Abstract
Background
The incidence of respiratory syncytial virus (RSV)–acute respiratory infection (ARI) in community-dwelling adults after the Omicron variant of the COVID-19 pandemic is unknown. Our aim was to assess the incidence of RSV-ARI in adults aged 18 to 64 years over 2 consecutive RSV seasons (October–April 2022–2024) in 4 US states.
Methods
This community-based prospective cohort study comprised 7501 participants in Minnesota, Wisconsin, Florida, and Arizona. We calculated RSV-ARI and RSV–lower respiratory tract disease (LRTD) incidence and attack rates. We reported unadjusted incidence by age group, gender, race and ethnicity, Charlson Comorbidity Index, socioeconomic status, residential state, and rural/urban setting.
Results
Seasons 1 and 2 had 2250 and 2377 ARI episodes, respectively, with an RSV-ARI positivity rate of 5.5% for season 1 and 5.8% for season 2 among those tested. In season 1, the overall incidence of RSV-ARI was 27.71 (95% CI, 22.82–33.34) per 1000 person-years (1.49% attack rate). Almost half (49.0%) had RSV-LRTD, with an incidence of 13.53 (95% CI, 10.19–17.61) per 1000 person-years (0.73% attack rate). In season 2, the RSV-ARI and RSV-LRTD incidence rates were 26.39 (95% CI, 21.73–31.75) per 1000 person-years (1.51% attack rate) and 12.43 (95% CI, 9.31–16.26) per 1000 person-years (0.72% attack rate). RSV-ARI incidence peaked in November 2022 and December 2023.
Conclusions
Our observations suggest that RSV-ARI incidence and seasonal pattern are shifting to prepandemic RSV epidemiology.
Keywords: acute respiratory infection, incidence, lower respiratory tract disease, respiratory syncytial virus
During 2 consecutive respiratory syncytial virus (RSV) seasons from 2022 to 2024, RSV–acute respiratory infection (ARI) incidences in community-dwelling adults aged 18 to 64 years who were residing in 4 US states suggest that the RSV-ARI incidence and seasonal pattern are shifting toward pre–COVID-19 pandemic RSV epidemiology.
A disruption of the typical epidemic patterns and seasonal shifts of respiratory syncytial virus (RSV) has occurred in the United States since the onset of the COVID-19 pandemic [1–4]. We recently reported the incidence of RSV–acute respiratory infection (ARI) before and during the COVID-19 pandemic in a prospective community-based cohort study of Minnesota (MN) residents aged ≥50 years by following them for 2 consecutive RSV seasons (April–October 2019–2021) [5]. The prepandemic overall incidence of RSV-ARI for October 2019 to April 2020 was 48.62 (95% CI, 36.92–62.86) per 1000 person-years (2.50% attack rate). However, there were no RSV-ARI cases identified from October 2020 to April 2021. In support of this observation, a recent study of hospitalized adults aged ≥18 years based on data from the RSV Hospitalization Surveillance Network found that RSV case counts were 4.1 and 5.2 times higher in 2018–2019 and 2019–2020 than in 2020–2021 (ie, after onset of the pandemic) [4]. The National Healthcare Safety Network depicts the timeline for the first and second Omicron waves as October 2021 to September 2022 [6]. To our knowledge, there has been no large multistate community-based prospective cohort study of RSV-ARI in community-dwelling adults after the Omicron period of the COVID-19 pandemic. To address these gaps, our aim was to assess the incidence of RSV-ARI and RSV–lower respiratory tract disease (LRTD) and its associated sociodemographic factors in a large community-based prospective cohort study of adults aged 18 to 64 years residing in MN, Wisconsin (WI), Florida (FL), and Arizona (AZ) during 2 consecutive RSV seasons: October 2022 to April 2023 and October 2023 to April 2024.
METHODS
Study Setting and Design
This is an ongoing large community-based prospective cohort study that was designed to measure the incidence of RSV in adults aged 18 to 64 years from October 2022 to April 2023 (season 1) and October 2023 to April 2024 (season 2). We recruited and followed Mayo Clinic primary care patients residing in 4 states: the upper Midwest (Mayo Clinic Rochester and Mayo Clinic Health System in MN and WI), the Mayo Clinic in Florida, and the Mayo Clinic in Arizona. Mayo Clinic Rochester, Mayo Clinic in Florida, and Mayo Clinic in Arizona represent academic practices while the Mayo Clinic Health System is a community-based practice [7]. Participants who met the ARI case definition (Supplementary Table 1) collected their own nasal and oropharyngeal swab specimens at home upon onset of ARI symptoms, which were picked up by a courier service (MedSpeed LLC). We followed similar study design and procedures as our previous studies [5, 8]. The study was approved by the Mayo Clinic Institutional Review Board (22-003660).
Study Cohort
Participants were selected from our electronic health record (EHR) of eligible Mayo Clinic primary care patients with at least 1 primary care clinic visit within 3 years (370 000 participants) via a stratified random sampling approach by region (Mayo Clinic Rochester/Mayo Clinic Health System, Mayo Clinic in Florida, Mayo Clinic in Arizona) and status of underlying medical conditions. We remotely enrolled those who agreed to participate between June 2022 and December 2022. Those who met the inclusion criteria received a mailed or electronic (Technology-Enabling Subject Recruitment System) invitation for participation [9]. Once the inclusion criteria were confirmed via electronic survey or institutional review board–approved scripted phone interview, participants provided consent by remote electronic consenting and DocuSign technology or mail [9].
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (1) adults aged 18 to 64 years at time of the consent with residency in southeast MN, southwest WI, FL (Jacksonville area), and AZ (Scottsdale area); (2) Mayo Clinic primary care patient with at least 1 primary care visit within 3 years prior to enrollment; (3) authorization to use the EHR for research in MN [10]; and (4) written informed consent.
Exclusion criteria were as follows: (1) development of ARI after 1 October 2022, before baseline data were collected; (2) opting out of self-swab or study procedures; (3) inability to ambulate, due to requirement of a 5-m walk test as an outcome measure for a different study; (4) cognitive impairment; (5) participants who planned to reside out of their residential counties >2 weeks during the fall/winter season (October–April) and/or >4 weeks during the summer season (May–September 2023), to avoid inaccurate estimations of RSV in those who seasonally migrate to other US regions for prolonged periods; (6) pregnancy, to avoid RSV detection bias as pregnancy is a high-risk condition for COVID-19 (but not RSV-ARI) and routine screening of asymptomatic pregnant persons during medical visits happened with a combined COVID-19/influenza/RSV test during the COVID-19 pandemic; (7) adults aged 50 to 64 years who participated in our previous study [5]; and (8) adjudication by the principal investigators for situations where the inclusion/exclusion criteria could not be fully operationalized.
Baseline Data Collection
We collected the same baseline data as our previous study [5, 8] via patient-reported information from phone interview and EHR review within 3 years of study enrollment. Data included sociodemographic information and Charlson Comorbidity Index (CCI; age unadjusted) [11–13]. We categorized age in groups (18–49, 50–59, 60–64 years). EHR self-described race and ethnicity were categorized as non-Hispanic White, Hispanic or Latino, African American, Asian, American Indian/Alaskan Native, Native Hawaiian/Pacific Islander, or unknown. We assessed socioeconomic status (SES) via a standardized and objective individual housing property data–based measure (the Housing-Based Socioeconomic Status [HOUSES] Index), incorporating 4 property variables (value, square footage, number of bedrooms, and number of bathrooms), as validated by epidemiologic studies [7, 8, 14–18]. A greater HOUSES Index quartile indicates a higher SES. Rurality was determined by the US Census Bureau's urban/rural classification [19].
ARI Case Definitions and Specimen Collection
The study procedures were recently reported [5, 8]. Instructions for self-swab specimen collection were provided in written/pictorial and video forms. Participants were provided a pulse oximeter upon study enrollment. Study participants informed research coordinators when they developed ARI symptoms. If participants met the case definition for ARI, they were assessed to determine if they met the case definition for LRTD (Supplementary Table 1) by telephone communication and/or EHR review for those who sought medical care. Swab specimens were obtained from anterior nares and oropharynx and placed in viral transport media (MicroTest M4RT Multi-Microbe Media; Remel, Inc), delivered to each site's laboratory via courier, and then transported to the Mayo Clinic Rochester laboratory and assessed with reverse transcriptase–polymerase chain reaction (RT-PCR) via the Simplexa Flu A/B and RSV Direct assay (DiaSorin Molecular) or Panther Fusion Flu A/B and RSV assay (Hologic, Inc). Testing was performed according to the manufacturer's instructions for use. Participants with positive results were given educational material on symptom management, transmission prevention, and when to seek medical care.
Statistical Analysis
Demographics were pulled from the EHR at the start of the surveillance period (1 October 2022 or the date when the participant completed baseline measurements if after 1 October) and again at the start of the second season of surveillance (1 October 2023). Incidence rates with exact Poisson 95% CIs were computed according to the number of first RSV-ARI cases per 1000 person-years of follow-up. The attack rate (percentage of participants with at least 1 RSV-ARI episode) was calculated with Clopper-Pearson exact 95% CIs [20]. We did not censor for RSV vaccination given the small number of participants (n = 208, 2.8% of the cohort) who were vaccinated during the study period, after the recommendations from the US Advisory Committee on Immunization Practices in June 2023 [21]. The overall duration of follow-up was based on (1) the start of the surveillance period until symptom onset of the first RSV-ARI occurred, (2) the end of the surveillance period (30 April of each year), or (3) the last follow-up (ie, death, residing out of the study region >2 weeks, time when opted out of the study, or moving out of the region), whichever came first. Characteristics based on the participant’s residential address (HOUSES Index, residential state, and rural status) were treated as time-varying factors, allowing participants to contribute to different strata depending on their change of address during the study period. For those participants who moved throughout the study period, their follow-up time was split across their residential addresses based on the amount of time at each address. If those who moved experienced an RSV-ARI episode, their follow-up stopped at the time of the episode, and they contributed their RSV-ARI episode to the numerator at the address where they were currently living at the time of symptom onset. CCI was a time-varying factor allowing participants to contribute to a different group if they developed a condition during the study period. All analyses were conducted in SAS statistical software version 9.4M7 (SAS Institute) while figures were created with ggplot2 (version 3.5.0) in R version 4.3.2 [22].
RESULTS
Participant Characteristics
Of 7504 accrued participants, 3 withdrew prior to season 1 and were excluded from all analyses (n = 7501), while 27 withdrew during season 1, 77 between seasons 1 and 2, and 129 during season 2 and were censored at the time of withdrawal (Supplementary Table 2). At the start of the surveillance for season 1, the median age was 47 years (IQR, 37–57); the majority were female (71.4%), non-Hispanic White (90.3%), and resided in urban settings (88.4%); and 37.7% had a CCI ≥1. There were 208 participants who received the RSV vaccine prior to or during the season 2 study period. The characteristics of the study participants are summarized in Table 1.
Table 1.
Characteristics of Study Participants (N = 7501)
| At the Start of Surveillance for Season 1 | No. (%) |
|---|---|
| Age, y | |
| Mean (SD) | 46.5 (11.7) |
| Median (IQR) | 47 (37–57) |
| 18–49 | 4169 (55.6) |
| 50–59 | 2064 (27.5) |
| 60–64 | 1268 (16.9) |
| Gender | |
| Female | 5356 (71.4) |
| Male | 2145 (28.6) |
| Race and ethnicity | |
| Non-Hispanic White | 6776 (90.3) |
| Hispanic or Latino | 252 (3.4) |
| African American | 141 (1.9) |
| Asian | 214 (2.9) |
| American Indian/Alaskan Native | 27 (0.4) |
| Native Hawaiian/Pacific Islander | 8 (0.1) |
| Unknown | 83 (1.1) |
| CCIa | |
| 0 | 4674 (62.3) |
| 1 | 1561 (20.8) |
| ≥2 | 1266 (16.9) |
| SES per HOUSES Index by quartileb | |
| 1 (lowest) | 1028 (14.1) |
| 2 | 1323 (18.1) |
| 3 | 1962 (26.8) |
| 4 (highest) | 3000 (41.0) |
| Missing | 188 |
| Geographic location | |
| Minnesota | 4229 (56.4) |
| Wisconsin | 1068 (14.2) |
| Florida | 1171 (15.6) |
| Arizona | 1033 (13.8) |
| Ruralityc | |
| Urban | 6605 (88.4) |
| Rural | 871 (11.6) |
| Missing | 25 |
Abbreviations: CCI, Charlson Comorbidity Index; SES, socioeconomic status.
aCCI excludes age as a variable [11–13].
bThe HOUSES Index is a validated, standardized, and objective individual housing asset–based SES index incorporating 4 property variables: value, square footage, number of bedrooms, and number of bathrooms [7, 8, 14–18]. A higher HOUSES Index quartile score indicates a higher SES level.
cRurality was determined per the US Census Bureau's urban/rural classification [19] .
Seasonal Incidence and Attack Rates of RSV-ARI: Seasons 1 and 2
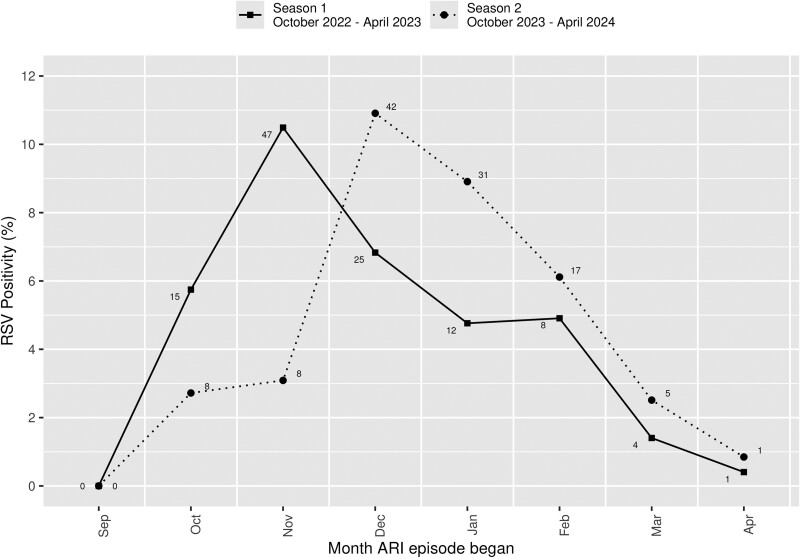
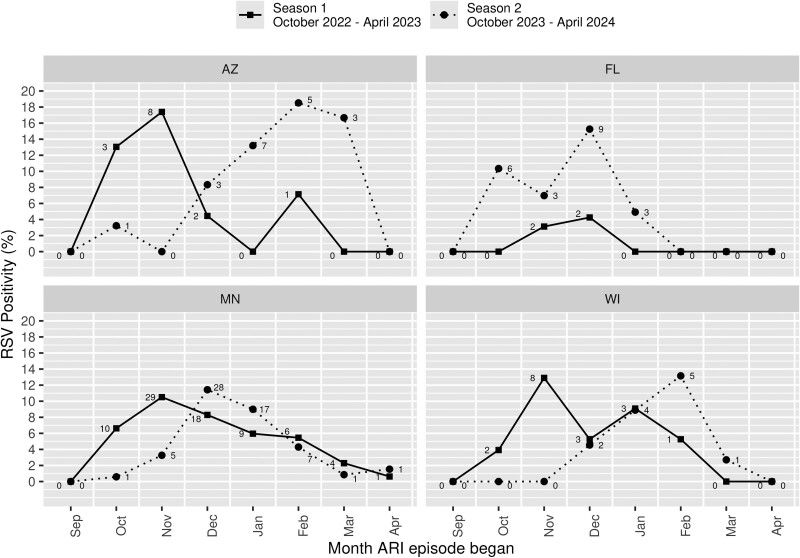
The incidence and attack rates of RSV-ARI during seasons 1 and 2 are summarized in Table 2. For season 1, an overall 1811 (24.1%) participants had 2250 ARI episodes with 2023 (89.9%) specimens that were tested with results (217 ARI episodes without specimen collection and 10 with no valid RT-PCR result). Of these, 112 (5.5%) ARI episodes were RSV positive (none tested RSV positive more than once in season 1). The incidence of RSV-ARI in season 1 was 27.71 (95% CI, 22.82–33.34) per 1000 person-years (1.49% attack rate). For season 2, an overall 1909 (25.8%) participants with 2377 ARI episodes submitted 1920 (80.8%) specimens that were tested with results (454 ARI episodes without specimen collection and 3 with no valid RT-PCR result). Of these, 112 (5.8%) ARI episodes were RSV positive (none tested RSV positive more than once in season 2). The incidence rate of RSV-ARI in season 2 was 26.39 (95% CI, 21.73–31.75) per 1000 person-years (1.51% attack rate). None of the participants who had RSV during season 1 or 2 contracted RSV more than once throughout the entire study period. In the overall cohort, RSV-ARI peaked in November 2022 and December 2023 (Figure 1), while there were regional differences (Figure 2) across the 4 states.
Table 2.
Seasonal Incidence and Attack Rates of RSV-ARI for Season 1 (1 October 2022–30 April 2023) and Season 2 (1 October 2023–30 April 2024)
| Season 1 (1 October 2022–30 April 2023) | Season 2 (1 October 2023–30 April 2024) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants, No.a | RSV-ARI, No.b | PYc | IR per 1000 PY (95% CI)d | Attack Rate, % (95% CI)e | Participants, No. | RSV-ARI, No. | PY | IR per 1000 PY (95% CI) | Attack Rate, % (95% CI) | |
| Overall | ||||||||||
| Unstandardized | 7501 | 112 | 4041.56 | 27.71 (22.82–33.34) | 1.49 (1.23–1.79) | 7397 | 112 | 4244.75 | 26.39 (21.73–31.75) | 1.51 (1.25–1.82) |
| Standardized age/gender-adjusted IRf | … | … | … | 26.46 (20.81–32.11) | … | … | … | … | 25.32 (19.65–30.98) | … |
| Age at start of season, y | ||||||||||
| 18–49 | 4169 | 69 | 2239.15 | 30.82 (23.98–39.00) | 1.66 (1.29–2.09) | 3929 | 49 | 2251.24 | 21.77 (16.10–28.78) | 1.25 (.92–1.65) |
| 50–59 | 2064 | 22 | 1117.80 | 19.68 (12.33–29.80) | 1.07 (.67–1.61) | 1989 | 29 | 1146.82 | 25.29 (16.94–36.32) | 1.46 (.98–2.09) |
| 60–64 | 1268 | 21 | 684.61 | 30.67 (18.99–46.89) | 1.66 (1.03–2.52) | 1479 | 34 | 846.68 | 40.16 (27.81–56.12) | 2.30 (1.60–3.20) |
| Gender | ||||||||||
| Male | 2145 | 27 | 1158.68 | 23.30 (15.36–33.90) | 1.26 (.83–1.83) | 2132 | 33 | 1228.08 | 26.87 (18.50–37.74) | 1.55 (1.07–2.17) |
| Female | 5356 | 85 | 2882.89 | 29.48 (23.55–36.46) | 1.59 (1.27–1.96) | 5265 | 79 | 3016.67 | 26.19 (20.73–32.64) | 1.50 (1.19–1.87) |
| Race and ethnicity | ||||||||||
| African American | 141 | 0 | 78.20 | 0.00 (.00–47.17) | 0.00 (.00–2.58) | 136 | 2 | 78.52 | 25.47 (3.08–92.01) | 1.47 (.18–5.21) |
| Asian | 214 | 0 | 117.36 | 0.00 (.00–31.43) | 0.00 (.00–1.71) | 210 | 1 | 120.18 | 8.32 (.21–46.36) | 0.48 (.01–2.62) |
| Native Hawaiian/Pacific Islander | 8 | 0 | 4.50 | 0.00 (.00–819.50) | 0.00 (.00–36.94) | 8 | 0 | 4.67 | 0.00 (.00–790.16) | 0.00 (.00–36.94) |
| American Indian/Alaskan Native | 27 | 1 | 14.43 | 69.29 (1.75–386.04) | 3.70 (.09–18.97) | 27 | 0 | 15.76 | 0.00 (.00–234.12) | 0.00 (.00–12.77) |
| Hispanic or Latino | 252 | 1 | 137.01 | 7.30 (.18–40.67) | 0.40 (.01–2.19) | 247 | 3 | 141.66 | 21.18 (4.37–61.89) | 1.21 (.25–3.51) |
| Non-Hispanic White | 6776 | 110 | 3644.15 | 30.19 (24.81–36.38) | 1.62 (1.34–1.95) | 6687 | 106 | 3836.86 | 27.63 (22.62–33.41) | 1.59 (1.30–1.91) |
| Unknown | 83 | 0 | 45.92 | 0.00 (.00–80.34) | 0.00 (.00–4.35) | 82 | 0 | 47.10 | 0.00 (.00–78.31) | 0.00 (.00–4.40) |
| HOUSES Index | ||||||||||
| Q1 | 1065 | 13 | 553.04 | 23.51 (12.52–40.20) | 1.22 (.65–2.08) | 1042 | 16 | 567.00 | 28.22 (16.13–45.83) | 1.54 (.88–2.48) |
| Q2 | 1366 | 25 | 711.71 | 35.13 (22.73–51.85) | 1.83 (1.19–2.69) | 1318 | 9 | 731.47 | 12.30 (5.63–23.36) | 0.68 (.31–1.29) |
| Q3 | 2001 | 33 | 1054.35 | 31.30 (21.54–43.96) | 1.65 (1.14–2.31) | 1965 | 33 | 1097.05 | 30.08 (20.71–42.24) | 1.68 (1.16–2.35) |
| Q4 | 3027 | 38 | 1621.91 | 23.43 (16.58–32.16) | 1.26 (.89–1.72) | 3034 | 50 | 1715.48 | 29.15 (21.63–38.43) | 1.65 (1.23–2.17) |
| State | ||||||||||
| MN | 4233 | 77 | 2253.13 | 34.17 (26.97–42.71) | 1.82 (1.44–2.27) | 4161 | 60 | 2375.37 | 25.26 (19.28–32.51) | 1.44 (1.10–1.85) |
| WI | 1069 | 17 | 570.25 | 29.81 (17.37–47.73) | 1.59 (.93–2.53) | 1050 | 12 | 602.32 | 19.92 (10.29–34.80) | 1.14 (.59–1.99) |
| FL | 1173 | 4 | 647.42 | 6.18 (1.68–15.82) | 0.34 (.09–.87) | 1159 | 21 | 662.13 | 31.72 (19.63–48.48) | 1.81 (1.13–2.76) |
| AZ | 1033 | 14 | 568.40 | 24.63 (13.47–41.33) | 1.36 (.74–2.26) | 1023 | 19 | 585.81 | 32.43 (19.53–50.65) | 1.86 (1.12–2.89) |
| Midwest: MN, WI | 5301 | 94 | 2823.38 | 33.29 (26.90–40.74) | 1.77 (1.44–2.17) | 5203 | 72 | 2977.70 | 24.18 (18.92–30.45) | 1.38 (1.08–1.74) |
| Southern: FL, AZ | 2206 | 18 | 1215.82 | 14.80 (8.77–23.40) | 0.82 (.48–1.29) | 2182 | 40 | 1247.94 | 32.05 (22.90–43.65) | 1.83 (1.31–2.49) |
| Rural status census | ||||||||||
| Rural | 897 | 10 | 471.19 | 21.22 (10.18–39.03) | 1.11 (.54–2.04) | 913 | 6 | 515.86 | 11.63 (4.27–25.32) | 0.66 (.24–1.42) |
| Urban | 6621 | 102 | 3555.87 | 28.68 (23.39–34.82) | 1.54 (1.26–1.87) | 6491 | 105 | 3698.93 | 28.39 (23.22–34.36) | 1.62 (1.32–1.95) |
| CCI, age unadjusted | ||||||||||
| 0 | 4674 | 61 | 2457.78 | 24.82 (18.98–31.88) | 1.31 (1.00–1.67) | 4516 | 65 | 2539.67 | 25.59 (19.75–32.62) | 1.44 (1.11–1.83) |
| 1 | 1686 | 28 | 858.35 | 32.62 (21.68–47.15) | 1.66 (1.11–2.39) | 1651 | 25 | 895.17 | 27.93 (18.07–41.23) | 1.51 (.98–2.23) |
| ≥2 | 1397 | 23 | 725.44 | 31.70 (20.10–47.57) | 1.65 (1.05–2.46) | 1471 | 22 | 809.91 | 27.16 (17.02–41.13) | 1.50 (.94–2.26) |
Time points when participants had missing rurality or HOUSES Index were excluded from the rurality- or HOUSES Index–stratified analysis.
Abbreviations: ARI, acute respiratory infection; CCI, Charlson Comorbidity Index; HOUSES Index, housing-based socioeconomic status index; IR, incidence rate; PY, person-years; RSV, respiratory syncytial virus.
aParticipants may contribute to >1 stratum of CCI, HOUSES Index, geographic location, or rurality.
bParticipants with RSV-ARI.
cPerson-years: sum of the follow-up periods at risk expressed in years censored at the first occurrence of the RSV-ARI episode.
dIncidence rate per 1000 person-years: number of first RSV-ARI episodes per 1000 person-years. 95% CI: exact Poisson 95% CI.
eAttack rate: percentage of participants with at least 1 RSV-ARI episode. 95% CI: Clopper-Pearson exact 95% CI.
fStandardized incidence rate: number of first RSV-ARI episodes per 1000 person-years standardized to the age-sex proportions of the 2020 US White population. 95% CI: normal approximation 95% CI.
Figure 1.
RSV-ARI for seasons 1 and 2 by month for the entire cohort. A few participants had ARI episodes with symptoms starting prior to surveillance each season (ie, in September) but were within the 7-day window when swabbed in early October and so were included. The numbers adjacent to the points refer to the number of positive RSV-ARI episodes. RSV positivity rate: the number of RSV-ARI–positive samples over the total number of ARI episodes with RSV results available. ARI, acute respiratory infection; RSV, respiratory syncytial virus.
Figure 2.
RSV-ARI for seasons 1 and 2 by month for each state. A few participants had ARI episodes with symptoms starting prior to surveillance each season (ie, in September) but were within the 7-day window when swabbed in early October and so were included. The numbers adjacent to the points refer to the number of positive RSV-ARI episodes. RSV positivity rate: the number of RSV-ARI–positive samples over the total number of ARI episodes with RSV results available. ARI, acute respiratory infection; RSV, respiratory syncytial virus.
Seasonal Incidence and Attack Rates of RSV-LRTD: Seasons 1 and 2
Table 3 summarizes the incidence and attack rates of RSV-LRTD overall and by sociodemographic characteristics of the participants during seasons 1 and 2. For the first season, 55 (49.1%) participants had RSV-LRTD with an incidence rate of 13.53 (95% CI, 10.20–17.61) per 1000 person-years (0.73% attack rate). In the second season, 53 (47.3%) participants had RSV-LRTD with an incidence rate of 12.43 (95% CI, 9.31–16.26) per 1000 person-years (0.72% attack rate).
Table 3.
Seasonal Incidence and Attack Rates of RSV-LRTD for Season 1 (1 October 2022–30 April 2023) and Season 2 (1 October 2023–30 April 2024)
| Season 1 (1 October 2022–30 April 2023) | Season 2 (1 October 2023–30 April 2024) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants, No.a | RSV- LRTD, No.b | PYc | IR per 1000 PY (95% CI)d | Attack Rate, % (95% CI)e | Participants, No. | RSV-ARI, No. | PY | IR per 1000 PY (95% CI) | Attack Rate, % (95% CI) | |
| Overall | ||||||||||
| Unstandardized | 7501 | 55 | 4064.04 | 13.53 (10.20–17.62) | 0.73 (.55–.95) | 7397 | 53 | 4264.02 | 12.43 (9.31–16.26) | 0.72 (.54–.94) |
| Standardized age/gender-adjusted IRf | … | … | … | 12.94 (8.95–16.93) | … | … | … | … | 10.87 (7.38–14.36) | … |
| Age at start of season, y | ||||||||||
| 18–49 | 4169 | 32 | 2253.95 | 14.20 (9.71–20.04) | 0.77 (.53–1.08) | 3929 | 18 | 2261.32 | 7.96 (4.72–12.58) | 0.46 (.27–.72) |
| 50–59 | 2064 | 13 | 1121.37 | 11.59 (6.17–19.82) | 0.63 (.34–1.07) | 1989 | 17 | 1150.82 | 14.77 (8.61–23.65) | 0.85 (.50–1.36) |
| 60–64 | 1268 | 10 | 688.72 | 14.52 (6.96–26.70) | 0.79 (.38–1.45) | 1479 | 18 | 851.88 | 21.13 (12.52–33.39) | 1.22 (.72–1.92) |
| Gender | ||||||||||
| Male | 2145 | 13 | 1164.25 | 11.17 (5.95–19.09) | 0.61 (.32–1.03) | 2132 | 15 | 1234.04 | 12.16 (6.80–20.05) | 0.70 (.39–1.16) |
| Female | 5356 | 42 | 2899.78 | 14.48 (10.44–19.58) | 0.78 (.57–1.06) | 5265 | 38 | 3029.98 | 12.54 (8.88–17.21) | 0.72 (.51–.99) |
| Race and ethnicity | ||||||||||
| African American | 141 | 0 | 78.20 | 0.00 (.00–47.17) | 0.00 (.00–2.58) | 136 | 1 | 78.84 | 12.68 (.32–70.67) | 0.74 (.02–4.03) |
| Asian | 214 | 0 | 117.36 | 0.00 (.00–31.43) | 0.00 (.00–1.71) | 210 | 1 | 120.18 | 8.32 (.21–46.36) | 0.48 (.01–2.62) |
| Native Hawaiian/Pacific Islander | 8 | 0 | 4.50 | 0.00 (.00–819.50) | 0.00 (.00–36.94) | 8 | 0 | 4.67 | 0.00 (.00–790.16) | 0.00 (.00–36.94) |
| American Indian/Alaskan Native | 27 | 1 | 14.43 | 69.29 (1.75–386.04) | 3.70 (.09–18.97) | 27 | 0 | 15.76 | 0.00 (.00–234.12) | 0.00 (.00–12.77) |
| Hispanic or Latino | 252 | 1 | 137.01 | 7.30 (.18–40.67) | 0.40 (.01–2.19) | 247 | 1 | 142.41 | 7.02 (.18–39.12) | 0.40 (.01–2.23) |
| Non-Hispanic White | 6776 | 53 | 3666.62 | 14.45 (10.83–18.91) | 0.78 (.59–1.02) | 6687 | 50 | 3855.07 | 12.97 (9.63–17.10) | 0.75 (.56–.98) |
| Unknown | 83 | 0 | 45.92 | 0.00 (.00–80.34) | 0.00 (.00–4.35) | 82 | 0 | 47.10 | 0.00 (.00–78.31) | 0.00 (.00–4.40) |
| HOUSES Index | ||||||||||
| Q1 | 1065 | 8 | 555.16 | 14.41 (6.22–28.39) | 0.75 (.32–1.47) | 1042 | 7 | 569.63 | 12.29 (4.94–25.32) | 0.67 (.27–1.38) |
| Q2 | 1366 | 12 | 716.45 | 16.75 (8.65–29.26) | 0.88 (.45–1.53) | 1318 | 5 | 732.52 | 6.83 (2.22–15.93) | 0.38 (.12–.88) |
| Q3 | 2001 | 14 | 1062.32 | 13.18 (7.20–22.11) | 0.70 (.38–1.17) | 1966 | 19 | 1101.94 | 17.24 (10.38–26.93) | 0.97 (.58–1.51) |
| Q4 | 3027 | 18 | 1629.54 | 11.05 (6.55–17.46) | 0.59 (.35–.94) | 3035 | 21 | 1725.43 | 12.17 (7.53–18.60) | 0.69 (.43–1.06) |
| State | ||||||||||
| MN | 4233 | 33 | 2270.09 | 14.54 (10.01–20.42) | 0.78 (.54–1.09) | 4161 | 28 | 2386.04 | 11.73 (7.80–16.96) | 0.67 (.45–.97) |
| WI | 1069 | 13 | 571.76 | 22.74 (12.11–38.88) | 1.22 (.65–2.07) | 1050 | 5 | 604.16 | 8.28 (2.69–19.31) | 0.48 (.15–1.11) |
| FL | 1173 | 1 | 648.76 | 1.54 (.04–8.59) | 0.09 (.00–.47) | 1159 | 10 | 666.37 | 15.01 (7.20–27.60) | 0.86 (.41–1.58) |
| AZ | 1033 | 8 | 571.07 | 14.01 (6.05–27.60) | 0.77 (.33–1.52) | 1023 | 10 | 588.33 | 17.00 (8.15–31.26) | 0.98 (.47–1.79) |
| Midwest: MN, WI | 5301 | 46 | 2841.85 | 16.19 (11.85–21.59) | 0.87 (.64–1.16) | 5203 | 33 | 2990.21 | 11.04 (7.60–15.50) | 0.63 (.44–.89) |
| Southern: FL, AZ | 2206 | 9 | 1219.82 | 7.38 (3.37–14.01) | 0.41 (.19–.77) | 2182 | 20 | 1254.70 | 15.94 (9.74–24.62) | 0.92 (.56–1.41) |
| Rural status census | ||||||||||
| Rural | 897 | 5 | 472.89 | 10.57 (3.43–24.67) | 0.56 (.18–1.30) | 913 | 3 | 516.68 | 5.81 (1.20–16.97) | 0.33 (.07–.96) |
| Urban | 6621 | 50 | 3576.63 | 13.98 (10.38–18.43) | 0.76 (.56–.99) | 6491 | 49 | 3717.38 | 13.18 (9.75–17.43) | 0.75 (.56–1.00) |
| CCI, age unadjusted | ||||||||||
| 0 | 4674 | 24 | 2472.07 | 9.71 (6.22–14.45) | 0.51 (.33–.76) | 4516 | 24 | 2553.15 | 9.40 (6.02–13.99) | 0.53 (.34–.79) |
| 1 | 1686 | 14 | 864.09 | 16.20 (8.86–27.18) | 0.83 (.45–1.39) | 1652 | 14 | 898.47 | 15.58 (8.52–26.14) | 0.85 (.46–1.42) |
| ≥2 | 1398 | 17 | 727.88 | 23.36 (13.61–37.39) | 1.22 (.71–1.94) | 1472 | 15 | 812.41 | 18.46 (10.33–30.45) | 1.02 (.57–1.68) |
Time points when participants had missing rurality or HOUSES Index were excluded from the rurality- or HOUSES Index–stratified analysis.
Abbreviations: CCI, Charlson Comorbidity Index; HOUSES Index, housing-based socioeconomic status index; IR, incidence rate; LRTD, lower respiratory tract disease; PY, person-years; RSV, respiratory syncytial virus.
aParticipants may contribute to >1 stratum of CCI, HOUSES Index, geographic location, or rurality.
bParticipants with RSV-LRTD.
cPerson-years: sum of the follow-up periods at risk expressed in years censored at the first occurrence of the RSV-LRTD episode.
dIncidence rate per 1000 person-years: number of first RSV-LRTD episodes per 1000 person-years. 95% CI: exact Poisson 95% CI.
eAttack rate: percentage of participants with at least 1 RSV-LRTD episode. 95% CI: Clopper-Pearson exact 95% CI.
fStandardized incidence rate: number of first RSV-LRTD episodes per 1000 person-years standardized to the age-sex proportions of the 2020 US White population. 95% CI: normal approximation 95% CI.
RSV-ARI Health Care Utilization
For the first season, 18 (16.1%) participants with RSV-ARI were medically attended within 30 days of follow-up. Health care utilizations for RSV-ARI included 12 (10.7%) outpatient visits (10 RSV-LRTD), 5 (4.5%) emergency department visits (4 RSV-LRTD), and 1 (0.9%) hospitalization without intensive care unit admission. For season 2, there were 34 (30.4%) medically attended participants: health care utilization for RSV-ARI included 28 (25.0%) outpatient visits (15 RSV-LRTD), 5 (4.5%) emergency department visits (2 RSV-LRTD), and 1 (0.9%) hospitalization without intensive care unit admission. For both seasons, there were no deaths in participants with RSV-ARI (data not shown).
Incidence Rate of RSV-ARI by Participant Characteristics
The incidence rates of RSV-ARI by participant characteristics are summarized in Table 2. In general, the RSV-ARI incidence rates were similar across most subgroups, considering the overlapping 95% CIs. There were a couple of notable differences observed in season 1. For instance, the American Indian/Alaskan Native group had a higher incidence rate when compared with other minority groups, although the 95% CI was wide. The RSV-ARI incidence rate was higher among participants residing in the Midwestern states (MN and WI with colder winters) than Southern states (FL and AZ with warmer winters), with an approximate 5-fold difference in incidence between FL (6.18 [95% CI, 1.68–15.83] per 1000 person-years) and the Midwest states (33.29 [95% CI, 26.90–40.74]). Those residing in an urban setting had a higher incidence rate as compared with a rural setting, albeit with overlapping 95% CIs. Participants with a CCI of 0 had a lower incidence rate vs a CCI ≥1, recognizing overlapping 95% CIs. For season 2, there were several notable differences. The incidence rate was higher in those aged 60 to 64 years (40.16 [95% CI, 27.81–56.12] per 1000 person-years) than 18 to 49 years (21.77 [95% CI, 16.10–28.78]). The African American and Hispanic/Latino groups had higher incidence rates than other minority groups, although both had wide 95% CIs. Those residing in an urban setting had a higher incidence rate vs a rural setting. Participants with a CCI of 0 had a lower RSV-ARI incidence rate when compared with a CCI ≥1.
Incidence Rate of RSV-LRTD by Participant Characteristics
The incidence rates of RSV-LRTD by participant characteristics are summarized in Table 3. In general, the incidence rates were similar by subgroups with some exceptions. There were a couple of notable differences for season 1. The American Indian/Alaskan Native group had a high incidence rate of RSV-LRTD, although with a wide 95% CI due to the group's underrepresentation in our cohort. The incidence rate of RSV-LRTD was higher among participants residing in the Midwestern states (MN and WI with colder winters) than Southern states (FL and AZ with warmer winters), with FL having an especially low incidence of RSV-LRTD (1.54 [95% CI, .04–8.59] per 1000 person-years) vs Midwestern states (16.19 [95% CI, 11.85–21.59]). Participants with a CCI of 0 had a lower incidence rate as compared with a CCI ≥1. For season 2, there were several notable differences. We found higher incidence rates with increasing age, noting overlapping 95% CIs. Those residing in an urban setting had higher incidence rates when compared with a rural setting. Participants with a CCI of 0 had a lower incidence rate of RSV-LRTD as compared with a CCI ≥1, despite overlapping 95% CIs.
DISCUSSION
This is the first community-based prospective cohort study during the post-Omicron period of the COVID-19 pandemic that assessed RSV-ARI incidences among 7501 adults aged 18 to 64 years, residing in MN, WI, FL, and AZ. The incidence rate of RSV-ARI was 27.72 per 1000 person-years for season 1 (peaked in November 2022) and 26.39 per 1000 person-years for season 2 (peaked in December 2023). While there was geographic and temporal variability over 2 consecutive RSV seasons, our findings suggest that the incidence rate of RSV-ARI and its patterns are shifting to prepandemic level and seasonality.
As shown in our study and others [2–5, 23], RSV epidemic patterns in the United States have been affected by regional variations and the COVID-19 pandemic. A meta-analysis of community-based adults from Europe and the United States with high-risk conditions found an RSV-ARI annual incidence rate of 37.6 (95% CI, 20.1–70.3) per 1000 persons per year and a seasonal incidence rate of 28.4 (95% CI, 11.4–70.9) per 1000 persons per season during surveillance from 1996 to 2013 [24]. While this meta-analysis reported pooled estimates from multiple studies with different study designs [25–31], to our knowledge, there has been only 5 true community-based prospective longitudinal surveillance studies based on at-home specimen collection during a span of 1992 to 2020, focusing on older adults or those at high risk [5, 26, 31–33]. Three of these studies took place in the United States, of which 2 were conducted by the same research group with different surveillance periods—in 1996 to 1998 [31] and 1999 to 2003 (estimated attack rate by RT-PCR, 2.0%–4.7%) [26]—and 1 was our previously reported study of older adults with surveillance from 2019 to 2020 (attack rate, 2.50% [95% CI, 1.90%–3.21%]) [5]. The other 2 prospective longitudinal surveillance studies were European community-based cohorts of RSV-ARI in adults aged ≥60 years (with and without medical conditions), one with surveillance from 1992 to 1994 [32] and the other from 2017 to 2019 (estimated attack rate by RT-PCR, 2.1% for season 1 [95% CI, 1.0%–3.7%] and 4.9% for season 2 [95% CI, 3.2%–7.1%]) [33]. It is important to recognize that these studies assessed RSV-ARI incidence or attack rates in older adults and/or those at high risk and are anticipated to have a higher incidence or attack rate than our study of a younger cohort (55.6% were aged 18–49 years), of which 62.3% had a CCI of 0. Thus, it is difficult to quantitatively compare these study results with ours, with a prepandemic incidence estimate of high-risk populations. As no multistate, prospective, community-based longitudinal surveillance study of RSV-ARI with at-home self-collected swab specimen has been conducted in adults aged 18 to 64 years, we are not able to directly compare our results with those previously published. However, as our study results are within the 95% CIs of the annual and seasonal incidence rates reported in the meta-analysis of adults at high risk described earlier [24] and close to the incidence rates by those pre–COVID-19 pandemic community-based studies on older adults and those at high risk [5, 26, 33], this is suggestive that the RSV-ARI incidence is shifting to prepandemic level and seasonality, as shown in Figure 1.
After the onset of the pandemic, deviations of RSV epidemiology in the United States occurred with an early national peak of RSV-positive tests in November 2022 [34]. The RSV epidemic season for 2022 to 2023 started in June 2022, thus earlier when compared with the prepandemic 2017–2020 RSV seasons (October) [3]. The RSV epidemic onset month for 2022 to 2023 was August for MN and WI, September for AZ, and May for FL [3]. Given that our surveillance period started in October 2022, our season 1 incidence rate did not capture the early RSV epidemic onset in certain regions. This may explain why our FL participants had a significantly lower RSV-ARI incidence rate as compared with those in the Midwestern states (approximately a 5-fold difference), although in season 2, this observation was no longer present. Data on US RSV hospitalization surveillance showed that the 2023–2024 RSV season began in October and peaked in December [35], which may suggest that our study findings (ie, an RSV-ARI peak in December 2023) reflect these national trends, recognizing the difference in geographic regions and RSV case definition in our cohort.
About half of RSV-ARI cases had RSV-LRTD, with an incidence of 13.53 per 1000 person-years (0.73% attack rate) in season 1 and 12.43 (0.72% attack rate) in season 2, suggesting a similar proportion of RSV-LRTD and RSV upper respiratory tract disease. Our incidence rates for RSV-LRTD were lower for those with a CCI of 0 as compared with a CCI ≥1. In season 2, incidences of RSV-LRTD were higher in older groups (14.77 per 1000 person-years in 50–59 years and 21.13 in 60–64 years) vs the youngest group (18–49 years; 7.96 per 1000 person-years), with overlapping 95% CIs. These observations indicate that people with chronic medical conditions or advancing age have greater vulnerability for developing RSV-LRTD and so warrant consideration of RSV vaccination.
Sociodemographic, clinical, and geographic/environmental factors were assessed in relation to the risk of RSV-ARI and RSV-LRTD. Sex, race/ethnicity, SES, and state of residence did not consistently affect the burden of RSV-ARI. For example, we found similar incidence rates of RSV-ARI and RSV-LRTD in non-Hispanic White patients in both seasons. The high RSV-ARI and RSV-LRTD incidence rates in season 1 for American Indian/Alaskan Native patients did not persist in season 2, although the group's sample size is small in our cohort and the estimated incidence rate is not precise. The literature suggests an association of poverty or lower SES with high risk of RSV-related hospitalization [36, 37]. In our study, belonging to the lowest SES by the HOUSES Index was not reflective of higher RSV incidence rates for both seasons. However, this discrepancy may be related to differences in SES measurements and RSV case definition. Additionally, participants in the older category (60–64 years), those with a CCI ≥1, and those residing in urban settings had higher incidence rates of RSV-ARI and RSV-LRTD, depending on the season.
Our study has several strengths. To our knowledge, this is the first prospective cohort study during the post-Omicron period of the COVID-19 pandemic that assessed the incidence and sociodemographic characteristics of RSV-ARI and RSV-LRTD in community-dwelling adults aged 18 to 64 years. Furthermore, this large community-based study did not rely on medically attended or hospitalization surveillance data; rather, the participants collected their own nasal and oropharyngeal swab specimens at home. In addition, our cohort consisted of primary care patients from 4 US states of different climates and geographic regions. Finally, we applied an innovative recruitment digital tool (Technology-Enabling Subject Recruitment System) enabling the efficient remote enrollment of participants, in addition to a mail option, recruiting approximately 7500 participants over 6 months [9].
Our study has several limitations. The RSV-ARI incidence is likely to be underestimated for several reasons. In participants with ARI, we were not able to collect specimens in about 10% in season 1 and 19% in season 2, primarily due to the missed 7-day window for specimen collection after symptom onset. Additionally, our study period began on 1 October 2022; thus, our currently reported rates might not capture the RSV epidemic onset in certain regions due to the impact of the COVID-19 pandemic. Furthermore, ascertainment of RSV infection in our study was based on participant self-obtained nasal and oropharyngeal swabs. As such, there is a possibility of RSV-ARI incidence underdetection, as additional specimens (eg, sputum, saliva, and serology) may have increased detection of RSV [38–40], given that previous studies found an increase of RSV detection by 1.5 to 2.2 times if the nasopharyngeal RT-PCR was paired with serology, saliva, or sputum for testing [39–41]. Other potential study limitations include the predominance of female participants, the single-institution study design (despite including participants from multiple geographic locations), the exclusion of pregnant participants, and the lack of a comprehensive evaluation of preexisting conditions. There are also limitations with the study involving the demographics of the empaneled primary care population, which overrepresents non-Hispanic White persons in all locations. As such, the generalizability of our study findings to other settings of racially diverse communities needs to be interpreted with caution, although we tried to diversify the populations by SES (HOUSES Index), residential state, and rurality to improve generalizability.
CONCLUSION
Our study results suggest that the RSV-ARI incidence rate and seasonal pattern are shifting toward pre–COVID-19 pandemic RSV epidemiology. This may pose a few important implications on vulnerable populations to RSV-ARI, especially those with chronic medical conditions who may share a disproportionately greater burden of RSV-ARI. As the age group in our study represents a crucial economic workforce in any given community, public health surveillance of RSV-ARI epidemiology and its impact at a population level is important.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Wendelyn Bosch, Division of Infectious Diseases, Mayo Clinic, Jacksonville, Florida, USA.
Lisa J Speiser, Division of Infectious Diseases, Mayo Clinic, Phoenix, Arizona, USA.
Chung-Il Wi, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Katherine S King, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, Minnesota, USA.
Traci L Natoli, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Kathy D Ihrke, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Matthew J Spiten, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Matthew J Binnicker, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota, USA.
Joseph D Yao, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota, USA.
Paul Y Takahashi, Division of Community Internal Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Robert J Pignolo, Divisions of Hospital Internal Medicine, Endocrinology, and Geriatric Medicine and Gerontology, Department of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Brandon H Hidaka, Northwest Wisconsin Family Medicine, Mayo Clinic Health System, Wisconsin, USA.
Randy M Foss, Southeast Minnesota Family Medicine, Mayo Clinic Health System, Minnesota, USA.
Jean-Yves Pirçon, GlaxoSmithKline, Wavre, Belgium.
Pouya Saeedi, GlaxoSmithKline, Wavre, Belgium.
Mohamed Oujaa, GlaxoSmithKline, Wavre, Belgium.
Young J Juhn, Department of Pediatric and Adolescent Medicine and Internal Medicine, Mayo Clinic and Mayo Clinic Health System, Rochester, Minnesota, USA.
Notes
Acknowledgments. The GSK authors thank Dominique Luyts for her valuable feedback and contributions to the study. We appreciate Jenny Weis, Nancy Hawley, and Kelly Okeson’s administrative support. Also, we appreciate the advice and input from many staff of the Precision Population Science Lab and Mayo Clinic Health System.
Author contributions. W. B., L. J. S., C.-I. W., P. Y. T., R. J. P., B. H. H., R. M. F., and Y. J. J. contributed to the study design, interpretation of data, and manuscript writing. K. S. K. contributed to the study design, interpretation of data, statistical analysis, and manuscript writing. T. L. N., K. D. I., and M. J. S. contributed to the study design, data collection, and manuscript writing. M. J. B. and J. D. Y. contributed to the study design, data interpretation, manuscript writing. J.-Y. P. contributed to the study design and interpretation of data and critically reviewed the manuscript. P. S. and M. O. contributed to the interpretation of data, and critically reviewed the manuscript.
Financial support. This work was supported by a research grant from GlaxoSmithKline to W. B., L. J. S., C.-I. W., K. S. K., T. L. N., K. D. I., M. J. S., P.Y.T., R. J. P., Y. J. J.
References
- 1. Rose EB, Wheatley A, Langley G, Gerber S, Haynes A. Respiratory syncytial virus seasonality—United States, 2014–2017. MMWR Morb Mortal Wkly Rep 2018; 67:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic—United States, 2020–2021. MMWR Morb Mortal Wkly Rep 2021; 70:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus—United States, 2017–2023. MMWR Morb Mortal Wkly Rep 2023; 72:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Havers FP, Whitaker M, Pham H, et al. 2209. RSV-associated hospitalizations in adults aged ≥18 years and the impact of the COVID-19 pandemic in the United States, October 2018–February 2022. Open Forum Infect Dis 2022; 9(suppl 2):ofac492.1828. [Google Scholar]
- 5. Juhn YJ, Wi CI, Takahashi PY, et al. Incidence of respiratory syncytial virus infection in older adults before and during the COVID-19 pandemic. JAMA Netw Open 2023; 6:e2250634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Navarrete J, Barone G, Qureshi I, et al. SARS-CoV-2 infection and death rates among maintenance dialysis patients during Delta and early Omicron waves—United States, June 30, 2021–September 27, 2022. MMWR Morb Mortal Wkly Rep 2023; 72:871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Juhn YJ, Beebe TJ, Finnie DM, et al. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health 2011; 88:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Juhn YJ, Wi CI, Ryu E, et al. Adherence to public health measures mitigates the risk of COVID-19 infection in older adults: a community-based study. Mayo Clin Proc 2021; 96:912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wi CI, King KS, Ryu E, et al. Application of innovative subject recruitment system for batch enrollment: a pilot study. J Prim Care Community Health 2023; 14:21501319231194967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol 2011; 173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 12. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–9. [DOI] [PubMed] [Google Scholar]
- 13. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi PY, Ryu E, Hathcock MA, et al. A novel housing-based socioeconomic measure predicts hospitalisation and multiple chronic conditions in a community population. J Epidemiol Community Health 2016; 70:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wi CI, St Sauver JL, Jacobson DJ, et al. Ethnicity, socioeconomic status, and health disparities in a mixed rural-urban US community—Olmsted County, Minnesota. Mayo Clin Proc 2016; 91:612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barwise A, Wi CI, Frank R, et al. An innovative individual-level socioeconomic measure predicts critical care outcomes in older adults: a population-based study. J Intensive Care Med 2021; 36:828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Felzer JR, Finney Rutten LJ, Wi CI, et al. Disparities in vaccination rates in solid organ transplant patients. Transpl Infect Dis 2023; 25:e14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryu E, Juhn YJ, Wheeler PH, et al. Individual housing-based socioeconomic status predicts risk of accidental falls among adults. Ann Epidemiol 2017; 27:415–20.e2. [DOI] [PubMed] [Google Scholar]
- 19.US Census Bureau. Urban and rural. Updated 28 June 2023. Available at: https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural.html. Accessed 12 July 2023.
- 20. Fleiss JL. Statistical methods for rates and proportions. 2nd ed. New York: Wiley, John & Sons, 1981. [Google Scholar]
- 21. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep 2023; 72:793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wickham H. Ggplot2: elegant graphics for data analysis. New York: Springer-Verlag, 2016. [Google Scholar]
- 23. Midgley CM, Haynes AK, Baumgardner JL, et al. Determining the seasonality of respiratory syncytial virus in the United States: the impact of increased molecular testing. J Infect Dis 2017; 216:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi T, Vennard S, Jasiewicz F, Brogden R, Nair H; RESCEU Investigators . Disease burden estimates of respiratory syncytial virus related acute respiratory infections in adults with comorbidity: a systematic review and meta-analysis. J Infect Dis 2022; 226(suppl 1):S17–21. [DOI] [PubMed] [Google Scholar]
- 25. D’Angelo CR, Kocherginsky M, Pisano J, et al. Incidence and predictors of respiratory viral infections by multiplex PCR in allogeneic hematopoietic cell transplant recipients 50 years and older including geriatric assessment. Leuk Lymphoma 2016; 57:1807–13. [DOI] [PubMed] [Google Scholar]
- 26. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 27. Flight WG, Bright-Thomas RJ, Tilston P, et al. Incidence and clinical impact of respiratory viruses in adults with cystic fibrosis. Thorax 2014; 69:247–53. [DOI] [PubMed] [Google Scholar]
- 28. McClure DL, Kieke BA, Sundaram ME, et al. Seasonal incidence of medically attended respiratory syncytial virus infection in a community cohort of adults ≥50 years old. PLoS One 2014; 9:e102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mikulska M, Del Bono V, Gandolfo N, et al. Epidemiology of viral respiratory tract infections in an outpatient haematology facility. Ann Hematol 2014; 93:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tischer J, Engel N, Fritsch S, et al. Virus infection in HLA-haploidentical hematopoietic stem cell transplantation: incidence in the context of immune recovery in two different transplantation settings. Ann Hematol 2015; 94:1677–88. [DOI] [PubMed] [Google Scholar]
- 31. Walsh EE, Falsey AR, Hennessey PA. Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. Am J Respir Crit Care Med 1999; 160:791–5. [DOI] [PubMed] [Google Scholar]
- 32. Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ 1997; 315:1060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Korsten K, Adriaenssens N, Coenen S, et al. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur Respir J 2021; 57:2002688. [DOI] [PubMed] [Google Scholar]
- 34. Rios-Guzman E, Simons LM, Dean TJ, et al. Deviations in RSV epidemiological patterns and population structures in the United States following the COVID-19 pandemic. Nat Commun 2024; 15:3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. RSV Hospitalization Surveillance Network: RSV-NET interactive dashboard. Updated 6 July 2024. Available at: https://www.cdc.gov/rsv/php/surveillance/rsv-net.html. Accessed 17 July 2024.
- 36. Zheng Z, Warren JL, Shapiro ED, Pitzer VE, Weinberger DM. Estimated incidence of respiratory hospitalizations attributable to RSV infections across age and socioeconomic groups. Pneumonia (Nathan) 2022; 14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holmen JE, Kim L, Cikesh B, et al. Relationship between neighborhood census-tract level socioeconomic status and respiratory syncytial virus-associated hospitalizations in US adults, 2015–2017. BMC Infect Dis 2021; 21:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Onwuchekwa C, Moreo LM, Menon S, et al. Underascertainment of respiratory syncytial virus infection in adults due to diagnostic testing limitations: a systematic literature review and meta-analysis. J Infect Dis 2023; 228:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis 2022; 9:ofac300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramirez J, Carrico R, Wilde A, et al. Diagnosis of respiratory syncytial virus in adults substantially increases when adding sputum, saliva, and serology testing to nasopharyngeal swab RT-PCR. Infect Dis Ther 2023; 12:1593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Y, Kulkarni D, Begier E, et al. Adjusting for case under-ascertainment in estimating RSV hospitalisation burden of older adults in high-income countries: a systematic review and modelling study. Infect Dis Ther 2023; 12:1137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.