Abstract
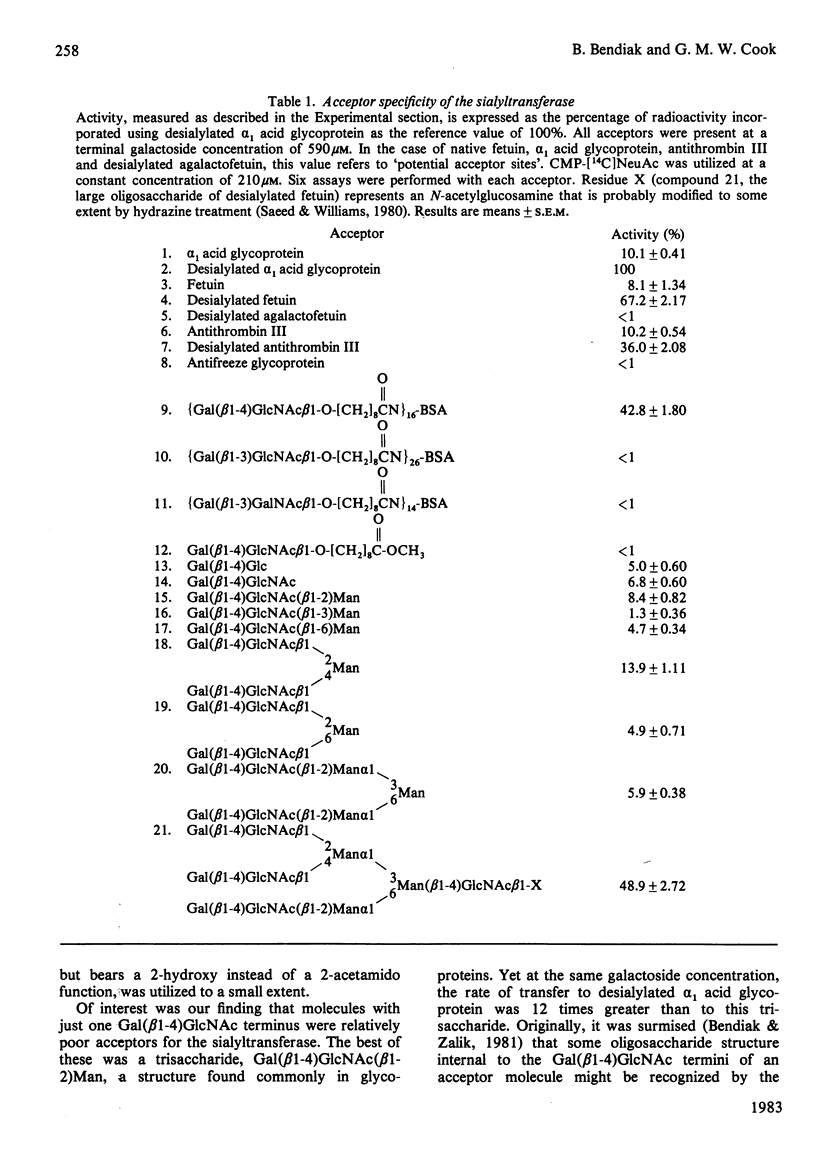
Using a number of branched and unbranched oligosaccharides, glycoproteins and artificial glycoproteins bearing Gal(beta 1-4)GlcNAc-R termini as acceptors (where R represents H, oligosaccharide, oligosaccharide-protein or fatty acid-protein), the comparative rates of transfer of NeuAc by the Gal(beta 1-4)GlcNAc(NeuAc-Gal) (alpha 2-6)-sialyltransferase of embryonic chicken liver were determined. Acceptor substrates were utilized at levels approximating physiological, near the Km value of the best acceptor, desialylated alpha 1 acid glycoprotein. The sialyltransferase has a marked preference for multi-branched acceptors. From the specificity data, it is concluded that the enzyme binds at least two Gal(beta 1-4)GlcNAc termini of an acceptor molecule, and that the relative orientation of the branches is an important factor determining the rate of catalysis by the enzyme. The use of oligosaccharides as acceptors to study sialyltransferase catalyses is emphasized. Results are discussed in the context of the mode of assembly of sialoside termini of known glycoprotein structures in vivo.
Full text
PDF

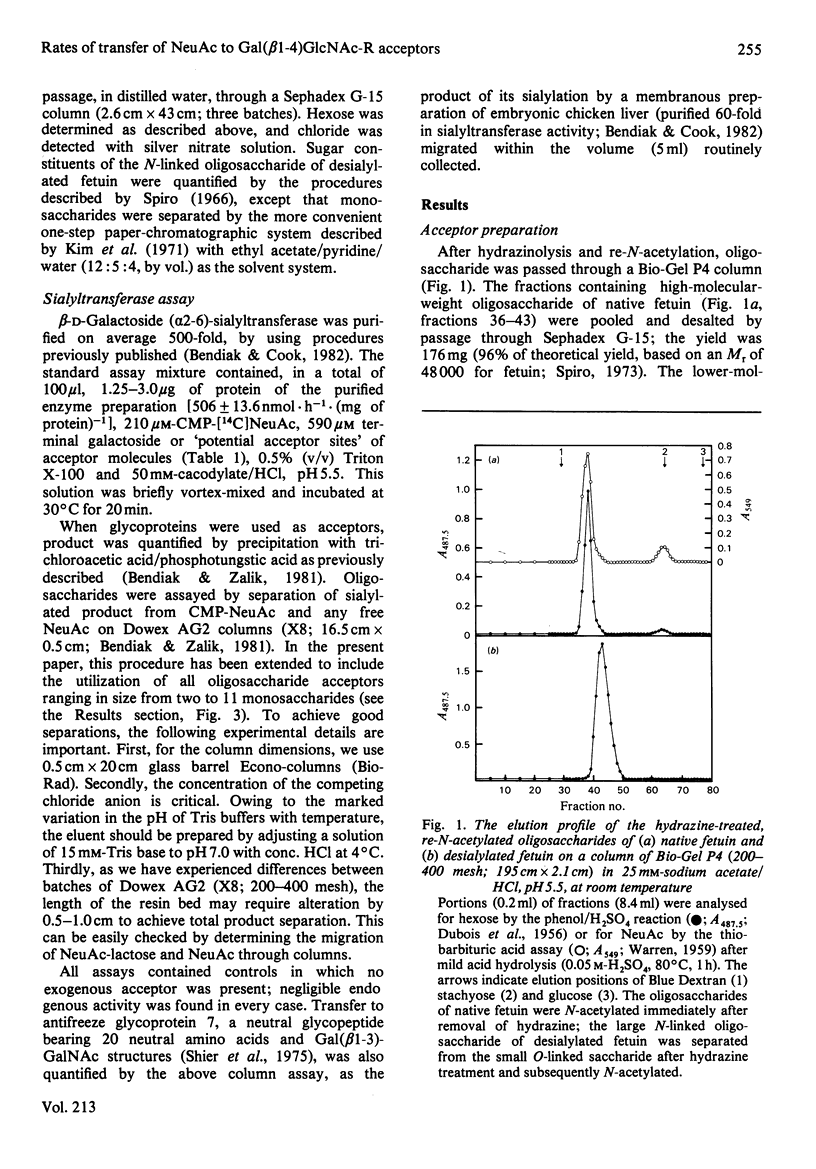
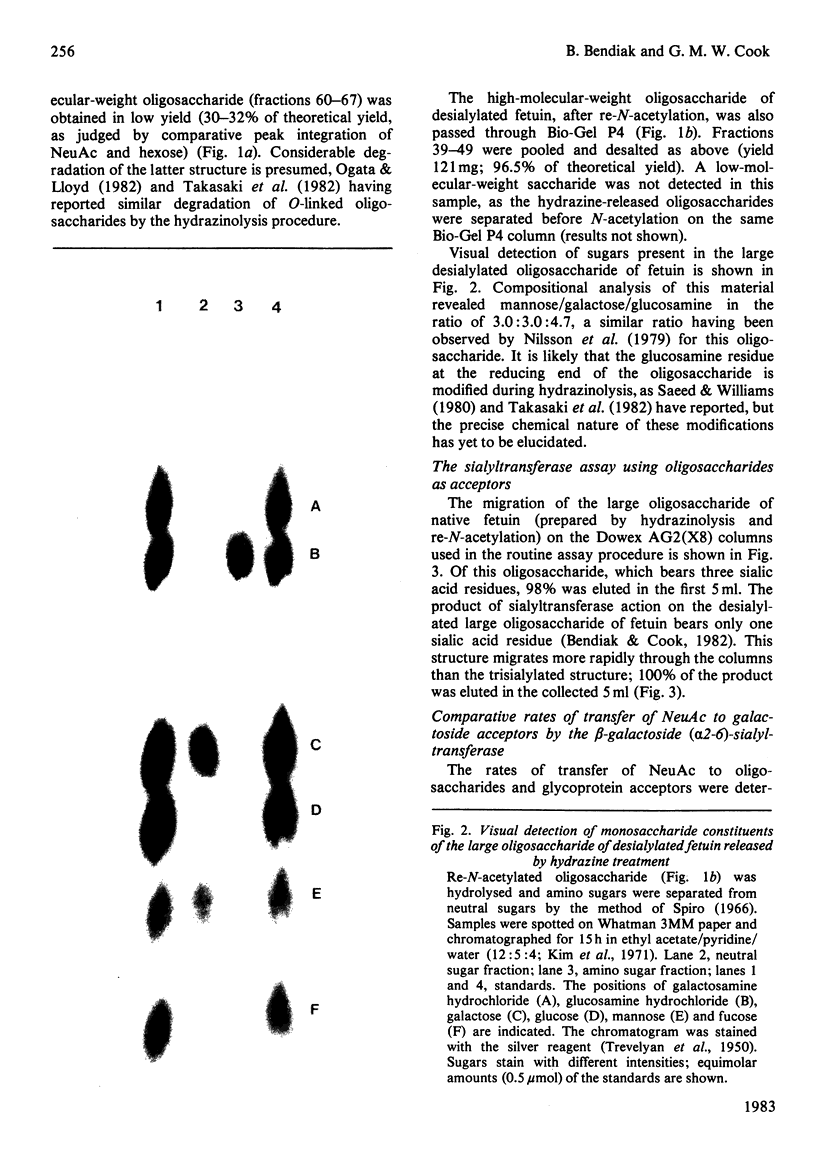
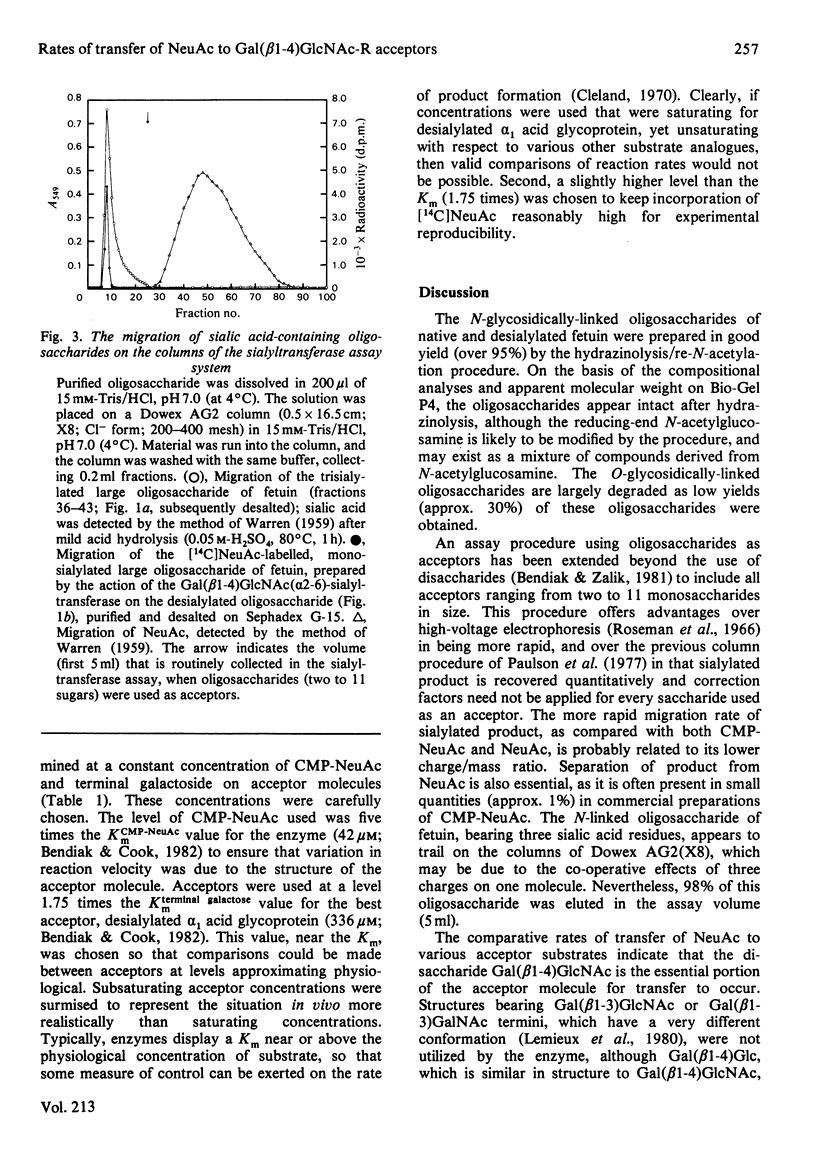


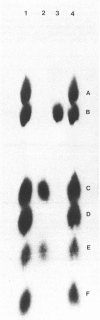
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aminoff D., Gathmann W. D., Baig M. M. Glycoproteins and blood group activity. Isolation and characterization of oligosaccharides of H+ hog submaxillary glycoprotein, and their comparison to those found in A+ and A-H- glycoproteins. J Biol Chem. 1979 Sep 25;254(18):8909–8913. [PubMed] [Google Scholar]
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7260–7269. [PubMed] [Google Scholar]
- Bendiak B., Cook G. M. Kinetic parameters of a beta-D-galactoside alpha 2 leads to 6 sialyltransferase from embryonic chicken liver. Eur J Biochem. 1982 Nov 15;128(2-3):355–362. [PubMed] [Google Scholar]
- Bendiak B., Zalik S. E. The specificity of sialyltransferase activity in smooth membrane fractions of embryonic chicken liver. Can J Biochem. 1981 Mar;59(3):171–180. doi: 10.1139/o81-025. [DOI] [PubMed] [Google Scholar]
- Bennett G., O'Shaughnessy D. The site of incorporation of sialic acid residues into glycoproteins and the subsequent fates of these molecules in various rat and mouse cell types as shown by radioautography after injection of [3H]N-acetylmannosamine. I. Observations in hepatocytes. J Cell Biol. 1981 Jan;88(1):1–15. doi: 10.1083/jcb.88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Carver J. P., Grey A. A. Determination of glycopeptide primary structure by 360-MHz proton magnetic resonance spectroscopy. Biochemistry. 1981 Nov 10;20(23):6607–6616. doi: 10.1021/bi00526a014. [DOI] [PubMed] [Google Scholar]
- Dorland L., Haverkamp J., Viliegenthart J. F., Strecker G., Michalski J. C., Fournet B., Spik G., Montreuil J. 360-MHz 1H nuclear-magnetic-resonance spectroscopy of sialyl-oligosaccharides from patients with sialidosis (mucolipidosis I and II). Eur J Biochem. 1978 Jun 15;87(2):323–329. doi: 10.1111/j.1432-1033.1978.tb12381.x. [DOI] [PubMed] [Google Scholar]
- Finne J. Occurrence of unique polysialosyl carbohydrate units in glycoproteins of developing brain. J Biol Chem. 1982 Oct 25;257(20):11966–11970. [PubMed] [Google Scholar]
- Fournet B., Montreuil J., Strecker G., Dorland L., Haverkamp J., Vliegenthart F. G., Binette J. P., Schmid K. Determination of the primary structures of 16 asialo-carbohydrate units derived from human plasma alpha 1-acid glycoprotein by 360-MHZ 1H NMR spectroscopy and permethylation analysis. Biochemistry. 1978 Nov 28;17(24):5206–5214. doi: 10.1021/bi00617a021. [DOI] [PubMed] [Google Scholar]
- Franzén L. E., Svensson S., Larm O. Structural studies on the carbohydrate portion of human antithrombin III. J Biol Chem. 1980 Jun 10;255(11):5090–5093. [PubMed] [Google Scholar]
- Fukuda M., Hakomori S. Carbohydrate structure of galactoprotein a, a major transformation-sensitive glycoprotein released from hamster embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):5451–5457. [PubMed] [Google Scholar]
- Hagopian A., Eylar E. H. Glycoprotein biosynthesis: studies on the receptor specificity of the polypeptidyl: N-acetylgalactosaminyl transferase from bovine submaxillary glands. Arch Biochem Biophys. 1968 Nov;128(2):422–433. doi: 10.1016/0003-9861(68)90048-9. [DOI] [PubMed] [Google Scholar]
- Inoue S., Iwasaki M., Matsumura G. Novel carbohydrate structures in trout egg glycoprotein: occurrence of a neuraminidase-resistant N-glycolylneuraminosyl-(2 leads to 3)-N-acetylgalactosamine linkage. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1295–1301. doi: 10.1016/s0006-291x(81)80152-0. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Nordberg J. Glycoprortein biosynthesis in small intestinal mucosa. I. A study of glycosyltransferases in microsomal subfractions. J Biol Chem. 1971 Sep 10;246(17):5466–5476. [PubMed] [Google Scholar]
- Mega T., Lujan E., Yoshida A. Studies on the oligosaccharide chains of human alpha 1-protease inhibitor. II. Structure of oligosaccharides. J Biol Chem. 1980 May 10;255(9):4057–4061. [PubMed] [Google Scholar]
- Miyagi T., Tsuiki S. Purification and characterization of beta-galactoside (alpha 2 leads to 6)sialyltransferase from rat liver and hepatomas. Eur J Biochem. 1982 Aug;126(2):253–261. [PubMed] [Google Scholar]
- Mizuochi T., Fujii J., Kurachi K., Kobata A. Structural studies of the carbohydrate moiety of human antithrombin III. Arch Biochem Biophys. 1980 Aug;203(1):458–465. doi: 10.1016/0003-9861(80)90199-x. [DOI] [PubMed] [Google Scholar]
- Mizuochi T., Yamashita K., Fujikawa K., Titani K., Kobata A. The structures of the carbohydrate moieties of bovine blood coagulation factor X. J Biol Chem. 1980 Apr 25;255(8):3526–3531. [PubMed] [Google Scholar]
- Montreuil J. Primary structure of glycoprotein glycans: basis for the molecular biology of glycoproteins. Adv Carbohydr Chem Biochem. 1980;37:157–223. doi: 10.1016/s0065-2318(08)60021-9. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Nordén N. E., Svensson S. Structural studies on the carbohydrate portion of fetuin. J Biol Chem. 1979 Jun 10;254(11):4545–4553. [PubMed] [Google Scholar]
- Ogata S., Lloyd K. O. Mild alkaline borohydride treatment of glycoproteins-a method for liberating both N- and O-linked carbohydrate chains. Anal Biochem. 1982 Jan 15;119(2):351–359. doi: 10.1016/0003-2697(82)90597-8. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Rearick J. I., Hill R. L. Enzymatic properties of beta-D-galactoside alpha2 leads to 6 sialytransferase from bovine colostrum. J Biol Chem. 1977 Apr 10;252(7):2363–2371. [PubMed] [Google Scholar]
- Rearick J. I., Sadler J. E., Paulson J. C., Hill R. L. Enzymatic characterization of beta D-galactoside alpha2 leads to 3 sialyltransferase from porcine submaxillary gland. J Biol Chem. 1979 Jun 10;254(11):4444–4451. [PubMed] [Google Scholar]
- Reddy M. S., Levine M. J., Tabak L. A. Structure of the carbohydrate chains of the proline-rich glycoprotein from human parotid saliva. Biochem Biophys Res Commun. 1982 Feb 11;104(3):882–888. doi: 10.1016/0006-291x(82)91331-6. [DOI] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Shier W. T., Lin Y., DeVries A. L. Structure of the carbohydrate of antifreeze glycoproteins from an antartic fish. FEBS Lett. 1975 Jun 15;54(2):135–138. doi: 10.1016/0014-5793(75)80060-3. [DOI] [PubMed] [Google Scholar]
- Slomiany A., Slomiany B. L. Structures of the acidic oligosaccharides isolated from rat sublingual glycoprotein. J Biol Chem. 1978 Oct 25;253(20):7301–7306. [PubMed] [Google Scholar]
- Sommers L. W., Hirschberg C. B. Transport of sugar nucleotides into rat liver Golgi. A new Golgi marker activity. J Biol Chem. 1982 Sep 25;257(18):10811–10817. [PubMed] [Google Scholar]
- Spik G., Strecker G., Fournet B., Bouquelet S., Montreuil J., Dorland L., van Halbeek H., Vliegenthart J. F. Primary structure of the glycans from human lactotransferrin. Eur J Biochem. 1982 Jan;121(2):413–419. doi: 10.1111/j.1432-1033.1982.tb05803.x. [DOI] [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G. Glycoprotein biosynthesis: studies on thyroglobulin. Thyroid sialyltransferase. J Biol Chem. 1968 Dec 25;243(24):6520–6528. [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Takasaki S., Mizuochi T., Kobata A. Hydrazinolysis of asparagine-linked sugar chains to produce free oligosaccharides. Methods Enzymol. 1982;83:263–268. doi: 10.1016/0076-6879(82)83019-x. [DOI] [PubMed] [Google Scholar]
- Takasaki S., Yamashita K., Suzuki K., Iwanaga S., Kobata A. The sugar chains of cold-insoluble globulin. A protein related to fibronectin. J Biol Chem. 1979 Sep 10;254(17):8548–8553. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weinstein J., de Souza-e-Silva U., Paulson J. C. Sialylation of glycoprotein oligosaccharides N-linked to asparagine. Enzymatic characterization of a Gal beta 1 to 3(4)GlcNAc alpha 2 to 3 sialyltransferase and a Gal beta 1 to 4GlcNAc alpha 2 to 6 sialyltransferase from rat liver. J Biol Chem. 1982 Nov 25;257(22):13845–13853. [PubMed] [Google Scholar]
- Zinn A. B., Marshall J. S., Carlson D. M. Carbohydrate structures of thyroxine-binding globulin and their effects on hepatocyte membrane binding. J Biol Chem. 1978 Oct 10;253(19):6768–6773. [PubMed] [Google Scholar]
- van den Eijnden D. H., Joziasse D. H., Dorland L., van Halbeek H., Vliegenthart J. F., Schmid K. Specificity in the enzymic transfer of sialic acid to the oligosaccharide branches of b1- and triantennary glycopeptides of alpha 1-acid glycoprotein. Biochem Biophys Res Commun. 1980 Feb 12;92(3):839–845. doi: 10.1016/0006-291x(80)90779-2. [DOI] [PubMed] [Google Scholar]