ABSTRACT
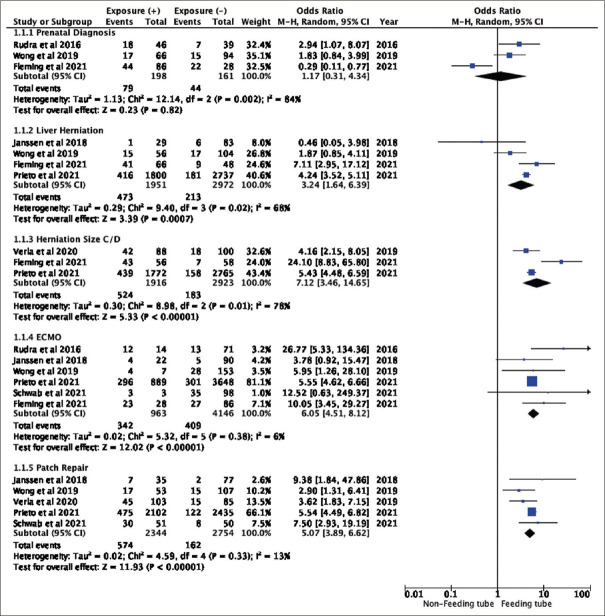
The early stages of life pose feeding challenges for infants with Congenital Diaphragmatic Hernia (CDH), necessitating feeding tube placement to prevent growth failure. Predicting the factors prompting this intervention has yielded inconclusive findings in prior research. Thus, this review explored prenatal, perinatal, and postnatal variables associated with feeding tube placement in CDH. Retrospective cohort or case-control reporting outcomes linked to prenatal, antenatal or postnatal predictors of feeding tube placement were included, following PRISMA 2020 guidelines. Reports, case series, conference abstracts, book sections, commentary, reviews, and editorials were excluded. Database searches were conducted in August 2023 encompassed Cochrane, MEDLINE, ProQuest, Wiley, and Google Scholar. Quality assessment using the Newcastle-Ottawa Scale and Review Manager 5.4 performed meta-analysis. Within eight studies, four exhibited a low risk of bias and the other was categorized as moderate. Analysis revealed significant effects for liver herniation (OR = 3.24, 95%CI 1.64-6.39, P = 0.0007), size of herniated defects classified as C or D (OR = 7.12, 95%CI 3.46-14.65, P < 0.00001), Extracorporeal Membrane Oxygenation treatment (ECMO) (OR = 6.05, 95%CI 4.51-8.12, P < 0.00001), and patch repair (OR = 5.07, 95%CI 3.89-6.62, P < 0.00001). ECMO treatment and patch repair surgery are robust predictors of feeding tube placement in CDH infants. Although liver herniation and size of herniated defect also showed associations, further studies are needed to address heterogeneity concerns. The review was registered in PROSPERO with the number CRD42023480109. No funding was received.
KEYWORDS: Congenital abnormalities, gastroenterology, extracorporeal membrane oxygenation, pediatrics
INTRODUCTION
Congenital diaphragmatic hernia (CDH) leads to lung and vascular issues through abdominal contents herniation into the thoracic cavity.[1] CDH prevalence ranges from 1 to 4 per 10,000 live births.[2] Despite progress in diagnosis and treatment, CDH maintains a high morbidity and mortality rate.[3] The early stages of life pose considerable feeding challenges for infants with CDH necessitating feeding tube placement to prevent growth failure[4,5] The primary enteral access beside nasogastric or nasojejunal tube feeding methods involves surgically placing gastrostomy or jejunostomy tubes.[6] Prior research has identified several predictors related to the necessity for enteral access; however, those data were still distinct, thus making it difficult to draw firm conclusions regarding which predictive elements have a significant role.[7]
Prenatal predictors for feeding tube placement can be identified antenatally and postnatally.[8,9] Antenatal assessment of CDH is based on several criteria such as observed-to-expected lung area-to-head circumference ratio (O/E-LHR), the intrathoracic position of the liver, and the ratio between intrathoracic liver and thoracic volume through magnetic resonance imaging (MRI). Postnatally, infants with lower APGAR scores, longer lengths of stay, and a higher proportion of flap closures, infants with a type C or D defect, and extracorporeal membrane oxygenation (ECMO) were related to a higher need for a GT placement.[9]
Predicting the factors prompting this intervention has yielded inconclusive findings in prior research. Thus, this review explored prenatal, perinatal, and postnatal variables associated with feeding tube placement in CDH infants.
MATERIALS AND METHODS
Retrospective cohort or case–control reporting outcomes linked to prenatal, antenatal, or postnatal predictors of feeding tube placement were included, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement.[10]
Registration of the review protocol
This review was registered in PROSPERO on November 13, 2023, with the registration number CRD42023480109.
Variable of interest
The purpose of this study was to identify prenatal, antenatal, and postnatal predictors that contribute to feeding tube placement in infants who have CDHs.
Eligibility criteria
The review consisted of published studies examining the factors associated with feeding tube placement in CDH infants, conducted from 2014 to 2023, encompassing retrospective cohort studies/retrospective reviews of medical records or case–control that are published in English, with reported outcomes on at least one of the prenatal/antenatal/postnatal predictors of feeding tube placement. Meanwhile, research publications that did not involve human subjects, reports, case series, conference abstracts, book sections, commentary, reviews, editorials, and articles with incomplete text were excluded.
Outcome of interest
The primary focus of this study was to identify predictors associated with feeding tube placement in live-born infants with CDH, either right/left-sided or bilateral, who have undergone feeding tube placement during hospitalization (gastrostomy, jejunostomy, or gastrojejunostomy), without restriction of age, gender, or race. Through identifying preliminary studies, systematic literature review, and expert consultation, several key predictors emerged as consistently reported in the literature. These include prenatal diagnoses of CDH, presence of size C/D defects, liver herniation, ECMO treatment, and utilization of patch repair. It is important to note that only outcomes with complete reporting, suitable for calculating odds ratios (ORs), were included in this study.
Search strategy and study selection
Electronic databases including Cochrane, MEDLINE, Wiley, ProQuest, and Google Scholar were used. Four independent authors identified papers using the specified search strategy in August 2023. The patient population, intervention, comparison, outcomes, timing, settings (PICOTS-SD) criteria and the full search strategy utilized are attached in the Supplementary Tables 1-4.
Supplementary Table 1.
PRISMA 2020 abstract checklist
| Section and topic | Item # | Checklist item | Location where the item is reported |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review | Page 1, Title Section, Line 1-3 |
| Background | |||
| Objectives | 2 | Provide an explicit statement of the main objective(s) or question(s) the review addresses | Page 1, Abstract Section, Line 8-9 |
| Methods | |||
| Eligibility criteria | 3 | Specify the inclusion and exclusion criteria for the review | Page 1, Abstract Section, Line 10-13 |
| Information sources | 4 | Specify the information sources (e.g., databases and registers) used to identify studies and the date when each was last searched | Page 1, Abstract Section, Line 13-14 |
| Risk of bias | 5 | Specify the methods used to assess the risk of bias in the included studies | Page 1, Abstract Section, Line 14-15 |
| Synthesis of results | 6 | Specify the methods used to present and synthesize results | Page 1, Abstract Section, Line 15 |
| Results | |||
| Included studies | 7 | Give the total number of included studies and participants and summarize relevant characteristics of studies | Page 1, Abstract Section, Line 16-17 |
| Synthesis of results | 8 | Present results for main outcomes, preferably indicating the number of included studies and participants for each. If meta-analysis was done, report the summary estimate and confidence/credible interval. If comparing groups, indicate the direction of the effect (i.e., which group is favored) | Page 1, Abstract Section, Line 17-20 |
| Discussion | |||
| Limitations of evidence | 9 | Provide a brief summary of the limitations of the evidence included in the review (e.g., study risk of bias, inconsistency, and imprecision) | Page 1, Abstract Section, Line 22-23 |
| Interpretation | 10 | Provide a general interpretation of the results and important implications | Page 1, Abstract Section, Line 21-22 |
| Other | |||
| Funding | 11 | Specify the primary source of funding for the review | Page 2, Abstract Section, Line 24-25 |
| Registration | 12 | Provide the register name and registration number | Page 2, Abstract Section, Line 24 |
|
| |||
| PRISMA 2020 manuscript checklist | |||
|
| |||
| Title | |||
| Title | 1 | Identify the report as a systematic review | Page 1, Title Section, Line 1-3 |
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for the abstract checklist | Page 1-2 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge | Page 3, Introduction Section, Line 34-50 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses | Page 3, Introduction Section, Line 51-53 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses | Page 4, Material and Methods Section, Line 64-71 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted | Page 5, Material and Methods Section, Line 83-84 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used | Page 5, Material and Methods Section, Line 84-86 +Supplementary files |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, | Page 5, Search Strategy and Study Selection Section, Line 87-92 |
| whether they worked independently, and if applicable, details of automation tools used in the process | |||
|
| |||
| PRISMA 2020 manuscript checklist | |||
|
| |||
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process | Page 5, Search Strategy and Study Selection Section, Line 93-98 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, and analyses), and if not, the methods used to decide which results to collect | Page 4-5, Data Collection Process Section, Line 72-81 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics and funding sources). Describe any assumptions made about any missing or unclear information | Page 4-5, Data Collection Process Section, Line 72-81 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process | Page 5-6, Assessment of Risk Bias Section, Line 99-105 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio and mean difference) used in the synthesis or presentation of results | Page 6, Effect Measures Section, Line 111-116 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)) | Page 6, Synthesis of Results and Statistical Analysis Section, Line 118-120 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions | Page 6, Synthesis of Results and Statistical Analysis Section, Line 120-123 | |
| 13c | Describe any methods used to tabulate or visually display the results of individual studies and syntheses | Page 6, Synthesis of Results and Statistical Analysis Section, Line 120-123 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used | Page 6-7, Synthesis of Results and Statistical Analysis Section, Line 124-129 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis and meta-regression) | N/A | |
| 13f | Describe any sensitivity analyses conducted to assess the robustness of the synthesized results | Page 6-7, Synthesis of Results and Statistical Analysis Section, Line 125-127 | |
| Reporting bias assessment | 14 | Describe any methods used to assess the risk of bias due to missing results in a synthesis (arising from reporting biases) | Page 6, Methods Section, Line 106-110+Supplementary file |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome | Page 6, Methods Section, Line 106-110+ Supplementary file |
| Results | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram | Page 7, PRISMA Section, Line 132-140+ Figure 1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded | Page 7, PRISMA Section, Line 132-140+ Figure 1 | |
| Study characteristics | 17 | Cite each included study and present its characteristics | Page 12, Results Section, Line 179-192 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study | Page 12, Quality Assessment Section, Line 194-198+Figure 2 |
|
| |||
| PRISMA 2020 manuscript checklist | |||
|
| |||
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots | Page 9-11, Table 1 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies | Page 14, Line 212-232+Figure 3 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect | Page 14, Line 212-232+Figure 3 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results | Page 14, Line 228-232 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results | N/A | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed | Page 13, Line 204-211 + Supplementary file |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed | Page 13, Line 204-211+Supplementary file |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence | Page 17-19, Discussion Section, Line 253-322 |
| 23b | Discuss any limitations of the evidence included in the review | Page 20, Strengths and Limitation Section, Line 324-328 | |
| 23c | Discuss any limitations of the review processes used | Page 20, Strengths and Limitation Section, Line 324-328 | |
| 23d | Discuss the implications of the results for practice, policy, and future research | Page 20, Conclusion Section, Line 328-329 | |
| Other information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered | Page 2, Line 24 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared | Page 2, Line 24 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol | N/A | |
| Support | 25 | Describe sources of financial or nonfinancial support for the review, and the role of the funders or sponsors in the review | Page 20, Line 337 |
| Competing interests | 26 | Declare any competing interests of review authors | Page 20, Line 338 |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; and any other materials used in the review | Data are publicly available and can be found in the articles of included studies |
*N/A: Not available. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021;372:n71
Supplementary Table 4.
Grades of recommendation, assessment, development, and evaluation evidence profile
| Outcome | Number of participants (studies) | Quality assessment | Summary findings | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| NOS | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence | OR total | 95% CI (lower–upper) | ||
| Prenatal diagnosis | Case: 79 Control: 44 (3 studies) | Not serious | Seriousa | Not serious | Seriousb | Not seriousc | Moderate | 1.17 | (0.31–4.34) |
| Liver herniation | Case: 473 Control: 213 (4 studies) | Not serious | Seriousa | Not serious | Seriousb | Not seriousc | Moderate | 3.24 | (1.64–6.39) |
| Herniation size C/D | Case: 524 Control: 183 (3 studies) | Not serious | Not serious | Not serious | Seriousb | Not seriousc | Moderate | 7.12 | (3.46–14.65) |
| ECMO | Case: 342 Control: 409 (6 studies) | Not serious | Not serious | Not serious | Seriousb | Not seriousc | Moderate | 6.05 | (4.51–8.12) |
| Patch repair | Case: 574 Control: 162 (5 studies) | Not serious | Not serious | Not serious | Seriousb | Not seriousc | Moderate | 5.07 | (3.89–6.62) |
aThere are some studies favoring control and others favoring case groups; therefore, the results depict inconsistent findings, bEffect estimates come from a small number of studies (only three to six studies included in each outcome), cPublication bias was assessed qualitatively, and no unpublished studies were found in the literature search, thus not affecting the publication bias. ECMO: Extracorporeal membrane oxygenation, OR: Odds ratio, CI: Confidence interval, NOS: Newcastle–Ottawa Scale, C/D: Size of herniated defect classified as C or D
Supplementary Table 2.
Participant, intervention or exposure, comparator, outcomes, time, setting, and study design
| Category | Descriptions |
|---|---|
| Patients | Live-born infants with congenital diaphragmatic hernia, either right/left-sided or bilateral, or either Bochdalek or Morgagni or total agenesis |
| Intervention or exposure | Predictors of feeding tube placement |
| Prenatal diagnosis | |
| Liver herniation | |
| Herniation size C/D | |
| ECMO | |
| Patch repair | |
| Comparator | No exposure of predictors |
| Outcomes | Neonates undergo invasive tube placement during hospitalization (gastrostomy, jejunostomy, or gastrojejunostomy) |
| Time | No publication year restrictions |
| Setting | Subjects visiting medical facilities |
| Study design | Observational studies (retrospective cohort studies/retrospective reviews of medical records or case–control study) |
ECMO: Extracorporeal membrane oxygenation, C/D: Size of herniated defect classified as C or D
Supplementary Table 3.
Search strategy
| Database | Query | Results |
|---|---|---|
| MEDLINE | “Hernias, Diaphragmatic, Congenital”[Mesh] | 4562 |
| Filter: Full text | ((((“Prenatal Diagnosis”[Mesh]) OR “Extracorporeal Membrane Oxygenation”[Mesh]) OR “Internal Hernia”[Mesh]) OR “Hypertension, Pulmonary”[Mesh]) OR “Gastroesophageal Reflux”[Mesh] | 143,354 |
| ((“Gastrostomy”[Mesh]) OR “Jejunostomy”[Mesh]) OR “Gastroenterostomy”[Mesh] | 21, 825 | |
| #1 AND #2 AND #3 | 79 | |
| ProQuest | “Congenital Diaphragmatic Hernia” | 5266 |
| Filter: Full text, document type: Article | (“Prenatal Diagnosis”) OR (“Extracorporeal Membrane Oxygenation”) OR (“Liver Herniation”) OR (“Pulmonary Hypertension”) OR (“Gastroesophageal Reflux”) OR (“chromosomal anomaly”) OR (“patch repair”) | 139,247 |
| (“Gastrostomy”) OR (“Jejunostomy”) OR (“Gastroenterostomy”) | 25,124 | |
| #1 AND #2 AND #3 | 103 | |
| Science direct Filter: Research articles, medicine and | “Congenital Diaphragmatic Hernia” | 18,065 |
| dentistry | (“Prenatal Diagnosis”) OR (“Extracorporeal Membrane Oxygenation”) OR (“Liver Herniation”) OR (“Pulmonary Hypertension”) OR (“Gastroesophageal Reflux”) OR (“chromosomal anomaly”) OR (“patch repair”) | 86,517 |
| (“Gastrostomy”) OR (“Jejunostomy”) OR (“Gastroenterostomy”) | 5679 | |
| #1 AND #2 AND #3 | 28 | |
| Google Scholar Filter: Advance search: With all the words | “Congenital Diaphragmatic Hernia”, “Feeding tube”, “Gastrostomy”, “Jejunostomy” | 47 |
| Cochrane Library | “Congenital Diaphragmatic Hernia” | 19,919 |
| Filter: Title, Abstract, Keyword, Trials | (“Prenatal Diagnosis”) OR (“Extracorporeal Membrane Oxygenation”) OR (“Liver Herniation”) OR (“Pulmonary Hypertension”) OR (“Gastroesophageal Reflux”) OR (“chromosomal anomaly”) OR (“patch repair”) | 27,653 |
| (“Gastrostomy”) OR (“Jejunostomy”) OR (“Gastroenterostomy”) | 8691 | |
| #1 AND #2 AND #3 | 26 | |
| Total | 283 | |
All authors performed article screening and retrieval. Every study that was obtained was imported into the Mendeley Reference Manager application. After examining the studies for duplication, the titles and abstracts were screened. The authors evaluated each paper independently and excluded any that did not fit the review’s requirements. Using the previously mentioned eligibility criteria, the chosen papers were reviewed in full-text evaluation, and relevant articles were added to the review. The authors resolved the observed differences.
Data collection process
After the included studies were examined, the data extracted were the first author, year of publication, country, study design, data source, participants’ demographic (including number of population and gender), number of exposed/unexposed populations, prenatal/antenatal/postnatal predictors, type of feeding tubes, time of feeding tube placement, OR, 95% CI, and P value. The data extraction was performed independently by all authors in Microsoft Excel.
Assessment of risk bias/quality assessment
The quality of each study was assessed using the Newcastle–Ottawa Scale (NOS) for cohort studies. The NOS tools comprised several categories for selection, comparability, and outcome or exposure. There is a star system that ranges from zero to nine stars. The overall score determined the thresholds: seven to nine stars indicated “Low risk of bias,” four to six indicated “Unclear risk of bias,” and three or fewer indicated “High risk of bias.” Every author assessed each article independently, and any differences were discussed until a consensus was reached.[11,12]
Confidence in cumulative evidence
Grades of Recommendations, Assessment, Development, and Evaluation (GRADE) were used to assess evidence certainty for each outcome. It considered study flaws, applicability, variation in results, precision, and publication bias. Evidence certainty was rated high, moderate, low, or very low.
Effect measures
The measures of all predictors were ORs that were calculated from the population number of exposed/unexposed groups. P value and 95% confidence interval (CI) were included for each item to show the results’ significance. P ≤ 0.05 was statistically significant, indicating a strong association between the predictor and the likelihood of feeding tube placement in infants with CDH. The data summary was constructed by all authors.
Synthesis of results and statistical analysis
Predictors were tabulated for each outcome to assess eligibility for synthesis. ORs determined effect sizes, calculated from exposed/nonexposed populations. Studies lacking these data were excluded.[13] Meta-analyses employed a random-effects model to accommodate variations in techniques. This model, using the inverse variance and Mantel–Haenszel methods, provided combined effect measures for predictor exposures, assuming exposure distribution across specific populations.
The I2 statistic assessed study heterogeneity, with values categorized as low (<25%), moderate (25%–50%), and high (>50%). Sensitivity analysis by deletion was initially performed as planned; however, the restricted number of studies available limited the credibility of the outcomes. Consequently, the sensitivity analysis was opted from the final analysis. Forest plots visually presented results, and funnel plots analyzed publication bias. Review Manager 5.4 performed synthesis and meta-analysis. All statistical analyses were done thoroughly by all authors.
RESULTS
Preferred Reporting Items for Systematic Reviews and Meta-analyses
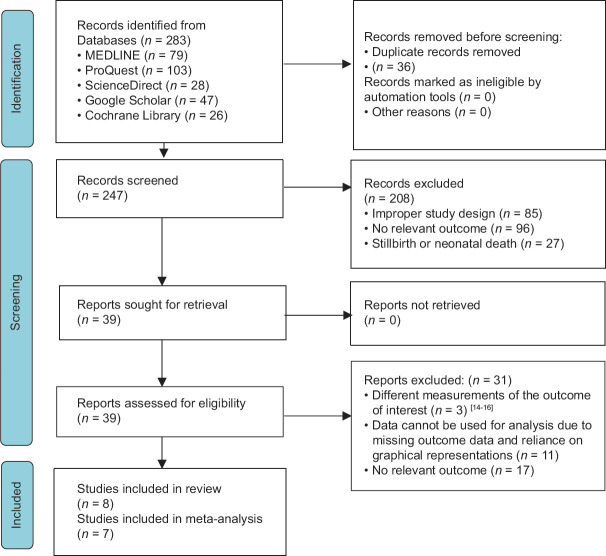
Following keyword searching, the search strategy produced 283 potentially pertinent studies [Figure 1]. Among 283 papers, 208 were excluded due to improper study design (n = 85), no relevant outcome, stillbirth/neonatal death (n = 96), and stillbirth/neonatal death (n = 27). Only 39 full-access articles were retrieved according to the eligibility criteria. Furthermore, three studies were excluded due to different measurement outcomes (n = 3), missing outcome data (n = 11), and no relevant outcome (n = 17).[14,15,16] Eight papers were included in the systematic review, but seven papers were only included in the meta-analysis. One paper was excluded from the meta-analysis due to not giving the number of exposed/unexposed groups.[17]
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 flow diagram of included studies
Table 1 lists the characteristics of the studies. Infants that were included in the study mainly come from developed countries and from tertiary care hospitals where neonatal care intensive units might be present. Wong et al.,[7] Rudra et al.,[19] and Fleming et al.,[21] utilized ultrasound techniques for prenatal/antenatal diagnosis of CDH, and all of the remaining studies utilized clinical and imaging findings for postnatal diagnosis of CDH. Among the studies examined, only those by Janssen et al.,[18] Rudra et al.,[19] Wong et al.,[7] and Fleming et al.[21] provided specific data on Morgagni hernia cases. Fleming et al.[21] reported two cases of Morgagni hernia, while Janssen et al.,[18] Rudra et al.,[19] Wong et al.[7] did not include cases of Morgagni. The remaining studies did not differentiate between Bochdalek, Morgagni, and total agenesis types of CDH.[17,20,22,23] Prenatal diagnosis, liver herniation, size of herniated defects, ECMO, and patch repair were the outcomes assessed that had the same outcome measure. Predictors were assessed from the medical records, registry, and research database. The majority of the studies observed patients for 1–2 years. The gastrostomy tube, jejunostomy tube, and gastrojejunostomy tube were feeding tubes that were utilized.
Table 1.
Study characteristics included in the analysis
| Author, year of publication, country | Types of study | Participants data source | Congenital diaphragmatic hernia type | Participants demographic | Prenatal/antenatal/postnatal predictors | Predictors assessment | Time of feeding tube placement and observation period | Type of feeding tube (%) | Implications of feeding tube placement | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Population included in the study (n) | Male and female gender, n (%) | |||||||||
| Pierog et al., 2014, United States[17] | Retrospective cohort study | NICU patient with CDH in a tertiary care pediatric hospital from January 1, 2007, to June 30, 2012 | Morgagni type, Bochdalek type Total Agenesis Each number population was not explicitly stated | 72 | Male: 37 (51) Female: 35 (49) | ECMO treatment | Medical records, DHREAMS | Time of placement: N/A Observation: Up to 1 year | Gastrostomy tube (70), jejunostomy (15), gastrojejunostomy tube (15) | Among nine patients with gastrostomy tubes, 2 (22.2%) underwent fundoplication at 1 year of age |
| Janssen et al., 2018, The Netherlands[18] | Retrospective cohort study | Surviving neonates who were treated in Amalia Children’s Hospital had complete information on surgical approach, type of repair, and ECMO treatment, born between January 2000 and March 2014 | Bochdalek type only Morgagni type and total agenesis were excluded | 112 | Male: 69 (62) Female: 43 (38) | Liver herniation, primary/patch repair, ECMO treatment | Medical records and surgical reports | Time of placement: 324 (36–864) days Observation: Up to 2 years | Gastrostomy tube (100) | Postfundoplication gastrostomy placement exhibited a nearly 3-fold increase following patch repair versus primary repair for severe GER |
| Rudra et al., 2016, United States[19] | Retrospective cohort study | Infants with CDH cared for at the Duke University Medical Center ICN from 1997 to 2013 | Morgagni type, Bochdalek type Total agenesis was excluded Each number population was not explicitly stated | 85 | Male: 58 (68) Female: 27 (32) | Prenatal diagnosis, ECMO treatment | Medical records | Time of placement: After hernia repair surgery (26, 93, and 159 days) Observation: Up to 1 year | Gastrostomy tube (100) | Infants with G-tubes exhibited a higher rate of in-hospital GER treatment (68% receiving PPIs) and a prolonged initial hospitalization compared to those without G-tubes |
| Wong et al., 2019, Canada[7] | Retrospective study | Infants with CDH treated at a single institution from January 1, 2000, to December 31, 2013 | Bochdalek type only Morgagni type and total agenesis were excluded | 160 | N/A | Prenatal diagnosis, liver herniation, patch repair, and ECMO treatment | N/A | Time of placement: N/A Observation: Up to 1 year | Gastrostomy tube (100) | With a mean discontinuation time of 2.31 years, tube feeding cessation rates did not differ between full and partial feeding groups. After 3 years, 50% of patients had discontinued, with three patients ending after 4 years |
| Verla et al., 2020, United States[20] | Retrospective review of medical records | All neonates treated for CDH at TCH, Houston, TX, USA, from February 2004 to May 2017 | Morgagni type, Bochdalek type Total agenesis Each number population was not explicitly stated | 188 | Male: 102 (54) Female: 86 (46) | Liver herniation, defect size C/D, patch repair, and ECMO treatment | Medical records | Time of placement: 168 (105–252) days Observation: Up to 6 months | Gastrostomy tube, gastrojejunostomy tube (% not specified) | G-tube placement was associated with delayed feeding achievement, extended hospitalization, and a requirement for supplemental enteral feeding |
| Fleming et al., 2021, United States[21] | Retrospective cohort study | Surviving infants with CDH at CHCO neonatal intensive care unit from 2010–2019 | Morgagni type, Bochdalek type Total agenesis was excluded Each number population was not explicitly stated | 2 Morgagni type 112 Bochdalek type | Male: 65 (57) Female: 49 (43) | Prenatal diagnosis, herniation size C/D, liver herniation, and ECMO treatment | Medical records | Time of placement: N/A Observation: N/A | Gastrostomy tube (100) | G-tube neonates exhibited lower Apgar scores, prolonged hospitalization, larger birth defects (C or D), and a higher prevalence of flap closures |
| Prieto et al., 2021, United States[22] | Retrospective cohort study | All live-born patients with CDH at participating tertiary referral centers, between 2007 and 2019, and surviving to hospital discharge. (Japan, Spain, England, Canada, Italy, Poland, the Netherlands, Australia, Scotland, Sweden, Malaysia, Italy, and Russia) | Morgagni type, Bochdalek type Total agenesis Each number population was not explicitly stated | 4537 | Male: 2693 (59) Female: 1844 (41) | Liver herniation, defect size C/D, patch repair, and ECMO treatment | The CDH study group database (CDH Registry) | Time of placement: 80 (57–108) days Observation: Up to 1 year | Gastrostomy tube, gastrojejunostomy tube | Enteral access procedures correlated with markers of severe conditions (right-sided defects, liver herniation, increased defect size, a prolonged supplemental oxygen requirement, and ECMO therapy) and increased comorbidity burden (chromosomal anomalies, cardiac defects, and GER) |
| Schwab et al., 2021, United States[23] | Retrospective study | All patients with a CDH who underwent surgical repair at UCSF Benioff Children’s Hospital between 2012 and 2020 | Morgagni type, Bochdalek type Total agenesis Each number population was not explicitly stated | 101 | Male: 44 (44) Female: 57 (56) | ECMO treatment and Patch/primary repair | N/A | Time of placement: 67 (50–88) days Observation: Up to 1 year | Gastrostomy tube (100) | Neonates requiring gastrostomy tubes exhibited lower Apgar scores, increased surgical interventions, prolonged ventilation dependence, delayed enteral initiation, and extended hospitalization |
NICU: Neonatal intensive care unit, ECMO: Extracorporeal membrane oxygenation treatment, CDH: Congenital diaphragmatic hernia, TCH: Texas Children's Hospital, CHCO: Children's Hospital Colorado, DHREAMS: Diaphragmatic Hernia Research and Exploration Advancing Molecular Science, GER: Gastroesophageal reflux, N/A: Not available, ICN: Intensive care nursery, PPIs: Proton Pump Inhibitors, UCSF: University of California San Francisco, C/D: Size of herniated defect classified as C or D
Quality assessment
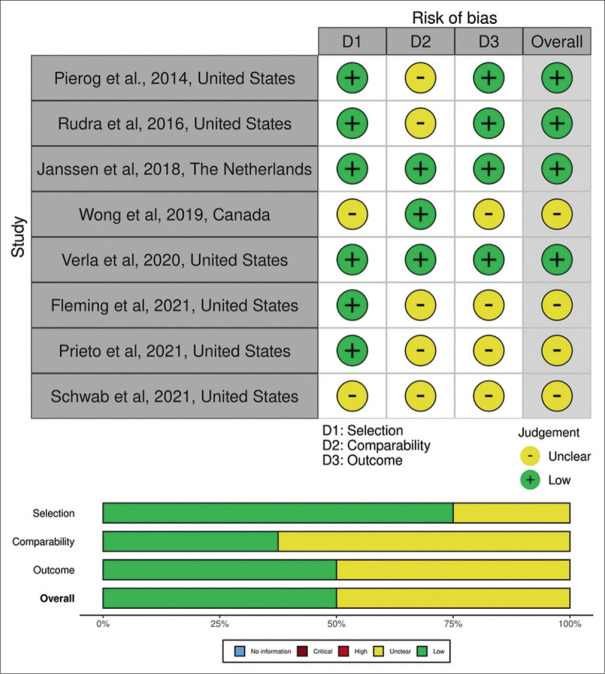
The Newcastle–Ottawa Quality Assessment Scale was used to evaluate each study. Eight studies were analyzed, with four exhibiting a low risk of bias[17,18,19,20] and the other categorized as moderate.[7,21,22,23] The overall results of the study quality assessment in the form of a visual traffic-light plot are shown in Figure 2, respectively.
Figure 2.
Risk-of-bias assessment and domain summary of included studies illustrated with Robvis tool
Confidence in cumulative evidence
According to the NOS assessment, there was a low-to-moderate risk of bias in the investigated studies, meaning that conceivable bias was unlikely to have a major impact on the outcomes. In prenatal diagnosis and liver herniation predictors, some studies favor control and others favor case groups, depicting inconsistent findings. Publication bias assessment was done qualitatively due to insufficient data. While imprecision was seen in all of the outcomes due to the minimum sample size, there was no discernible indirectness. Eventually, the GRADE profile showed a medium quality of evidence, as illustrated in the Supplementary Tables 1-4.
Data extraction
Except for the study by Pierog et al.,[17] every study provided the number of exposed and unexposed individuals [Figure 3]. The OR was generated from the calculation of the number of exposed/unexposed groups. Studies that have the same predictor reported are classified into the same group.
Figure 3.
Meta-analysis results for prenatal, antenatal, and postnatal predictors of feeding tube placement in infants with congenital diaphragmatic hernia (forest plot). *Event = Predictor Exposure + Feeding Tube Placement
Final results
The analysis was shown in the forest plot and revealed significant effects for each predictor of tube placement such as liver herniation (OR = 3.24, 95% CI 1.64–6.39, P = 0.0007), size of herniated defects classified as C or D (OR = 7.12, 95% CI 3.46–14.65, P < 0.00001), ECMO treatment (OR = 6.05, 95% CI 4.51–8.12, P < 0.00001), and patch repair (OR = 5.07, 95% CI 3.89–6.62, P < 0.00001) [Figure 3]. These results suggest that there are robust associations between those predictors to the feeding tube placement. However, prenatal diagnosis did not show statistically significant overall effects (OR = 1.17, 95% CI 0.31-4.34, P = 0.82), indicating that there is no strong evidence for an association between prenatal diagnosis and feeding tube placement.
The ECMO treatment and patch repair as predictors for feeding tube placement was considered to have a low heterogeneity, with I2 results of 6% (P = 0.38) and 13% (P = 0.33), respectively. On the other hand, prenatal diagnosis, liver herniation, and herniation size C/D as predictors were considered to have moderate to high levels of heterogeneity, with I² values of 84% (P = 0.002), 68% (P = 0.02), and 78% (P = 0.01), respectively.
The publication bias was represented as a funnel plot diagram and shown in Figure 4. The funnel plot diagrams of prenatal, antenatal, and postnatal predictors of feeding tube placement in infants with CDH showed that the distribution of studies was symmetric, with studies generally distributed evenly around the estimated average effect size. The position of the papers in the funnel plot increases as SE decreases, showing high power in comparison to other papers.
Figure 4.
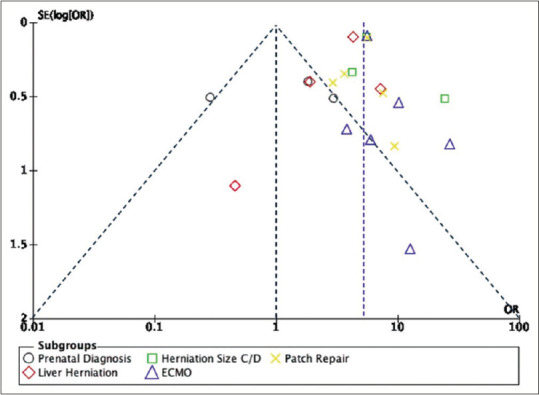
Publication bias as funnel plot diagram for prenatal, antenatal, and postnatal predictors of feeding tube placement in infants with congenital diaphragmatic hernia
The small number of relevant studies made it difficult to perform a thorough sensitivity assessment, even though a sensitivity analysis was taken into consideration to gauge the reliability of our findings. Our capacity to carry out a meaningful sensitivity analysis was limited since the sensitivity analysis led to the exclusion of one study, reducing the dataset to only two or three studies for analysis.
DISCUSSION
CDH newborns exhibit a 25% higher calorie intake, highlighting the prevalent issue of feeding intolerance, necessitating advancements in enteral feeding.[15,16] In the review, all studies incorporated a range of feeding tube procedures. The primary indication for feeding tube placement across the studies revolved around the inability to achieve adequate oral nutrition or transition from nasogastric tube feedings. This is due to feeding intolerance, presumed to be associated with reflux and/or dysmotility.[17,22] Male-to-female ratios in studies like Rudra et al.[19] [68:32], Janssen et al.[18] [62:38], Prieto et al.[22] [59:41], Fleming et al.[21] [57:43], and Verla et al.[20] [54:46] varied. Sferra et al.’s 2023 study with 7288 CDH patients, including 41.8% of females, revealed higher incidences of intrathoracic liver herniation and pulmonary hypertension in females. Females also exhibited shorter survival rates, leading to potential challenges in follow-up due to higher mortality rates.[24]
Furthermore, the timing of feeding tube placement varied, ranging from the earliest reported by Schwab et al.[23] (67 days) to the latest by Janssen et al.[18] (324 days), and the follow-up periods ranged from 6 months to 1 year, with several studies by Fleming et al.[21] and Prieto et al.[22] did not state the duration. This could potentially limit the ability to observe long-term predictive factors of tube feeding requirements. All papers were considered retrospective observational studies, some took the form of cohort,[17,18,19,20,21,22] and others were designed as a review of the medical record.[7,20] The types of CDH contribute to some degree of heterogeneity. For example, Morgagni hernias, characterized by a defect at the anterior part of the diaphragm, are often associated with Down syndrome featuring hypotonia, impaired coordination of swallowing muscles, and structural anomalies in the oropharynx and esophagus. These factors disrupt the oral phase of swallowing, predisposing individuals to aspiration or incomplete bolus formation, and increasing the likelihood of feeding tube placement.[25,26]
Our study found that diagnosis of CDH prenatally has no association with the need for feeding tube placement (OR = 1.17, 95% CI 0.31–4.34, P < 0.002), which contrasts with studies by Wong et al.,[7] Rudra et al.,[19] and Fleming et al.[21] This finding is possibly due to variations in herniation size, stomach–liver position, percent predictive lung volume (PPLV), total lung volume (TLV), lung-to-head ratio (LHR), and observed/expected lung-to-head ratio (O/E LHR) across study populations. These parameters were measured prenatally using fetal ultrasound and MRI, with cutoff values indicative of more severe lung issues if PPLV <21%, TLV <30 ml, LHR <1.2, and O/E LHR <55%. Differences in these parameters between study populations may contribute to the varying likelihoods of feeding tube placement observed in meta-analyses.[21]
Liver herniation in CDH exhibits a strong association with feeding tube placement (OR = 3.24, 95% CI 1.69–6.39, P < 0.002). This suggests a 3.24 times higher likelihood of requiring feeding tubes compared with no liver herniation, which aligns with Fleming et al.[21] and Prieto et al.[22] Liver herniation linked to pulmonary hypoplasia and hypertension, disrupts parallel liver and lung growth, impacting physiological function and necessitating feeding tubes.[27] Similarly, the size of herniated defects classified as C or D is associated with feeding tube placement (OR = 7.12, 95% CI 3.46–14.65, P < 0.00001). This result is consistent with Fleming et al.,[20] Verla et al.,[21] and Prieto et al.[22] A “Size C defect” signifies a diaphragmatic absence exceeding 50%, while a “Size D defect” indicates a complete absence of the hemidiaphragm. Size C and D defects indicate a more extensive herniation of abdominal contents, which often correlates with more severe respiratory distress and a higher likelihood of feeding tube placement compared to size A (<10% diaphragmatic tissue absence) and B (<50% diaphragmatic tissue absence) defects.[27]
Infants with CDH who underwent ECMO are significantly more prone to feeding tube placement (OR = 6.05, 95% CI 4.51–8.12, P < 0.00001). This finding suggests that infants who underwent ECMO are 6.05 times more likely to necessitate feeding tube placement, consistent with the results reported by Jaillard et al.[28] Infants with CDH who exhibit challenges in maintaining preductal saturation >85%, postductal saturation >70%, peak inspiratory pressure >28 cm H2O or mean airway pressure >17 cm H2O, respiratory and metabolic acidosis often necessitate ECMO support. However, ECMO results in weariness and weakening of the muscles as well as a delay in the preparedness for oral feeding due to prolonged immobilization and physiological stress. Consequently, the placement of tube feedings helps optimize nutritional intake, supporting both growth and recovery.[29]
Our findings suggest that the placement of feeding tubes is significantly correlated with CDH infants who had patch repair surgery (OR = 5.07, 95% CI 3.89–6.62, P < 0.00001). Infants who undergo patch repair procedures are over five times more likely to necessitate gastrostomy or other feeding tube interventions. Our study aligns with Bourezma et al., highlighting a significant association. The mechanism underlying this process is that patch repair alters diaphragmatic function and affects the ability of infants to breathe and swallow, influencing the oral feeding process, thus feeding tube placement might help to keep nutritional adequacy.[30]
The possible reason for the studies’ heterogeneity is that studies might have varied populations in terms of gestational age, prenatal care, or severity of diaphragmatic hernia. Overall, the funnel plots exhibit a symmetric distribution of studies. This symmetry suggests that publication bias is unlikely to substantially influence the results. Moreover, the spread of the study along the funnel plots gives insight into the higher precision and statistical power of the included studies in Figure 4.
Strengths and limitations of the study
The study’s strength lies in its comprehensive approach, analyzing various predictors and exploring potential variations across clinical scenarios. However, acknowledging limitations, including high heterogeneity in results due to diverse CDH types like Bochdalek and Morgagni hernias, is crucial. Future research should prioritize diverse populations, fostering collaborative efforts among multiple centers for a larger sample size.
CONCLUSION
ECMO treatment and patch repair surgery are robust predictors of feeding tube placement in CDH infants. Although liver herniation and the size of the herniated defect C/D are also more likely to require feeding tube placement, further studies are needed to address heterogeneity concerns. Understanding these predictors offers valuable insights for clinical decision-making and avoids needless lengthy hospital stays.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors extend sincere gratitude to the esteemed colleagues within the Department of Pediatric Surgery, notably neonatal surgery, pediatric gastroenterology surgery, and pediatric urogenital surgery division, at Padjadjaran University. In addition, we express our appreciation to the Atma Jaya University of Indonesia for their invaluable support and contributions to this work.
REFERENCES
- 1.Dumpa V, Chandrasekharan P. Treasure Island (FL): StatPearls Publishing; 2023. [[Last accessed on 2023 Oct 25]]. Congenital Diaphragmatic Hernia. Availabe form: http://www.ncbi.nlm.nih.gov/books/NBK556076/ [PubMed] [Google Scholar]
- 2.Burgos CM, Frenckner B. Addressing the hidden mortality in CDH: A population-based study. J Pediatr Surg. 2017;52:522–5. doi: 10.1016/j.jpedsurg.2016.09.061. [DOI] [PubMed] [Google Scholar]
- 3.Hagadorn JI, Brownell EA, Herbst KW, Trzaski JM, Neff S, Campbell BT. Trends in treatment and in-hospital mortality for neonates with congenital diaphragmatic hernia. J Perinatol. 2015;35:748–54. doi: 10.1038/jp.2015.46. [DOI] [PubMed] [Google Scholar]
- 4.Basurto D, Russo FM, Van der Veeken L, Van der Merwe J, Hooper S, Benachi A, et al. Prenatal diagnosis and management of congenital diaphragmatic hernia. Best Pract Res Clin Obstet Gynaecol. 2019;58:93–106. doi: 10.1016/j.bpobgyn.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Braegger C, Decsi T, Dias JA, Hartman C, Kolacek S, Koletzko B, et al. Practical approach to paediatric enteral nutrition: A comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2010;51:110–22. doi: 10.1097/MPG.0b013e3181d336d2. [DOI] [PubMed] [Google Scholar]
- 6.Löser C, Aschl G, Hébuterne X, Mathus-Vliegen EM, Muscaritoli M, Niv Y, et al. ESPEN guidelines on artificial enteral nutrition – Percutaneous endoscopic gastrostomy (PEG) Clin Nutr. 2005;24:848–61. doi: 10.1016/j.clnu.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Wong MK, Haliburton B, Graham A, Lapidus-Krol E, Moraes TJ, Marcon MA, et al. Requirement and duration of tube feed supplementation among congenital diaphragmatic hernia patients. J Pediatr Surg. 2019;54:895–8. doi: 10.1016/j.jpedsurg.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Cordier AG, Laup L, Letourneau A, Le Sache N, Fouquet V, Senat MV, et al. Prenatal stomach position predicts gastrointestinal morbidity at 2 years in fetuses with left-sided congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2021;57:959–67. doi: 10.1002/uog.22086. [DOI] [PubMed] [Google Scholar]
- 9.Jancelewicz T, Brindle ME. Prediction tools in congenital diaphragmatic hernia. Semin Perinatol. 2020;44:151165. doi: 10.1053/j.semperi.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Page MJ, Bossuyt PM, Boutron I, Hoffman TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. [[Last accessed on 2023 Oct 25]];The BMJ. 2021 372:n71. doi: 10.1136/bmj.n71. Available from: https://www.bmj.com/content/372/bmj.n71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 12.Kadhim K, Middeldorp ME, Elliott AD, Agbaedeng T, Gallagher C, Malik V, et al. Prevalence and assessment of sleep-disordered breathing in patients with atrial fibrillation: A systematic review and meta-analysis. Can J Cardiol. 2021;37:1846–56. doi: 10.1016/j.cjca.2021.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Tenny S, Hoffman MR. Treasure Island (FL): StatPearls Publishing; 2023. [[Last accessed on 2023 Oct 25]]. Odds Ratio. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431098/ [Google Scholar]
- 14.Rosenberg J, Amaral JG, Sklar CM, Connolly BL, Temple MJ, John P, et al. Gastrostomy and gastrojejunostomy tube placements: Outcomes in children with gastroschisis, omphalocele, and congenital diaphragmatic hernia. Radiology. 2008;248:247–53. doi: 10.1148/radiol.2481061193. [DOI] [PubMed] [Google Scholar]
- 15.Herranz Barbero A, Iglesias-Platas I, Prat-Ortells J, Clotet Caba J, Moreno Hernando J, Castañón García-Alix M, et al. Transpyloric tube placement shortens time to full feeding in left congenital diaphragmatic hernia. J Pediatr Surg. 2023;58:2098–104. doi: 10.1016/j.jpedsurg.2023.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Muratore CS, Utter S, Jaksic T, Lund DP, Wilson JM. Nutritional morbidity in survivors of congenital diaphragmatic hernia. J Pediatr Surg. 2001;36:1171–6. doi: 10.1053/jpsu.2001.25746. [DOI] [PubMed] [Google Scholar]
- 17.Pierog A, Aspelund G, Farkouh-Karoleski C, Wu M, Kriger J, Wynn J, et al. Predictors of low weight and tube feedings in children with congenital diaphragmatic hernia at 1 year of age. J Pediatr Gastroenterol Nutr. 2014;59:527–30. doi: 10.1097/MPG.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 18.Janssen S, Heiwegen K, van Rooij IA, Scharbatke H, Roukema J, de Blaauw I, et al. Factors related to long-term surgical morbidity in congenital diaphragmatic hernia survivors. J Pediatr Surg. 2018;53:508–12. doi: 10.1016/j.jpedsurg.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 19.Rudra S, Adibe OO, Malcolm WF, Smith PB, Cotten CM, Greenberg RG. Gastrostomy tube placement in infants with congenital diaphragmatic hernia: Frequency, predictors, and growth outcomes. Early Hum Dev. 2016;103:97–100. doi: 10.1016/j.earlhumdev.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verla MA, Style CC, Mehollin-Ray AR, Fallon SC, Vogel AM, Fernandes CJ, et al. Prenatal imaging features and postnatal factors associated with gastrointestinal morbidity in congenital diaphragmatic hernia. Fetal Diagn Ther. 2020;47:252–60. doi: 10.1159/000501555. [DOI] [PubMed] [Google Scholar]
- 21.Fleming H, Dempsey AG, Palmer C, Dempsey J, Friedman S, Galan HL, et al. Primary contributors to gastrostomy tube placement in infants with congenital diaphragmatic hernia. J Pediatr Surg. 2021;56:1949–56. doi: 10.1016/j.jpedsurg.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Prieto JM, Harting MT, Calvo RY, Carroll JM, Sykes AG, Ignacio RC, et al. Identifying risk factors for enteral access procedures in neonates with congenital diaphragmatic hernia: A novel risk-assessment score. J Pediatr Surg. 2021;56:1130–4. doi: 10.1016/j.jpedsurg.2021.02.029. [DOI] [PubMed] [Google Scholar]
- 23.Schwab ME, Burke S, Klarich MK, Vu LT. Factors and growth trends associated with the need for gastrostomy tube in neonates with congenital diaphragmatic hernia. J Pediatr Gastroenterol Nutr. 2021;73:555–9. doi: 10.1097/MPG.0000000000003203. [DOI] [PubMed] [Google Scholar]
- 24.Sferra SR, Guo M, Salazar AJG, Penikis AB, Engwall-Gill AJ, Ebanks A, et al. Sex-Specific Differences in Congenital Diaphragmatic Hernia Mortality. The Journal of Pediatrics. 2020;259:113481. doi: 10.1016/j.jpeds.2023.113481. doi: 10.1016/j.jpeds.2023.113481. [DOI] [PubMed] [Google Scholar]
- 25.Zalla JM, Stoddard GJ, Yoder BA. Improved mortality rate for congenital diaphragmatic hernia in the modern era of management: 15 year experience in a single institution. J Pediatr Surg. 2015;50:524–7. doi: 10.1016/j.jpedsurg.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravel A, Mircher C, Rebillat AS, Cieuta-Walti C, Megarbane A. Feeding problems and gastrointestinal diseases in down syndrome. Arch Pediatr. 2020;27:53–60. doi: 10.1016/j.arcped.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Ott KC, Bi M, Scorletti F, Ranginwala SA, Marriott WS, Peiro JL, et al. The interplay between prenatal liver growth and lung development in congenital diaphragmatic hernia. Front Pediatr. 2022;10:983492. doi: 10.3389/fped.2022.983492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaillard SM, Pierrat V, Dubois A, Truffert P, Lequien P, Wurtz AJ, et al. Outcome at 2 years of infants with congenital diaphragmatic hernia: A population-based study. Ann Thorac Surg. 2003;75:250–6. doi: 10.1016/s0003-4975(02)04278-9. [DOI] [PubMed] [Google Scholar]
- 29.Dresen E, Naidoo O, Hill A, Elke G, Lindner M, Jonckheer J, et al. Medical nutrition therapy in patients receiving ECMO: Evidence-based guidance for clinical practice. J Parenter Enteral Nutr. 2023;47:220–35. doi: 10.1002/jpen.2467. [DOI] [PubMed] [Google Scholar]
- 30.Bourezma M, Mur S, Storme L, Cailliau E, Vaast P, Sfeir R, et al. Surgical risk factors for delayed oral feeding autonomy in patients with left-sided congenital diaphragmatic hernia. J Clin Med. 2023;12:2415. doi: 10.3390/jcm12062415. [DOI] [PMC free article] [PubMed] [Google Scholar]