Abstract
This study showed that a severe acute respiratory syndrome coronavirus 2 infection reduced the risk of reinfection among vaccinated individuals by 0.50 (95% CI, 0.39–0.64) over a 1-year period, after accounting for unreported infections using avidity-based serology. Reciprocally, chronic symptoms increased from a baseline of 21% (95% CI, 16%–28%) among infection-naïve individuals to 43% (95% CI, 30%–61%) in reinfected individuals.
Keywords: COVID-19, Omicron, post-COVID symptoms, SARS-CoV-2, serology
This study using avidity-based serology found that SARS-CoV-2 reinfection risk decreases with prior infection, yet increases the likelihood of chronic post-COVID symptoms in fully vaccinated individuals, amid challenges tracking reinfections during the Omicron variant surge.
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) protects against reinfection by a similar viral strain but carries a risk of developing chronic post–coronavirus disease 2019 (COVID) symptoms [1]. As Omicron emerged late in 2021, tracking the cumulative health consequences of reinfections became challenging due to reduced viral testing and the fact that conventional serology lacks the ability to discern infections from reinfections [2]. Moreover, in contrast to nucleocapsid (N) immunoglobulin G (IgG) antibodies that wane, N antibody avidity is boosted after each reinfection and remains stable for months, independent of age, vaccine status, or disease severity [3–5]. Thus, changes in antibody avidity can be used to track reinfections up to a year [6]. The objectives of this study were to estimate the risk of reinfection and cumulative risk of chronic post-COVID symptoms after SARS-CoV-2 infections in a cohort of vaccinated healthy adults, accounting for unreported reinfections using avidity-based serology.
METHODS
The cohort consisted of 989 individuals nested within a larger longitudinal cohort of school staff [7, 8] who received at least 2 doses of SARS-CoV-2 vaccines before enrollment between January and April 2022 (baseline). Participants were surveyed annually to report all viral nucleic acid amplification (NAAT) or rapid antigen (RAT) testing results (with dates, type of testing, positive/negative) since the beginning of the pandemic (January 2020) through March 31, 2023. At baseline, about 27% of the school staff in the study had been infected with SARS-CoV-2, 80% were classroom staff (eg, teachers, assistants, etc.), and ∼99% received at least 2 doses of COVID-19 vaccines [8]. To give some context, the Vancouver metropolitan area in BC (British Columbia, Canada) where participants resided experienced its largest pandemic wave with Omicron BA.1/BA.2 variants between December 2021 and July 2022 where most residents of BC were infected (N seropositivity rose from <10% to >60% over this period, based on population-based serology estimates reported by the BC Centre for Disease Control) [9]. Therefore, this study was positioned to examine the health consequences of reinfections after the public SARS-CoV-2 vaccination campaign in BC, in the context of the first Omicron (BA.1/BA.2) wave.
In addition to a detailed history of SARS-CoV-2 infections documented by viral testing, participants also provided a blood sample to define their immune status between those who were never infected (referred to as the vaccine immunity group) and those with at least 1 previous infection (hybrid immunity group) at baseline, based on a positive serology (≥1.00) using the Elecsys Anti-SARS-CoV-2 antinucleocapsid assay (Roche Diagnostics). At follow-up (∼1 year later), participants provided another blood sample for serology and antibody avidity testing, and were asked to report whether they experienced new-onset chronic post-COVID symptoms lasting at least 3 months. Details of how infection statuses were classified in each individual are in the Supplementary Methods.
For post-COVID symptoms, participants were specifically asked if they had newly experienced at least 1 of the chronic symptoms detailed in the Supplementary Methods for at least 3 months in the last year (for those who never reported infection) or since their COVID-19 infection (for those who reported an infection). The sample size was set by the original cohort, with no sample size calculations a priori. The study was approved by the University of British Columbia Children's and Women's Research Ethics Board (H20-03593). Written consent was obtained from all participants.
RESULTS
The hybrid (≥2 doses of vaccine + a positive N serology at the beginning of the follow-up period, referred to as baseline; n = 198) and vaccine (≥2 vaccine doses; negative serology at baseline; n = 791) immunity groups were similar in terms of age, sex distribution, total number of vaccine doses received, number of children present in the household, presence of 1 or more preexisting comorbidities, and tobacco smoking status at follow-up (Supplementary Table 1).
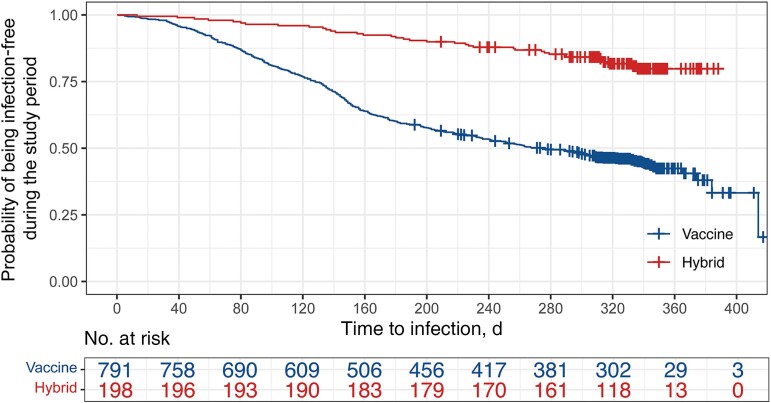
Overall, 48% (476/989) of individuals reported an infection diagnosed by viral testing (NAAT or RAT), and 3.5% (35/989) of individuals reported >1 infection during the 1-year study period. The incidence of infection during this follow-up period, by positive viral testing, remained significantly lower (19%; 95% CI, 14%–26%; n = 37/198) for the hybrid immunity group compared with the vaccine immunity group (56%; 95% CI, 51%–61%; n = 439/791; adjusted incidence ratio: 0.34; 95% CI, 0.24–0.46; P < .0001; after adjusting for age, sex, children in the household, comorbidity, and smoking). Moreover, viral test–confirmed infections were delayed in the hybrid compared with the vaccine immunity group (median [IQR], 6.2 [4.4–9.1] vs 4.5 [2.7–6.4] months from baseline serology to infection; adjusted log-rank test P < .0001) (Figure 1). Estimates for each variable between groups with available viral testing data are in Supplementary Table 2.
Figure 1.
Kaplan-Meier curves of the probability of being infection-free during the follow-up period based on viral testing between individuals in the hybrid and vaccine immunity groups. The number of participants at risk over time was adjusted for censored cases (crosses; multivariate Cox proportional hazards model–adjusted hazard ratio, 0.25; 95% CI, 0.18–0.35).
In total, 886/989 (90%) participants completed serology testing at follow-up, and 654 were serology positive, of whom 416 (64%) reported an infection by viral testing (NAAT or RAT) during the study follow-up period. Of the individuals who were serology negative (n = 232/886), 17 (7.3%) reported an infection by viral testing during the study follow-up period. Of the remaining 103 who did not complete serology testing at follow-up, 43 (41%) reported an infection by viral testing during the follow-up period. Using serology and high antibody avidity measures between baseline and follow-up, we were able to classify the infection status of 849/886 (95%) cases (Supplementary Table 1). When reporting infections based on both serology and avidity testing, 41% (95% CI, 31%–53%; n = 54/133) of individuals in the hybrid group had been reinfected during the follow-up period, compared with 70% (95% CI, 64%–76%; n = 498/716) in the vaccine immunity group.
When combining all data from viral testing, serology, and avidity-based serology testing (Supplementary Figure 1), 622 individuals had an infection during the follow-up period: 35% (95% CI, 29%–42%; n = 70/198) in the hybrid immunity group and 70% (95% CI, 67%–73%; n = 552/791) in the vaccine immunity group. Based on avidity antibody testing, we identified an additional 17% (33/198) infected individuals in the hybrid group who had not been diagnosed based on viral testing and/or conventional serology testing alone. The rate of infection remained lower in the hybrid compared with the vaccine group after adjusting for age, sex, children in the household, comorbidities, and smoking (adjusted incidence ratio, 0.50; 95% CI, 0.39–0.64; P < .0001).
Of 989 study participants, 955 (97%) completed the post-COVID/chronic symptom questionnaire at follow-up. Of those, 32% (n = 303/955) reported chronic post-COVID-like symptoms. When comparing individuals by infection status (based on viral testing, conventional serology plus avidity-based serology), the risk of experiencing 1 or more post-COVID symptoms was comparable between virus-naïve individuals from the vaccine group who were infected for the first time during the follow-up period (33%; 95% CI, 29%–38%; n = 177/536), individuals in the hybrid immunity group who did not get reinfected (40%; 95% CI, 30%–53%; n = 49/122), and individuals who were reinfected during the follow-up period (43%; 95% CI, 30%–61%; n = 30/70). By way of comparison, a significantly lower proportion of individuals who were never infected, supported by negative serology or negative viral testing data, reported 1 or more chronic symptoms at follow-up (21%; 95% CI, 16%–28%; n = 47/227). Post-COVID symptoms between infected groups are detailed in Table 1 and were similar between uninfected, infected, and reinfected individuals.
Table 1.
Frequency of Post-COVID-Like Symptoms by Infection Status (n = 955)
| Uninfecteda | Infected at Baselineb | Uninfected at Baseline and Infected During Follow-upc | Infected at Baseline and Reinfected During Follow-upd | |
|---|---|---|---|---|
| % [95% CI] who reported ≥1 symptom | n = 47/227 21 [16–28] |
n = 49/122 40 [31–49] |
n = 177/536 33 [29–37] |
n = 30/70 43 [30–61] |
| Symptom, No. (%) | ||||
| Fatigue | 27 (58) | 25 (51) | 115 (65) | 18 (60) |
| Decreased energy to exercise | 28 (60) | 27 (55) | 108 (61) | 18 (60) |
| Shortness of breath | 13 (28) | 10 (20) | 69 (14) | 6 (20) |
| Chest pain | 5 (11) | 8 (16) | 25 (14) | 4 (13) |
| Abdominal pain | 6 (13) | 5 (10) | 19 (11) | 3 (10) |
| Palpitations | 13 (28) | 5 (10) | 37 (21) | 6 (20) |
| Trouble sleeping | 25 (53) | 24 (49) | 91 (51) | 13 (43) |
| Headache | 20 (43) | 22 (45) | 72 (41) | 12 (40) |
| Weakness | 13 (28) | 8 (6.6) | 54 (31) | 11 (37) |
| Loss of taste and smell | 7 (15) | 7 (16) | 41 (23) | 3 (10) |
| Hoarse voice/change in voice | 14 (30) | 7 (16) | 45 (25) | 3 (10) |
| Rashes | 6 (13) | 4 (8.2) | 29 (16) | 5 (17) |
| Discoloration of fingers and toes | 3 (6) | 1 (2.0) | 5 (2.8) | 0 |
| Dizziness | 9 (19) | 9 (7.4) | 48 (27) | 6 (20) |
| Numbness/paresthesia | 6 (13) | 6 (18) | 20 (11) | 2 (7) |
| Difficulty concentrating, “brain fog” | 28 (60) | 25 (51) | 109 (62) | 19 (63) |
Abbreviation: COVID, coronavirus disease 2019.
aInfection-naïve individuals (negative serology both at baseline and at follow-up) and no evidence of infection during the follow-up period*.
bInfected before baseline (positive serology at baseline) and no evidence of second infection during the follow-up period*.
cInfected individuals (negative serology at baseline) who got infected for the first time during the follow-up period*.
dIndividuals infected before baseline (positive serology at baseline) who got reinfected during the follow-up period*.
*By the combined serology, avidity-based and viral testing data classification (Supplementary Figure 1).
DISCUSSION
This study found that a SARS-CoV-2 infection delayed and protected against reinfection up to a year, but that reciprocally the risk of experiencing post-COVID symptoms increased significantly among vaccinated adults. The risk of new-onset chronic symptoms rose significantly in individuals infected once, but nonsignificantly after reinfections, as compared with a group of vaccinated adults who were never infected. This suggests that initial infections may have greater health impact compared to reinfections with closely related variants. Consistent with previous findings, this study confirmed that hybrid immunity resulted in lower infection rates. The quantified estimated protection from reinfection during the Omicron BA.1/BA.2 waves is consistent with a population-based national study conducted in a similar period in Qatar demonstrating that infection documented by viral testing with the same Omicron lineages as the most likely lineages at baseline in the current study (BA.1 or BA.2) prevented self-reported reinfection with 49.9% (95% CI, 47.6%–52.1%) effectiveness [10]. This study adds to these previous findings, and to the best of our knowledge is the first to account for unreported or asymptomatic infections in a population, using a novel avidity-based serology testing method. The present study included adults vaccinated with the wild-type vaccine and infected with the BA.1 or BA.2 strain. The impact of updated vaccines and later variants of SARS-CoV-2 may change the epidemiology of reinfections and reduce the prevalence of post-COVID symptoms. Limitations of the current study include the use of self-reported viral testing results, potential unmeasured confounding, and a relatively small number of individuals in the hybrid immunity group who became reinfected. There is also the possibility that a small number of infection-naive individuals were incorrectly classified due to the waning of N antibodies or because they were tested too soon (ie, within 10–14 days) after infection. Effectively, this could underestimate differences between groups. The measure of at least one post-COVID symptoms was highly sensitive but is not particularly specific and could have captured non-post-COVID-19-related symptoms; however, this approach aligns with National Academies of Science, Engineering and Medicine (NASEM) [11]. Altogether, infected adults were less likely to be reinfected but more likely to report chronic symptoms. These findings add to our understanding of the epidemiology and health consequences of SARS-CoV-2 infections in an era when reinfections commonly occur without reporting.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Author contributions. P.M.L. and L.C.M. obtained funding. L.G., A.W.W., D.M.G., L.C.M., and P.M.L. designed the study. L.G. developed the avidity assay with help from B.A. and M.P. L.G., J.S., and M.P. processed the serology samples. L.G. and J.S. ran immunological assays. P.M.L., L.G., and A.W.W. wrote the manuscript. L.G., A.W.W., M.P., M.A.I., and F.C. conducted data and statistical analyses. L.G. generated the figures. P.M.L. provided study oversight. All authors reviewed and approved the final manuscript.
Data availability. De-identified participant data and data dictionaries will be made available after publication through requests to the Government of Canada's COVID-19 Immunity Task Force.
Financial support . This work was supported by the Government of Canada via its COVID-19 Immunity Task Force (to P.M.L. and L.C.M. as co-principal applicants; award # AWD-016994). P.M.L. and L.C.M. received a salary award from the British Columbia Children’s Hospital (BCCH) Foundation through the Investigator Grant Award Program.
Contributor Information
Liam Golding, BC Children's Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada; Department of Pediatrics, Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Allison W Watts, BC Children's Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada; Department of Pediatrics, Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Mark Pitblado, BC Children's Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada.
Felicity Clemens, British Columbia Centre for Disease Control, Vancouver, British Columbia, Canada.
Marina Viñeta Paramo, BC Children's Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada; Department of Pediatrics, Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Jacob Shew, BC Children's Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada; Department of Pediatrics, Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Michael A Irvine, British Columbia Centre for Disease Control, Vancouver, British Columbia, Canada.
Bahaa Abu-Raya, BC Children's Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada; Department of Pediatrics, Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada; Department of Pediatrics, Dalhousie University, Halifax, Nova Scotia, Canada.
David M Goldfarb, BC Children's Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Louise C Mâsse, BC Children's Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada; School of Population and Public Health, University of British Columbia, Vancouver, British Columbia, Canada.
Pascal M Lavoie, BC Children's Hospital Research Institute, University of British Columbia, Vancouver, British Columbia, Canada; Department of Pediatrics, Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
References
- 1. Mizrahi B, Sudry T, Flaks-Manov N, et al. Long COVID outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ 2023; 380:e072529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kahlert CR, Strahm C, Gusewell S, et al. Post-acute sequelae after SARS-CoV-2 infection by viral variant and vaccination status: a multicenter cross-sectional study. Clin Infect Dis 2023; 77:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benner SE, Patel EU, Laeyendecker O, et al. SARS-CoV-2 antibody avidity responses in COVID-19 patients and convalescent plasma donors. J Infect Dis 2020; 222:1974–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garcia L, Woudenberg T, Rosado J, et al. Kinetics of the SARS-CoV-2 antibody avidity response following infection and vaccination. Viruses 2022; 14:1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lofstrom E, Eringfalt A, Kotz A, et al. Dynamics of IgG-avidity and antibody levels after COVID-19. J Clin Virol 2021; 144:104986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Golding L, Watts AW, Shew J, Viñeta Paramo M, Abu-Raya B, Lavoie PM. A novel anti-nucleocapsid antibody avidity method for identifying SARS-CoV-2 reinfections. J Infect Dis 2024; 230:e579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldfarb DM, Masse LC, Watts AW, et al. SARS-CoV-2 seroprevalence among Vancouver public school staff in British Columbia, Canada: a cross-sectional study. BMJ Open 2022; 12:e057846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watts AW, Masse LC, Goldfarb DM, et al. SARS-CoV-2 cross-sectional seroprevalence study among public school staff in metro Vancouver after the first Omicron wave in British Columbia, Canada. BMJ Open 2023; 13:e071228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skowronski DM, Kaweski SE, Irvine MA, et al. Serial cross-sectional estimation of vaccine-and infection-induced SARS-CoV-2 seroprevalence in British Columbia, Canada. CMAJ 2022; 194:E1599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chemaitelly H, Tang P, Coyle P, et al. Protection against reinfection with the Omicron BA.2.75 subvariant. N Engl J Med 2023; 388:665–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Global Health; Board on Health Sciences Policy; Committee on Examining the Working Definition for Long COVID . A Long COVID Definition: A Chronic, Systemic Disease State With Profound Consequences. Fineberg HV, Brown L, Worku T, Goldowitz I, eds. The National Academies Press; 2024. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.