Abstract
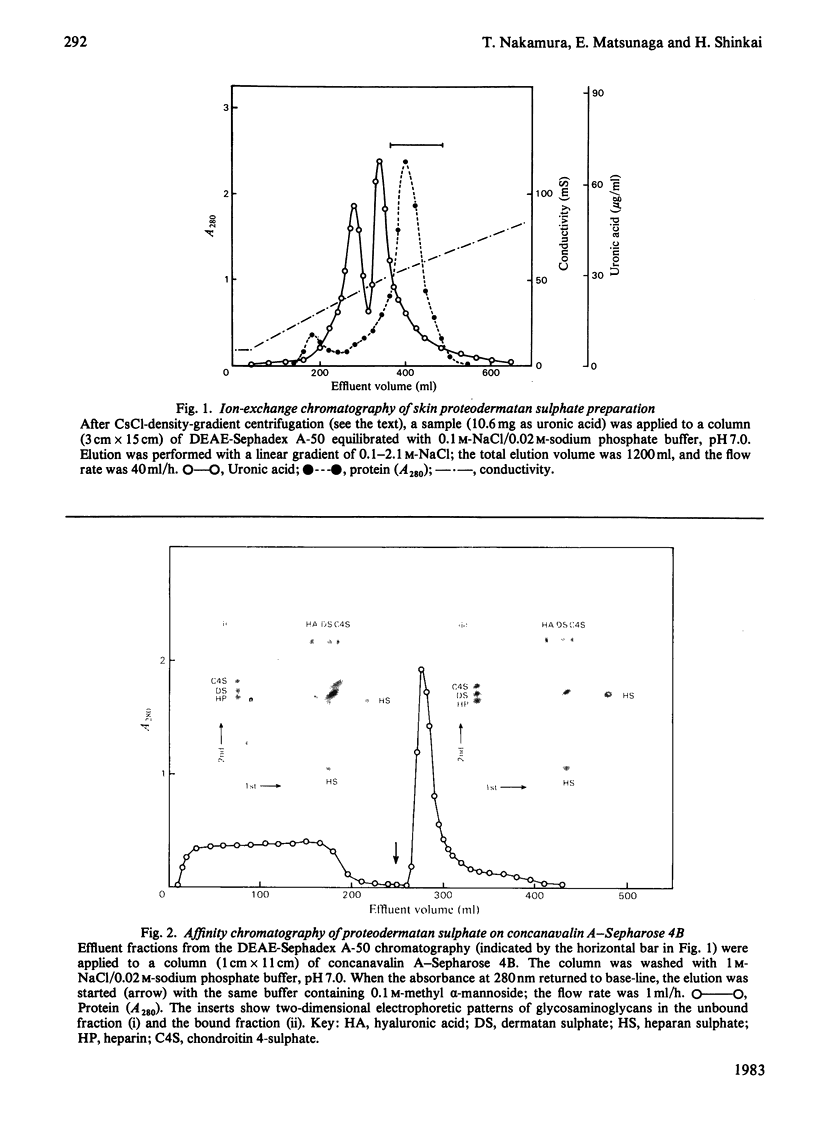
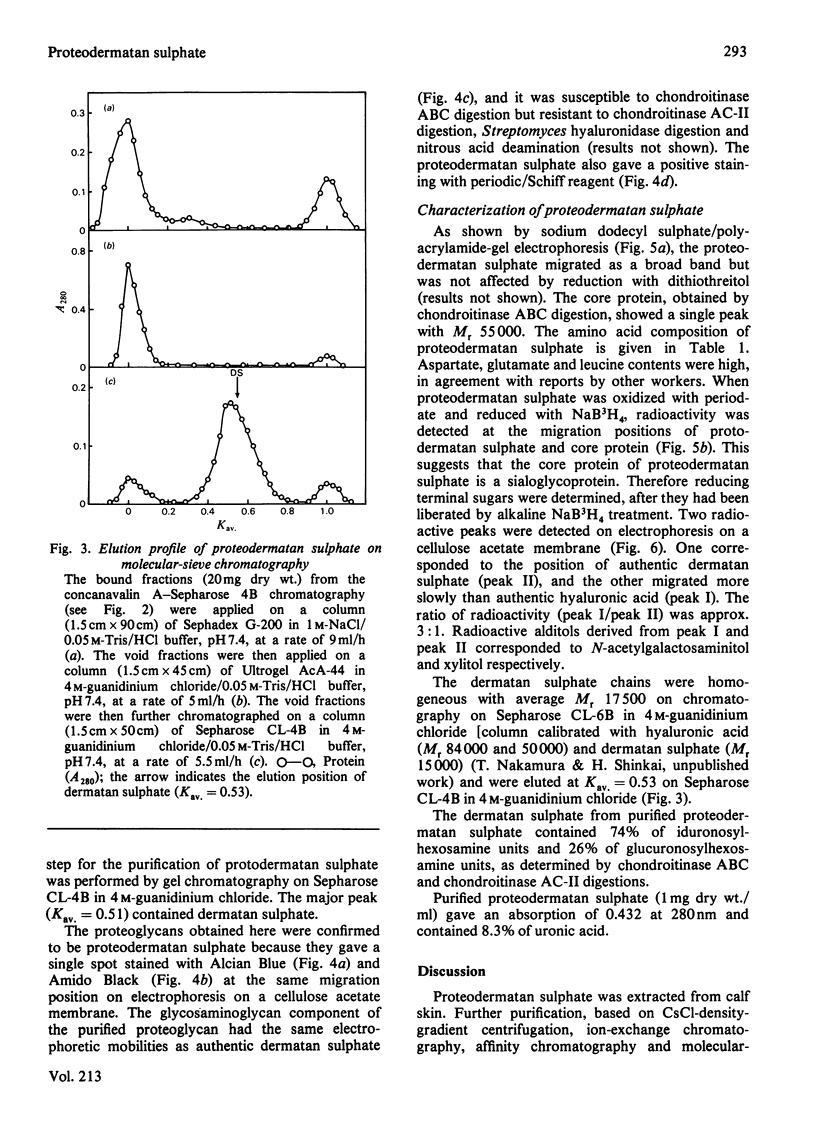
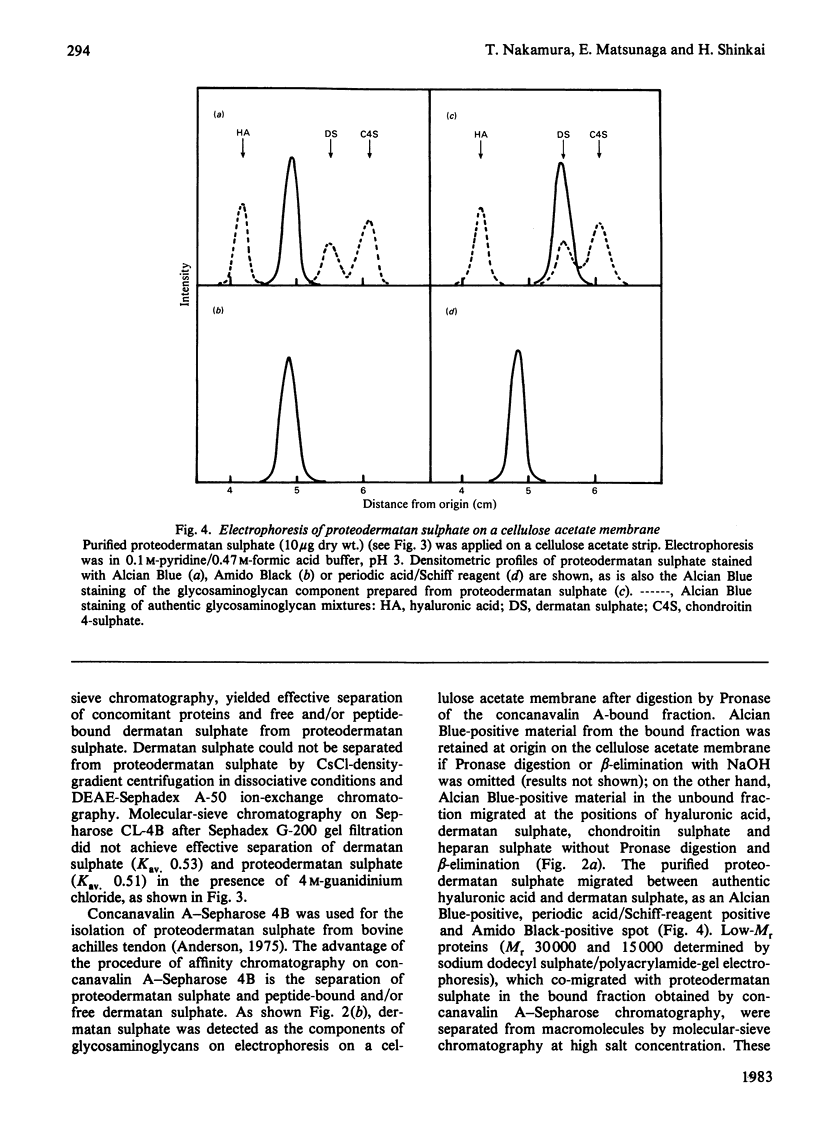
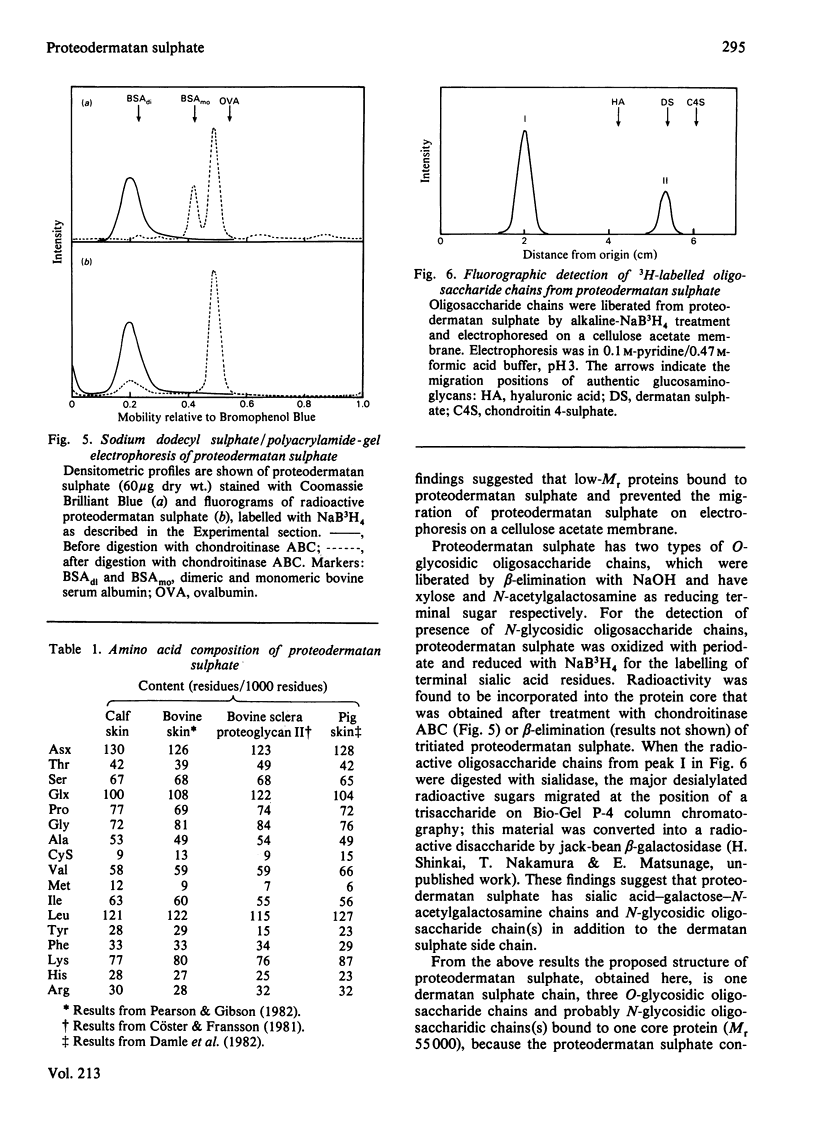
A proteodermatan sulphate was isolated from 0.15 M-NaCl and 0.45 M-NaCl extracts of newborn-calf skin. The proteoglycan was separated from collagen and hyaluronic acid by precipitation with cetylpyridinium chloride and CsCl-density-gradient centrifugation. Further purification was performed by ion-exchange, affinity and molecular-sieve chromatography. The proteoglycan bound to concanavalin A-Sepharose in 1 M-NaCl. It gave a positive reaction with periodic acid/Schiff reagent and contained 8.3% of uronic acid. The dermatan sulphate, the only glycosaminoglycan component, was composed of 74% iduronosylhexosamine units and 26% glucuronosylhexosamine units. The Mr was assessed to be 15000-20000 by gel chromatography. The core protein was found to be a sialoglycoprotein that had O-glycosidic oligosaccharides with N-acetylgalactosamine at the reducing termini. The molar ratio of oligosaccharide chains to dermatan sulphate was approx. 3:1. From these results the proposed structure of proteodermatan sulphate is: one dermatan sulphate chain (average Mr 17500), three O-glycosidic oligosaccharide chains and probably N-glycosidic oligosaccharide chain(s) bound to one core-protein molecule (Mr 55000).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. C. Isolation of a glycoprotein and proteodermatan sulphate from bovine achilles tendon by affinity chromatography on concanavalin A-Sepharose. Biochim Biophys Acta. 1975 Feb 27;379(2):444–455. doi: 10.1016/0005-2795(75)90151-8. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cöster L., Fransson L. A. Isolation and characterization of dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981 Jan 1;193(1):143–153. doi: 10.1042/bj1930143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle S. P., Cöster L., Gregory J. D. Proteodermatan sulfate isolated from pig skin. J Biol Chem. 1982 May 25;257(10):5523–5527. [PubMed] [Google Scholar]
- Ehrlich K. C., Radhakrishnamurthy B., Berenson G. S. Isolation of a chondroitin sulfate--dermatan sulfate proteoglycan from bovine aorta. Arch Biochem Biophys. 1975 Nov;171(1):361–369. doi: 10.1016/0003-9861(75)90043-0. [DOI] [PubMed] [Google Scholar]
- Fujii N., Nagai Y. Isolation and characterization of a proteodermatan sulfate from calf skin. J Biochem. 1981 Nov;90(5):1249–1258. doi: 10.1093/oxfordjournals.jbchem.a133589. [DOI] [PubMed] [Google Scholar]
- Hata R., Nagai Y. A rapid and micro method for separation of acidic glycosaminoglycans by two-dimensional electrophoresis. Anal Biochem. 1972 Feb;45(2):462–468. doi: 10.1016/0003-2697(72)90208-4. [DOI] [PubMed] [Google Scholar]
- Hata R., Nagai Y. Distribution of acidic glycosaminoglycans in tadpole back skin. Biochim Biophys Acta. 1973 Apr 28;304(2):408–412. doi: 10.1016/0304-4165(73)90260-2. [DOI] [PubMed] [Google Scholar]
- Hök M., Riesenfeld J., Lindahl U. N-[3H]Acetyl-labeling, a convenient method for radiolabeling of glycosaminoglycans. Anal Biochem. 1982 Jan 15;119(2):236–245. doi: 10.1016/0003-2697(82)90580-2. [DOI] [PubMed] [Google Scholar]
- Kapoor R., Phelps C. F., Cöster L., Fransson L. A. Bovine aortic chondroitin sulphate- and dermatan sulphate-containing proteoglycans. Isolation, fractionation and chemical characterization. Biochem J. 1981 Aug 1;197(2):259–268. doi: 10.1042/bj1970259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- Kondo K., Seno N., Anno K. Mucopolysaccharides from chicken skin of three age groups. Biochim Biophys Acta. 1971 Sep 21;244(3):513–522. doi: 10.1016/0304-4165(71)90068-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto I., Nagase S. Isolation and characterization of proteodermatansulfate from rat skin. J Biochem. 1980 Dec;88(6):1793–1803. doi: 10.1093/oxfordjournals.jbchem.a133154. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nagai Y. Developmental changes in the synthesis of glycosaminoglycans and collagen in embryonic chick skin. J Biochem. 1980 Feb;87(2):629–637. doi: 10.1093/oxfordjournals.jbchem.a132787. [DOI] [PubMed] [Google Scholar]
- Ohya T., Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970 Mar 18;198(3):607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Oike Y., Kimata K., Shinomura T., Nakazawa K., Suzuki S. Structural analysis of chick-embryo cartilage proteoglycan by selective degradation with chondroitin lyases (chondroitinases) and endo-beta-D-galactosidase (keratanase). Biochem J. 1980 Oct 1;191(1):193–207. doi: 10.1042/bj1910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C. H., Gibson G. J. Proteoglycans of bovine periodontal ligament and skin. Occurrence of different hybrid-sulphated galactosaminoglycans in distinct proteoglycans. Biochem J. 1982 Jan 1;201(1):27–37. doi: 10.1042/bj2010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter W. H. Application of nitrous acid deamination of hexosamines to the simultaneous GLC determination of neutral and amino sugars in glycoproteins. Anal Biochem. 1975 Jan;63(1):27–43. doi: 10.1016/0003-2697(75)90186-4. [DOI] [PubMed] [Google Scholar]
- SCHILLER S., SLOVER G. A., DORFMAN A. A method for the separation of acid mucopolysaccharides: its application to the isolation of heparin from the skin of rats. J Biol Chem. 1961 Apr;236:983–987. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki S., Kobata A. Microdetermination of individual neutral and amino sugars and N-acetylneuraminic acid in complex saccharides. J Biochem. 1974 Oct;76(4):783–789. [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]



