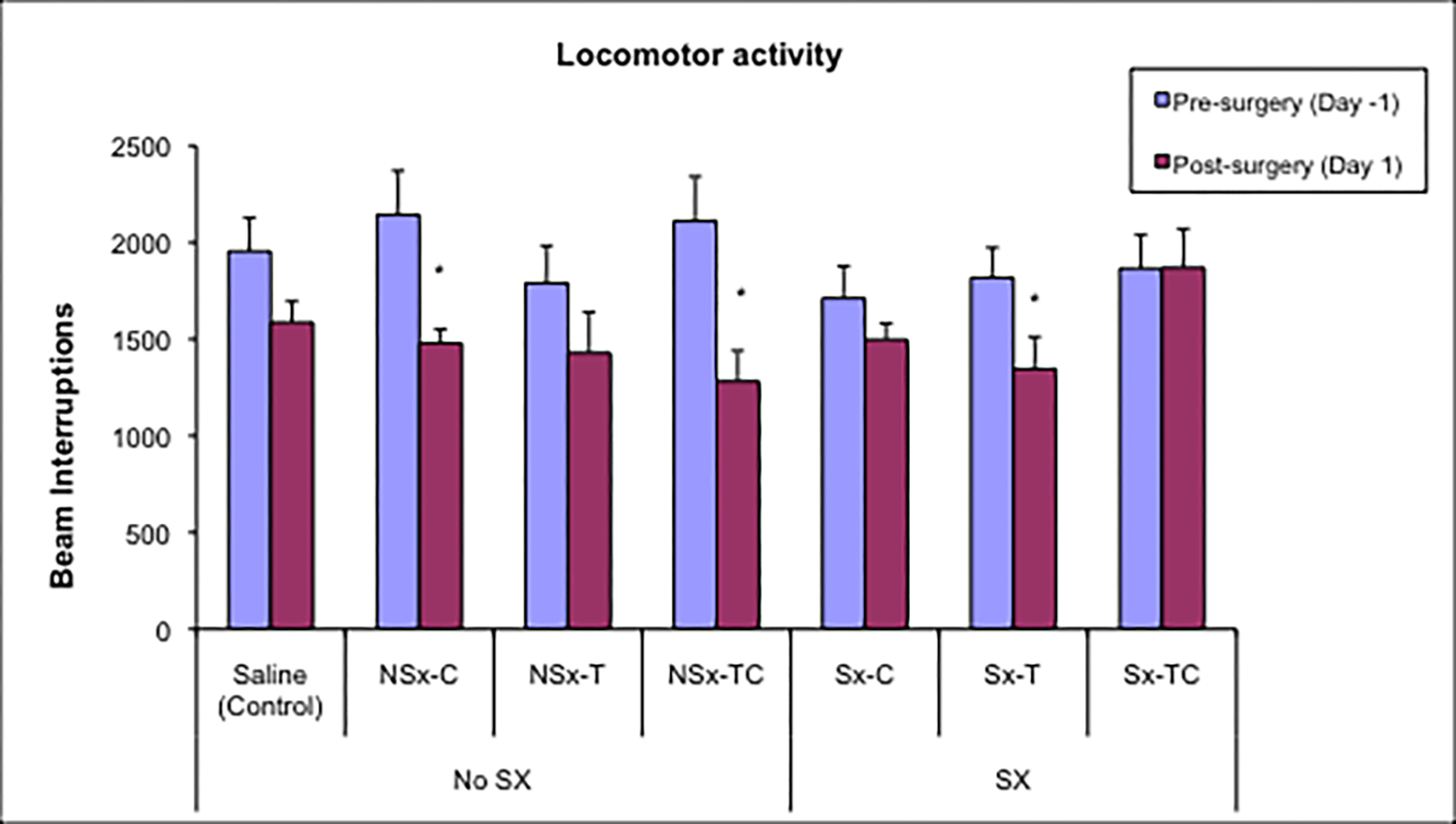
* Day 1 post-surgery differs significantly from pre-surgery baseline level at p<0.05
Running Wheel Activity.
Figure 2 shows the amplitude of wheel activity of the groups. Pre-surgery, there were no significant differences among the groups. All surgery groups had decreased wheel activity post-operatively on the day of surgery as compared to their baseline activity levels. On day 1 post-surgery, the Sx-C and Sx-TC groups continued to have decreased wheel activity compared to baseline, and activity returned to baseline levels by day 2 post-surgery. Wheel activity in the NSx-T group was increased 2 days post-surgery compared to baseline. The Sx-T group started running later and stopped earlier on the day of surgery and each day post-surgery as compared to baseline and the saline control (data not shown).
Figure 2. Amplitude of wheel activity among treatment groups.
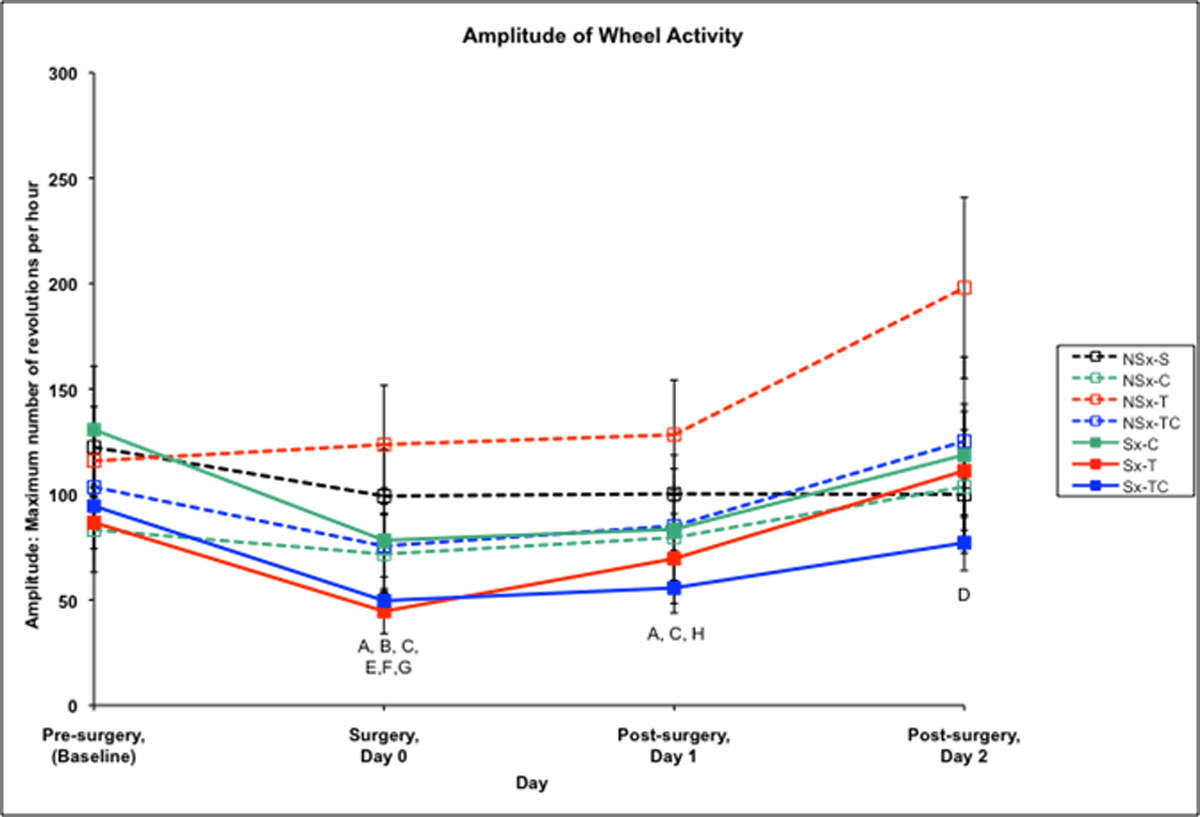
A-Sx-C group differs from baseline at p<0.05
B-Sx-T group differs from baseline at p<0.05
C-Sx-TC group differs from baseline at p<0.05
D-NSx-T group differs from baseline at p<0.05
E-NSx-TC group differs from baseline at p<0.05
F-Saline group differs from baseline at p<0.05
G-Sx-T group differs from saline control at p<0.05
H-Sx-TC differs from saline control at p<0.05
Body weight.
There were no significant differences in body weights among groups at any time point. Within each treatment group, body weights increased significantly from one time point to the next (p<0.01, data not shown).
Feed consumption.
Feed consumption averaged 19.7 grams/rat/day pre-surgery for all groups. Among the surgery groups, feed consumption was significantly decreased on the day of surgery compared to their baseline levels in both the Sx-T and Sx-TC groups (p<0.05). On day 1 post-surgery, all surgery groups (Sx-C, Sx-T, and Sx-TC) were significantly decreased compared to saline control. On day 2 post-surgery, feed consumption in all surgery groups (Sx-T, Sx-C, and Sx-TC) did not differ from baseline however Sx-TC and NSx-C were significantly increased compared to saline control. Feed consumption in the non-surgery drug groups did not differ significantly from baseline at any of the post-surgery days.
Water consumption.
Pre-surgery water consumption averaged 21.2 ml/rat/day pre-surgery for all groups. Among non-surgery groups, water consumption significantly increased in the NSx-T and NSx-TC groups on the day of surgery compared to their baseline levels. All non-surgery groups had increased water consumption on day 2 post-surgery compared to baseline. Among the surgery groups, water consumption was significantly increased in Sx-C and Sx-T groups on day 2 post-surgery compared to baseline.
Clinical Observations.
No clinical signs of pain were observed in the non-surgery drug groups at any time during the study. The Sx-T group appeared sedated post-surgery (day of surgery and day 1 post-surgery). All groups appeared to be normal by day 2 post-surgery.
Fecal Corticosterone.
No significant differences in corticosterone levels were seen across treatment groups or between pre-surgery and post-surgery.
Necropsy.
Skin Incision.
In the surgery groups, no histopathological differences were noted in the skin between the analgesic treatment groups in regards to severity of inflammation and degree of healing. Minimal to moderate exudate and cell inflammation was present at the epidermal surgical site across all surgery groups.
Liver.
Minimal lesions were observed in the liver and appeared unrelated to analgesia or surgery.
Kidneys.
Minimal lesions were observed in the kidney and appeared unrelated to analgesia or surgery.
Discussion
The multimodal therapeutic approach potentially enables increased analgesic effects while decreasing the required dosage of each individual drug, leading to potential decrease in individual drug side effects [19]. NSAIDs provide longer lasting analgesia while opioids provide more euphoric effects to relieve anxiety and stress [7]. The combination of carprofen and tramadol was chosen for this multimodal therapy evaluation in rats because the combination of opioids and NSAIDs has been used successfully in human and veterinary patients to control postoperative pain [20], [21], [22], [19]. Tramadol, an opioid-like drug, was chosen for clinical evaluation because there is limited literature reporting its clinical use in rats. In addition, it is not controlled by the DEA and has fewer side effects than most opioids [23], [11]. Carprofen was chosen for evaluation because it has been shown to provide analgesia post-operatively and is the current drug of choice for analgesia in our laboratory animal program [4–6]. This is the first study to evaluate the effects of multimodal therapy using tramadol and carprofen in a rat surgery model.
Body weight, locomotor activity, feed and water consumption have all been shown to be depressed following a surgical procedure due to surgical stress and postoperative pain [24],[25],[26],[27]. Reduction in feed and water consumption may further exacerbate the stress response to surgery [5]. These parameters were used, in addition to running wheel activity, clinical observations and fecal corticosterone levels, as a measure of analgesia among both surgery and non-surgery treatment groups. The Sx-T group showed decreased open field locomotor activity on day 1 post-surgery compared to pre-surgery which may suggest the rats experienced some level of postoperative pain. All surgery groups (Sx-C, Sx-T, and Sx-TC) showed decreased running wheel activity on the day of surgery and running wheel activity remained decreased in the Sx-C and Sx-TC groups 1 day post-surgery, compared to their respective baseline levels. Decreased running wheel activity on the day of surgery was also observed in the NSx-TC and saline control groups, which may explained by the effects of anesthesia however this was not observed in other groups. As a group, the Sx-T rats started running later on days 0, 1, and 2 post-surgery compared to their baseline levels. This activity pattern may be explained by a lack of analgesia effect, forcing the rats to delay activity levels and stop activity sooner or by the tramadol sedative effect that was reported in clinical observations made post-surgery. Feed consumption was significantly decreased in two of the surgery groups, Sx-T and Sx-TC, on the day of surgery compared to baseline which may suggest rats were experiencing some postoperative pain, as reported by Flecknell et al. [24]. All surgery groups (Sx-T, Sx-C, and Sx-TC) had significantly decreased feed consumption day 1 post-surgery compared to the saline control. By 2 days post-surgery, however, the Sx-TC group had significantly increased feed consumption compared to the saline control group, which may suggest the rats had recovered from surgery and perhaps consuming increased amount of feed to make up for the decreased feed consumption previously seen the day of surgery and day 1 post-surgery. These findings suggest that rats in the tramadol treatment surgery groups may not have had adequate levels of analgesia as both locomotor activity and feed consumption were decreased post-surgery. There were no significant differences in body weights among the 7 groups. However, as might be expected, body weights differed significantly from one time point to the next within each group as male Sprague-Dawley (Crl:CD (SD)) rats of this age are still growing. Necropsy demonstrated no differences in severity of inflammation or degree of healing at skin incision sites among the surgery groups. None of the lesions from any of the examined tissues were considered analgesic-exposure related.
The tramadol dose of 12.5 mg/kg was selected based on a pilot study that evaluated dosage and route of administration using the hot plate and tail flick assays (to be reported). In the current study, analgesics were evaluated in a rat surgery model because surgical procedures are accompanied by tissue damage and may change nociceptive threshold. Additionally, the analgesic requirements for relieving postoperative pain may differ from that needed to inhibit nociceptive reflex in the absence of injury [28]. Clinical observations made in the surgery and non-surgery tramadol groups indicated sedative effects the day of surgery. Drug-induced sedation can mask the presence of pain by dampening the overt signs even though pain may not be attenuated [3]. Sedation was also observed in a previous pilot study, particularly at tramadol dosages of 25 and 50 mg/kg IP and SC (to be reported). A similar finding was reported at 15 mg/kg IP tramadol when evaluating motor function in rodents using the rotarod [29]. Tramadol may not provide effective analgesia in a rat laparotomy model due to a high first-pass effect, low plasma protein binding and the possible requirement for more frequent dosing intervals [30], [7].
The carprofen dose of 5 mg/kg was selected on the basis of previous studies of NSAIDs in rat laparotomy models [31], [4]. The use of NSAIDs in laboratory animal medicine is advantageous as they are not controlled drugs [28]. Carprofen has been shown to provide sufficient analgesia in acute post-operative pain in dogs [32], [3]. Liles and Flecknell (1994) demonstrated rats that underwent laparotomy had less reduction in food and water intake and body weight when treated with carprofen at 5 mg/kg (once daily or four times daily) or 10 mg/kg SC (once daily) and that feed intake was significantly greater compared to saline control in the rats that received a single dose of carprofen at either 5 or 10 mg/kg pre-surgery [4]. Carprofen has a half-life of 8 hours, however research by Mathews demonstrated that administering it every 24 hours in dogs was effective [33]. Carprofen has a long duration of action and has been shown to be effective for up to 18 hours in dogs [34]. The long duration of effect may be due to its ability to better penetrate inflamed tissue than healthy tissue [35]. Carprofen has a COX-1 sparing effect and does not inhibit prostaglandin synthesis like other NSAIDs, and therefore has been shown to produce fewer gastrointestinal lesions versus aspirin [36].
The multimodal combination of 12.5 mg/kg tramadol and 5 mg/kg carprofen appeared to provide some level of analgesia, as locomotor activity level remained the same from pre-surgery to post-surgery in the open field test. The multimodal group also had increased food consumption compared to saline control by day 2 post-surgery. Slingsby et al. previously demonstrated the combination of another opioid, pethidine, together with carprofen provided the same analgesia effect as carprofen alone in dogs that underwent neutering [13]. The opioid provided a shorter duration of action versus caprofen [34]. Liles et al. demonstrated the combination of carprofen 5 mg/kg and buprenorphine 0.05 mg/kg resulted in increased body weight, locomotor activity, and food and water intake among rats that received laparotomy compared to saline controls, and that this was as effective as two doses of buprenorphine 0.05 mg/kg [4].
Carprofen 5 mg/kg SC given once before surgery and at 12 and 24 hours post-surgery appeared more effective in controlling pain in a rat laparotomy model compared to tramadol 12.5 mg/kg IP given at the same time intervals. The use of the combination of carprofen 5 mg/kg SC and tramadol 12.5 mg/kg IP, known as multimodal therapy, provided improved analgesic effect compared to tramadol alone but not compared to carprofen alone. Further research is needed to evaluate the use of other potential drug combinations using the multimodal analgesia approach.
Figure 3. Feed consumption (g/day) among treatment groups.
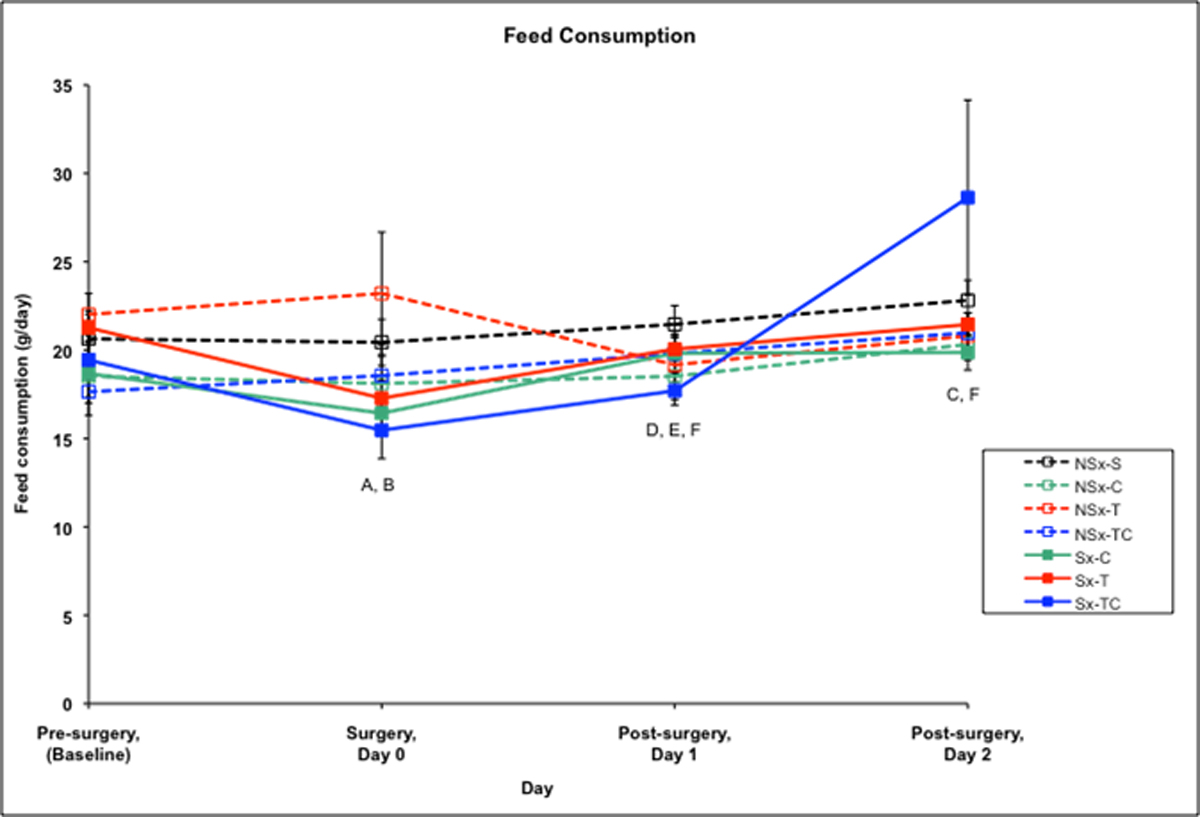
A-Sx-T group differs from baseline at p<0.05
B-Sx-TC group differs from baseline at p<0.05
C-NSx-C group differs from baseline at p<0.05
D-Sx-C group differs from saline control at p<0.05
E-Sx-T group differs from saline control at p<0.05
F-Sx-TC group differs from saline control at p<0.05
Figure 4. Water consumption (ml/day) among treatment groups.
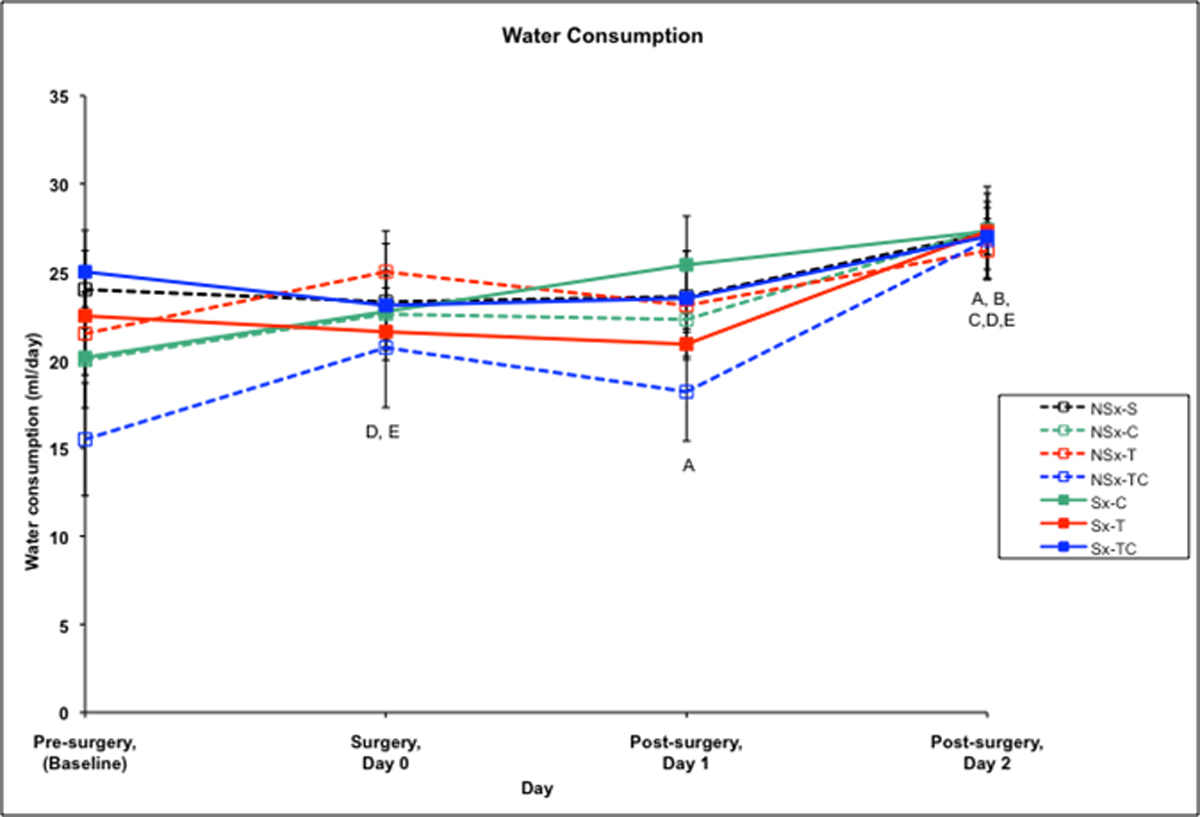
A-Sx-C group differs from baseline at p<0.05
B-Sx-T group differs from baseline at p<0.05
C-NSx-C group differs from baseline at p<0.05
D-NSx-T group differs from baseline at p<0.05
E-NSx-TC group differs from baseline at p<0.05
Acknowledgements
We thank Ms. Danielle Waxer, Mr. James Clark, Ms. Page Myers, Ms. Sandy Hackney, Mr. Joe Hensley, and Mr. Rodney Chavis for their assistance, support and care of the research and the animals, Dr. Paul Flecknell for his consultation, Dr. Larry Wright for his assistance in literature evaluation, The Brookfield Zoo Endocrinology Lab, Dr. Mark Cesta for pathology and Dr. Gregory Cannon. This research was supported by the Intramural Research Program of the NIH and the NIEHS.
References
- 1.AWA, AWA regulations, CFR, Title 9 (Animals and Animal Products), Chapter 1. Animal Welfare Act. 2005. [Google Scholar]
- 2.NRC, Institutional Policies and Responsibilities. Guide for the Care and Use of Laboratory Animals, ed. I.o.L.A. Resources. 1996, Washington D.C.: National Academy Press. [Google Scholar]
- 3.Lascelles BD, Butterworth SJ, and Waterman AE, Postoperative analgesic and sedative effects of carprofen and pethidine in dogs. Vet Rec, 1994. 134(8): p. 187–91. [DOI] [PubMed] [Google Scholar]
- 4.Liles JH and Flecknell PA, A comparison of the effects of buprenorphine, carprofen and flunixin following laparotomy in rats. J Vet Pharmacol Ther, 1994. 17(4): p. 284–90. [DOI] [PubMed] [Google Scholar]
- 5.Flecknell PA, Orr HE, Roughan JV, and Stewart R, Comparison of the effects of oral or subcutaneous carprofen or ketoprofen in rats undergoing laparotomy. Vet Rec, 1999. 144(3): p. 65–7. [DOI] [PubMed] [Google Scholar]
- 6.Roughan JV and Flecknell PA, Behaviour-based assessment of the duration of laparotomy-induced abdominal pain and the analgesic effects of carprofen and buprenorphine in rats. Behav Pharmacol, 2004. 15(7): p. 461–72. [DOI] [PubMed] [Google Scholar]
- 7.Papich M, Pharmacological considerations for Opiate Analgesic and Nonsteroidal Anti inflammatory Drugs. Veterinary Clinics of North America: Small Animal Practice. Vol. 30. 2000. [DOI] [PubMed] [Google Scholar]
- 8.Guneli E, Yavasoglu NUK, Apaydin S, Uyar M, and Uyar M, Analysis of the antinociceptive effect of systemic administration of tramadol and dexmedetomidine combination on rat models of acute and neuropathic pain. Pharmacology Biochemistry and Behavior, 2007. 88(1): p. 9–17. [DOI] [PubMed] [Google Scholar]
- 9.Clark JA Jr., Myers PH, Goelz MF, Thigpen JE, and Forsythe DB, Pica behavior associated with buprenorphine administration in the rat. Lab Anim Sci, 1997. 47(3): p. 300–3. [PubMed] [Google Scholar]
- 10.Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, and Vaught JL, Opioid and Nonopioid Components Independently Contribute to the Mechanism of Action of Tramadol, an Atypical Opioid Analgesic. Journal of Pharmacology and Experimental Therapeutics, 1992. 260(1): p. 275–285. [PubMed] [Google Scholar]
- 11.Papich M, Tramadol. 2nd ed. Saunders Handbook of Veterinary Drugs. 2007, St. Louis: Saunders Elsevier. [Google Scholar]
- 12.Raffa R, The Basic Science Aspect of Tramadol Hydrochloride. Pain Rev, 1996. 3: p. 249–271. [Google Scholar]
- 13.Slingsby LS and Waterman-Pearson AE, Analgesic effects in dogs of carprofen and pethidine together compared with the effects of either drug alone. Vet Rec, 2001. 148(14): p. 441–4. [DOI] [PubMed] [Google Scholar]
- 14.Cloutier S and Newberry R, Tickled Pink: Playful handling as social enrichment for laboratory rats. AWI Quarterly, 2009. 58: p. 24–25. [Google Scholar]
- 15.Roughan JV and Flecknell PA, Evaluation of a short duration behaviour-based post-operative pain scoring system in rats. Eur J Pain, 2003. 7(5): p. 397–406. [DOI] [PubMed] [Google Scholar]
- 16.Neter J, Jutner M, Nachtsheim C, and Wasserman W, Applied Linear Statistical Models. Fourth Edition ed. 1996, Boston: WCB McGraw-Hill. [Google Scholar]
- 17.Conover W, Practical Nonparametric Statistics, ed. I. John Wiley and Sons. 1971, New York. [Google Scholar]
- 18.Marler M, Gehrman P, Martin J, and Ancoli-Israel S, The sigmoidally transformed cosine curve: a methematical model for circadian rhythms with symmetric non-sinusoidal shapes. Statistics in Medicine, 2006. 25: p. 3893–3904. [DOI] [PubMed] [Google Scholar]
- 19.Picard P, Bazin J, Conio N, Ruiz F, and Schoeffler P, Ketorolac potentiates morphine in postoperative patient-controlled analgesia. Pain, 1997. 73(3): p. 401–406. [DOI] [PubMed] [Google Scholar]
- 20.McQuay HJ, Carroll D, Watts PG, Juniper RP, and Moore RA, Codeine 20 mg increases pain relief from ibuprofen 400 mg after third molar surgery. A repeat-dosing comparison of ibuprofen and an ibuprofen-codeine combination. Pain, 1989. 37(1): p. 7–13. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson T, Rude C, Randberg F, Johansen T, Lang-Jensen T, and Jensen N, Postoperative pain treated with piroxicam and buprenorphine, each drug alone or in a combination. Pain, 1990. 41(Supplement 1): p. S144. [Google Scholar]
- 22.Wideman GL, Keffer M, Morris E, Doyle RT Jr., Jiang JG, and Beaver WT, Analgesic efficacy of a combination of hydrocodone with ibuprofen in postoperative pain. Clin Pharmacol Ther, 1999. 65(1): p. 66–76. [DOI] [PubMed] [Google Scholar]
- 23.DEA. U.S. Drug Enforcement Administration. Tramadol; 2010; Available from: http://www.justice.gov/dea. [Google Scholar]
- 24.Flecknell P and Liles J, Evaluation of locomotor activity and food and water consumption as a method of assessing post-operative pain in rodents. Animal Pain, ed. Short Cand Van Poznak A. 1991, New York: Churchill Livingstone. [Google Scholar]
- 25.Flecknell PA and Liles JH, The effects of surgical procedures, halothane anaesthesia and nalbuphine on locomotor activity and food and water consumption in rats Lab Anim, 1991. 25(1): p. 50–60. [DOI] [PubMed] [Google Scholar]
- 26.Krugner-Higby L, Smith L, Clark M, Heath TD, Dahly E, Schiffman B, Hubbard-VanStelle S, Ney D, and Wendland A, Liposome-encapsulated oxymorphone hydrochloride provides prolonged relief of postsurgical visceral pain in rats. Comp Med, 2003. 53(3): p. 270–9. [PubMed] [Google Scholar]
- 27.Clark MD, Krugner-Higby L, Smith LJ, Heath TD, Clark KL, and Olson D, Evaluation of liposome-encapsulated oxymorphone hydrochloride in mice after splenectomy. Comp Med, 2004. 54(5): p. 558–63. [PubMed] [Google Scholar]
- 28.Stewart LSA and Martin WJ, Evaluation of postoperative analgesia in a rat model of incisional pain. Contemporary Topics in Laboratory Animal Science, 2003. 42(1): p. 28–34. [PubMed] [Google Scholar]
- 29.Loram L, Mitchell D, Skosana M, and Fick L, Tramadol is more effective than morphine and amitriptyline against ischaemic pain but not thermal pain in rats. Pharmacological Research, 2007. 56: p. 80–85. [DOI] [PubMed] [Google Scholar]
- 30.Wu WN, McKown LA, Codd EE, and Raffa RB, In vitro metabolism of the analgesic agent, tramadol-N-oxide, in mouse, rat, and human. European Journal of Drug Metabolism and Pharmacokinetics, 2002. 27(3): p. 193–197. [DOI] [PubMed] [Google Scholar]
- 31.Liles JH and Flecknell PA, The effects of surgical stimulus on the rat and the influence of analgesic treatment. Br Vet J, 1993. 149(6): p. 515–25. [DOI] [PubMed] [Google Scholar]
- 32.Nolan A and Reid J, Comparison of the postoperative analgesic and sedative effects of carprofen and papaveretum in the dog. Vet Rec, 1993. 133(10): p. 240–2. [DOI] [PubMed] [Google Scholar]
- 33.Mathews KA, Nonsteroidal anti-inflammatory analgesics in pain management in dogs and cats. Can Vet J, 1996. 37(9): p. 539–45. [PMC free article] [PubMed] [Google Scholar]
- 34.Lascelles BD, Cripps PJ, Jones A, and Waterman-Pearson AE, Efficacy and kinetics of carprofen, administered preoperatively or postoperatively, for the prevention of pain in dogs undergoing ovariohysterectomy. Vet Surg, 1998. 27(6): p. 568–82. [DOI] [PubMed] [Google Scholar]
- 35.Lees P, May SA, and Mckellar QA, Pharmacology and Therapeutics of Nonsteroidal Antiinflammatory Drugs in the Dog and Cat. 1. General Pharmacology. Journal of Small Animal Practice, 1991. 32(4): p. 183–193. [Google Scholar]
- 36.Reimer ME, Johnston SA, Leib MS, Duncan RB Jr., Reimer DC, Marini M, and Gimbert K, The gastroduodenal effects of buffered aspirin, carprofen, and etodolac in healthy dogs. J Vet Intern Med, 1999. 13(5): p. 472–7. [DOI] [PubMed] [Google Scholar]


