Abstract
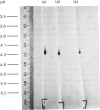
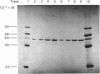
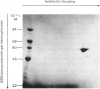
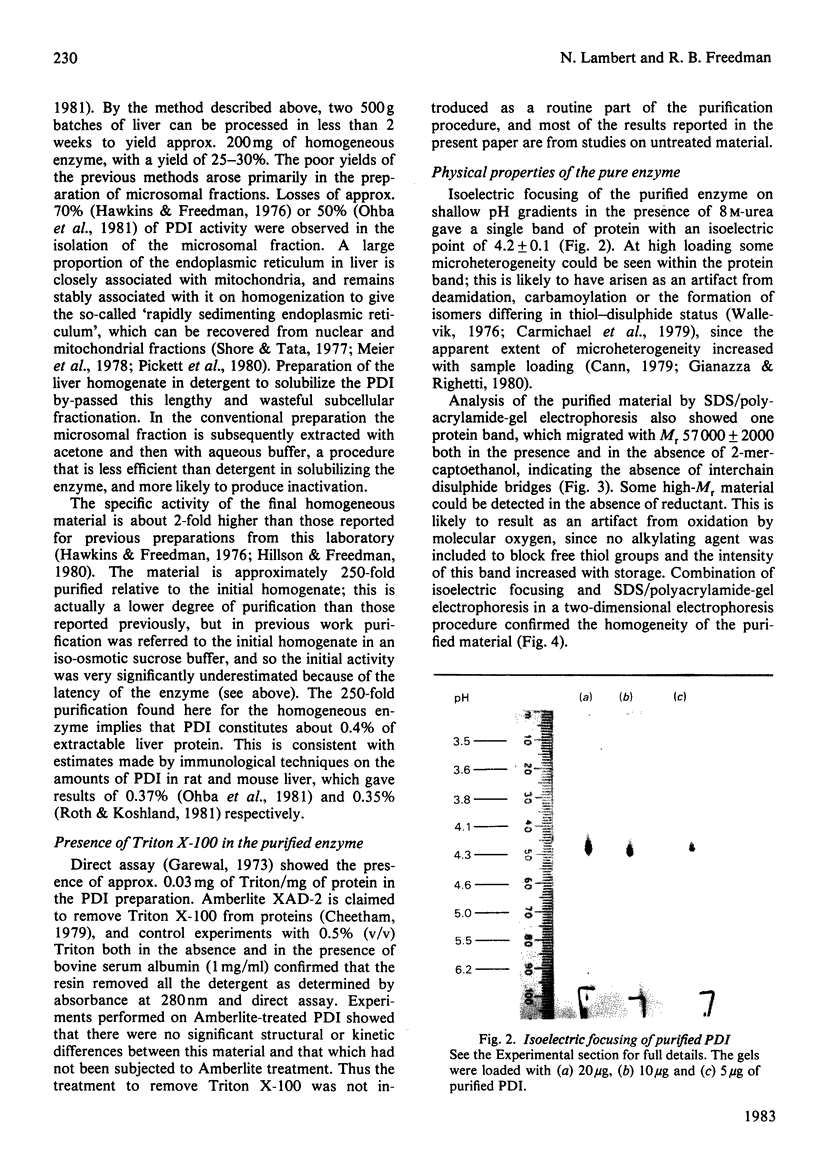
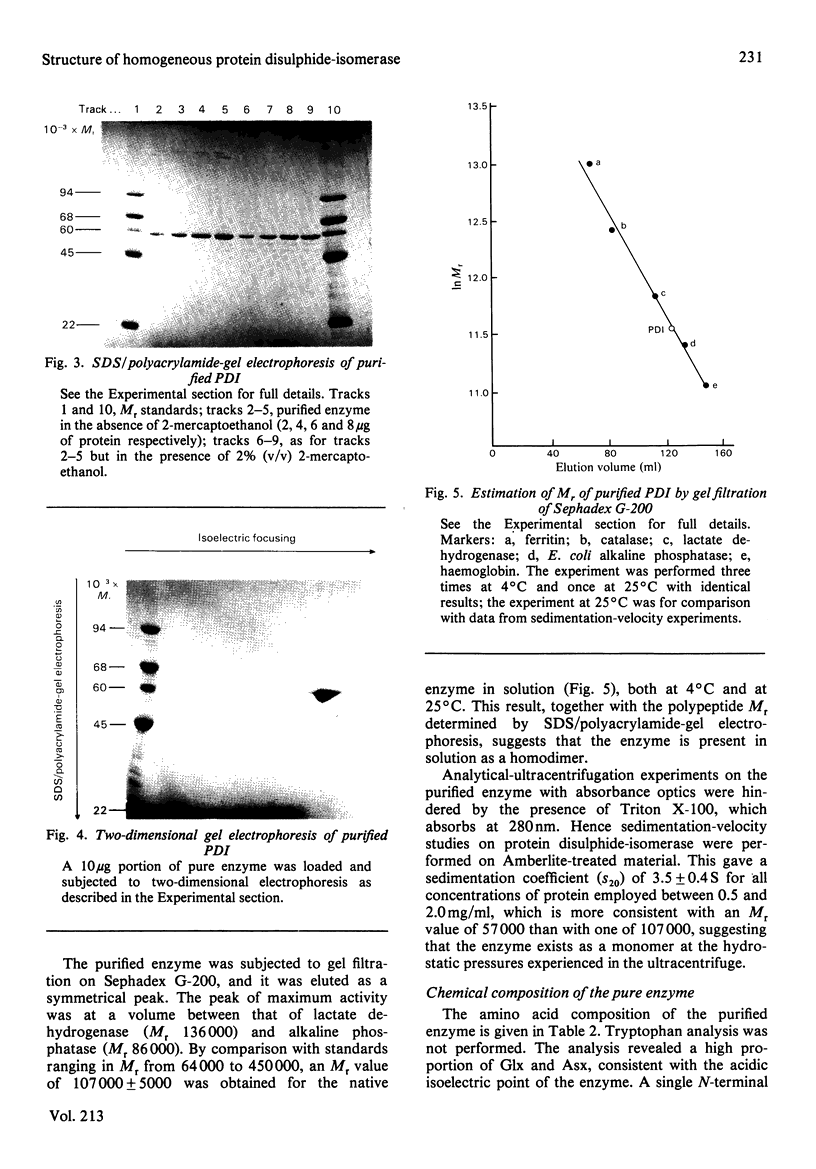
Protein disulphide-isomerase from bovine liver was purified to homogeneity as judged by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, two-dimensional electrophoresis and N-terminal amino acid analysis. The preparative procedure, a modification of that of Carmichael, Morin & Dixon [(1977) J. Biol. Chem. 252, 7163-7167], is much faster and higher-yielding than previous procedures, and the final purified material is of higher specific activity. The enzyme has Mr 57 000 as determined by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, both in the presence and in the absence of thiol compounds. Gel-filtration studies on Sephadex G-200 indicate an Mr of 107 000, suggesting that the native enzyme is a homodimer with no interchain disulphide bonds. Ultracentrifugation studies give a sedimentation coefficient of 3.5S, implying that the enzyme sediments as the monomer. The isoelectric point, in the presence of 8 M-urea, is 4.2, and some microheterogeneity is detectable. The amino acid composition is comparable with previous analyses of this enzyme from bovine liver and of other preparations of thiol:protein disulphide oxidoreductases whose relation to protein disulphide-isomerase has been controversial. The enzyme contains a very high proportion of Glx + Asx residues (27%). The N-terminal residue is His. The pure enzyme has a very small carbohydrate content, determined as 0.5-1.0% by the phenol/H2SO4 assay. Unless specific steps are taken to remove it, the purified enzyme contains a small amount (5 mol/mol of enzyme) of Triton X-100 carried through the purification.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge S., Bohley P., Kirschke H., Langner J., Marquardt I., Wiederanders B., Hanson H. The identity of the insulin degrading thiol-protein disulfide oxidoreductase (glutathione-insulin transhydrogenase) with the sulfhydryl-disulfide interchange enzyme. FEBS Lett. 1973 Dec 1;37(2):238–240. doi: 10.1016/0014-5793(73)80468-5. [DOI] [PubMed] [Google Scholar]
- Ansorge S., Bohley P., Kirschke H., Langner J., Wiederanders B., Hanson H. Metabolism of insulin and glucagon. Glutathione-insulin transhydrogenase from microsomes of rat liver. Eur J Biochem. 1973 Jan 3;32(1):27–35. doi: 10.1111/j.1432-1033.1973.tb02574.x. [DOI] [PubMed] [Google Scholar]
- Brockway B. E., Forster S. J., Freedman R. B. Protein disulphide-isomerase activity in chick-embryo tissues. Correlation with the biosynthesis of procollagen. Biochem J. 1980 Dec 1;191(3):873–876. doi: 10.1042/bj1910873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann J. R. Multibanded isoelectric focusing patterns produced by macromolecular interactions. Methods Enzymol. 1979;61:142–147. doi: 10.1016/0076-6879(79)61012-1. [DOI] [PubMed] [Google Scholar]
- Carmichael D. F., Keefe M., Pace M., Dixon J. E. Interchangeable forms of thiol:protein disulfide oxidoreductase. J Biol Chem. 1979 Sep 10;254(17):8386–8390. [PubMed] [Google Scholar]
- Carmichael D. F., Morin J. E., Dixon J. E. Purification and characterization of a thiol:protein disulfide oxidoreductase from bovine liver. J Biol Chem. 1977 Oct 25;252(20):7163–7167. [PubMed] [Google Scholar]
- Cheetham P. S. Removal of triton X-100 from aqueous solution using amberlite XAD-2. Anal Biochem. 1979 Jan 15;92(2):447–452. doi: 10.1016/0003-2697(79)90683-3. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. Critical values for testing the significance of amino acid composition indexes. Anal Biochem. 1980 Jul 1;105(2):233–238. doi: 10.1016/0003-2697(80)90450-9. [DOI] [PubMed] [Google Scholar]
- De Lorenzo F., Goldberger R. F., Steers E., Jr, Givol D., Anfinsen B. Purification and properties of an enzyme from beef liver which catalyzes sulfhydryl-disulfide interchange in proteins. J Biol Chem. 1966 Apr 10;241(7):1562–1567. [PubMed] [Google Scholar]
- Freedman R. B. How many distinct enzymes are responsible for the several cellular processes involving thiol:protein-disulphide interchange? FEBS Lett. 1979 Jan 15;97(2):201–210. doi: 10.1016/0014-5793(79)80085-x. [DOI] [PubMed] [Google Scholar]
- Freedman R. B., Newell A., Walklin C. M. Paradoxical detergent effects on microsomal protein disulphide isomerase. FEBS Lett. 1978 Apr 1;88(1):49–52. doi: 10.1016/0014-5793(78)80604-8. [DOI] [PubMed] [Google Scholar]
- GIVOL D., GOLDBERGER R. F., ANFINSEN C. B. OXIDATION AND DISULFIDE INTERCHANGE IN THE REACTIVATION OF REDUCED RIBONUCLEASE. J Biol Chem. 1964 Sep;239:PC3114–PC3116. [PubMed] [Google Scholar]
- GOLDBERGER R. F., EPSTEIN C. J., ANFINSEN C. B. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J Biol Chem. 1963 Feb;238:628–635. [PubMed] [Google Scholar]
- GOLDBERGER R. F., EPSTEIN C. J., ANFINSEN C. B. PURIFICATION AND PROPERTIES OF A MICROSOMAL ENZYME SYSTEM CATALYZING THE REACTIVATION OF REDUCED RIBONUCLEASE AND LYSOZYME. J Biol Chem. 1964 May;239:1406–1410. [PubMed] [Google Scholar]
- Garewal H. S. A procedure for the estimation of microgram quantities of triton X-100. Anal Biochem. 1973 Aug;54(2):319–324. doi: 10.1016/0003-2697(73)90359-x. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins H. C., Freedman R. B. Thiol-protein disulphide oxidoreductases. Differences between protein disulphide-isomerase and glutathione-insulin transhydrogenase activities in ox liver. Biochem J. 1976 Nov;159(2):385–393. doi: 10.1042/bj1590385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillson D. A., Freedman R. B. Resolution of protein disulphide-isomerase and glutathione-insulin transhydrogenase activities by covalent chromatography. Biochem J. 1980 Nov 1;191(2):373–388. doi: 10.1042/bj1910373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibbetson A. L., Freedman R. B. Thiol-protein disulphide oxidoreductases. Assay of microsomal membrane-bound glutathione-insulin transhydrogenase and comparison with protein disulphide-isomerase. Biochem J. 1976 Nov;159(2):377–384. doi: 10.1042/bj1590377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janolino V. G., Swaisgood H. E. Isolation and characterization of sulfhydryl oxidase from bovine milk. J Biol Chem. 1975 Apr 10;250(7):2532–2538. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert N., Freedman R. B. Kinetics and specificity of homogeneous protein disulphide-isomerase in protein disulphide isomerization and in thiol-protein-disulphide oxidoreduction. Biochem J. 1983 Jul 1;213(1):235–243. doi: 10.1042/bj2130235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P. J., Spycher M. A., Meyer U. A. Isolation of a subfraction of rough endoplasmic reticulum closely associated with mitochondria. Evidence for its role in cytochrome P450 synthesis. Exp Cell Res. 1978 Feb;111(2):479–483. doi: 10.1016/0014-4827(78)90197-0. [DOI] [PubMed] [Google Scholar]
- Mills E. N., Lambert N., Freedman R. B. Identification of protein disulphide-isomerase as a major acidic polypeptide in rat liver microsomal membranes. Biochem J. 1983 Jul 1;213(1):245–248. doi: 10.1042/bj2130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin J. E., Carmichael D. F., Dixon J. E. Characterization, kinetics and comparative properties of thiol:protein disulfide oxidoreductase. Arch Biochem Biophys. 1978 Aug;189(2):354–363. doi: 10.1016/0003-9861(78)90222-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohba H., Harano T., Omura T. Intracellular and intramembranous localization of a protein disulfide isomerase in rat liver. J Biochem. 1981 Mar;89(3):889–900. doi: 10.1093/oxfordjournals.jbchem.a133272. [DOI] [PubMed] [Google Scholar]
- Ohba H., Harano T., Omura T. Presence of two different types of protein-disulfide isomerase on cytoplasmic and luminal surfaces of endoplasmic reticulum of rat liver cells. Biochem Biophys Res Commun. 1977 Aug 8;77(3):830–836. doi: 10.1016/s0006-291x(77)80053-3. [DOI] [PubMed] [Google Scholar]
- Pickett C. B., Montisano D., Eisner D., Cascarano J. The physical association between rat liver mitochondria and rough endoplasmic reticulum. I. Isolation, electron microscopic examination and sedimentation equilibrium centrifugation analyses of rough endoplasmic reticulum-mitochondrial complexes. Exp Cell Res. 1980 Aug;128(2):343–352. doi: 10.1016/0014-4827(80)90070-1. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Koshland M. E. Role of disulfide interchange enzyme in immunoglobulin synthesis. Biochemistry. 1981 Nov 10;20(23):6594–6599. doi: 10.1021/bi00526a012. [DOI] [PubMed] [Google Scholar]
- Shore G. C., Tata J. R. Two fractions of rough endoplasmic reticulum from rat liver. I. Recovery of rapidly sedimenting endoplasmic reticulum in association with mitochondria. J Cell Biol. 1977 Mar;72(3):714–725. doi: 10.1083/jcb.72.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Bowen J., Tewksbury D., Johnson D., Pannell R. Isolation of albumin from whole human plasma and fractionation of albumin-depleted plasma. Biochem J. 1976 Aug 1;157(2):301–306. doi: 10.1042/bj1570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENETIANER P., STRAUB F. B. THE MECHANISM OF ACTION OF THE RIBONUCLEASE-REACTIVATING ENZYME. Biochim Biophys Acta. 1964 Jul 8;89:189–190. doi: 10.1016/0926-6569(64)90123-3. [DOI] [PubMed] [Google Scholar]
- VENETIANER P., STRAUB F. B. The enzymic reactivation of reduced ribonuclease. Biochim Biophys Acta. 1963 Jan 8;67:166–168. doi: 10.1016/0006-3002(63)91812-2. [DOI] [PubMed] [Google Scholar]
- Varandani P. T. Insulin degradation. XII. The amino acid composition, amino-terminal, and carbohydrate content of beef pancreatic glutathione-insulin transhydrogenase. Biochim Biophys Acta. 1974 Dec 18;371(2):577–581. [PubMed] [Google Scholar]
- Wallevik K. SS-interchanged and oxidized isomers of bovine serum albumin separated by isoelectric focusing. Biochim Biophys Acta. 1976 Jan 20;420(1):42–56. doi: 10.1016/0005-2795(76)90343-3. [DOI] [PubMed] [Google Scholar]