Abstract
Mycobacterium abscessus infection is challenging to treat. Extrapulmonary M. abscessus infections (EP-MAB) are less common than pulmonary M. abscessus infections. To evaluate treatment regimens, we retrospectively analyzed consecutive microbiologically confirmed EP-MAB cases diagnosed in France during 2012–2020. We studied 45 patients with EP-MAB, including 14 bone and joint infections, 10 skin and soft tissue infections, and 8 lymph node infections. Most (62%) patients had no reported immunodeficiency. In 27 patients, EP-MAB followed healthcare-associated (44%) or environmental (16%) injuries. Of the 45 isolates, 25 were subspecies abscessus, 10 bolletii, and 9 massiliense; 1 was unidentified. Cure was achieved for 36 (80%) patients who received a median antimicrobial regimen of 6 months; 22 (55%) also underwent surgery. Four patients died, and 5 were unavailable for follow-up. EP-MAB predominantly affects immunocompetent patients after an injury; outcomes are favorable. We propose a >6-month regimen of antimicrobial therapy with consideration for surgery and regular patient reassessment.
Keywords: nontuberculous mycobacteria, tuberculosis and other mycobacteria, Mycobacterium abscessus, emerging communicable diseases, healthcare-associated infection, travel medicine, extrapulmonary, France
Nontuberculous mycobacteria (NTM) are found in the environment (1,2). They are increasingly recognized as causative agents of infections regardless of patient age or immune status, and in several countries they surpass tuberculosis in terms of prevalence (3–5).
Mycobacterium abscessus is a rapidly growing NTM (6,7), subcategorized as 3 subspecies: abscessus, massiliense, and bolletii. Subspecies affect pulmonary infection outcomes (8) because they correlate with the expression of the erm(41) gene (9), conferring inducible resistance to macrolides. Resistance has been noted for M. abscessus subsp. bolletii and M. abscessus subsp. abscessus sequevar T28 isolates, and susceptibility has been noted for M. abscessus subsp. massiliense and M. abscessus subsp. abscessus sequevar C28 isolates (10). Infection with those subspecies contributes to a poorer clinical outcome, restricting the effectiveness of macrolides, although they are recommended for pulmonary infections for all M. abscessus strains (10).
M. abscessus primarily causes pulmonary infections, particularly in patients with bronchiectasis (10). Despite multidrug antimicrobial therapy, cure rates remain <50%, and mortality rates are high (11). Extrapulmonary M. abscessus infections (EP-MAB) are rare, documented through localized outbreaks in single centers (12–17) or case series (18).
In France, M. abscessus accounts for 20%–25% of NTM clinical isolates received by the French National Reference Centre for Mycobacteria (CNR-MyRMA; https://cnrmyrma.fr) for identification and antimicrobial susceptibility testing in an infection context (E. Cambau, unpub. data). Although previous guidelines (19) provided advice about extrapulmonary NTM infections and proposed macrolide-based antimicrobial regimens and surgery and new guidelines have recently been updated for pulmonary M. abscessus infections (10), there are no specific recommendations for EP-MAB treatment duration. To investigate EP-MAB medical management (e.g., diagnostis, treatments, and outcomes), we retrospectively studied consecutive patients with microbiologically confirmed EP-MAB. In accordance with French law, our study protocol received approval by the “Comité d’éthique de la recherche AP-HP Centre” (IRB registration no. 00011928, Réf. 2020–12–04).
Material and Methods
Case Eligibility Criteria
We reviewed cases involving extrapulmonary infections associated with M. abscessus strains among all strains registered in the CNR-MyRMA database during January 2012–April 2020. Cases were eligible if they met the criteria of clinical signs/symptoms dependent on the site of the infection and >1 M. abscessus isolate was concurrently recovered from a sample from an extrapulmonary site (e.g., skin biopsy sample, articular fluid, blood). Multisite infections were defined as those involving 2 nonadjacent organs with concordant clinical, microbiological, or histologic criteria. Multisite infections that included pulmonary localization were eligible, and cases of pulmonary infection alone were not. The inclusion was assessed by authors B.H.P. and A.P.
Data Retrieval
We sent a questionnaire gathering epidemiologic, clinical, biological, radiologic, therapeutic, and follow-up data to physicians and microbiologists who reported a case. When needed, we also contacted them by phone or mail to request medical reports.
For injury-related infections, we set the geographic origin as the location where the injury occurred. For infections not associated with injury, the geographic origin was the location of the patient when initial symptoms were noted and, if that information was unavailable, the location where the diagnostic specimen was obtained. Healthcare-associated infections were defined as those following medical, surgical, or aesthetic procedures performed before the infection and at the same location. Antimicrobial regimens were recorded if administered for >7 days. We presented qualitative variables as medians (ranges). We considered patients cured if clinical reports indicated such; no microbiological evidence was required.
Identification and Antibiotic Susceptibility
We conducted mycobacterial identification and antimicrobial susceptibility testing (AST) in accordance with standard practice (10,20) and strain identification by using the GenoType Mycobacterium CM kit (Hain Lifescience, https://www.hain-lifescience.de), IVD MALDI Biotyper (Brucker Daltronics, https://www.bruker.com), and mass spectrometry Microflex LT MALDI-TOF MS (Brucker Daltronics), analyzed with the Mycobacteria Library MBT Compass3, (Bruker Daltonics) subspecies. We determined erm(41) sequevars by using the Genotype NTM-DR test (Hain Lifesciences, Bruker Daltronics) (21) complemented with hsp65 or rpoB PCR Sanger sequencing when necessary. We performed AST in calcium-supplemented Mueller-Hinton medium and used Sensititre Myco AST Plate (Thermo Scientific, https://www.thermofisher.com) to determine MICs (10,22). To detect inducible clarithromycin resistance, we determined clarithromycin MICs after 3–5 days and after 14 days of incubation (9,23). We conducted molecular detection of mutations associated with antimicrobial resistance by targeting rrl for macrolides and rrs for aminoglycosides (24). We identified the erm(41) sequevar through PCR sequencing (24).
Results
During 2012–2020, CNR-MyRMA received 3,684 NTM strains for identification, including 736 (20%) M. abscessus strains, among which 56 (8%) were associated with EP-MAB (Figure 1). The average annual ratio of extrapulmonary to pulmonary strains was 0.07 (range 0.02–0.15) (Appendix Figure). Clinical data were collected for 47 (84%) strains isolated from the 45 patients (Table; Appendix Table 1).
Figure 1.
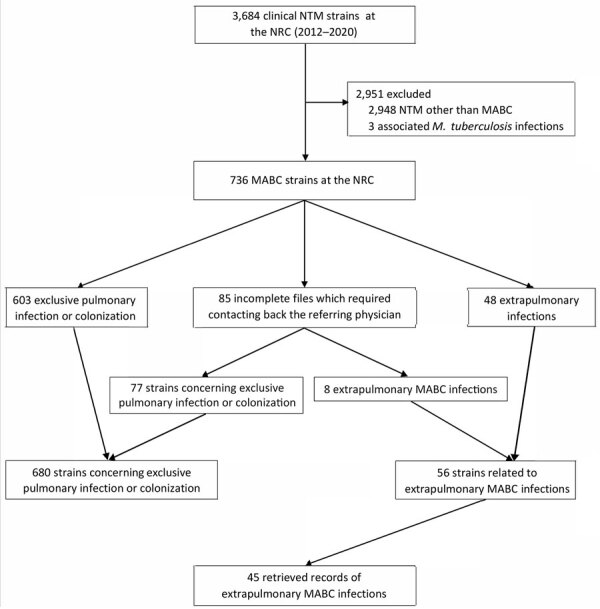
Flowchart of selection for study of extrapulmonary Mycobacterium abscessus infections, France, 2012–2020. NRC, national reference center; NTM, nontuberculous mycobacteria; MABC, Mycobacterium abscessus complex.
Table. Characteristics of 45 extrapulmonary Mycobacterium abscessus infections, France, 2012–2020*.
| Variable |
Infections |
Total |
||
|---|---|---|---|---|
| Bone/joint |
Skin/soft tissue |
Lymph node |
||
| Patients, no. (%) | 14 (30) | 10 (22) | 8 (18) | 45 (100) |
| Mean age, y, ± SD | 50.2 ± 26.6 | 60.8 ± 21.2 | 43.7 ± 25.3 | 51.4 ± 24.5 |
| M/F sex ratio |
1.33 |
0.66 |
1 |
0.88 |
| Geographic origin, no. (%) | ||||
| Metropolitan France | 3 | 4 | 2 | 13 (29) |
| Overseas French territories | 4 | 3 | 2 | 13 (29) |
| Foreign countries |
7 |
3 |
4 |
19 (42) |
| Underlying immune disorder, no. (%) | ||||
| No known immunodeficiency | 13 | 4 | 5 | 28 (62) |
| Immunosuppressive treatment | 1 | 5 | 2 | 9 (20) |
| Solid tumor | 0 | 0 | 1 | 4 (9) |
| Malignant hemopathy |
0 |
1 |
0 |
2 (4) |
| Clinical features, no. (%) | ||||
| B symptoms† | 7 | 4 | 2 | 17 (38) |
| Skin involvement |
4 |
10 |
2 |
22 (49) |
| Initial injury | 12 | 3 | 3 | 27 (60) |
| Median months to diagnosis [range] | 4.5 [0.25–72] | 1.5 [1–72] | 1 [0.25–3] | 3 [0.25–72] |
| Environmental | 4 | 1 | 2 | 7 (16) |
| Healthcare associated |
8 |
2 |
1 |
20 (44) |
| M. abscessus subspecies and erm(41) sequevars, no. (%) | ||||
| abscessus T28 | 7 | 5 | 1 | 19 (40) |
| abscessus C28 | 2 | 1 | 0 | 6 (13) |
| bolletii | 2 | 2 | 4 | 12 (26) |
|
massiliense
|
3 |
2 |
2 |
9 (19) |
| Medical management, no. (%) | ||||
| Surgery + antimicrobial regimen | 11 | 3 | 3 | 22 (49) |
| Surgery alone | 1 | 0 | 1 | 3 (7) |
| Antimicrobial regimen alone | 2 | 6 | 3 | 13 (29) |
| Media months of antimicrobial regimen duration [range] |
6 [2–12] |
6 [3–15] |
6 [1–6] |
6 [1–long-term] |
| Outcome, no. (%) | ||||
| Cure | 13 | 7 | 7 | 33 (73) |
| Relapse then cured | 0 | 0 | 0 | 3 (7) |
| Not available for follow-up | 1 | 2 | 1 | 5 (11) |
| Death | 0 | 1 | 0 | 4 (9) |
*Values are no. (%) except as indicated. †Fever, night sweats, or weight loss.
Clinical and Biological Features
The 45 cases were distributed as 14 (31%) bone and joint infections (BJIs), 10 (22%) skin and soft tissue infections (SSTIs), 8 (18%) lymph node infections (LNIs), 4 (9%) bacteremia or catheter-related infections, 4 (9%) multisite infections, 3 (7%) breast infections, and 2 (4%) biliary tract infections. The median time between initial signs/symptoms and diagnosis was 2 months (range 1 week–2 years); 89% of cases were diagnosed in <6 months. The clinical signs were cutaneous lesions in 22 (46%) patients and palpable lymph nodes in 6 (13%) patients. Fever was noted for 9 (20%) patients, asthenia for 8 (17%), and night sweats for 2 (4%). BJIs were 7 monoarthritis, 4 lower limb osteitis, 2 spondylodiscitis, and 1 hip arthroplasty. Few details were available for SSTI descriptions; 7 cases were reported as cutaneous nodules. LNIs involved cervical lymph nodes in 4 patients, mediastinal lymph nodes in 3, and inguinal lymph nodes in 1. Median neutrophil count was 4.1 × 109 cells/L (range 0.93–11.64 × 109 cells/L), median lymphocyte count was 1.2 × 109 cells/L (range 0.1–3.3 × 109 cells/L), and median monocyte count was 0.6 × 109 cells/L (range 0.08–1.77 × 109 cells/L). Median C-reactive protein concentration was 29 mg/L (range <5–230 mg/L). Thoracic computed tomography was available for 21 (47%) patients and revealed parenchymal infiltrates (including mostly condensations, nodules, or both) in 15 patients. After other diseases were excluded, pulmonary involvement was confirmed for only 2 of the 15 patients. For 2 of 4 patients with multisite M. abscessus infection, a thoracic computed tomography scan showed mediastinal lymph nodes in one and bilateral pulmonary nodules in the other. For the 2 patients with microbiologically proven spondylodiscitis, respiratory samples were also M. abscessus despite the absence of pulmonary signs or known underlying bronchopulmonary disease, suggesting probable colonization.
Epidemiology of Patients with Extrapulmonary Infections
The median patient age was 51.4 years (range 1–98 years); 6 (13%) were <18 years of age. The M:F sex ratio was 0.88. Underlying disease was documented for 19 (42%) patients, including 16 with immunodeficiency acquired by treatment or disease (Figure 2), 1 with inborn error of immunity (NEMO [nuclear factor κB essential modulator] mutation), and 2 with prior chronic pulmonary diseases. The remaining 26 patients were classified as immunocompetent, 2 of whom were negative for inborn immunodeficiency.
Figure 2.
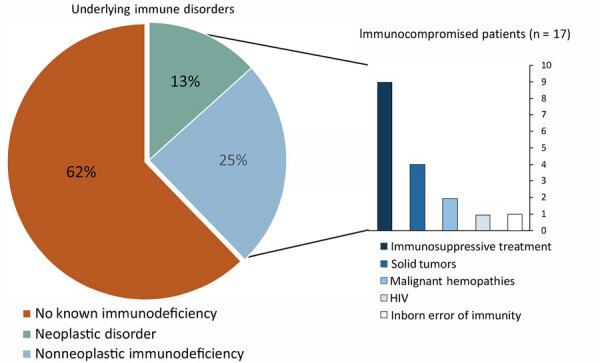
Medical conditions associated with extrapulmonary M. abscessus infections, France, 2012–2020.
Penetrating injury was suspected as the portal of entry for 27 (60%) patients, including 20 (44%) with healthcare-related cases and 7 (16%) associated with an environmental source. The median time after injury was 3 months (range 1 week–5 years). Of the 14 BJIs, 11 (79%) followed an initial injury, which was either skin injury (all 4 osteitis) or articular infiltration (3 of 7 monoarthritis). The 3 breast infections were associated with healthcare injury, 2 after bilateral prosthetic implant procedures performed in Mauritius and 1 after breast biopsy. Of 4 patients who experienced bacteremia, 3 had an implantable venous access device. Of 10 SSTIs, 3 were associated with injury, 2 of them on tattoos.
Travel-related infection affected 32 (71%) patients, of which 13 (29%) of 45 cases were diagnosed in overseas France (6 in French West Indies, 4 in La Réunion Island, 1 in French Guyana, 1 in New Caledonia, and 1 in French Polynesia), 10 in Africa, 3 in the Americas, 3 in East Asia, 2 in Europe, and 1 in the Middle East. The remaining 13 (29%) patients were metropolitan France residents with no travel history that could be associated with M. abscessus infection.
Microbiological Results
The 47 strains collected for the 45 cases were distributed as 25 (53%) M. abscessus subsp. abscessus that included 19 (40%) erm(41) sequevar T28 and 6 (13%) sequevar C28, 12 (26%) M. abscessus subsp. bolletii, and 9 (19%) M. abscessus subsp. massiliense (Appendix Table 2). Subspecies identification was missing for 1 strain isolated in 2012. For the patient who experienced 2 relapses (patient 42), the same M. abscessus subsp. bolletii strain was isolated. All 9 strains of M. abscessus subsp. massiliense and 6 M. abscessus subsp. abscessus erm(41) sequevar C28 were susceptible to macrolides with a clarithromycin 90% MIC of 0.5 mg/L after 3–5 days and 1 mg/L after 14 days of incubation. For the 31 strains of M. abscessus subsp. bolletii and M. abscessus subsp. abscessus erm(41) sequevar T28, the clarithromycin 90% MIC was 8 mg/L after 3–5 days and >16 mg/L after 14 days of incubation. Sequences of rrl and rrs were available for all identified strains, and both were exhibited as a wild-type genotype.
Treatment
Before diagnosis, none of the patients had been treated with a drug that targeted M. abscessus. Antimycobacterial agents were administered to 35 (77%) patients, including 22 (49%) who also underwent local surgery or local care; 3 (7%) patients underwent local surgery without antimicrobial drugs; 3 (7%) received local care alone; and 4 (9%) received no treatment (Appendix). Of the 35 initial antimicrobial drug regimens, 26 (74%) involved a combination of >3 drugs, 8 a combination of 2 drugs, and 1 was monotherapy. All regimens included macrolides for >3 weeks: clarithromycin (21 [62%]), azithromycin (11 [32%]), or sequential treatment with both (2 [6%]). For 30 (86%) patients, treatment started with an induction phase including >1 intravenous antimicrobial drug, and 4 (12%) patients received oral antimicrobial drugs only. For patients with BJIs, SSTIs, and LNIs, the median duration of the antimicrobial regimen was 6 months and the median duration of the induction phase was 6 weeks.
Outcomes
Cure rate with no relapse was 73% (33 patients), including 4 patients who received neither antimicrobial drugs nor surgery: 3 infections resolved with local care alone, and 1 patient with LNI experienced spontaneous resolution after 1 month. During the follow-up period, 4 (9%) patients experienced relapses (3 of whom eventually experienced cure and the other was not available for follow-up) and 4 (9%) patients died (3 died before receiving treatment for M. abscessus infection). Of the 4 who died, 2 had M. abscessus bacteremia and received supportive care for cancer, 1 had M. abscessus multisite infection diagnosed with a concomitant JC virus infection, and 1 had an SSTI and died of limb ischemia at 6 months of treatment. The 4 deaths were unrelated to M. abscessus infection. Five (11%) patients were unavailable for follow-up after 1 month of treatment. Overall cure was observed for 36 (80%) patients; median duration of patient follow-up was 32.5 months (1st quartile 26.25–3rd quartile 45.0 months). No explanatory factor was associated with the few infections that led to a negative outcome.
Immune reconstitution syndrome was noted for 1 patient (patient 25). The patient had an SSTI of the lower limb, and immune reconstitution syndrome affected the draining abdominal lymph nodes. The patient’s condition improved after administration of steroids.
Discussion
M. abscessus is a challenging-to-treat pathogen (10), and EP-MAB is rarely described. In our series, EP-MAB often affected immunocompetent patients after injury, and for some patients, outcomes were favorable after antimicrobial therapy complemented with surgery.
In France, we benefit from the microbiological expertise of university hospital clinical microbiology national network reporting to CNR-MyRMA and from the monthly NTM treatment consilium organized by CNR-MyRMA. The collaborative network probably contributes to the widespread use of macrolides and the avoidance of antimicrobial monotherapy.
Although NTM are known as opportunistic pathogens with infections occurring in patients with underlying conditions or immunodeficiencies (10), in our series of EP-MAB, most (62%) patients had no reported immunodeficiency, although a few (15%) had undergone immunologic testing. Underlying inborn errors of immunity in children and adults have been revealed by NTM infections (25). We think that among patients with new EP-MAB infections, it is worthwhile to screen for immunodeficiencies (e.g., HIV serology testing or autoantibodies against interferon-γ [26]) as first-line tests in association with a clinical examination by a trained physician.
Unlike pulmonary infections, for which exposures are mainly unknown (10), in our study, the EP-MAB trigger was identified for 60% of the patients as a penetrating injury associated with healthcare or environmental inoculation. No outbreak was identified. Only the 3 patients with SSTIs remembered an injury, showing that small cutaneous injuries are almost unnoticed, as described previously (27–29). Considering the literature (13,14,30) and our data, M. abscessus infections might result from lack of antiseptic and aseptic procedures before joint infiltration, surgery, and aesthetic procedures including surgical tourism and thus might be avoidable.
Guidelines for treatment of NTM infections have recently been updated but only for pulmonary disease (10). Consequently, the recommendations for treatment of extrapulmonary NTM infections are those of the 2007 guidelines (19), which proposed a macrolide-based antimicrobial regimen and surgery but had no recommendations for treatment duration. With a cure rate of 80%, our study suggests that EP-MAB may have a much more favorable outcome than pulmonary M. abscessus diseases, which could result from the short time to diagnosis, because the median time to diagnosis observed in our study (2 months) aligns with findings from previous studies reporting outbreaks (13,14,31). Such a short time frame could be attributed to clinical similarities with tuberculosis, the ease of accessing sample sites, and the rapid growth ability of M. abscessus. It might contribute to the overall better prognosis for EP-MAB than for pulmonary M. abscessus infections. The clinical microbiology network reporting to the CNR-MyRMA together with a monthly NTM treatment consilium probably contributes to the use of macrolides and the avoidance of antimicrobial monotherapy. The high proportion of surgeries could be attributed to specific infection sites, primarily involving BJIs and SSTIs. Some EP-MAB resolved spontaneously or with local care or surgery alone, a practice already described for LNIs in children (32). Overall, despite the relatively short duration of antimicrobial drug regimens, EP-MAB seems to have a favorable outcome with no sequelae and rare relapses, which contrasts with pulmonary infections, despite longer treatment durations (10). The discrepancy underscores the need for prospective studies specific for EP-MAB, specifically the optimal antimicrobial combinations, treatment duration, and criteria for associated or exclusive surgery.
The increase in cases reported to CNR-MyRMA during 2012–2020 was similar to reports from other northern countries (33–36), which could be attributed to awareness among physicians, along with improvements and standardization of the microbiological diagnosis of NTM (37). The increases may also indicate a growing prevalence of M. abscessus infections. Our findings underscore the value of increasing knowledge and monitoring of M. abscessus. In our cohort, the high number of infections originating from overseas territories of France or from countries in Africa suggests a higher risk for M. abscessus infection in tropical regions, consistent with previous reports (13,14,31,38). Factors such as climate change, globalization of trade, and medical tourism in tropical regions could contribute to the increased M. abscessus infections. Therefore, patients with EP-MAB should be asked about their detailed travel history (13). However, we did not identify any outbreaks, and isolates were not genotypically related (data not shown); 30% of the patients had infections diagnosed in metropolitan France, some of them having never traveled abroad. In temperate countries, M. abscessus has also been widely reported, primarily affecting patients with pulmonary infections, especially cystic fibrosis (39), but the M. abscessus environmental reservoir remains largely unknown.
Infection sites were diverse, localized primarily to 3 clinical manifestations: BJIs, SSTIs, and LNIs. Clinical manifestations varied, but classic tuberculosis symptoms (e.g., fever, night sweats, asthenia, or lymph node involvement), were reported for <40% of patients. Conversely, cutaneous signs were observed for 46% of patients, probably linked to a high proportion of SSTIs and BJIs. Enhanced clinical description of skin lesions could help clinicians determine severity and guide treatment decisions, particularly the need for surgery.
One of the main limitations of our study is its retrospective nature. Another limitation is that despite centralization at CNR-MyRMA, EP-MAB cases are not mandatorily reported, resulting in our data not being exhaustive. Less severe cases may not require specific medical management and microbiological diagnosis.
The results from our cohort of EP-MAB enables us to draw the following conclusions: there are no distinct underlying host characteristics but rather an association with penetrating injury and travel to tropical regions, and prognosis is generally favorable when multiple antimicrobial drugs are administered, with surgery if needed. We could not assess differences in outcomes based on clinical localization, causal subspecies, or underlying diseases. Such an assessment would require a collaborative study involving a larger number of cases, categorized by infection sites. While we wait for prospective data to become available, our data may support the benefits of treating EP-MAB with an antimicrobial regimen of >6 months including a macrolide, supplemented with surgery for BJIs and regular patient reassessment.
Additional information for study of extrapulmonary Mycobacterium abscessus infections, France, 2012–2020.
Acknowledgments
We thank all the patients who participated in this study. We also acknowledge the technical work done by the CNR-MyRMA team (Odile Vissouarn and all the technicians of the CNR-MyRMA during 2012–2020). We further acknowledge all the physicians of the Mabsc Study Group who contributed to these data by means of their involvement in the management of patients, expertise in diagnostic or characterization of mycobacterial isolates, listed alphabetically by location: M. Cihanek (Aix); C. Andrejak (Amiens); H. Cordel (Avicennes); R. Barruet (Beauvais); C. Chirouze (Besançon); A. Lesueur (Bligny); V. Castaigns, M.C. Receveur (Bordeaux); A.L. Roux (Boulogne); A. Desdoits, S. Deshayes, A. Laquievre (Caen); E. Beillard, L. Epelboin (Cayenne); M.E. Chanard (Centre Est); C. Bernard, C. Ragot (Clamart); V. Grobost, M. Vidal (Clermont-Ferrand); W. Vindrios (Créteil); J. Bador (Dijon); C. Etienne (Grasse); J. Claudeon, E. Curlier, U. Françoise, S. Guyomard, K. Pailla, E. Rossigneux (Guadeloupe); M. Gueye (Guyane); B. Hillion (Lagny); N. Allou, O. Belmonte, B. Kuli, R. Manaquin, L. Raffray, M. Verduyn (La Réunion); N. Crochette (Le Mans); V. Vieillefond (Levallois Perret); A.F. Georgel (Lille); P. Miailhes, C. Pariset, M.M. Ponsoda, E. Pricaz, A. Sénéchal, G. Singier (Lyon); L. Delapierre, H. Pegliasco (Marseille); O. Grossi (Nantes); G. Foulon (Neuilly); N. Ehret, J.M. Turmel (Martinique); K. Risso (Nice); I. Bourlaud, P. Lureau (Niort); J. Colot (Nouvelle-Calédonie); C. Bernigaud, S. Bulifon, J. Bustamente, A. Canellas, E. Caumes, S. Chelabi, A. Contejean, F. Defournier, V. Delcey, L. Escaut, R. Gueneau, M. Humbert, V. Lalande, J. Lourtet, O. Paccoud, J. Pastre, G. Simonneau, C. Verdet, N. Veziris, V. Zeller (Paris); F. Chaix (Polynésie Française); D. Minette (Reims); V. Fabre (Rodez); I. Michelet (Rouen); M. Lagrange (Saint Denis); H. Tchero (Saint-Martin); P. Boyer (Strasbourg); E. Catherinot, I. Marroun (Suresnes); E. Oehler (Tahiti); K. Delavigne, M. Gauthier (Toulouse); G. Dewulf (Valenciennes); M. Amara, X. Brickley, A. Greder Belan, M. Groh, J. Vendroux (Versailles); E. Giroudon (Villefranche); E. Chachaty, E. Gallois, F. Griscelli (Villejuif).
The authors received no financial support for the research, authorship, or publication of this article. CNR-MyRMA is supported by Santé Publique France (Ministry of Health) by quinquennial grants for the periods 2009–2014 and 2015–2022. B.H.P., F.M., A.P., Z.A., E.B., E.C., and O.L. declare that they have no conflict of interest. F.L. received honoraria from Gilead, F2G, and Pfizer with no link to this work.
E.C., O.L., F.M., F.L., and E.B. designed the study. B.H.P. and A.P. were responsible for patients’ recruitment and management. E.C., F.M., and Z.A. performed bacteriologic analysis. B.H.P., E.C., O.L., and A.P. drafted the manuscript. All authors contributed to revise the manuscript and all gave their approval for publication.
Biography
Dr. Heid-Picard is a physician specialist in immunology and infectious diseases and a PhD student researching inborn errors of immunity and immune responses to viruses at the Imagine Institute, Paris.
Footnotes
Suggested citation for this article: Heid-Picard B, Mougari F, Pouvaret A, Lanternier F, Awad Z, Bille E, et al. Extrapulmonary Mycobacterium abscessus infections, France, 2012–2020. Emerg Infect Dis. 2024 Nov [date cited]. https://doi.org/10.3201/eid3011.240459
Preliminary results from this study were displayed as oral communications at the 31st European Congress of Clinical Microbiology and Infectious Diseases (July 9–12, 2021, Vienna, Austria; abstract no. 04396) and at the 24èmes Journées Nationales d’Infectiologie (June 7–9, 2023, Grenoble, France; abstract no. PADS02-03).
These authors contributed equally to this article.
Mabsc Study Group members are listed at the end of this article.
References
- 1.Falkinham JO. Impact of human activities on the ecology of nontuberculous mycobacteria. Future Microbiol. 2010;5:951–60. 10.2217/fmb.10.53 [DOI] [PubMed] [Google Scholar]
- 2.Falkinham JO III. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis. 2011;17:419–24. 10.3201/eid1703.101510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Reyn CF, Waddell RD, Eaton T, Arbeit RD, Maslow JN, Barber TW, et al. Isolation of Mycobacterium avium complex from water in the United States, Finland, Zaire, and Kenya. J Clin Microbiol. 1993;31:3227–30. 10.1128/jcm.31.12.3227-3230.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Groote MA, Pace NR, Fulton K, Falkinham JO III. Relationships between Mycobacterium isolates from patients with pulmonary mycobacterial infection and potting soils. Appl Environ Microbiol. 2006;72:7602–6. 10.1128/AEM.00930-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Runyon EH. Identification of mycobacterial pathogens utilizing colony characteristics. Am J Clin Pathol. 1970;54:578–86. 10.1093/ajcp/54.4.578 [DOI] [PubMed] [Google Scholar]
- 7.Lyle Cummins S, Williams EM. An “acid-fast” other than Koch’s bacillus cultivated from sputum. Tubercle. 1933;15:49–53. 10.1016/S0041-3879(33)80019-5 [DOI] [Google Scholar]
- 8.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183:405–10. 10.1164/rccm.201003-0395OC [DOI] [PubMed] [Google Scholar]
- 9.Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53:1367–76. 10.1128/AAC.01275-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56:2000535. 10.1183/13993003.00535-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirsaeidi M, Machado RF, Garcia JGN, Schraufnagel DE. Nontuberculous mycobacterial disease mortality in the United States, 1999-2010: a population-based comparative study. PLoS One. 2014;9:e91879. 10.1371/journal.pone.0091879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong SH, Kim SY, Huh HJ, Ki CS, Lee NY, Kang CI, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49–56. 10.1016/j.ijid.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 13.Furuya EY, Paez A, Srinivasan A, Cooksey R, Augenbraun M, Baron M, et al. Outbreak of Mycobacterium abscessus wound infections among “lipotourists” from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46:1181–8. 10.1086/529191 [DOI] [PubMed] [Google Scholar]
- 14.Cardoso AM, Martins de Sousa E, Viana-Niero C, Bonfim de Bortoli F, Pereira das Neves ZC, Leão SC, et al. Emergence of nosocomial Mycobacterium massiliense infection in Goiás, Brazil. Microbes Infect. 2008;10:1552–7. 10.1016/j.micinf.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 15.Leao SC, Tortoli E, Viana-Niero C, Ueki SYM, Lima KVB, Lopes ML, et al. Characterization of mycobacteria from a major Brazilian outbreak suggests that revision of the taxonomic status of members of the Mycobacterium chelonae-M. abscessus group is needed. J Clin Microbiol. 2009;47:2691–8. 10.1128/JCM.00808-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu HS, Chang SC, Shen EP, Hu FR. Nontuberculous mycobacterial ocular infections—comparing the clinical and microbiological characteristics between Mycobacterium abscessus and Mycobacterium massiliense. PLoS One. 2015;10:e0116236. 10.1371/journal.pone.0116236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiwari TSP, Ray B, Jost KC Jr, Rathod MK, Zhang Y, Brown-Elliott BA, et al. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis. 2003;36:954–62. 10.1086/368192 [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Jung IY, Song JE, Kim EJ, Kim JH, Lee WJ, et al. Profiles of extrapulmonary nontuberculous mycobacteria infections and predictors for species: a multicenter retrospective study. Pathogens. 2020;9:949. 10.3390/pathogens9110949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. ; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 20.Cornaglia G, Courcol R, Herrmann J-L, Kahlmeter G. European Manual of Clinical Microbiology [cited 2024 Oct 1]. https://www.decitre.fr/livres/european-manual-of-clinical-microbiology-9782878050264.html
- 21.Mougari F, Loiseau J, Veziris N, Bernard C, Bercot B, Sougakoff W, et al. ; French National Reference Center for Mycobacteria. Evaluation of the new GenoType NTM-DR kit for the molecular detection of antimicrobial resistance in non-tuberculous mycobacteria. J Antimicrob Chemother. 2017;72:1669–77. 10.1093/jac/dkx021 [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. CLSI M24, susceptibility testing of mycobacteria, Nocardia spp., and other aerobic Actinomycetes [cited 2023 Apr 21]. https://clsi.org/standards/products/microbiology/documents/m24 [PubMed]
- 23.Maurer FP, Castelberg C, Quiblier C, Böttger EC, Somoskövi A. Erm(41)-dependent inducible resistance to azithromycin and clarithromycin in clinical isolates of Mycobacterium abscessus. J Antimicrob Chemother. 2014;69:1559–63. 10.1093/jac/dku007 [DOI] [PubMed] [Google Scholar]
- 24.Bastian S, Veziris N, Roux AL, Brossier F, Gaillard JL, Jarlier V, et al. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother. 2011;55:775–81. 10.1128/AAC.00861-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin Immunol. 2014;26:454–70. 10.1016/j.smim.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Döffinger R, Helbert MR, Barcenas-Morales G, Yang K, Dupuis S, Ceron-Gutierrez L, et al. Autoantibodies to interferon-γ in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis. 2004;38:e10–4. 10.1086/380453 [DOI] [PubMed] [Google Scholar]
- 27.Grubbs J, Bowen C. Mycobacterium abscessus infection following home dermabrasion. Cutis. 2019;104:79–80. [PubMed] [Google Scholar]
- 28.Dickison P, Howard V, O’Kane G, Smith SD. Mycobacterium abscessus infection following penetrations through wetsuits. Australas J Dermatol. 2019;60:57–9. 10.1111/ajd.12915 [DOI] [PubMed] [Google Scholar]
- 29.Carter KK, Lundgren I, Correll S, Schmalz T, McCarter T, Stroud J, et al. First United States outbreak of Mycobacterium abscessus hand and foot disease among children associated with a wading pool. J Pediatric Infect Dis Soc. 2019;8:291–6. 10.1093/jpids/piy036 [DOI] [PubMed] [Google Scholar]
- 30.Pereira RT, Malone CM, Flaherty GT. Aesthetic journeys: a review of cosmetic surgery tourism. J Travel Med. 2018;•••:25. [DOI] [PubMed] [Google Scholar]
- 31.Baker AW, Maziarz EK, Lewis SS, Stout JE, Anderson DJ, Smith PK, et al. Invasive Mycobacterium abscessus complex infection after cardiac surgery: epidemiology, management, and clinical outcomes. Clin Infect Dis. 2021;72:1232–40. 10.1093/cid/ciaa215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loizos A, Soteriades ES, Pieridou D, Koliou MG. Lymphadenitis by non-tuberculous mycobacteria in children. Pediatr Int. 2018;60:1062–7. 10.1111/ped.13708 [DOI] [PubMed] [Google Scholar]
- 33.Cristancho-Rojas C, Varley CD, Lara SC, Kherabi Y, Henkle E, Winthrop KL. Epidemiology of Mycobacterium abscessus. Clin Microbiol Infect. 2024;30:712–7. 10.1016/j.cmi.2023.08.035 [DOI] [PubMed] [Google Scholar]
- 34.Valadas E. Nontuberculous mycobacteria: clinical importance and relevance to bacille Calmette-Guérin vaccination. Clin Infect Dis. 2004;39:457–8. 10.1086/422326 [DOI] [PubMed] [Google Scholar]
- 35.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–6. 10.1164/rccm.201002-0310OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brode SK, Marchand-Austin A, Jamieson FB, Marras TK. Pulmonary versus nonpulmonary nontuberculous mycobacteria, Ontario, Canada. Emerg Infect Dis. 2017;23:1898–901. 10.3201/eid2311.170959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tagliani E, Kohl TA, Ghodousi A, Groenheit R, Holicka Y, Niemann S, et al. Appeal from the European tuberculosis reference laboratory network (ERLTB-Net) for improving the diagnosis of infections due to nontuberculous mycobacteria. Clin Microbiol Infect. 2024;30:4–6. 10.1016/j.cmi.2023.06.005 [DOI] [PubMed] [Google Scholar]
- 38.Baker AW, Lewis SS, Alexander BD, Chen LF, Wallace RJ Jr, Brown-Elliott BA, et al. Two-phase hospital-associated outbreak of Mycobacterium abscessus: investigation and mitigation. Clin Infect Dis. 2017;64:902–11. 10.1093/cid/ciw877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipworth S, Hough N, Weston N, Muller-Pebody B, Phin N, Myers R, et al. Epidemiology of Mycobacterium abscessus in England: an observational study. Lancet Microbe. 2021;2:e498–507. 10.1016/S2666-5247(21)00128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information for study of extrapulmonary Mycobacterium abscessus infections, France, 2012–2020.


