Abstract
Background
Amphetamine dependence is a public health problem with medical, psychiatric, cognitive, legal and socioeconomic consequences. To date, no pharmacological treatment has been approved for this disorder, and psychotherapy remains the mainstay of treatment. In recent years, psychostimulants have been investigated as a possible replacement therapy.
Objectives
To evaluate the efficacy and safety of psychostimulant medications for amphetamine abuse or dependence. The influences of type of drug, type of dependence, comorbid disorders, clinical trial risk of bias and publication of data were also studied.
Search methods
Relevant trials were searched in the following sources: PubMed (January 1966 to 6 June 2012), EMBASE (January 1988 to 6 June 2012), CENTRAL (The Cochrane Library, Issue 5 of 12, May 2012), PsycINFO (January 1985 to 6 June 2012) and the Specialised Register of the Cochrane Drug and Alcohol Group (June 2012). We also searched the reference lists of retrieved trials, the list of studies citing the included trials and the main electronic registers of ongoing trials (ClinicalTrials.gov, International Clinical Trials Registry Platform and EU Clinical Trials Register). Finally, we contacted investigators to request information about unpublished trials. Searches included non-English language literature.
Selection criteria
All randomised, placebo‐controlled, parallel‐group clinical trials investigating the efficacy or safety of psychostimulants for amphetamine dependence or abuse conducted in an outpatient setting.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration.
Main results
Eleven studies were included in the review (791 participants). Studied psychostimulants included dexamphetamine, bupropion, methylphenidate and modafinil. No significant differences were found between psychostimulants and placebo for any of the studied efficacy outcomes. Overall retention in studies was low (50.4%). Psychostimulants did not reduce amphetamine use (mean difference (MD) ‐0.26, 95% confidence interval (CI) ‐0.85 to 0.33) or amphetamine craving (MD 0.07, 95% CI ‐0.44 to 0.59) and did not increase sustained abstinence (relative risk (RR) 1.12, 95% CI 0.84 to 1.49). The proportion of adverse events inducing dropout was similar for psychostimulants and placebo (risk difference (RD) 0.01, 95% CI ‐0.03 to 0.04). The main findings did not change in any subgroup analysis.
Authors' conclusions
Results of this review do not support the use of psychostimulant medications at the tested doses as a replacement therapy for amphetamine abuse or dependence. Future research could change this conclusion, as the numbers of included studies and participants are limited and information on relevant outcomes, such as efficacy according to the severity of dependence or craving, is still missing.
Keywords: Humans, Amphetamine‐Related Disorders, Amphetamine‐Related Disorders/drug therapy, Benzhydryl Compounds, Benzhydryl Compounds/therapeutic use, Bupropion, Bupropion/therapeutic use, Central Nervous System Stimulants, Central Nervous System Stimulants/adverse effects, Central Nervous System Stimulants/therapeutic use, Dextroamphetamine, Dextroamphetamine/therapeutic use, Methylphenidate, Methylphenidate/therapeutic use, Modafinil, Randomized Controlled Trials as Topic
Plain language summary
Efficacy of psychostimulant drugs for amphetamine abuse or dependence
Amphetamine dependence constitutes a public health problem with many consequences and complications. Amphetamine abuse refers to a maladaptive and hazardous pattern of use considered to be less severe than dependence. To date, no pharmacological treatment has been approved for amphetamine abuse or dependence, and psychotherapy remains the best treatment option.
Long‐term amphetamine use reduces dopamine levels in the brain. Drugs increasing dopamine and mimicking the effects of amphetamines with lower abuse liability could be used as replacement therapy in amphetamine dependence. Several psychostimulants have been studied recently for this purpose.
In this review, the efficacy and safety of psychostimulants for amphetamine abuse or dependence were studied. We found eleven studies enrolling 791 amphetamine‐dependent participants and assessing the effects of four different psychostimulants: dexamphetamine, bupropion, methylphenidate and modafinil. Psychosocial interventions were additionally provided to all participants. The studies were conducted in the USA, Australia or Northern Europe, and study length ranged from 8 to 20 weeks.
Psychostimulants did not reduce amphetamine use or amphetamine craving and also did not increase sustained abstinence in comparison with placebo. Retention in treatment was similar and low with both treatments. Psychostimulants also did not increase the risk of adverse events that were intense enough to induce dropouts.
Research with larger and longer trials is needed to determine whether psychostimulants can be a useful replacement therapy for patients with amphetamine abuse or dependence. The design of future trials should consider the level of dependence at study entry, the potency and the dose of the psychostimulant administered, the length of the trial and the representativeness of included participants.
Summary of findings
Summary of findings for the main comparison. Psychostimulants for amphetamine abuse or dependence.
| Psychostimulants for amphetamine abuse or dependence | ||||||
| Patient or population: Amphetamine abuse or dependence Settings: Outpatients Intervention: Psychostimulants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Psychostimulants | |||||
| Amphetamine use (UA) Negative urinalyses across the study Follow‐up: 8‐12 weeks | The mean of the proportion of amphetamine‐negative UA ranged in the control groups from 0.56 to 33.1 | The mean of the proportion of amphetamine‐negative UA ranged in the intervention groups from 0.33 to 36.85 | 473 (7 studies) | ⊕⊝⊝⊝ very low1,2,3,4,5 |
MD ‐0.26 (‐0.85 to 0.33) |
|
| Sustained abstinence Negative urinalyses for at least 3 consecutive weeks Follow‐up: mean 8‐12 weeks | Study population | RR 1.12 (0.84 to 1.49) | 559 (6 studies) | ⊕⊝⊝⊝ very low1,2,3,4,5 | ||
| 220 per 1000 | 247 per 1000 (185 to 328) | |||||
| Moderate | ||||||
| 285 per 1000 | 319 per 1000 (239 to 425) | |||||
| Retention to treatment Number of participants who competed treatment Follow‐up: 8‐20 weeks | Study population | RR 1.01 (0.9 to 1.14) | 791 (11 studies) | ⊕⊕⊝⊝ low2,3,4,5,6 | ||
| 489 per 1000 | 494 per 1000 (440 to 557) | |||||
| Moderate | ||||||
| 378 per 1000 | 382 per 1000 (340 to 431) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The outcome is not influenced by lack of blinding, but high attrition in trials has been noted. 2No statistical heterogeneity was found. 3The studied intervention includes different types of psychostimulants and different doses. 495% CI is wide, and the intervention effect over this outcome can range from no benefit to small effect. 5Funnel plot not suggested publication bias. Statistical power is low in using tests to detect publication bias for this comparison in this review. 6This comparison includes studies with treatment length ranging from 8 to 20 weeks.
Background
Description of the condition
Globally, amphetamines rank as the second most used illicit drug after cannabis, followed by cocaine and opiates. Methamphetamine and amphetamine are the most widely consumed synthetic stimulants (WHO Technical Briefs 2011). The United Nations Office on Drugs and Crime estimated in 2010 a prevalence of 0.3 to 1.2 per cent, or between 14 million and 52.5 million global users of amphetamines (excluding ecstasy). On the other hand, the number of ecstasy‐group users is smaller and ranged in 2010 between 10.5 million and 28 million people worldwide (0.2% to 0.6% of all adults aged 15 to 64) (UNODC 2012).
Amphetamine dependence is a consequence of long‐term amphetamine use and is considered a major global health problem. Smoked or injected amphetamines more commonly lead to dependence than does the oral form.
Amphetamine abuse refers to a maladaptive and hazardous pattern of use considered to be less severe than dependence. Amphetamine dependence occurs when an individual uses one or more of the amphetamine substances in a maladaptive way, resulting in at least three of the following symptoms: a need for increased quantities of amphetamines to achieve the desired subjective effect (tolerance); the presence of withdrawal symptoms such as depression, fatigue, insomnia or hypersomnia, increased appetite or agitation; use of amphetamines in larger amounts or for longer duration; a persistent, unsuccessful attempt to control use of the substance; increased amount of time spent using or obtaining amphetamines; giving up important activities in deference to the use of amphetamines; and continued amphetamine use despite related physical, emotional, occupational, legal or relational difficulties (DSM IV TR).
Dopamine dysfunction has been postulated as the main neurobiological mechanism involved in amphetamine dependence. Although amphetamine use is associated with an increase in dopamine in the nucleus accumbens, in response to long‐term amphetamine use a hypo‐dopaminergic state has been observed (Chang 2007; Shearer 2008). Otherwise, cessation and appearance of withdrawal symptoms can be explained by functional dopamine hypoactivity in the striatum (Koob 2009; Rossetti 1992).
The presence of other comorbidities in amphetamine‐dependent patients is not an exception. Amphetamine dependence is associated with depressive, anxiety and psychotic disorders (Salo 2011), with attention deficit hyperactivity disorder (ADHD) (Wilens 2004) and with antisocial personality disorder (Glasner‐Edwards 2010). The high rate of comorbidities is expected to increase the difficulty of managing these patients (Glasner‐Edwards 2009). In addition, amphetamine misuse has been associated with sexual risk behaviours and increased risk of human immunodeficiency virus (HIV) (Colfax 2010).
Description of the intervention
Amphetamine‐type stimulants (ATSs) are a group of drugs comprising synthetic stimulants such as the amphetamine‐group substances (like amphetamine and methamphetamine) and the ecstasy‐group substances (like 3,4‐methylenedioxymethamphetamine, also called MDMA). In this review, the word "amphetamines" stands for all them.
Amphetamines increase synaptic dopamine (DA), norepinephrine (NE) and serotonin (5‐HT) concentrations by inhibiting the presynaptic membrane transporters (DAT, NET and SERT, respectively). Also they can reverse the action of the transporters facilitating neurotransmitter efflux into the synaptic cleft and can displace newly synthesised neurotransmitters from the vesicle stores. Finally, they inhibit monoamine oxidase, the enzyme responsible for the metabolism of the neurotransmitters (Howell 2008; Robinson 1985; Zahniser 2009). These drugs have in common that they excite the central nervous system (CNS) and speed up body functions. Amphetamines induce euphoria, increase alertness, decrease appetite and fatigue, increase heart rate, blood pressure and breathing rate, constrict blood vessels, dilate pupils and release glucose and lipids into the bloodstream. These substances can be taken orally, snorted, smoked or injected intravenously, and their effects may appear in 30 to 40 minutes and last for 4 to 8 hours.
Methamphetamine is typically characterised as a more potent psychostimulant than non‐methylated amphetamine. At comparable doses, higher levels get into the brain because it is more lipophilic (NIDA 2006). Nevertheless, published data support both drugs as having a similar profile of effects and equivalent abuse potential (Kirkpatrick 2012 b). Short‐ and long‐term effects of methamphetamine are similar to those produced by cocaine (another potent psychostimulant), but the effects of methamphetamine last longer and can be stronger (Newton 2005).
No typical profile of an amphetamine user is known, and amphetamines are used for different purposes. Amphetamines can be used by students or drivers to stay awake, by athletes to enhance performance and at parties or clubs (club drugs) to increase sociability (WHO Technical Briefs 2011).
This review will be focused on the use of psychostimulant drugs as a substitution therapy for amphetamine abuse or dependence. By definition, medications used as maintenance therapy should have similar properties (mechanism of action, behavioural effects) to the abused drug, but with less addictive potential. The objective is to avoid illicit parenteral drug use by providing orally administered legal compounds. The rationale for use of psychostimulants to treat amphetamine dependence is based on previous successful results of replacement therapy for nicotine (Eisenberg 2008) or opiate dependence (Amato 2005).
To replace amphetamines for patients with amphetamine abuse or dependence, two different strategies should be considered: first, the use of milder psychostimulants with lower abuse potential, like caffeine, bupropion or modafinil; second, the use of sustained‐release formulations of classical psychostimulants like methylphenidate or dexamphetamine. Such formulations allow once‐ or twice‐daily dosing and therefore improve compliance (Herin 2010). Immediate‐release formulations of potent psychostimulants, a priori, are a less desirable option because of their potential for abuse.
How the intervention might work
Psychostimulants may substitute the use of amphetamines by reducing amphetamine withdrawal (McGregor 2008) and craving (Newton 2006), thereby leading to abstinence. Additionally, the use of psychostimulant drugs simultaneously with amphetamines can reduce the euphoriant and reinforcing effects of amphetamines (De la Garza 2010), which also could promote reduction of amphetamine use. Finally, long‐term increases in DA in the nucleus accumbens achieved by other psychostimulants different from the abused drug could improve the established dopaminergic dysfunction in amphetamine‐dependent patients (Xi 2008).
Previous preclinical and human laboratory studies have shown promising results for psychostimulants (amphetamine substitution, attenuation of amphetamine reinforcing and subjective effects and reduction of amphetamine self‐administration). Those results encouraged the performance of several clinical studies with these drugs, most of them carried out in recent years. Several experts on the topic have suggested that doses of psychostimulants used to replace amphetamines in dependent patients should be higher than those used to treat other disorders such as ADHD, obesity or narcolepsy. A possible explanation for this fact could be that chronic stimulant abuse decreases sensitivity to those medications. Additionally, the severity of the dependence may explain the different utility of particular psychostimulants in specific subgroups of patients. Strong psychostimulants may be appropriate for severely dependent patients, while mild psychostimulants could be useful for patients with a less severe disorder (Herin 2010).
Why it is important to do this review
To date, no pharmacological treatment has been approved for amphetamine abuse or dependence, although different kinds of drugs have been tested (Chen 2010; Karila 2010; Srisurapanont 2001). On the other hand, psychosocial interventions have shown modest results (Knapp 2007; Lee 2008; Shearer 2007), suggesting the need for a medication that could enhance their effectiveness.
Two previous Cochrane reviews (Shoptaw 2009; Srisurapanont 2001) have investigated participants with amphetamine‐related disorders-one including all different medications studied to that moment for amphetamine dependence (mainly antidepressants), and the other focused on treatment for amphetamine withdrawal symptoms, with both reviews highlighting the need for continued research in this area.
Indirect dopamine agonists used to treat psychostimulant abuse or dependence showed promising results in a recent systematic review, but conclusions were based mainly on cocaine‐dependent participants because the evidence for amphetamine abuse or dependence was limited (Pérez‐Mañá 2011).
Objectives
To evaluate the efficacy and safety of psychostimulant medications for amphetamine abuse or dependence. The influences of type of drug, type of dependence, comorbid disorders, clinical trial risk of bias and publication of data were also studied.
Methods
Criteria for considering studies for this review
Types of studies
All randomised, placebo‐controlled, parallel‐group clinical trials investigating the efficacy or safety of psychostimulants for amphetamine dependence or abuse conducted in an outpatient setting.
Types of participants
Participants with amphetamine abuse or dependence, according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM IV TR or previous versions). Patients with additional comorbidities were also included (and were studied later in subgroup analyses).
Types of interventions
Experimental intervention
Any psychostimulant medication alone or in combination with psychosocial interventions. Psychostimulants were any drugs with acute CNS stimulant effects defined as increased CNS activity resulting in fatigue relief, increased locomotor activity and anorexia in healthy participants (Boutrel 2004; King 2005; Kosman 1968). The criteria adopted to classify drugs as psychostimulants were the same as those used in two previous reviews on cocaine dependence. Drugs included had at least one published study showing psychostimulant effects (Castells 2007; Castells 2010). We did not limit the review to those drugs with CNS‐stimulating effects that directly target DA neurotransmission; at that time, xanthines were also included. The list of psychostimulants used for the search included classical (strong) psychostimulants, like amphetamine or methylphenidate, and mild psychostimulants, like bupropion, modafinil or caffeine.
Amphetamine withdrawal may last for up to 4 weeks; therefore we decided to exclude studies that lasted less than 4 weeks.
Control intervention
Placebo alone or in combination with psychosocial interventions.
Types of outcome measures
Primary outcomes
Efficacy primary outcomes.
Amphetamine use (definition 1). Mean (standard deviation (SD)) negative urinalyses (UA) across the study.
Amphetamine use (definition 2). Amphetamine concentration in hair analysis.
Sustained amphetamine abstinence. Number of participants who achieved sustained amphetamine abstinence.
Secondary outcomes
Efficacy secondary outcomes.
Self‐reported amphetamine use. The mean (SD) of days of amphetamine use across the study.
Retention in treatment. Number of participants who completed the treatment.
Amphetamine craving. Assessed by validated scales such as a visual analog scale (VAS) or the Brief Substance Craving Scale (BSCS) (Drobes 1999; Somoza 1995). The mean (SD) craving score at study end.
Depressive symptoms severity. Assessed by validated scales such as Hamilton Depression Rating Scale (HDRS or HAM‐D) (Hamilton 1960). The mean (SD) depression score at study end.
Anxiety symptoms severity. Assessed by validated scales such as Hamilton Anxiety Scale (HAM‐A) (Hamilton 1959). The mean (SD) anxiety score at study end.
Overall functioning. Assessed by validated scales such as Clinical Global Impression (CGI) rating scales (Guy 1976).The mean (SD) global impression scale score at study end.
Safety secondary outcomes.
Number of participants who dropped out because of any adverse event (AE).
Number of participants who dropped out because of cardiovascular adverse events.
Number of participants who dropped out because of psychiatric adverse events.
Search methods for identification of studies
Electronic searches
Relevant trials were obtained from the following sources.
PubMed (January 1966 to 6 June 2012).
EMBASE (January 1988 to 6 June 2012).
CENTRAL (The Cochrane Library, Issue 5 of 12, May 2012).
PsycINFO (January 1985 to 6 June 2012).
Cochrane Drug and Alcohol Group Specialised Register (June 2012).
Databases were searched using a strategy developed by incorporating the filter for identification of RCTs (Higgins 2011) combined with selected MeSH terms and free text terms related to amphetamine dependence. The PubMed search strategy was used with the other databases by inserting appropriate controlled vocabulary as applicable. Access was always performed through Ovid SP.
The search strategy used in the different databases is shown in Appendix 1; Appendix 2; Appendix 3; Appendix 4; and Appendix 5.
We searched for ongoing clinical trials and unpublished studies via Internet searches on the following Websites.
http://apps.who.int/trialsearch/
http://clinicaltrials.gov/
https://www.clinicaltrialsregister.eu/
Searching other resources
The reference lists of retrieved studies and relevant review articles were inspected to identify additional studies.
For each included study, a citation search was performed in ISI Web of Knowledge to identify any later studies that may have cited it.
Investigators were contacted through requests for information about unpublished trials.
All searches included non English language literature, and studies with English language abstracts were assessed for inclusion.
Data collection and analysis
Selection of studies
One review author (CP‐M) inspected the search titles and abstracts. The full text of each potentially relevant article to be included in the review was requested, and two review authors assessed the studies independently for inclusion (CP‐M, XC). Doubts were discussed with all review authors if no agreement could be reached between the two assessors.
Data extraction and management
From full papers, concrete information was extracted by two review authors (CP‐M, XC), who used a piloted data extraction sheet. Any disagreement was resolved by consensus and, if necessary, was discussed by all review authors. Study authors were contacted by e‐mail with requests for missing information on at least two different occasions.
Assessment of risk of bias in included studies
Two review authors assessed the risk of bias independently (CP‐M, XC), and if no agreement could be reached between them, doubts were discussed by all review authors.
The risk of bias assessment for RCTs in this review was performed using the criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The recommended approach for assessing risk of bias in studies included in a Cochrane review involves use of a two‐part tool to address specific domains, namely, sequence generation (selection bias),allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessor (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias) and other sources of bias. The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement related to the risk of bias for that entry, in terms of low, high or unclear risk. To make these judgements, we used the criteria indicated by the Cochrane Handbook for Systematic Reviews of Interventions as adapted for the field of addiction. See Appendix 6 for details.
The domains of sequence generation,allocation concealment (avoidance of selection bias), and other sources of bias were addressed by the tool by a single entry for each study.
Blinding of participants, personnel and outcome assessor (avoidance of performance bias and detection bias) was considered separately for objective outcomes (e.g. dropout, use of substance of abuse as measured by urinalysis) and subjective outcomes (e.g. participant self‐reported use of substance and side effects).
Incomplete outcome data (avoidance of attrition bias) were considered for all outcomes except dropout from treatment, which very often is the primary outcome measure in trials on addiction.
Measures of treatment effect
We used Review Manager 5.2 (RevMan) to perform the statistical analysis. We calculated mean difference (MD) using Hedges’ method for continuous outcomes and risk ratio (RR) for dichotomous ones. The risk difference (RD) was preferred over RR for outcomes such as dropouts due to any/cardiovascular/psychiatric adverse events if several studies were found to have 0 events for both psychostimulant and placebo groups, to avoid overestimation of treatment effect. Individual study weights were calculated as the inverse of the variance.
Uncertainty of all measures was expressed by means of their 95% confidence intervals (CI).
Unit of analysis issues
In studies with multiple and correlated interventions (e.g. studies with one control group and multiple experimental ones), the experimental groups were combined into a single group and were included in the meta‐analysis as a single pair‐wise comparison. This approach was used, for instance, when one study compared two different doses of a single psychostimulant drug against placebo. In this situation, both active intervention arms were combined, and only one comparison psychostimulant versus placebo was included in the meta‐analysis. For dichotomous outcomes, both the sample sizes and the numbers of people with events were summed across groups. For continuous outcomes, means and standard deviations were combined using the statistical formulae available in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
The intention‐to‐treat (ITT) sample size was used as the denominator for categorical variables such as retention or sustained amphetamine abstinence.
For continuous data, the sample size introduced in RevMan was the sample size used in each article to calculate that mean and SD. In cases of missing SDs, we used other information to determine them, such as confidence intervals, standard errors, t‐values, P‐values or F‐values as reported. If available information was insufficient, the SD was imputed from those available in other studies of the review (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed by means of the I2 statistic and the Chi2 test for heterogeneity.The cut points were I2 > 50% and P of the Chi2 test < 0.1.
Assessment of reporting biases
Funnel plots (plots of the effect estimate from each study against the standard error) were used to assess the potential for bias related to the size of the trials, which could indicate possible publication bias. If asymmetry was found, the Egger test (Egger 1997) was conducted.
Data synthesis
The outcomes of the individual trials were combined through meta‐analysis where possible (comparability of interventions and outcomes between trials) with the use of a random‐effects model, as some variability was expected in the studies included.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were carried out. The pooled effect and between‐study heterogeneity were calculated within each subgroup.
Type of studied psychostimulant: dextroamphetamine, modafinil, bupropion, etc.
Type of amphetamine dependence: amphetamine, methamphetamine, MDMA, etc.
Study length. The influence of study length was planned to be studied by means of bivariate random‐effects meta‐regression using the restricted maximum likelihood method if at least 10 studies were available (or by stratification in two groups according to the median study length). As most of the studies lasted 12 weeks, this subgroup analysis was not performed.
Comorbidities as inclusion criteria (ADHD, opioid dependence, alcohol dependence, etc).
Published versus unpublished data.
Risk of bias of included studies, assessed by means of the Cochrane risk of bias instrument: high risk versus intermediate or low risk of bias in the domain Incomplete Outcome Data. (High‐risk studies were expected according to this domain, as attrition is high in drug dependence trials.)
Sensitivity analysis
For safety outcomes, when studies with 0 events were identified and RD was used in the main analysis, a sensitivity analysis was carried out using the RR instead of the RD. Effects measured by the RR can be easily interpreted, although bias due to exclusion of studies with 0 events in this case cannot be ruled out.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies.
Results of the search
Despite all the reports obtained from the different databases, most were excluded after review authors read the title or the title and abstract. Twenty were retrieved in full text for more detailed evaluation. Of those 20, 9 studies were excluded and 11 met the inclusion criteria of this review. Seven studies are ongoing. See flow diagram in Figure 1.
1.
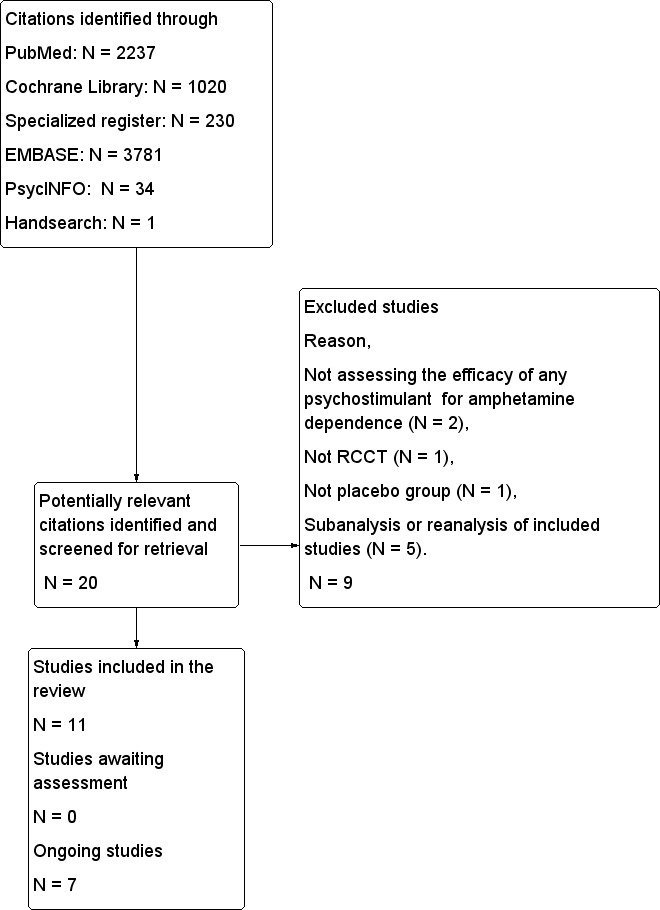
Flow diagram for selection of studies.
Included studies
Eleven studies were included in the review. Studies were conducted by university researchers with only one exception (Galloway 2011), and pharmaceutical industry helped to fund three of them (Heinzerling 2010; Konstenius 2010; Shearer 2009).
Participants
A total of 791 participants were enrolled. Almost two‐thirds (64.9%) were male and had a mean age of 35.9 years. Nine studies (Anderson 2012; Das 2009; Elkashef 2008 a; Galloway 2011; Heinzerling 2010; Longo 2010; Mancino 2011; Shearer 2009; Shoptaw 2008) enrolled methamphetamine‐dependent participants, and two enrolled amphetamine‐dependent participants (Konstenius 2010; Tiihonen 2007). Duration of use ranged from 7 to 15.9 years, and 59% of participants used the drug intrapulmonarily (ip). Comorbid ADHD was rather prevalent amongst participants enrolled because this condition was an inclusion criterion in one study (Konstenius 2010). Conversely, other non‐nicotine dependencies were infrequent, probably because patients with these comorbid disorders were excluded. Indeed, only two studies enrolled opioid‐dependent participants (Das 2009: 1 participant; Shearer 2009: 10 participants), and one included alcohol‐dependent participants (Anderson 2012: 7 participants). Participants did not have psychotic disorders, and only one study included participants with major depression (Anderson 2012: 15 participants).
A detailed description of the baseline characteristics of participants included in these studies can be found in Table 2.
1. Baseline characteristics of the participants included in the RCT of the meta‐analysis.
|
Sample size n |
791 |
|
Gender % male |
64.9 |
|
Age Mean age (years) |
35.9 |
|
Race % Caucasian % African‐American % Other |
70.5 3.8 25.7 |
|
Employment status % currently employed |
58.7 |
|
Type of dependence % methamphetamine dependence % amphetamine dependence |
92.7 7.3 |
|
Mean days of use/month Range |
9.3‐19.5 |
|
Mean length of amphetamine use (years) Range of mean lifetime amphetamine use |
7‐15.9 |
|
Route of use % ip % iv % in % oral |
59.0 22.8 17.6 0.6 |
|
Comorbidities % nicotine dependent % ADHD % opioid dependent % alcohol dependent % major depression % psychotic disorders |
72.9 13.9 1.6 1,1 0.8 0 |
Abbreviations: ADHD = attention deficit hyperactivity disorder, ip = intrapulmonary, iv = intravenous, in = intranasal. Baseline participant characteristics are presented for those trials reporting this information. Gender, age and type of dependence were available for all studies, whereas opioid dependence and alcohol dependence from 9 studies, psychotic disorders and major depression from 8, employment status from 7, lifetime amphetamine use and ADHD from 6, race from 5 studies and nicotine dependence days of amphetamine use in a month and route of amphetamine use in 4.
Interventions and settings
Four psychostimulants have been investigated for the treatment of amphetamine dependence, namely, modafinil, bupropion, dexamphetamine and methylphenidate. The most frequently studied psychostimulant was modafinil (4 studies: Anderson 2012; Heinzerling 2010; Mancino 2011; Shearer 2009) followed by bupropion (3 studies: Das 2009; Elkashef 2008 a; Shoptaw 2008). Both dexamphetamine (Galloway 2011; Longo 2010) and methylphenidate (Konstenius 2010; Tiihonen 2007) were studied in two trials.
Psychosocial interventions were provided in all studies, in addition to the study intervention. Cognitive‐behavioural therapy (CBT) was provided in five studies. CBT was the only psychological intervention provided in one study, but it was administered together with contingency management (CM) in three studies and with counselling and motivational enhancement therapy in the other two. The remaining seven studies provided one of the following psychotherapies: counselling, individual motivational psychotherapy, individual skills training program, unstructured psychosocial treatment, cognitive‐behavioural intervention or unspecified psychotherapy. Five studies were single site and five multiple site, and in one of them, the number of centres implied was not specified. More than half of the studies (seven studies) were conducted in the USA, two studies were conducted in Australia (Longo 2010; Shearer 2009), one in Sweeden (Konstenius 2010) and one in Finland (Tiihonen 2007). Study length ranged from 8 to 20 weeks, and most (seven) studies lasted for 12 weeks.
All studies were conducted in an outpatient setting, as this was an inclusion criterion of the review.
Excluded studies
Nine studies were excluded from the review (see Characteristics of excluded studies and Figure 1). Five were re‐analyses or sub‐analyses of included studies (55.6%). In two, the intervention studied was not a psychostimulant to treat amphetamine dependence. Finally, one study was not a randomised controlled clinical trial (RCCT), and another did not include a placebo group.
Risk of bias in included studies
A detailed description of the risk of bias for each study can be found in the corresponding risk of bias tables (Characteristics of included studies). This information is summarised in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
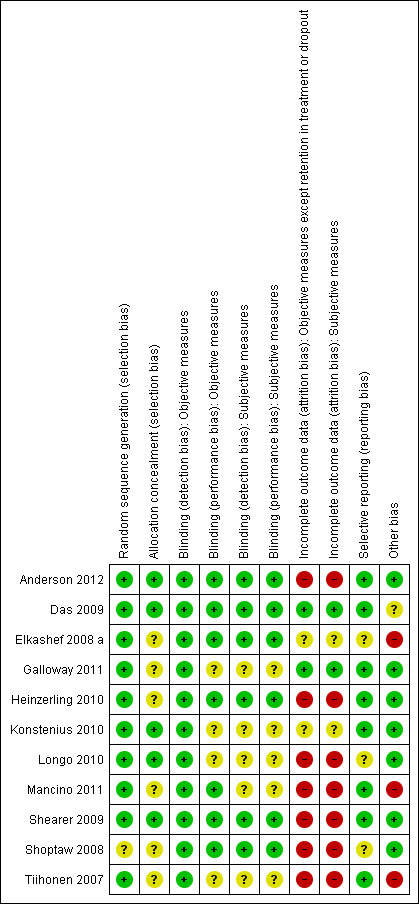
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Sequence generation
All studies were RCTs with parallel design. The method used to generate a random sequence was well described or was obtained through contact with authors in all but one study (Shoptaw 2008).
Allocation
Concealment of allocation was unclear in half of the studies (Elkashef 2008 a; Galloway 2011; Heinzerling 2010; Mancino 2011; Shoptaw 2008; Tiihonen 2007).
Blinding
Classical psychostimulants such as dexamphetamine or methylphenidate have powerful behavioural effects that may jeopardise blinding when these drugs are compared with placebo (Martin 1971; Makris 2007), leading to the possibility of both performance and detection bias. Performance bias arises from systematic differences between groups in the care provided. This type of bias can affect any study endpoint. For this reason, all outcomes assessed in this review in studies using those drugs were judged to have an unclear risk of performance bias. Blinding failure can also cause detection bias, which consists of systematic differences between groups in the the way study endpoints are measured. Objective outcomes were considered free of detection bias because assessment of these outcomes was considered not to be influenced by blinding failure. In studies with dexamphetamine and methylphenidate, the possibility that blinding failure could affect the assessment of subjective outcomes could not be ruled out; consequently, detection bias was rated "unclear" for subjective outcomes. In studies with bupropion and modafinil (mild psychostimulants), no differences between conditions (intervention and placebo) were reported when participants were asked to guess the medication they received (Das 2009; Shearer 2009). Therefore, we considered that blinding could be maintained in studies testing those medications.
Incomplete outcome data
To address attrition bias, we collected for each study discontinuation rate, reasons for dropout with each treatment and statistical methods used for data imputation. The treatment discontinuation rate was low (< 20%) in only two studies (Das 2009; Galloway 2011), both of which were considered to have low attrition bias. For the remaining studies, the discontinuation rate was moderate (> 20% to 50%) or high (> 50%), and attrition bias was deemed "unclear" or "high" after the other factors mentioned above were considered.
Selective reporting
The clinical trial protocol was available for most studies. Information about the design and about outcomes assessed (relevant protocol information) between the trial protocol and the article was identical in most of these studies; therefore, the risk of reporting bias was deemed to be low in all of them. However, in two cases, outcomes in the registered protocol were not the same as in the published report; therefore, the risk of selective reporting bias was considered to be unclear (Elkashef 2008 a; Shoptaw 2008). Another study had an unclear risk because the protocol was not available (Longo 2010).
Other potential sources of bias
Seven studies were free of other sources of bias. In one study, the risk of bias was deemed unclear because annual income was different between groups and participants were paid for participation (Das 2009). In one study, ADHD was not balanced between groups (Elkashef 2008 a), another study terminated because of lack of funding (Mancino 2011) and one was an interim analysis of a longer trial with age baseline differences (Tiihonen 2007). For these reasons, the last three were considered to have high risk of bias.
Effects of interventions
See: Table 1
Efficacy primary outcomes
Amphetamine use assessed by the mean (SD) of the proportion of amphetamine‐free UA across the study per participant
(01) Any psychostimulant vs placebo
Seven studies (Anderson 2012; Das 2009; Galloway 2011; Heinzerling 2010; Konstenius 2010; Mancino 2011; Shoptaw 2008), 473 participants: MD ‐0.26 (95% CI ‐0.85 to 0.33); this result was not statistically significant, see Analysis 1.1
1.1. Analysis.
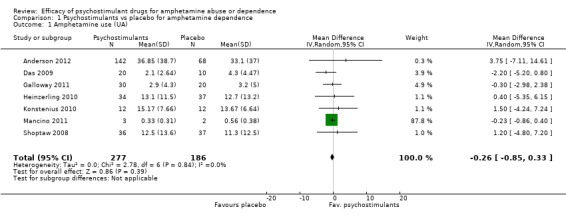
Comparison 1 Psychostimulants vs placebo for amphetamine dependence, Outcome 1 Amphetamine use (UA).
Amphetamine use assessed by the mean (SD) concentration of amphetamine in hair analysis at the end of the study
(01) Any psychostimulant vs placebo
Only one study (Longo 2010), 22 participants, assessed amphetamine use in hair: MD 0.53, 95% CI ‐6.02 to 7.08), the result was not statistically significant, see Analysis 1.2
1.2. Analysis.
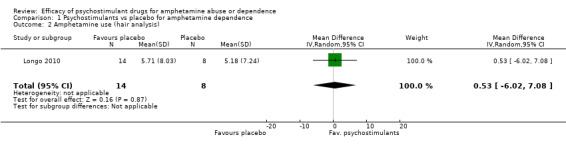
Comparison 1 Psychostimulants vs placebo for amphetamine dependence, Outcome 2 Amphetamine use (hair analysis).
Sustained amphetamine abstinence. Number of participants who achieved at least 3 weeks of sustained abstinence
(01) Any psychostimulant vs placebo
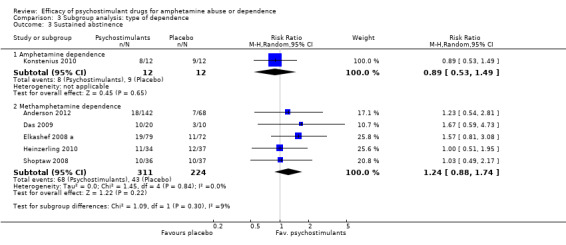
Six studies (Anderson 2012; Das 2009; Elkashef 2008 a; Heinzerling 2010; Konstenius 2010; Shoptaw 2008), 559 participants: RR 1.12 (95% CI 0.84 to 1.49); this result was not statistically significant, see Analysis 1.3
1.3. Analysis.

Comparison 1 Psychostimulants vs placebo for amphetamine dependence, Outcome 3 Sustained abstinence.
Efficacy secondary outcomes
Self‐reported amphetamine use assessed by the mean (SD) of days of amphetamine use across the study
(01) Any psychostimulant vs placebo
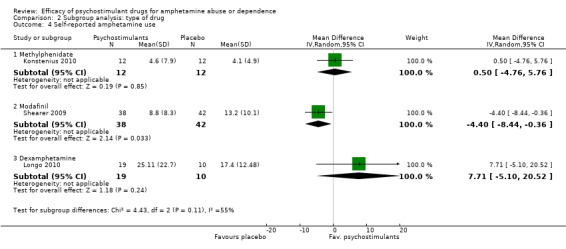
Three studies (Konstenius 2010; Longo 2010; Shearer 2009), 133 participants: MD ‐0.81 (95% CI ‐6.16 to 4.54); this result was not statistically significant. High heterogeneity was found (I2 = 55%); see Analysis 1.4
1.4. Analysis.
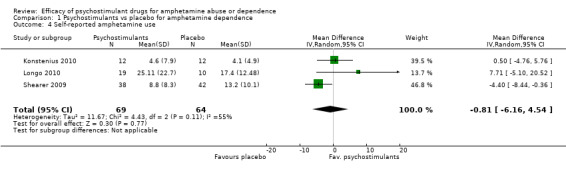
Comparison 1 Psychostimulants vs placebo for amphetamine dependence, Outcome 4 Self‐reported amphetamine use.
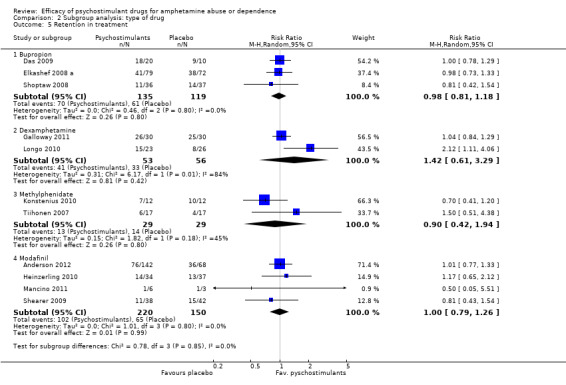
Retention in treatment. Number of participants who completed treatment
(01) Any pschychostimulant vs placebo
This outcome was available from all eleven studies (791 participants): RR 1.01 (95% CI 0.90 to 1.14), the result was not statistically significant; see Analysis 1.5
1.5. Analysis.
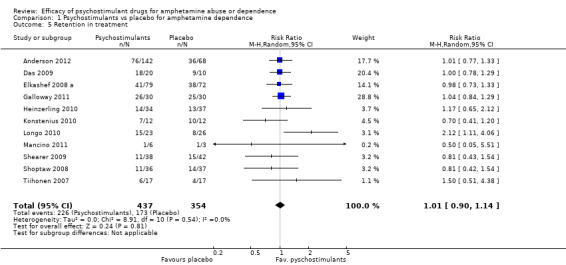
Comparison 1 Psychostimulants vs placebo for amphetamine dependence, Outcome 5 Retention in treatment.
Craving
(01) Any psychostimulant vs placebo
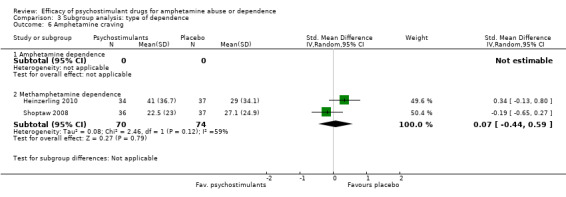
Two studies (Heinzerling 2010; Shoptaw 2008), 144 participants: SMD 0.07 (95% CI ‐0.44 to 0.59), the result was not statistically significant. High heterogeneity was found (I2 = 59%); see Analysis 1.6
1.6. Analysis.
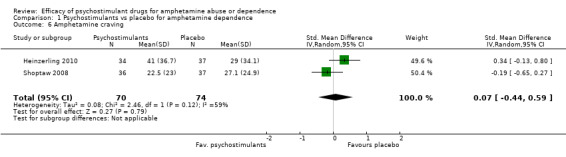
Comparison 1 Psychostimulants vs placebo for amphetamine dependence, Outcome 6 Amphetamine craving.
Safety secondary outcomes
Dropouts due to any adverse event
(01) Any psychostimulant vs placebo
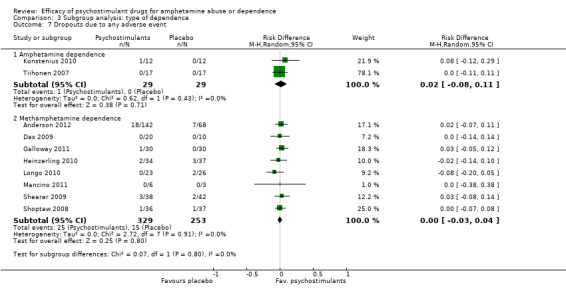
Ten studies (Anderson 2012; Das 2009; Galloway 2011; Heinzerling 2010; Konstenius 2010; Longo 2010; Mancino 2011; Shearer 2009; Shoptaw 2008; Tiihonen 2007), 640 participants: RD 0.01 (95% CI ‐0.03 to 0.04); this result was not statistically significant; see Analysis 1.7
1.7. Analysis.
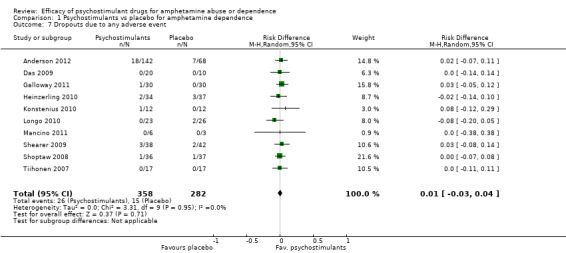
Comparison 1 Psychostimulants vs placebo for amphetamine dependence, Outcome 7 Dropouts due to any adverse event.
Dropouts due to cardiovascular adverse events
(01) Any psychostimulant vs placebo
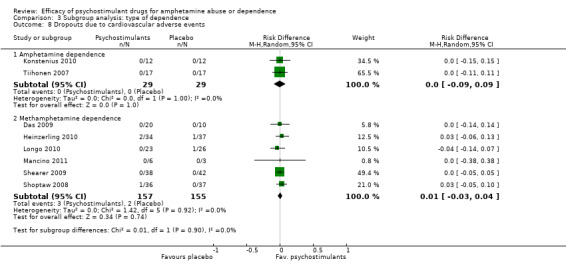
Eight studies (Das 2009; Heinzerling 2010; Konstenius 2010; Longo 2010; Mancino 2011;Shearer 2009; Shoptaw 2008; Tiihonen 2007), 3700 participants: RD 0.01 (95% CI ‐0.03 to 0.04); this result was not statistically significant, see Analysis 1.8
1.8. Analysis.
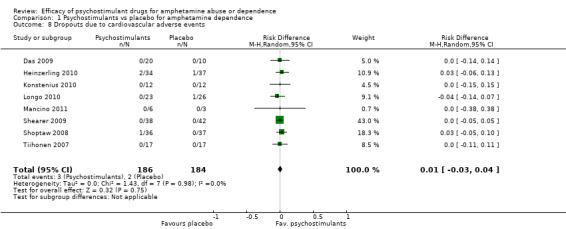
Comparison 1 Psychostimulants vs placebo for amphetamine dependence, Outcome 8 Dropouts due to cardiovascular adverse events.
Dropouts due to psychiatric adverse events
(01) Any psychostimulant vs placebo
Seven studies (Das 2009; Heinzerling 2010; Konstenius 2010; Longo 2010; Mancino 2011; Shoptaw 2008; Tiihonen 2007), 290 participants: RD ‐0.02 (95% CI ‐0.06 to 0.02); this result was not statistically significant; see Analysis 1.9
1.9. Analysis.
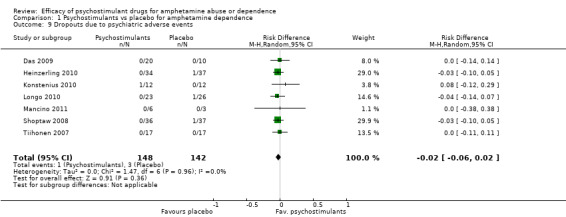
Comparison 1 Psychostimulants vs placebo for amphetamine dependence, Outcome 9 Dropouts due to psychiatric adverse events.
Subgroup analyses
Subgroup analyses are reported only for the main outcome of the review, "Amphetamine use (UA)", although this outcome did not show heterogeneity. Only two primary outcomes had statistical heterogeneity ("self‐reported use" and "craving"), but they involved only three and two studies, respectively, limiting the utility of a further analysis of the influence of moderating variables. Still, subgroup analyses of all outcomes were performed, all of which showed no differences between subgroups, for further details see Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.9;Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7; Analysis 3.8; Analysis 3.9; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.9; Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7; Analysis 5.8; Analysis 5.9; Analysis 6.2; Analysis 6.3; Analysis 6.4; Analysis 6.5; Analysis 6.6; Analysis 6.7; Analysis 6.8; Analysis 6.9
2.2. Analysis.

Comparison 2 Subgroup analysis: type of drug, Outcome 2 Amphetamine use (hair analysis).
2.3. Analysis.

Comparison 2 Subgroup analysis: type of drug, Outcome 3 Sustained abstinence.
2.4. Analysis.
Comparison 2 Subgroup analysis: type of drug, Outcome 4 Self‐reported amphetamine use.
2.5. Analysis.
Comparison 2 Subgroup analysis: type of drug, Outcome 5 Retention in treatment.
2.6. Analysis.
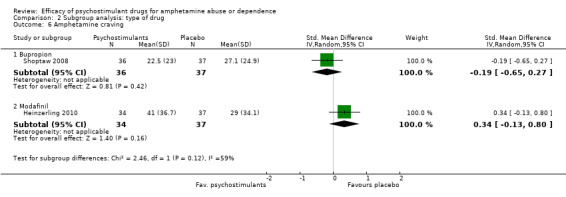
Comparison 2 Subgroup analysis: type of drug, Outcome 6 Amphetamine craving.
2.7. Analysis.

Comparison 2 Subgroup analysis: type of drug, Outcome 7 Dropouts due to any adverse event.
2.8. Analysis.
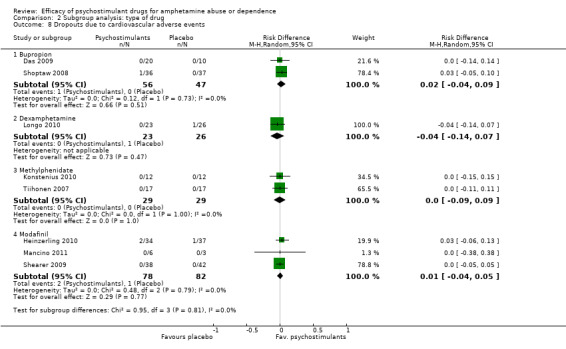
Comparison 2 Subgroup analysis: type of drug, Outcome 8 Dropouts due to cardiovascular adverse events.
2.9. Analysis.
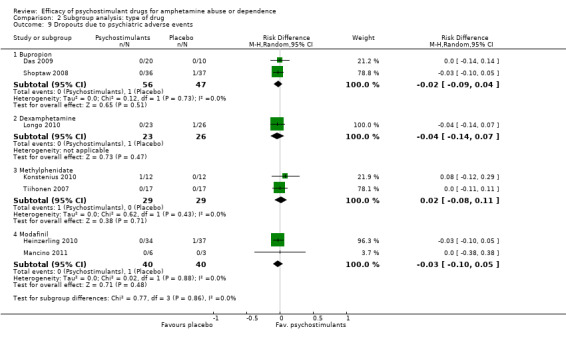
Comparison 2 Subgroup analysis: type of drug, Outcome 9 Dropouts due to psychiatric adverse events.
3.2. Analysis.
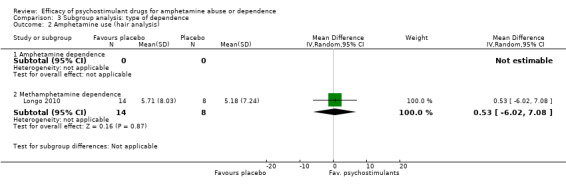
Comparison 3 Subgroup analysis: type of dependence, Outcome 2 Amphetamine use (hair analysis).
3.3. Analysis.
Comparison 3 Subgroup analysis: type of dependence, Outcome 3 Sustained abstinence.
3.4. Analysis.

Comparison 3 Subgroup analysis: type of dependence, Outcome 4 Self‐reported amphetamine use.
3.5. Analysis.

Comparison 3 Subgroup analysis: type of dependence, Outcome 5 Retention in treatment.
3.6. Analysis.
Comparison 3 Subgroup analysis: type of dependence, Outcome 6 Amphetamine craving.
3.7. Analysis.
Comparison 3 Subgroup analysis: type of dependence, Outcome 7 Dropouts due to any adverse event.
3.8. Analysis.
Comparison 3 Subgroup analysis: type of dependence, Outcome 8 Dropouts due to cardiovascular adverse events.
3.9. Analysis.
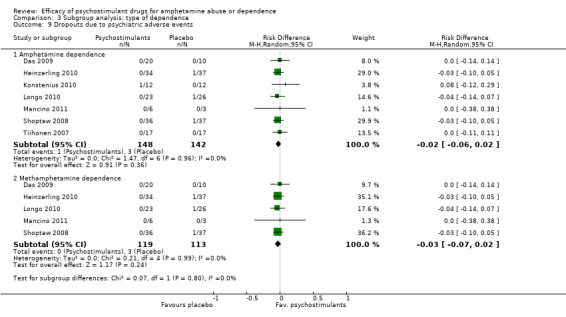
Comparison 3 Subgroup analysis: type of dependence, Outcome 9 Dropouts due to psychiatric adverse events.
4.2. Analysis.

Comparison 4 Subgroup analysis: comorbid ADHD as inclusion criterion, Outcome 2 Amphetamine use (hair analysis).
4.3. Analysis.
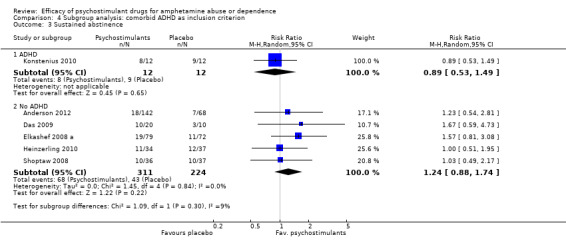
Comparison 4 Subgroup analysis: comorbid ADHD as inclusion criterion, Outcome 3 Sustained abstinence.
4.4. Analysis.

Comparison 4 Subgroup analysis: comorbid ADHD as inclusion criterion, Outcome 4 Self reported amphetamine use.
4.5. Analysis.

Comparison 4 Subgroup analysis: comorbid ADHD as inclusion criterion, Outcome 5 Retention in treatment.
4.6. Analysis.
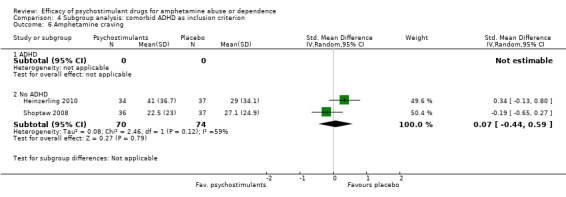
Comparison 4 Subgroup analysis: comorbid ADHD as inclusion criterion, Outcome 6 Amphetamine craving.
4.7. Analysis.
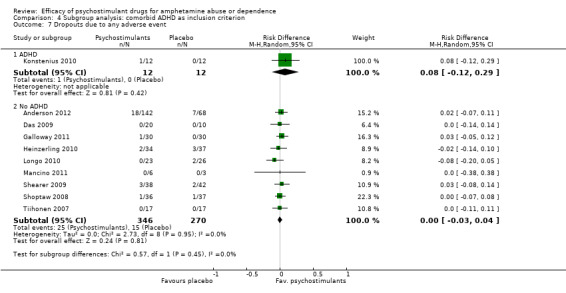
Comparison 4 Subgroup analysis: comorbid ADHD as inclusion criterion, Outcome 7 Dropouts due to any adverse event.
4.8. Analysis.
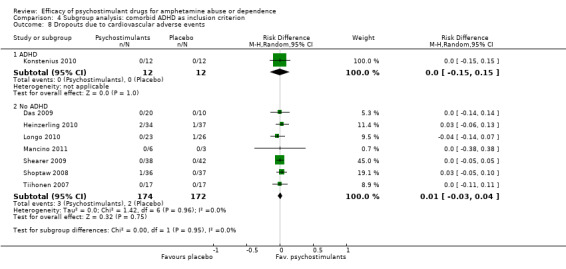
Comparison 4 Subgroup analysis: comorbid ADHD as inclusion criterion, Outcome 8 Dropouts due to cardiovascular adverse events.
4.9. Analysis.
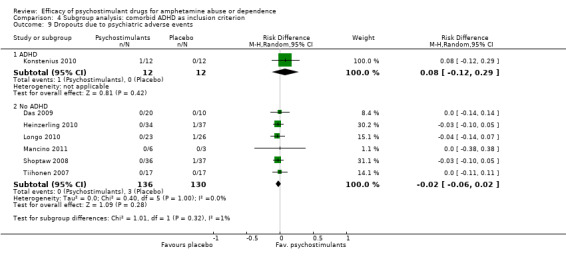
Comparison 4 Subgroup analysis: comorbid ADHD as inclusion criterion, Outcome 9 Dropouts due to psychiatric adverse events.
5.2. Analysis.

Comparison 5 Subgroup analysis: data publication, Outcome 2 Amphetamine use (hair analysis).
5.3. Analysis.
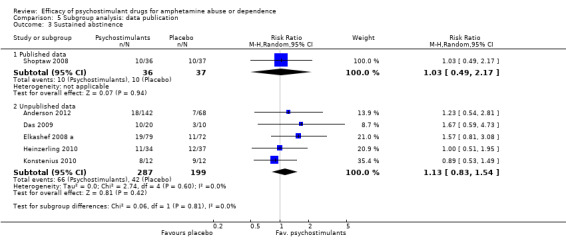
Comparison 5 Subgroup analysis: data publication, Outcome 3 Sustained abstinence.
5.4. Analysis.
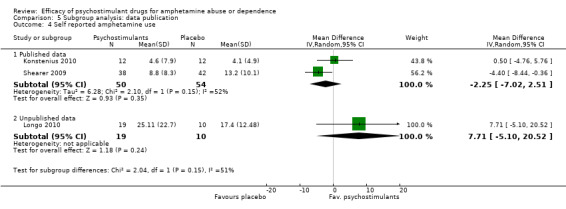
Comparison 5 Subgroup analysis: data publication, Outcome 4 Self reported amphetamine use.
5.5. Analysis.
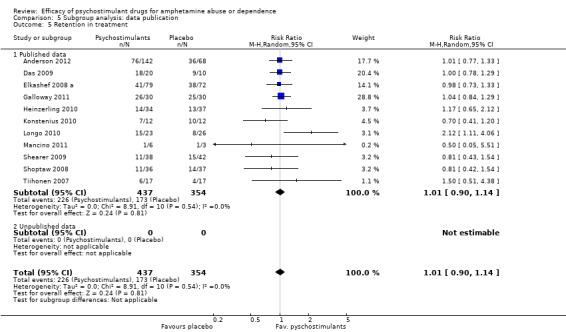
Comparison 5 Subgroup analysis: data publication, Outcome 5 Retention in treatment.
5.6. Analysis.
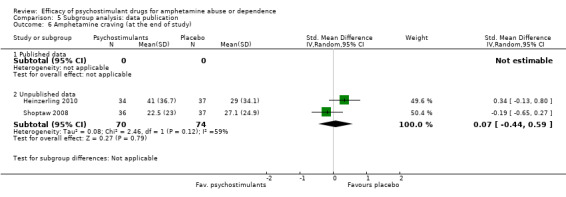
Comparison 5 Subgroup analysis: data publication, Outcome 6 Amphetamine craving (at the end of study).
5.7. Analysis.

Comparison 5 Subgroup analysis: data publication, Outcome 7 Dropouts due to any adverse event.
5.8. Analysis.

Comparison 5 Subgroup analysis: data publication, Outcome 8 Dropouts due to cardiovascular adverse events.
5.9. Analysis.
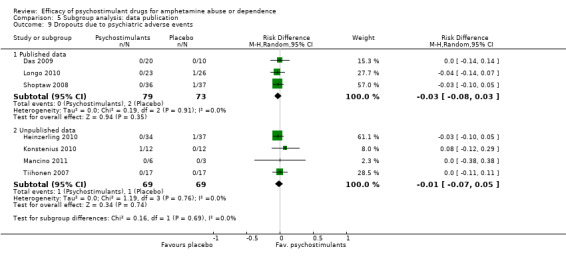
Comparison 5 Subgroup analysis: data publication, Outcome 9 Dropouts due to psychiatric adverse events.
6.2. Analysis.
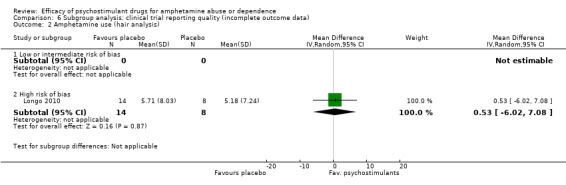
Comparison 6 Subgroup analysis: clinical trial reporting quality (incomplete outcome data), Outcome 2 Amphetamine use (hair analysis).
6.3. Analysis.
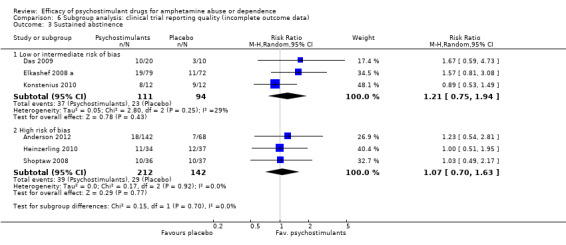
Comparison 6 Subgroup analysis: clinical trial reporting quality (incomplete outcome data), Outcome 3 Sustained abstinence.
6.4. Analysis.
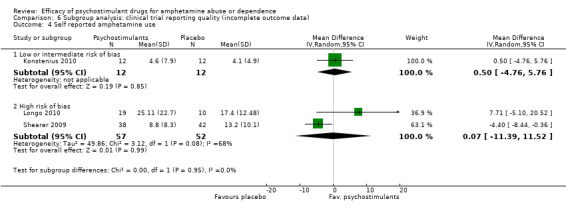
Comparison 6 Subgroup analysis: clinical trial reporting quality (incomplete outcome data), Outcome 4 Self reported amphetamine use.
6.5. Analysis.

Comparison 6 Subgroup analysis: clinical trial reporting quality (incomplete outcome data), Outcome 5 Retention in treatment.
6.6. Analysis.

Comparison 6 Subgroup analysis: clinical trial reporting quality (incomplete outcome data), Outcome 6 Amphetamine craving.
6.7. Analysis.

Comparison 6 Subgroup analysis: clinical trial reporting quality (incomplete outcome data), Outcome 7 Dropouts due to any adverse event.
6.8. Analysis.
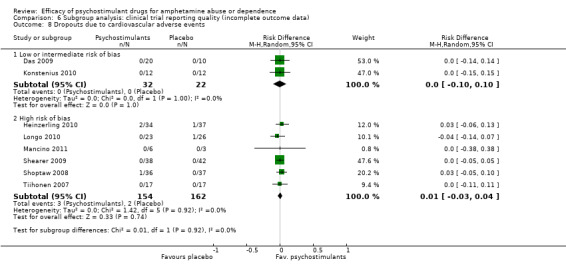
Comparison 6 Subgroup analysis: clinical trial reporting quality (incomplete outcome data), Outcome 8 Dropouts due to cardiovascular adverse events.
6.9. Analysis.
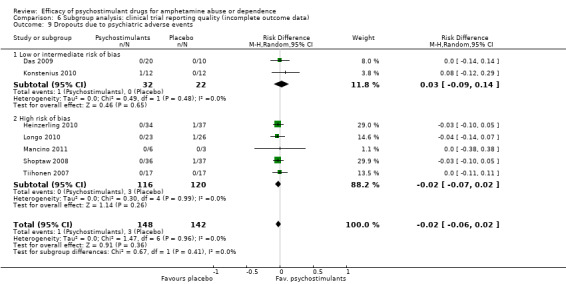
Comparison 6 Subgroup analysis: clinical trial reporting quality (incomplete outcome data), Outcome 9 Dropouts due to psychiatric adverse events.
Amphetamine use assessed by the mean (SD) of the proportion of amphetamine‐free UA across the study per participant
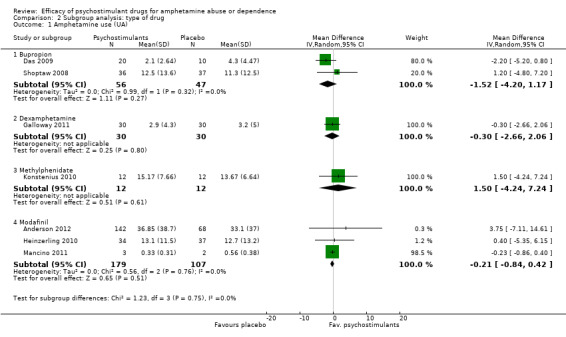
(2) Subgroup analysis: type of drug
Bupropion vs placebo, two studies (Das 2009; Shoptaw 2008), 103 participants: MD ‐1.52 (95% CI ‐4.20 to 1.17); this result was not statistically significant
Dexamphetamine vs placebo, one study (Galloway 2011), 60 participants: MD ‐0.30 (95% CI ‐2.66 to 2.06); this result was not statistically significant.
Methylphenidate vs placebo, one study (Konstenius 2010), 24 participants: MD 1.50 (95% CI ‐4.24 to 7.24); this result was not statistically significant.
Modafinil vs placebo, three studies (Anderson 2012; Heinzerling 2010; Mancino 2011), 286 participants: MD ‐0.21 (95%CI ‐0.84 to 0.42); this result was not statistically significant.
For all see Analysis 2.1
2.1. Analysis.
Comparison 2 Subgroup analysis: type of drug, Outcome 1 Amphetamine use (UA).
(3) Subgroup analysis: type of dependence
Amphetamine dependence: psychostimulants vs placebo, one study (Konstenius 2010), 24 participants: MD 1.50 (95% CI ‐4.24 to 7.24); this result was not statistically significant.
Methamphetamine dependence: psychostimulants vs placebo, six studies (Anderson 2012; Das 2009; Galloway 2011; Heinzerling 2010; Mancino 2011; Shoptaw 2008), 449 participants: MD ‐0.28 (95% CI ‐0.87 to 0.31); this result was not statistically significant. No heterogeneity was found.
For both see Analysis 3.1
3.1. Analysis.

Comparison 3 Subgroup analysis: type of dependence, Outcome 1 Amphetamine use (UA).
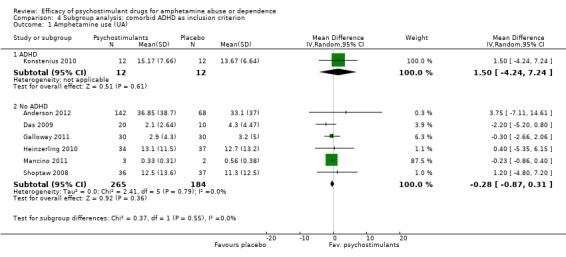
(4) Subgroup analysis: comorbid ADHD as inclusion criterion
ADHD: psychostimulants vs placebo, one study (Konstenius 2010), 24 participants: MD 1.50 (95% CI ‐4.24 to 7.24); this result was not statistically significant.
No ADHD: psychostimulants vs placebo, six studies (Anderson 2012; Das 2009; Galloway 2011; Heinzerling 2010; Mancino 2011; Shoptaw 2008), 449 participants: MD ‐0.28 (95% CI ‐0.87 to 0.31); this result was not statistically significant. No heterogeneity was found.
For both see Analysis 4.1
4.1. Analysis.
Comparison 4 Subgroup analysis: comorbid ADHD as inclusion criterion, Outcome 1 Amphetamine use (UA).
(5) Subgroup analysis: data publication
Published data: psychostimulants vs placebo, three studies (Galloway 2011; Mancino 2011; Shoptaw 2008), 138 participants: MD 0.22 (95% CI ‐0.83 to 0.39); this result was not statistically significant.
Unpublished data: psychostimulants vs placebo, four studies (Anderson 2012; Das 2009; Heinzerling 2010; Konstenius 2010), 335 participants: MD ‐0.86 (95% CI ‐3.21 to 1.50); this result was not statistically significant.
For both see Analysis 5.1
5.1. Analysis.

Comparison 5 Subgroup analysis: data publication, Outcome 1 Amphetamine use (UA).
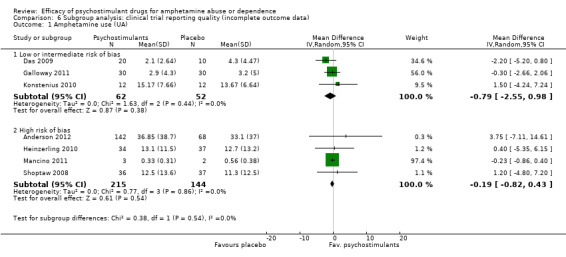
(6) Subgroup analysis: clinical trial reporting quality (incomplete outcome data)
Low or intermediate risk of bias: psychostimulants vs placebo, three studies (Das 2009; Galloway 2011; Konstenius 2010), 114 participants: MD ‐0.79 (95% CI ‐2.55 to 0.98); this result was not statistically significant.
High risk of bias: psychostimulants vs placebo, one study (Mancino 2011), 5 participants: MD ‐0.19, 95% CI‐0.82 to 0.43); this result was not statistically significant.
For both see Analysis 6.1
6.1. Analysis.
Comparison 6 Subgroup analysis: clinical trial reporting quality (incomplete outcome data), Outcome 1 Amphetamine use (UA).
We planned to determine the influence of study length over treatment outcomes. Nevertheless, we could not finally run this analysis because between‐study variability of this co‐variable was not large enough. Indeed, most studies (7 out of 11) had the same study length (12 weeks).
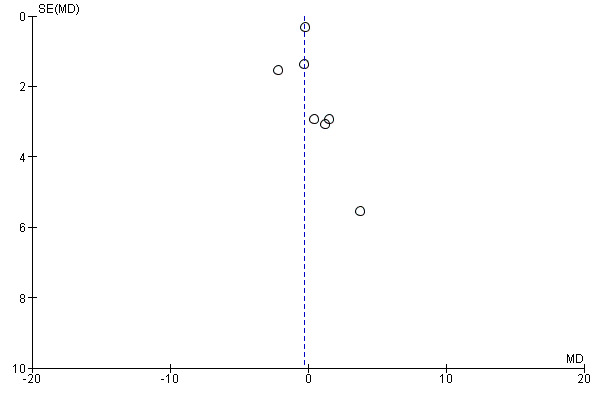
Reporting bias analyses
Funnel plots of the efficacy primary outcomes were drawn (Figure 4; Figure 5), with the exception of amphetamine use as assessed in hair (only one study assessed that). A funnel plot was also performed for one of the most relevant outcomes of this kind of study: "retention in treatment" (Figure 6). None showed asymmetry suggestive of reporting bias.
4.
Funnel plot of comparison: 1 Psychostimulants vs placebo for amphetamine dependence, outcome: 1.1 Amphetamine use (UA).
5.

Funnel plot of comparison: 1 Psychostimulants vs placebo for amphetamine dependence, outcome: 1.3 Sustained abstinence.
6.
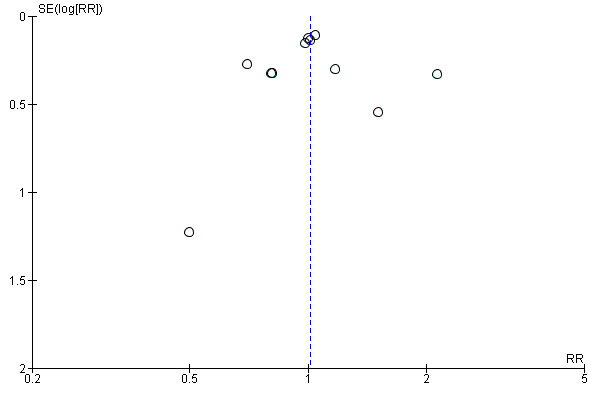
Funnel plot of comparison: 1 Psychostimulants vs placebo for amphetamine dependence, outcome: 1.5 Retention in treatment.
Reporting bias could also arise as the result of selective outcome reporting. To explore this, a subgroup analysis was performed while taking into account whether or not data were published (or obtained from authors), and, as mentioned previously, the effect of the intervention was similar in both subgroups.
Sensitivity analyses
The sensitivity analysis of the outcome "dropouts due to any adverse event" was conducted while excluding three studies with 0 events from the primary analysis (Das 2009; Mancino 2011; Tiihonen 2007). Therefore, seven studies were included (Anderson 2012; Galloway 2011; Heinzerling 2010; Konstenius 2010; Longo 2010; Shearer 2009; Shoptaw 2008), 567 participants: RR 1.18 (95% CI 0.63 to 2.20); this result was not statistically significant, see Analysis 7.1
7.1. Analysis.
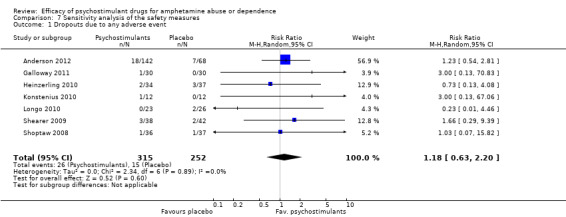
Comparison 7 Sensitivity analysis of the safety measures, Outcome 1 Dropouts due to any adverse event.
The sensitivity analysis of the outcome "dropouts due to cardiovascular adverse events" was conducted while excluding five studies with 0 events from the primary analysis (Das 2009; Konstenius 2010; Mancino 2011;Shearer 2009; Tiihonen 2007). Therefore, three studies were included (Heinzerling 2010; Longo 2010; Shoptaw 2008), 193 participants: RR 1.50 (95% CI 0.30 to 7.58); this result was not statistically significant, see Analysis 7.2
7.2. Analysis.
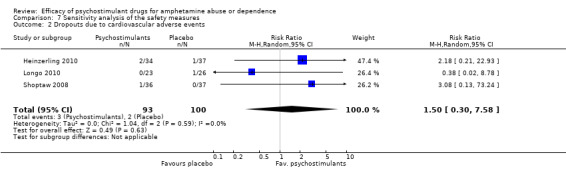
Comparison 7 Sensitivity analysis of the safety measures, Outcome 2 Dropouts due to cardiovascular adverse events.
The sensitivity analysis of the outcome "dropouts due to psychiatric adverse events" was conducted while excluding three studies with 0 events from the primary analysis (Das 2009; Mancino 2011; Tiihonen 2007). Therefore, four studies were included (Heinzerling 2010; Konstenius 2010; Longo 2010; Shoptaw 2008), 217 participants: RR 0.62 (95% CI 0.13 to 2.99); this result was not statistically significant, see Analysis 7.3
7.3. Analysis.
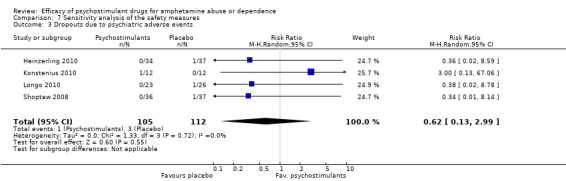
Comparison 7 Sensitivity analysis of the safety measures, Outcome 3 Dropouts due to psychiatric adverse events.
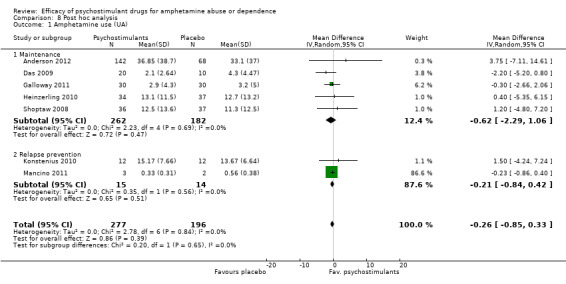
Post hoc analysis
Two studies (Konstenius 2010; Mancino 2011) had a relapse prevention approach, and the remaining studies had a maintenance treatment approach. In Mancino 2011, participants completed 2 weeks of residential phase treatment to achieve initial abstinence before the outpatient phase. In Konstenius 2010, participants were required to stay abstinent 2 weeks before inclusion.
A post hoc analysis was performed by splitting the available trials into two subgroups: maintenance versus relapse prevention treatment. No differences were found between psychostimulants and placebo in any of both subgroups for any outcome, as in the primary analysis. We present only data for the outcome "Amphetamine use (UA)".
Subcategory 01: maintenance: psychostimulants vs placebo, five studies (Anderson 2012; Das 2009; Galloway 2011; Heinzerling 2010; Shoptaw 2008), 444 participants: MD ‐0.62 (95% CI ‐2.29 to 1.06); this result was not statistically significant,
Subcategory 02: relapse prevention: psychostimulants vs placebo, two studies (Konstenius 2010; Mancino 2011), 29 participants: ‐MD 0.21, 95% CI ‐0.84 to 0.42); this result was not statistically significant.
For both see see Analysis 8.1Figure 7;
8.1. Analysis.
Comparison 8 Post hoc analysis, Outcome 1 Amphetamine use (UA).
7.

Forest plot of comparison: 8 Post hoc analysis, outcome: 8.1 Amphetamine use (UA).
Discussion
Summary of main results
During recent years, several psychostimulants have been tested to treat amphetamine dependence. The objective of this systematic review was to summarise available evidence of the use of psychostimulants in amphetamine abusers or dependents. It was found that psychostimulant maintenance for amphetamine dependence has been infrequently studied, as shown by the fact that only 11 clinical trials (791 participants) investigating four drugs with psychostimulant effect have been carried out. Neither psychostimulants as a group nor any single drug was found to reduce amphetamine use (by means of UA), attain sustained amphetamine abstinence or improve treatment retention. Only two studies individually showed a favourable result for one outcome (Longo 2010: retention improved with dexamphetamine; Shearer 2009: self‐reported use improved with modafinil). Data for anxiety symptoms, depressive symptoms and overall functioning could not be meta‐analysed because they were not reported in a way that allowed aggregation.
Regarding safety, no statistically significant differences between psychostimulants and placebo were found in the dropout rate due to AEs. This finding suggests that psychostimulants are well tolerated by amphetamine‐dependent patients. It must be noted that this safety outcome is rather unspecific because it provides no information on the type of AE that causes treatment discontinuation. However, the way AEs are usually reported in clinical trials shows large between‐study heterogeneity, precluding the possibility of pooling together the results of specific types of AEs from different studies. Furthermore, mild and transient side effects that do not cause participants to discontinue treatment are not included in this safety outcome; therefore, our review cannot rule out the possibility that the rate of this type of AE is different between psychostimulants and placebo. It is interesting to note that no cases of study medication abuse were reported in the included trials; this is one of the main concerns when psychostimulants are used to treat amphetamine‐dependent patients.
A series of subgroup analyses was performed and showed that results were consistent across all of them. No differences in efficacy, safety and retention were found between psychostimulants and placebo on any of these analyses. Grouping the included RCCTs into different categories in the subgroup analysis did not result in any difference in the main analysis, probably because no heterogeneity to be explained was previously found. It should be taken into account that in the subgroup analysis performed, we studied the effects of the intervention within the planned subgroups, but we did not compare the effects of the intervention between the different subgroups because this was not the aim of this systematic review.
With the available data at this moment, it seems that although it is effective in treating nicotine and opiate dependence, replacement therapy does not seem to be useful for treating amphetamine dependence. This finding contrasts with those of psychostimulant maintenance for cocaine dependence, for which hopeful results were found on cocaine abstinence, particularly in methadone‐maintained dual opioid‐cocaine-dependent participants (Castells 2010). The fact that these participants were receiving methadone enabled that study retention was high. Conversely, the retention rate in most studies included in the present review was relatively low; therefore, treatment compliance was also low, and so it was not possible to demonstrate the therapeutic effect of the studied intervention.
It should be kept in mind that the results of this meta‐analysis have been obtained with only a few studies with small sample sizes, undertaken to test psychostimulants with different stimulant potencies (strong psychostimulants like dexamphetamine and methylphenidate and mild psychostimulants like bupropion and modafinil) and restricted ranges of doses.
Overall completeness and applicability of evidence
The external validity of the review is limited by the inclusion/exclusion criteria of the studies. Studies were conducted in the USA, Australia or Northern Europe. Generalisation of the results to other countries should be made with caution because social, cultural and health system differences can actively affect the overall treatment outcome. Furthermore, most studies included participants dependent upon methamphetamine because amphetamine dependence is rather infrequent. In all studies, participants were dependent on amphetamine‐type stimulants (amphetamine or methamphetamine), and no abusers were included. It is likely that the results obtained for methamphetamine‐dependent participants can be extrapolated to amphetamine‐dependent participants because, as was mentioned previously, these drugs have similar physiological and behavioural effects (Martin 1971).
Regarding MDMA, some important differences arise when it is compared with methamphetamine. MDMA has higher SERT selectivity, but methamphetamine acts mainly in the catecholamine transporters: NET and DAT (Baumann 2012). In physiological and behavioural terms, MDMA displays similarities to methamphetamine (Kirkpatrick 2012 a), but the dependence syndrome for ecstasy may not be of the same nature as for the other amphetamine‐type stimulants (Degenhardt 2010). Therefore, in our opinion, the results of this review should not be extrapolated to ecstasy‐dependent patients.
Finally, it must be noted that the representativeness of participants included in this systematic review is relatively low, fundamentally because patients with comorbid psychiatric disorders (major depression, psychotic disorders, other drug dependences) were usually excluded from the included trials, but comorbidities were frequent amongst amphetamine‐dependent clinical samples (Glasner‐Edwards 2010; Salo 2011). In fact, in only one study, other dependencies or psychiatric comorbidities were an inclusion criterion (ADHD: Konstenius 2010). It must be acknowledged that it is unlikely that psychostimulants could be efficacious in treating dual‐dependent patients when they have not proved so in selected samples.
Quality of the evidence
To grade the quality of evidence, a summary of findings table was prepared using the GRADE methodology.
Evidence was classified as very low for the outcomes "amphetamine use (UA)" and "sustained abstinence". Evidence was downgraded in both cases because of risk of bias (mainly for high attrition), imprecision of results obtained and indirectness. The imprecision and therefore the wide 95% CI calculated are consequences of the limited number of included studies and the small sample size in all of them. Furthermore, no included trial reported adequate allocation concealment, so it is unclear whether foreknowledge of the forthcoming allocations was prevented. Finally, attrition was high in most of the studies, and so was the possibility of bias due to incomplete outcome data (high risk or unclear in all but two studies). In our opinion, the high attrition in the included studies further reduces confidence in the results obtained for amphetamine use. We consider that reasons for missing data in addiction trials are likely to be related to true outcome. Indirectness arises from the fact that we pooled together the results of studies that investigated different drugs and doses. To our knowledge, no study has yet investigated the pharmacodynamic equivalence between psychostimulants; therefore the presence of a dose‐response relationship could not be investigated. Classifiying evidence as very low denotes that we are highly uncertain about the estimate calculated. Therefore, the publication of any new study could change substantially the results and conclusions of this review.
Evidence was classified as low for "retention in treatment". In this case, evidence was downgraded only as the result of indirectness and inconsistency because no risk of bias due to high attrition was associated with this outcome. Rating the evidence as low indicates that our confidence in the results of our review in terms of this outcome is poor. Thus, future research is likely to change the pooled estimate as calculated.
Potential biases in the review process
Reporting bias can jeopardise the validity of any meta‐analysis. We have tried to limit the influence of reporting bias by screening several data sets and requesting unpublished results from the contact authors. Indeed in this review, a considerable quantity of unpublished data was included after authors of the studies had been contacted (data from 10 of 11 studies). We have also carried out funnel plots for the outcomes "amphetamine use (UA)", "sustained abstinence" and "retention in treatment", and they do not suggest publication bias. Nevertheless, it must be noted that the number of studies included in this review was low, and so it was the sensitivity of the funnel plot that was used to identify the possibility of publication bias.
A subgroup analysis was conducted that took into account whether or not the data used were published. When published and unpublished data were compared, no differences were found, providing additional support for the lack of publication bias.
The fact that all studies were funded by public institutions may explain why studies with negative findings were published.
Agreements and disagreements with other studies or reviews
Several narrative reviews, including those on psychostimulant medications, are available (Brackins 2011; Elkashef 2008 b; Herin 2010; Karila 2010; Moeller 2008; Shearer 2008), but only one previous systematic review and meta‐analysis has been identified (Pérez‐Mañá 2011). This previous study assessed the efficacy of indirect dopamine agonists (some of them psychostimulants) in comparison with placebo for cocaine or amphetamine dependence. It showed favourable results only in cocaine‐dependent participants. In that moment, only four studies conducted in amphetamine‐dependent participants were available (all of them are included in this review). Performing the search three years later has led to the retrieval of seven additional studies. Although the statistical power of this review is greater than in the previous one (Pérez‐Mañá 2011) (11 studies vs 4 studies and 791 participants vs 357 participants), similar conclusions have been reached: Evidence in randomised clinical trials still does not support the use of psychostimulants for amphetamine dependence.
Authors' conclusions
Implications for practice.
Overall, the results of this systematic review do not support the use of psychostimulants to treat amphetamine abuse or dependence. No drug at the tested doses has shown to be efficacious for the treatment of amphetamine dependence.
Implications for research.
Future research should try to include outcomes more predictive of long‐term abstinence, such us "end of treatment abstinence" or "sustained abstinence" instead of "overall abstinence across the study" (McCann 2012). It should be mentioned that the outcome sustained abstinence was a preplanned endpoint in only one of the studies included in this review (Shoptaw 2008).
Subgroup analysis of two included studies with bupropion (Elkashef 2008 a; Shoptaw 2008) has shown favourable results in participants with light to moderate consumption of amphetamines before screening or at baseline. The degree of amphetamine consumption at the beginning of the trial (Dean 2009) and the achievement of early abstinence (Brensilver 2012) are good predictors of future response. The potency of the psychostimulant drug is another important factor to consider. It seems reasonable to test stronger psychostimulants in severely addicted participants, while mild psychostimulants could be reserved for those with lower rates of consumption. It could also be argued that the doses of psychostimulants used in these trials are not high enough to replace the effects of the abused drug in this kind of population (Herin 2010).
It could be desirable to carry out studies with longer follow‐up, as it has been suggested that 12‐week clinical trials are not long enough to assess treatment efficacy in chronic conditions like addiction (Whinchell 2012). These studies may also provide necessary data about long‐term safety.
Finally, the efficacy of psychostimulants in methadone‐maintained dual heroin‐amphetamine-dependent patients should be investigated because psychostimulant maintenance has shown promising results in dual cocaine‐heroin- dependent participants (Castells 2010). To our knowledge, no study has yet investigated this intervention in dual heroin‐amphetamine-dependent participants.
In summary, future research should consider carefully the level of dependence at study entry, the potency and dose of the psychostimulant administered, the length of the trial and the representativeness of included participants.
Acknowledgements
We want to thank Zuzana Mitrova for her help in performing the searches in the different databases and during the editorial process. We also want to thank all authors who sent us unpublished data from their studies.
Appendices
Appendix 1. PUBMED search strategy
1. Substance‐Related Disorders [MeSH]
2. (abstinen*[tiab] OR dependen*[tiab] OR addict*[tiab] OR withdraw*[tiab] OR misus*[tiab] OR use*[tiab] OR abus*[tiab])
3. (#1) OR #2
4. Amphetamines[MeSH]
5. (amphetamine[tw] OR amfetamine[tw] OR methamphetamine[tw] OR MDMA[tw] OR ecstasy[tw] OR dextroamphetamine[tw])
6. (#4) OR #5
7. randomized controlled trial [pt]
8. controlled clinical trial [pt]
9. randomized [tiab]
10. placebo [tiab]
11. drug therapy [sh]
12. randomly [tiab]
13. trial [tiab]
14. groups [tiab]
15. (((((((#7) OR #8) OR #9) OR #10) OR #11) OR #12) OR #13) OR #14
16. animals [mh] NOT humans [mh]
17. (#15) NOT #16
18. ((#3) AND #6) AND #17
Appendix 2. EMBASE search strategy
1. 'drug dependence'/exp
2. 'drug abuse'/exp
3. 'withdrawal syndrome'/exp
4. dependen*:ab,ti OR addict*:ab,ti OR overdos*:ab,ti OR abstin*:ab,ti OR abstain:ab,ti OR withdraw*:ab,ti OR abus*:ab,ti OR use*:ab,ti OR
misus*:ab,ti
5. #1 OR #2 OR #3 OR #4
6. 'amphetamine derivative'/exp
7. amphetamine:ab,ti OR amfetamine:ab,ti OR methamphetamine:ab,ti OR mdma:ab,ti OR ecstasy:ab,ti OR dextroamphetamine:ab,ti
8. #6 OR #7
9. 'crossover procedure'/exp
10. 'double blind procedure'/exp
11. 'single blind procedure'/exp
12. 'controlled clinical trial'/exp
13. 'clinical trial'/exp
14. placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti
15. random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR(cross:ab,ti AND over:ab,ti)
16. 'randomized controlled trial'/exp
17. #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16
18. #5 AND #8 AND #17
19. #5 AND #8 AND #17 AND [embase]/lim
Appendix 3. CENTRAL search strategy
1. MeSH descriptor Substance‐Related Disorders explode all trees
2. (abstinen* OR dependen* OR addict* OR withdraw* OR misus* OR use* OR abus*):ti,ab,kw
3. (#1 OR #2)
4. MeSH descriptor Amphetamines explode all trees
5. (amphetamine OR amfetamine OR methamphetamine OR MDMA OR ecstasy OR dextroamphetamine) :ti,ab,kw
6. (#4 OR #5) 7. (#3 AND #6)
Appendix 4. Specialized register search strategy
(amphetamine* OR amfetamine OR methamphetamine OR MDMA OR ecstasy OR dextroamphetamine)
Appendix 5. PsycINFO search strategy
(*amphetamine OR *amfetamine) AND (abuse* OR dependen* OR misuse or addict*) AND random*
Appendix 6. Criteria for risk of bias in RCTs
| Item | Judgment | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; and minimization |
| High risk | The investigators describe a non‐random component in the sequence generation process such as odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; and availability of the intervention | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, Web‐based, and pharmacy‐controlled, randomisation); sequentially numbered drug containers of identical appearance; and sequentially numbered, opaque, sealed envelopes |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following methods was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or were not sequentially numbered); alternation or rotation; date of birth; case record number; and any other explicitly unconcealed procedure | |
| Unclear risk | Insufficient information to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or is not described in sufficient detail to allow a definite judgement | |
| 3. Blinding of participants and providers (performance bias). Objective outcomes |
Low risk |
No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| 4. Blinding of participants and providers (performance bias). Subjective outcomes |
Low risk |
Blinding of participants and providers and unlikely that the blinding could have been broken |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 5. Blinding of outcome assessor (detection bias). Objective outcomes |
Low risk |
No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| 6. Blinding of outcome assessor (detection bias). Subjective outcomes |
Low risk |
No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 7. Incomplete outcome data (attrition bias). For all outcomes except retention in treatment or dropout |
Low risk |
No missing outcome data Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias) Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size Missing data have been imputed using appropriate methods All randomly assigned participants are reported/analysed in the group to which they were allocated by randomisation irrespective of non‐compliance and co‐interventions (intention to treat) |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated; no reasons for missing data provided; number of dropouts not reported for each group) | |
| 8. Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way The study protocol is not available, but it is clear thatpublished reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon) |
| High risk | Not all of the study’s prespecified primary outcomes have been reported One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect) One or more outcomes of interest in the review are reported incompletely, so that they cannot be entered into a meta‐analysis The study report fails to include results for a key outcome that would be expected to have been reported for such a study |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk |
Data and analyses
Comparison 1. Psychostimulants vs placebo for amphetamine dependence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amphetamine use (UA) | 7 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.85, 0.33] |
| 2 Amphetamine use (hair analysis) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐6.02, 7.08] |
| 3 Sustained abstinence | 6 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.84, 1.49] |
| 4 Self‐reported amphetamine use | 3 | 133 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐6.16, 4.54] |
| 5 Retention in treatment | 11 | 791 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.14] |
| 6 Amphetamine craving | 2 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.44, 0.59] |
| 7 Dropouts due to any adverse event | 10 | 640 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.04] |
| 8 Dropouts due to cardiovascular adverse events | 8 | 370 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.04] |
| 9 Dropouts due to psychiatric adverse events | 7 | 290 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
Comparison 2. Subgroup analysis: type of drug.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amphetamine use (UA) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Bupropion | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐4.20, 1.17] |
| 1.2 Dexamphetamine | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.66, 2.06] |
| 1.3 Methylphenidate | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 1.5 [‐4.24, 7.24] |
| 1.4 Modafinil | 3 | 286 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.84, 0.42] |
| 2 Amphetamine use (hair analysis) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Dexamphetamine | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐6.02, 7.08] |
| 3 Sustained abstinence | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Bupropion | 3 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.87, 2.14] |
| 3.2 Modafinil | 2 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.83] |
| 3.3 Methylphenidate | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.53, 1.49] |
| 4 Self‐reported amphetamine use | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Methylphenidate | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐4.76, 5.76] |
| 4.2 Modafinil | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐4.40 [‐8.44, ‐0.36] |
| 4.3 Dexamphetamine | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 7.71 [‐5.10, 20.52] |
| 5 Retention in treatment | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Bupropion | 3 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.18] |
| 5.2 Dexamphetamine | 2 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.61, 3.29] |
| 5.3 Methylphenidate | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.42, 1.94] |
| 5.4 Modafinil | 4 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.79, 1.26] |
| 6 Amphetamine craving | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Bupropion | 1 | 73 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.65, 0.27] |
| 6.2 Modafinil | 1 | 71 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.13, 0.80] |
| 7 Dropouts due to any adverse event | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Bupropion | 2 | 103 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.07, 0.07] |
| 7.2 Dexamphetamine | 2 | 109 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.12, 0.10] |
| 7.3 Methylphenidate | 2 | 58 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.08, 0.11] |
| 7.4 Modafinil | 4 | 370 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.07] |
| 8 Dropouts due to cardiovascular adverse events | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Bupropion | 2 | 103 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.09] |
| 8.2 Dexamphetamine | 1 | 49 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.14, 0.07] |
| 8.3 Methylphenidate | 2 | 58 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 8.4 Modafinil | 3 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.05] |
| 9 Dropouts due to psychiatric adverse events | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Bupropion | 2 | 103 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.09, 0.04] |
| 9.2 Dexamphetamine | 1 | 49 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.14, 0.07] |
| 9.3 Methylphenidate | 2 | 58 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.08, 0.11] |
| 9.4 Modafinil | 2 | 80 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.10, 0.05] |
Comparison 3. Subgroup analysis: type of dependence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amphetamine use (UA) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Amphetamine dependence | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 1.5 [‐4.24, 7.24] |
| 1.2 Methamphetamine dependence | 6 | 449 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.87, 0.31] |
| 2 Amphetamine use (hair analysis) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Amphetamine dependence | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Methamphetamine dependence | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐6.02, 7.08] |
| 3 Sustained abstinence | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Amphetamine dependence | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.53, 1.49] |
| 3.2 Methamphetamine dependence | 5 | 535 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.88, 1.74] |
| 4 Self‐reported amphetamine use | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Amphetamine dependence | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐4.76, 5.76] |
| 4.2 Methamphetamine dependence | 2 | 109 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐11.39, 11.52] |
| 5 Retention in treatment | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Amphetamine dependence | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.42, 1.94] |
| 5.2 Methamphetamine dependence | 9 | 733 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.91, 1.16] |
| 6 Amphetamine craving | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Amphetamine dependence | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Methamphetamine dependence | 2 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.44, 0.59] |
| 7 Dropouts due to any adverse event | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Amphetamine dependence | 2 | 58 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.08, 0.11] |
| 7.2 Methamphetamine dependence | 8 | 582 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.04] |
| 8 Dropouts due to cardiovascular adverse events | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Amphetamine dependence | 2 | 58 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 8.2 Methamphetamine dependence | 6 | 312 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.04] |
| 9 Dropouts due to psychiatric adverse events | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Amphetamine dependence | 7 | 290 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 9.2 Methamphetamine dependence | 5 | 232 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.07, 0.02] |
Comparison 4. Subgroup analysis: comorbid ADHD as inclusion criterion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amphetamine use (UA) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 ADHD | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 1.5 [‐4.24, 7.24] |
| 1.2 No ADHD | 6 | 449 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.87, 0.31] |
| 2 Amphetamine use (hair analysis) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 ADHD | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 No ADHD | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐6.02, 7.08] |
| 3 Sustained abstinence | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 ADHD | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.53, 1.49] |
| 3.2 No ADHD | 5 | 535 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.88, 1.74] |
| 4 Self reported amphetamine use | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 ADHD | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐4.76, 5.76] |
| 4.2 No ADHD | 2 | 109 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐11.39, 11.52] |
| 5 Retention in treatment | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 ADHD | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.41, 1.20] |
| 5.2 No ADHD | 10 | 767 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.16] |
| 6 Amphetamine craving | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 ADHD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 No ADHD | 2 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.44, 0.59] |
| 7 Dropouts due to any adverse event | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 ADHD | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.12, 0.29] |
| 7.2 No ADHD | 9 | 616 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.04] |
| 8 Dropouts due to cardiovascular adverse events | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 ADHD | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.15, 0.15] |
| 8.2 No ADHD | 7 | 346 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.04] |
| 9 Dropouts due to psychiatric adverse events | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 ADHD | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.12, 0.29] |
| 9.2 No ADHD | 6 | 266 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
Comparison 5. Subgroup analysis: data publication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amphetamine use (UA) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Published data | 3 | 138 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.83, 0.39] |
| 1.2 Unpublished data | 4 | 335 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐3.21, 1.50] |
| 2 Amphetamine use (hair analysis) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Published data | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Unpublished data | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐6.02, 7.08] |
| 3 Sustained abstinence | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Published data | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.49, 2.17] |
| 3.2 Unpublished data | 5 | 486 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.83, 1.54] |
| 4 Self reported amphetamine use | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Published data | 2 | 104 | Mean Difference (IV, Random, 95% CI) | ‐2.25 [‐7.02, 2.51] |
| 4.2 Unpublished data | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 7.71 [‐5.10, 20.52] |
| 5 Retention in treatment | 11 | 791 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.14] |
| 5.1 Published data | 11 | 791 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.14] |
| 5.2 Unpublished data | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Amphetamine craving (at the end of study) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Published data | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Unpublished data | 2 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.44, 0.59] |
| 7 Dropouts due to any adverse event | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Published data | 5 | 292 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.04, 0.05] |
| 7.2 Unpublished data | 5 | 348 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.05, 0.07] |
| 8 Dropouts due to cardiovascular adverse events | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Published data | 2 | 103 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.09] |
| 8.2 Unpublished data | 6 | 267 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 9 Dropouts due to psychiatric adverse events | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Published data | 3 | 152 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.08, 0.03] |
| 9.2 Unpublished data | 4 | 138 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
Comparison 6. Subgroup analysis: clinical trial reporting quality (incomplete outcome data).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amphetamine use (UA) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Low or intermediate risk of bias | 3 | 114 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐2.55, 0.98] |
| 1.2 High risk of bias | 4 | 359 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.82, 0.43] |
| 2 Amphetamine use (hair analysis) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Low or intermediate risk of bias | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 High risk of bias | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐6.02, 7.08] |
| 3 Sustained abstinence | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Low or intermediate risk of bias | 3 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.75, 1.94] |
| 3.2 High risk of bias | 3 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.70, 1.63] |
| 4 Self reported amphetamine use | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Low or intermediate risk of bias | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐4.76, 5.76] |
| 4.2 High risk of bias | 2 | 109 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐11.39, 11.52] |
| 5 Retention in treatment | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Low or intermediate risk of bias | 6 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.13] |
| 5.2 High risk of bias | 5 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.83, 1.53] |
| 6 Amphetamine craving | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Low or intermediate risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 High risk of bias | 2 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.44, 0.59] |
| 7 Dropouts due to any adverse event | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Low or intermediate risk of bias | 3 | 114 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| 7.2 High risk of bias | 7 | 526 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 8 Dropouts due to cardiovascular adverse events | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Low or intermediate risk of bias | 2 | 54 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.10, 0.10] |
| 8.2 High risk of bias | 6 | 316 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.04] |
| 9 Dropouts due to psychiatric adverse events | 7 | 290 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 9.1 Low or intermediate risk of bias | 2 | 54 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.09, 0.14] |
| 9.2 High risk of bias | 5 | 236 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.02] |
Comparison 7. Sensitivity analysis of the safety measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts due to any adverse event | 7 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.63, 2.20] |
| 2 Dropouts due to cardiovascular adverse events | 3 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.30, 7.58] |
| 3 Dropouts due to psychiatric adverse events | 4 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.13, 2.99] |
Comparison 8. Post hoc analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amphetamine use (UA) | 7 | 473 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.85, 0.33] |
| 1.1 Maintenance | 5 | 444 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐2.29, 1.06] |
| 1.2 Relapse prevention | 2 | 29 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.84, 0.42] |
| 2 Amphetamine use (hair analysis) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐6.02, 7.08] |
| 2.1 Maintenance | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐6.02, 7.08] |
| 3 Sustained abstinence | 6 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.84, 1.49] |
| 3.1 Maintenance | 5 | 535 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.88, 1.74] |
| 3.2 Relapse prevention | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.53, 1.49] |
| 4 Self reported amphetamine use | 3 | 133 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐6.16, 4.54] |
| 4.1 Maintenance | 3 | 133 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐6.16, 4.54] |
| 5 Retention in treatment | 11 | 791 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.14] |
| 5.1 Maintenance | 9 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.16] |
| 5.2 Relapse prevention | 2 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.41, 1.17] |
| 6 Amphetamine craving | 2 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.44, 0.59] |
| 6.1 Maintenance | 2 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.44, 0.59] |
| 7 Dropouts due to any adverse event | 10 | 640 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.04] |
| 7.1 Maintenance | 8 | 607 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.04] |
| 7.2 Relapse prevention | 2 | 33 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.11, 0.24] |
| 8 Dropouts due to cardiovascular adverse events | 8 | 370 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.04] |
| 8.1 Maintenance | 6 | 337 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.04] |
| 8.2 Relapse prevention | 2 | 33 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 9 Dropouts due to psychiatric adverse events | 7 | 290 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 9.1 Maintenance | 5 | 257 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 9.2 Relapse prevention | 2 | 33 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.11, 0.24] |
8.2. Analysis.
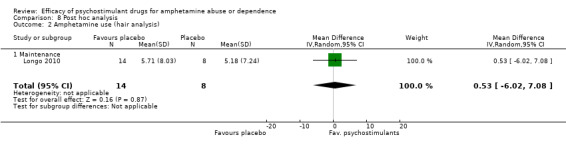
Comparison 8 Post hoc analysis, Outcome 2 Amphetamine use (hair analysis).
8.3. Analysis.

Comparison 8 Post hoc analysis, Outcome 3 Sustained abstinence.
8.4. Analysis.
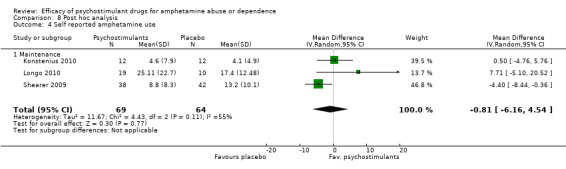
Comparison 8 Post hoc analysis, Outcome 4 Self reported amphetamine use.
8.5. Analysis.
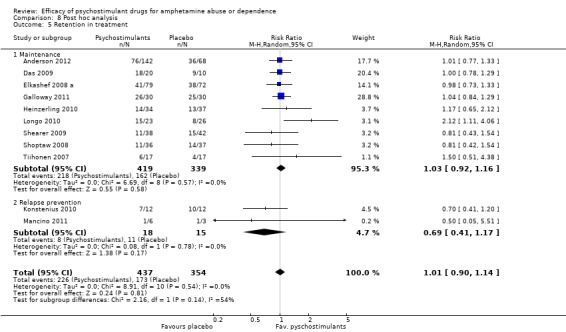
Comparison 8 Post hoc analysis, Outcome 5 Retention in treatment.
8.6. Analysis.
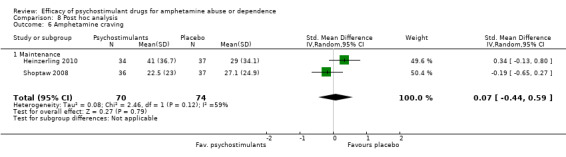
Comparison 8 Post hoc analysis, Outcome 6 Amphetamine craving.
8.7. Analysis.
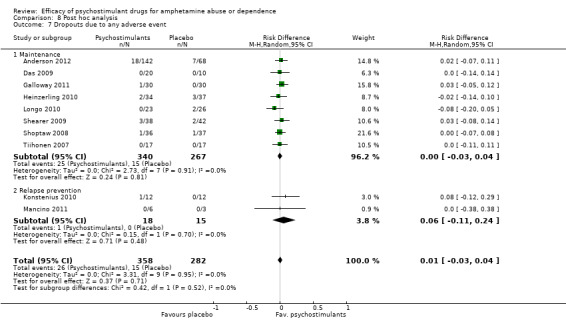
Comparison 8 Post hoc analysis, Outcome 7 Dropouts due to any adverse event.
8.8. Analysis.

Comparison 8 Post hoc analysis, Outcome 8 Dropouts due to cardiovascular adverse events.
8.9. Analysis.
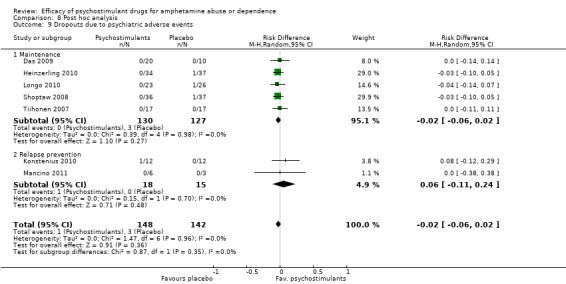
Comparison 8 Post hoc analysis, Outcome 9 Dropouts due to psychiatric adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 2012.
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial Stastitical analysis: modified ITT |
|
| Participants | n = 210 methamphetamine‐dependent outpatients (DSM‐IV), 20 with ADHD, 7 alcohol dependents Mean age: 39 years Gender: 124 men Race: African‐American: 10, Caucasian: 148, Other: 52 Employed: not reported (NR) History: < 18 days of methamphetamine use during last month: 84, > 18 days of methamphetamine use during last month: 126, lifetime methamphetamine use: NR Route of methamphetamine use: NR |
|
| Interventions | Three parallel groups: 1. Modafinil 200 mg qd (fixed posology), N = 72 2. Modafinil 400 mg qd (fixed posology), N = 70 2. Placebo, N = 68 + CBT (36 sessions) + HIV counselling + motivational enhancement therapy (1 session) Duration: 12 weeks Multiple site (USA) |
|
| Outcomes | Amphetamine use assessed with three‐times‐weekly UA Sustained abstinence (defined as at least 3 weeks of continuous abstinence) Retention in treatment Craving Depressive symptoms assessed by means of HAM‐D Overall functioning assessed by means of CGI Dropouts due to adverse events |
|
| Notes | Author's affiliation: university and other public institutions Funding: public Assessment of compliance: self‐report, pill count, modafinil and metabolite in urine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adapative urn randomisation used |
| Allocation concealment (selection bias) | Low risk | Central allocation. Using telephone. Pharmacy controlled |
| Blinding (detection bias): Objective measures Objective measures | Low risk | Outcome or outcome measurement was not likely to be influenced by lack of blinding |
| Blinding (performance bias): Objective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Blinding (detection bias): Subjective measures Subjective measures | Low risk | Study medication and matched placebo have identical appearance. Blinding can be achieved when the study medication with mild behavioural effects (modafinil) is compared with placebo |
| Blinding (performance bias): Subjective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Incomplete outcome data (attrition bias): Objective measures except retention in treatment or dropout Objective outcomes | High risk | High attrition in all study groups (globally 53%). Missing outcome data balanced in numbers across intervention groups. Reasons for dropping out not reported. Analysis performed without imputation methods |
| Incomplete outcome data (attrition bias): Subjective measures Subjective measures | High risk | High attrition in all study groups (globally 53%). Missing outcome data balanced in numbers across intervention groups. Reasons for dropping out not reported. Analysis performed without imputation methods |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and the study report includes all outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Das 2009.
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial Stastitical analysis: ITT |
|
| Participants | n = 30 methamphetamine‐dependent outpatients (SCID) who have sex with men. 1 opioid dependent Mean age: 36.5 years Gender: 30 men Race: African‐American: 3, Caucasian: 16, Other: 11 Employed: 10 History: days of methamphetamine use during last month: NR, lifetime methamphetamine use: NR Route of methamphetamine use: 26 ip, 14 in, 15 iv, 7 oral, 7 rectal |
|
| Interventions | Two parallel groups: 1. Bupropion XL 300 mg qd. (fixed posology), N = 20 2. Placebo, N = 10 + Counseling (12 sessions) Duration: 12 weeks Single site (USA) |
|
| Outcomes | Amphetamine use assessed with one‐time‐weekly UA Sustained abstinence (defined as at least 3 weeks of continuous abstinence) Retention in treatment Depressive symptoms assessed by means of Center for Epidemiologic Studies Depression Rating Scale (CES‐D) Dropouts due to adverse events |
|
| Notes | Author's affiliation: university and other public institutions Funding: public Assessment of compliance: MEMS caps and self‐report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization code" |
| Allocation concealment (selection bias) | Low risk | Pharmacy staff and biostatistician who prepared allocation did not have contact with study participants |
| Blinding (detection bias): Objective measures Objective measures | Low risk | Outcomes assessed by means of objective measures are unlikely to be influenced by lack of blinding |
| Blinding (performance bias): Objective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Blinding (detection bias): Subjective measures Subjective measures | Low risk | Study medication and matched placebo have identical appearance. Blinding can be achieved when the study medication with mild behavioural effects (bupropion) is compared with placebo |
| Blinding (performance bias): Subjective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Incomplete outcome data (attrition bias): Objective measures except retention in treatment or dropout Objective outcomes | Low risk | Very low attrition in both study groups. Missing outcome data balanced in numbers across intervention groups. Missing data have been imputed using appropriate methods (worst case scenario) |
| Incomplete outcome data (attrition bias): Subjective measures Subjective measures | Low risk | Very low attrition in both study groups. Missing outcome data balanced in numbers across intervention groups. Data analysed with and without imputation methods |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and the study report includes all outcomes |
| Other bias | Unclear risk | Different numbers of partners. Different annual income between groups, and participants are paid for participation |
Elkashef 2008 a.
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial Stastitical analysis: nearly ITT |
|
| Participants | n = 151 methamphetamine‐dependent outpatients (DSM‐IV). 20 with ADHD Mean age: 36 years Gender: 101 men Race: African‐American: 4, Caucasian: 112, Other: 35 Employed: NR History: days of methamphetamine use during last month: NR, lifetime methamphetamine use: 10.2 years Route of methamphetamine use: 98 ip, 25 in, 28 iv, 0 oral, 0 rectal |
|
| Interventions | Two parallel groups: 1. Bupropion 300 mg tid. (fixed posology), N = 79 2. Placebo, N = 72 + CBT (1 session per week) + CM + groupal CBT (3 sessions per week) Duration: 12 weeks Multiple site (USA) |
|
| Outcomes | Amphetamine use assessed with one‐time‐weekly UA Sustained abstinence (defined as at least 3 weeks of continuous abstinence) Self‐reported amphetamine use Retention in treatment Craving assessed by means of BSCS Depressive symptoms assessed by means of HAM‐D Addiction Severity Index |
|
| Notes | Author's affiliation: university and other public institutions Funding: public Assessment of compliance: weekly tablet count |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adaptive urn randomisation was used to balance groups |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (detection bias): Objective measures Objective measures | Low risk | Outcome or outcome measurement was not likely to be influenced by lack of blinding |
| Blinding (performance bias): Objective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Blinding (detection bias): Subjective measures Subjective measures | Low risk | Study medication and matched placebo have identical appearance, and blinding can be achieved when the study medication with mild behavioural effects (bupropion) is compared with placebo |
| Blinding (performance bias): Subjective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Incomplete outcome data (attrition bias): Objective measures except retention in treatment or dropout Objective outcomes | Unclear risk | Moderate attrition in both study groups (globally 48%). Missing outcome data balanced in numbers across intervention groups but reasons not reported. Missing data have not been imputed |
| Incomplete outcome data (attrition bias): Subjective measures Subjective measures | Unclear risk | Moderate attrition in both study groups (globally 48%). Missing outcome data balanced in numbers across intervention groups but reasons not reported. Missing data have not been imputed |
| Selective reporting (reporting bias) | Unclear risk | Some reported outcomes are not included in Clinicaltrials.gov |
| Other bias | High risk | ADHD was not balanced between groups (8% bupropion vs 19% placebo) |
Galloway 2011.
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial Stastitical analysis: ITT |
|
| Participants | n = 60 methamphetamine‐dependent outpatients (DSM‐IV‐TR), 9 with ADHD Mean age: 37.3 years Gender: 34 men Race: African‐American: NR, Caucasian: 41, Other: NR Employed: NR History: days of methamphetamine use during past month: 17.1, lifetime methamphetamine use: NR Route of methamphetamine use: 44 ip as primary route |
|
| Interventions | Two parallel groups: 1. Dexamphetamine 60 mg qd (fixed posology), N = 30 2. Placebo, N = 30 + individual motivational psychotherapy (9 sessions) Duration: 8 weeks Single site (USA) |
|
| Outcomes | Amphetamine use assessed with two‐times‐weekly UA Self‐reported amphetamine use Retention in treatment Craving Dropouts due to adverse events |
|
| Notes | Author's affiliation: public but not university Funding: public Assessment of compliance: unused capsules count |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An urn randomisation method was used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. Method of concealment is not described |
| Blinding (detection bias): Objective measures Objective measures | Low risk | Outcome or outcome measurement was not likely to be influenced by lack of blinding |
| Blinding (performance bias): Objective measures | Unclear risk | Given that the studied intervention has powerful behavioural effects, it is likely that blinding was broken; this could have yielded to the provision of additional interventions, depending on the treatment the participant was receiving |
| Blinding (detection bias): Subjective measures Subjective measures | Unclear risk | Study medication and matched placebo have identical appearance, but it is unclear that blinding can be achieved when the study medication with powerful behavioural effects (dexamphetamine) is compared with placebo |
| Blinding (performance bias): Subjective measures | Unclear risk | Given that the studied intervention has powerful behavioural effects, it is likely that blinding was broken; this could have yielded to the provision of additional interventions, depending on the treatment the participant was receiving |
| Incomplete outcome data (attrition bias): Objective measures except retention in treatment or dropout Objective outcomes | Low risk | Low attrition in both study groups (globally 15%). Missing outcome data balanced in numbers across intervention groups, similar reasons for missing data across groups. No imputation methods used |
| Incomplete outcome data (attrition bias): Subjective measures Subjective measures | Low risk | Low attrition in both study groups (globally 15%). Missing outcome data balanced in numbers across intervention groups, similar reasons for missing data across groups. No imputation methods used |
| Selective reporting (reporting bias) | Low risk | More outcomes present in Clinicaltrials.gov than in the published report. But typical outcomes for those studies are reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Heinzerling 2010.
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial Stastitical analysis: ITT |
|
| Participants | n = 71 methamphetamine‐dependent outpatients (DSM‐IV‐TR) Mean age: 38.4 years Gender: 50 men Race: African‐American: 4, Caucasian: 36, Other: 29 Employed: 54 History: days of methamphetamine use during past month: 9.3 days, lifetime methamphetamine use: 14.5 years Route of methamphetamine use: 49 ip, 17 in, 4 iv, 1 oral |
|
| Interventions | Two parallel groups: 1. Modafinil 400 mg qd (fixed posology), N = 34 2. Placebo, N = 37 + CBT + CM (12 sessions) Duration: 12 weeks Multiple site (USA) |
|
| Outcomes | Amphetamine use assessed with three‐times‐weekly UA Sustained abstinence (defined as at least 3 weeks of continuous abstinence) Retention in treatment Depressive symptoms assessed by means of BDI‐II Craving Dropouts due to adverse events |
|
| Notes | Author's affiliation: university Co‐funding: public and private Assessment of compliance: pill count and self‐report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An urn randomisation procedure used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (detection bias): Objective measures Objective measures | Low risk | Outcome or outcome measurement was not likely to be influenced by lack of blinding |
| Blinding (performance bias): Objective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Blinding (detection bias): Subjective measures Subjective measures | Low risk | Study medication and matched placebo have identical appearance, and blinding can be achieved when the study medication with mild behavioural effects (modafinil) is compared with placebo |
| Blinding (performance bias): Subjective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Incomplete outcome data (attrition bias): Objective measures except retention in treatment or dropout Objective outcomes | High risk | High attrition in both study groups (globally 62%). Missing outcome data balanced in numbers across intervention groups. Reasons for missing data partially described. No imputation methods used |
| Incomplete outcome data (attrition bias): Subjective measures Subjective measures | High risk | High attrition in both study groups (globally 62%). Missing outcome data balanced in numbers across intervention groups. Reasons for missing data partially described. No imputation methods used |
| Selective reporting (reporting bias) | Low risk | More outcomes present in Clinicaltrials.gov than in the published report. But typical outcomes for those studies are reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Konstenius 2010.
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial. Relapse prevention trial Stastitical analysis: ITT |
|
| Participants | n = 24 amphetamine‐dependent outpatients (DSM‐IV) with ADHD, abstinent for a minimum of 2 weeks Mean age: 37.4 years Gender: 18 men Race: African‐American: NR, Caucasian: NR, Other: NR Employed: 5 History: days of methamphetamine use during past month: NR, lifetime methamphetamine use: 13.9 years Route of methamphetamine use: NR |
|
| Interventions | Two parallel groups: 1. Methylphenidate 18 to 72 mg qd (flexible posology), N = 12 2. Placebo, N = 12 + individual skills training program (12 sessions) Duration: 12 weeks Single site (Sweden) |
|
| Outcomes | Amphetamine use assessed with two‐times‐weekly UA Sustained abstinence (defined as at least 3 weeks of continuous abstinence) Retention in treatment Craving Depressive symptoms assessed by means of BDI‐II Anxiety symptoms assessed by BAI Dropouts due to adverse events |
|
| Notes | Author's affiliation: university Co‐funding: public and private Assessment of compliance: pill count |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Ranzomisation performed with Trombul software |
| Allocation concealment (selection bias) | Low risk | Randomisation done by an independent pharmacist. Randomisation list was kept at the pharmacy until the end of the trial and was collected and opened thereafter |
| Blinding (detection bias): Objective measures Objective measures | Low risk | Outcome or outcome measurement was not likely to be influenced by lack of blinding |
| Blinding (performance bias): Objective measures | Unclear risk | Given that the studied intervention has powerful behavioural effects, it is likely that blinding was broken, which could have yielded to the provision of additional interventions, depending on the treatment the participant was receiving |
| Blinding (detection bias): Subjective measures Subjective measures | Unclear risk | It is unclear whether blinding can be achieved when the study medication with powerful behavioural effects (methylphenidate) is compared with placebo |
| Blinding (performance bias): Subjective measures | Unclear risk | Given that the studied intervention has powerful behavioural effects, it is likely that blinding was broken, which could have yielded to the provision of additional interventions, depending on the treatment the participant was receiving |
| Incomplete outcome data (attrition bias): Objective measures except retention in treatment or dropout Objective outcomes | Unclear risk | Moderate attrition in both study groups (globally 29%). Missing outcome data not balanced in numbers across intervention groups. Reasons for missing data across groups not reported. Imputation by worst case scenario |
| Incomplete outcome data (attrition bias): Subjective measures Subjective measures | Unclear risk | Moderate attrition in both study groups (globally 29%). Missing outcome data not balanced in numbers across intervention groups. Reasons for missing data across groups not reported. Imputation methods not reported |
| Selective reporting (reporting bias) | Low risk | The report includes expected outcomes (current controlled trials) |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Longo 2010.
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial Stastitical analysis: ITT |
|
| Participants | n = 49 methamphetamine‐dependent outpatients (DSM‐IV) Mean age: 31.9 years Gender: 30 men Race: NR Employed: 24 History: NR Route of methamphetamine use: 42 iv |
|
| Interventions | Two parallel groups: 1. Dexamphetamine mean of 80 mg qd (flexible posology), N = 23 2. Placebo, N = 26 + CBT (4 sessions) Duration: 12 weeks maintenance and 4 weeks dose reduction Single site (Australia) |
|
| Outcomes | Amphetamine use assessed with hair samples (baseline, month 3, follow‐up) Self‐reported amphetamine use Retention in treatment Dropouts due to adverse events |
|
| Notes | Author's affiliation: university Funding: public Assessment of compliance: dispensing under pharmacist supervision |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer randomisation list was used to select random permuted blocks |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by pharmacy assistant not involved in dosing or dispensing the medication |
| Blinding (detection bias): Objective measures Objective measures | Low risk | Outcome or outcome measurement was not likely to be influenced by lack of blinding |
| Blinding (performance bias): Objective measures | Unclear risk | Given that the studied intervention has powerful behavioural effects, it is likely that blinding was broken, which could have yielded to the provision of additional interventions, depending on the treatment the participant was receiving |
| Blinding (detection bias): Subjective measures Subjective measures | Unclear risk | It is unclear whether blinding can be achieved when the study medication with powerful behavioural effects (dexamphetamine) is compared with placebo |
| Blinding (performance bias): Subjective measures | Unclear risk | Given that the studied intervention has powerful behavioural effects, it is likely that blinding was broken, which could have yielded to the provision of additional interventions, depending on the treatment the participant was receiving |
| Incomplete outcome data (attrition bias): Objective measures except retention in treatment or dropout Objective outcomes | High risk | High attrition (globally 53%). Attrition was higher in the placebo group. Reasons for missing data reported and similar; nevertheless some participants dropped out because they believed they were on placebo. No imputation methods used |
| Incomplete outcome data (attrition bias): Subjective measures Subjective measures | High risk | High attrition (globally 53%). Attrition was higher in the placebo group. Reasons for missing data reported and similar; nevertheless some participants dropped out because they believed they were on placebo. No imputation methods used |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available in a register. Lack of typical outcomes like drug use assessed by means of UA |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Mancino 2011.
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial. Relapse prevention trial Stastitical analysis: not ITT, not PP |
|
| Participants | n = 9 methamphetamine‐dependent outpatients (DSM‐IV) Mean age: 32.5 years Gender: 5 men Race: NR Employed: NR History: NR Route of methamphetamine use: NR |
|
| Interventions | Two parallel groups: 1. Modafinil 400 mg qd, N = 6 2. Placebo, N = 3 + Psychotherapy not specified (weekly) Duration: 8 weeks Single site (USA) |
|
| Outcomes | Amphetamine use assessed with three‐times‐weekly UA Withdrawal symptoms Retention in treatment Dropouts due to adverse events |
|
| Notes | Author's affiliation: university Funding: NR Assessment of compliance: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (detection bias): Objective measures Objective measures | Low risk | Outcome or outcome measurement was not likely to be influenced by lack of blinding |
| Blinding (performance bias): Objective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Blinding (detection bias): Subjective measures Subjective measures | Unclear risk | Blinding theoretically can be achieved when a study medication with mild behavioural effects (modafinil) is compared with placebo. Nevertheless, information on whether medications used were identical in appearance is insufficient |
| Blinding (performance bias): Subjective measures | Unclear risk | Information is insufficient to permit judgement |
| Incomplete outcome data (attrition bias): Objective measures except retention in treatment or dropout Objective outcomes | High risk | High attrition (globally 78%). Reasons for missing data across groups not reported. Imputation methods not reported |
| Incomplete outcome data (attrition bias): Subjective measures Subjective measures | High risk | High attrition (globally 78%). Reasons for missing data across groups not reported. Imputation methods not reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and the study publication includes all outcomes. Information obtained from Clinicaltrials.gov |
| Other bias | High risk | Terminated because of lack of funding.The article is not published in a journal (no peer review process is involved for data included in the register) |
Shearer 2009.
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial Stastitical analysis: ITT |
|
| Participants | n = 80 methamphetamine‐dependent outpatients (DSM‐IV) who used amphetamines 2 to 3 days per week or more often. 10 with opioid dependence Mean age: 36 years Gender: 50 men Race: NR Employed: 42 History: days of methamphetamine use during past month: 19.5, lifetime methamphetamine use: 7 years Route of methamphetamine use: NR ip, NR in, 50 iv, NR oral, NR rectal |
|
| Interventions | Two parallel groups: 1. Modafinil 200 mg qd (fixed posology), N = 38 2. Placebo, N = 42 + cognitive‐behavioural intervention (4 sessions) Duration: 10 weeks Multiple‐site trial (Australia) |
|
| Outcomes | Amphetamine use assessed with one‐time‐weekly UA Self‐reported amphetamine use Retention in treatment Dropouts due to adverse events |
|
| Notes | Author's affiliation: university and other public institutions Co‐funding: public and private Assessment of compliance: MEMS bottles and modafinilic acid in urine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables, in blocks |
| Allocation concealment (selection bias) | Low risk | Central allocation, pharmacy controlled |
| Blinding (detection bias): Objective measures Objective measures | Low risk | Outcome or outcome measurement was not likely to be influenced by lack of blinding |
| Blinding (performance bias): Objective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Blinding (detection bias): Subjective measures Subjective measures | Low risk | Study medication and matched placebo have identical appearance, and blinding can be achieved when the study medication with mild behavioural effects (modafinil) is compared with placebo |
| Blinding (performance bias): Subjective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Incomplete outcome data (attrition bias): Objective measures except retention in treatment or dropout Objective outcomes | High risk | High attrition in both study groups (globally 68%). Reasons for missing data across groups reported and similar. Imputation by worst case scenario and last observation carried forward. No imputation for missing data due to treatment dropout |
| Incomplete outcome data (attrition bias): Subjective measures Subjective measures | High risk | High attrition in both study groups (globally 68%). Reasons for missing data across groups reported and similar. Imputation by worst case scenario and last observation carried forward. No imputation for missing data due to treatment dropout |
| Selective reporting (reporting bias) | Low risk | The report includes expected outcomes (cited in Clinicaltrials.gov) |
| Other bias | Low risk | The study is apparently free of other sources of bias |
Shoptaw 2008.
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial Stastitical analysis: ITT |
|
| Participants | n = 73 methamphetamine‐dependent outpatients (DSM‐IV TR) Mean age: 34.6 years Gender: 47 men Race: African‐American: 2, Caucasian: 41, Other: 30 Employed: 57 History: days of methamphetamine use during past month: 15.7 days, lifetime methamphetamine use: 9.6 years Route of methamphetamine use: 47 ip, 16 in, 9 iv, 1 oral, 0 rectal |
|
| Interventions | Two parallel groups: 1. Bupropion SR 150 mg bid (fixed posology), N = 36 2. Placebo, N = 37 + CBT + CM ( 12 sessions) Duration: 12 weeks Multisite trial (USA) |
|
| Outcomes | Amphetamine use assessed with three‐times‐weekly UA Sustained abstinence (defined as at least 3 weeks of continuous abstinence) Retention in treatment Depressive symptoms assessed by means of BDI Dropouts due to adverse events |
|
| Notes | Author's affiliation: university Funding: public Assessment of compliance: weekly pill counts, reports of medication taking |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (detection bias): Objective measures Objective measures | Low risk | Outcome or outcome measurement was not likely to be influenced by lack of blinding |
| Blinding (performance bias): Objective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Blinding (detection bias): Subjective measures Subjective measures | Low risk | Study medication and matched placebo have identical appearance, and blinding can be achieved when the study medication with mild behavioural effects (modafinil) is compared with placebo |
| Blinding (performance bias): Subjective measures | Low risk | Given that the studied intervention has mild behavioural effects, it is unlikely that blinding was broken |
| Incomplete outcome data (attrition bias): Objective measures except retention in treatment or dropout Objective outcomes | High risk | High attrition in both study groups (globally 66%). Missing outcome data balanced in numbers across intervention groups, similar reasons for missing data across groups. No imputation methods used |
| Incomplete outcome data (attrition bias): Subjective measures Subjective measures | High risk | High attrition in both study groups (globally 66%). Missing outcome data balanced in numbers across intervention groups, similar reasons for missing data across groups. No imputation methods used |
| Selective reporting (reporting bias) | Unclear risk | Fewer outcomes present in Clinicaltrials.gov than in the published report |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tiihonen 2007.
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial Stastitical analysis: ITT |
|
| Participants | n = 53 amphetamine‐dependent outpatients (DSM‐IV) Mean age: 35.63 years Gender: 24 men Race: African‐American: 0, Caucasian: 34, Other: 0 Employed: NR History: days of methamphetamine use during past month: NR, lifetime methamphetamine use: 15.9 Route of methamphetamine use: 0 ip, 0 in, 34 iv, 0 oral, 0 rectal |
|
| Interventions | Three parallel groups: 1. Methylphenidate OROS 54 mg qd (fixed posology), N = 17 2. Aripiprazole 15 mg qd, N = 19 3. Placebo, N = 17 + unstructured psychosocial treatment Duration: 20 weeks Number of centres: NR (Finland) |
|
| Outcomes | Amphetamine use assessed with two‐times‐weekly UA Retention in treatment Dropouts due to adverse events |
|
| Notes | Author's affiliation: university and other public institutions Funding: public Assessment of compliance: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a randomised plan generator in blocks of six participants |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (detection bias): Objective measures Objective measures | Low risk | Outcome or outcome measurement was not likely to be influenced by lack of blinding |
| Blinding (performance bias): Objective measures | Unclear risk | Given that the studied intervention has powerful behavioural effects, it is likely that blinding was broken, which could have yielded to the provision of additional interventions, depending on the treatment the participant was receiving |
| Blinding (detection bias): Subjective measures Subjective measures | Unclear risk | Study medication and matched placebo have identical appearance, but it is unclear whether blinding can be achieved when the study medication with powerful behavioural effects (methylphenidate) is compared with placebo |
| Blinding (performance bias): Subjective measures | Unclear risk | Given that the studied intervention has powerful behavioural effects, it is likely that blinding was broken, which could have yielded to the provision of additional interventions, depending on the treatment the participant was receiving |
| Incomplete outcome data (attrition bias): Objective measures except retention in treatment or dropout Objective outcomes | High risk | High attrition in both study groups (globally 71%). Missing outcome data balanced in numbers across intervention groups. Reasons for missing data across groups not reported. Imputation by worst case scenario |
| Incomplete outcome data (attrition bias): Subjective measures Subjective measures | High risk | High attrition in both study groups (globally 71%). Reasons for missing data across groups not reported. No imputation methods used |
| Selective reporting (reporting bias) | Low risk | The report includes expected outcomes (cited in Current Controlled Trials) |
| Other bias | High risk | Interim analysis of a longer trial. Age baseline differences between groups |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brensilver 2013 | Subanalysis of an included study (Shoptaw 2008) |
| Christian 2007 | Not a randomised clinical trial |
| Dean 2009 | Subanalysis of an included study (Shoptaw 2008) |
| Hartz 2001 | Not assessing the efficacy of any psychostimulant for amphetamine dependence |
| Marinelli‐Casey 2008 | Not assessing the efficacy of any psychostimulant for amphetamine dependence |
| McCann 2012 | Re‐analysis of an included study (Elkashef 2008 a) |
| Shearer 2001 | No placebo group |
| Shearer 2010 | Subanalysis of an included study (Shearer 2009) |
| Whinchell 2012 | Re‐analysis of an included study (Elkashef 2008 a) |
Characteristics of ongoing studies [ordered by study ID]
Akhondzadeh L.
| Trial name or title | Slow‐release methylphenidate in the treatment of methamphetamine dependence |
| Methods | Randomised, double‐blind, placebo‐controlled study; 12‐week trial; phase II to III |
| Participants | Methamphetamine‐dependent outpatients (DSM‐IV‐TR) |
| Interventions | 1. Sustained‐released methylphenidate 54 mg/d 2. Placebo |
| Outcomes | Amphetamine use Craving Addiction Severity Index |
| Starting date | March 2012 |
| Contact information | Shahin Akhondzadeh s.akhond@sina.tums.ac.ir |
| Notes |
Franck J.
| Trial name or title | Clinical trial of sustained‐release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adult criminal offenders with amphetamine addiction |
| Methods | Single‐centre double‐blind randomised placebo‐controlled with parallel groups: 24 weeks' duration |
| Participants | Prison inmates with ADHD and amphetamine addiction (DSM‐IV) |
| Interventions | 1. Sustained‐release methylphenidate 18 to 180 mg qd 2. Placebo + Relapse prevention |
| Outcomes | Amphetamine and other drug use Relapse to crime ADHD symptoms Psychiatric symptoms Craving Self‐reported drug use Plasma concentration of methylphenidate |
| Starting date | April 2007 |
| Contact information | Johan Frank johan.franck@ki.se |
| Notes |
Galloway GP a.
| Trial name or title | A dose‐ranging study of modafinil for methamphetamine dependence |
| Methods | Randomised, double‐blind, dose‐ranging study; 4‐week trial, phase II |
| Participants | Methamphetamine‐dependent participants |
| Interventions | 1. Modafinil 100‐mg, 400‐mg or 600‐mg tablets qd 2. Placebo |
| Outcomes | Amphetamine use |
| Starting date | December 2009 |
| Contact information | Gantt Galloway Gantt@cpmcri.org |
| Notes |
Galloway GP b.
| Trial name or title | A randomised, placebo‐controlled trial of modafinil for methamphetamine dependence |
| Methods | Randomised, double‐blind, 4‐week trial, phase II |
| Participants | Methamphetamine‐dependent outpatients |
| Interventions | 1. Modafinil 600‐mg capsule 2. Placebo + Motivational enhancement therapy |
| Outcomes | Amphetamine use |
| Starting date | October 2011 |
| Contact information | Kathleen Garrison garrisk@cpmcri.org |
| Notes |
Gorgon L.
| Trial name or title | Phase II, double‐blind, placebo‐controlled trial of bupropion for methamphetamine dependence |
| Methods | Double‐blind, placebo‐controlled, parallel‐assignment, phase II |
| Participants | Methamphetamine‐dependent participants (DSM‐IV), at least one positive urine specimen after the start of screening |
| Interventions | 1. Bupropion 150 mg for the first 3 days. Increased to 150 mg bid until taper 2. Placebo |
| Outcomes | Abstinence at the end Sustained abstinence |
| Starting date | May 2008 |
| Contact information | Liza Gorgon lgorgon@nih.gov |
| Notes |
Heinzerling K.
| Trial name or title | Study of medical treatment for methamphetamine addiction |
| Methods | Randomised, double‐blind, 12‐week trial, phase II |
| Participants | Methamphetamine‐dependent participants (DSM‐IV) |
| Interventions | 1. Bupropion 300 mg qd 2. Placebo + CBT |
| Outcomes | Clinical phenotype of frequency of baseline MA use in 30 days preceding the baseline period using self‐report and results of thrice‐weekly urine drug screens for MA metabolites during baseline |
| Starting date | January 2009 |
| Contact information | Keith Heinzerling Kheinzerling@mednet.ucla.edu |
| Notes |
Ling W.
| Trial name or title | Methylphenidate to treat methamphetamine dependence |
| Methods | Randomised, double‐blind, parallel‐assignment, 4‐year trial, phase II |
| Participants | Methamphetamine‐dependent participants (DSM‐IV‐TR) |
| Interventions | 1. Methylphenidate 18 mg/d during week 1; 36 mg/d during week 2; 54 mg/d during remainder of study 2. Placebo + CBT |
| Outcomes | Amphetamine use Retention in treatment |
| Starting date | October 2010 |
| Contact information | Jasmin Hernandez jashernandez@ucla.edu Maureen Hillhouse hillhous@ucla.edu |
| Notes |
Differences between protocol and review
Epidemiological data on consumption of amphetamines were updated in this review.
The subgroup analysis taking into account study length was not performed. A post hoc subgroup analysis was conducted. It analysed the effects of psychostimulants in participants who were already abstinent (relapse prevention trials) separately from the effects of psychostimulants administered to participants who were actively using amphetamines.
MD was used instead of SMD for continuous outcomes. Although different techniques were used to assess whether a urine sample was positive or negative for amphetamines, we changed standardised mean difference (SMD) to mean difference (MD), and heterogeneity did not increase. MD was finally selected because it can be interpreted more easily.
Contributions of authors
All authors have contributed to the protocol design. CP‐M and XC wrote the different sections of the protocol.
CP‐M and XC assessed the studies for inclusion, extracted the data and assessed the risk of bias of included studies. CP‐M wrote the different sections of the review, and all review authors' comments and suggestions were incorporated into the final document.
Sources of support
Internal sources
The authors received no funding for this project, Not specified.
External sources
The authors received no funding for this project, Not specified.
Declarations of interest
None.
New
References
References to studies included in this review
Anderson 2012 {published and unpublished data}
- Anderson AL, Li SH, Biswas K, McSherry F, Holmes T, Iturriaga E, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend 2012;120(1‐3):135‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2009 {published and unpublished data}
- Das M, Santos D, Matheson T, Santos GM, Chu P, Vittinghoff E, et al. Feasibility and acceptability of a phase II randomized pharmacologic intervention for methamphetamine dependence in high‐risk men who have sex with men. AIDS 2010;24(7):991‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elkashef 2008 a {published and unpublished data}
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology 2008;33(5):1162‐70. [DOI] [PubMed] [Google Scholar]
Galloway 2011 {published data only (unpublished sought but not used)}
- Galloway GP, Buscemi R, Coyle JR, Flower K, Siegrist JD, Fiske LA, et al. A randomized, placebo‐controlled trial of sustained‐release dextroamphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther 2011;89(2):276‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heinzerling 2010 {published and unpublished data}
- Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, Shoptaw S. Randomized, double‐blind, placebo‐controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend 2010;109(1‐3):20‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konstenius 2010 {published and unpublished data}
- Konstenius M, Jayaram‐Lindström N, Beck O, Franck J. Sustained release methylphenidate for the treatment of ADHD in amphetamine abusers: a pilot study. Drug Alcohol Depend 2010;108(1‐2):130‐3. [DOI] [PubMed] [Google Scholar]
Longo 2010 {published and unpublished data}
- Longo M, Wickes W, Smout M, Harrison S, Cahill S, White JM. Randomized controlled trial of dexamphetamine maintenance for the treatment of methamphetamine dependence. Addiction 2010;105(1):146‐54. [DOI] [PubMed] [Google Scholar]
Mancino 2011 {published and unpublished data}
- Mancino M. Modafinil for methamphetamine dependence. Clinicaltrials.gov (NCT00859573). Results published in April 15, 2011.
Shearer 2009 {published and unpublished data}
- Shearer J, Darke S, Rodgers C, Slade T, Beek I, Lewis J, et al. A double‐blind, placebo‐controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction 2009;104(2):224‐33. [DOI] [PubMed] [Google Scholar]
Shoptaw 2008 {published and unpublished data}
- Shoptaw S, Heinzerling KG, Rotheram‐Fuller E, Steward T, Wang J, Swanson AN, et al. Randomized, placebo‐controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend 2008;96(3):222‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiihonen 2007 {published and unpublished data}
- Tiihonen J, Kuoppasalmi K, Föhr J, Tuomola P, Kuikanmäki O, Vorma H, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry 2007;164(1):160‐2. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brensilver 2013 {published data only}
- Brensilver M, Heinzerling KG, Swanson AN, Telesca D, Furst BA, Shoptaw SJ. Cigarette smoking as a target for potentiating outcomes for methamphetamine abuse treatment. Drug Alcohol Rev 2013;32(1):96‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christian 2007 {published data only}
- Christian DR, Huber A, Brecht ML, McCann MJ, Marinelli‐Casey P, Lord RH, et al. Methamphetamine Treatment Project, Galloway GP. Methamphetamine users entering treatment: characteristics of the methamphetamine treatment project sample. Subst Use Misuse 2007;42(14):2207‐22. [DOI] [PubMed] [Google Scholar]
Dean 2009 {published data only}
- Dean AC, London ED, Sugar CA, Kitchen CM, Swanson AN, Heinzerling KG, et al. Predicting adherence to treatment for methamphetamine dependence from neuropsychological and drug use variables. Drug Alcohol Depend 2009;105(1‐2):48‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartz 2001 {published data only}
- Hartz DT, Frederick‐Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated‐measures, within‐subject analysis . Drug Alcohol Depend 2001;63(3):269‐76. [DOI] [PubMed] [Google Scholar]
Marinelli‐Casey 2008 {published data only}
- Marinelli‐Casey P, Gonzales R, Hillhouse M, Ang A, Zweben J, Cohen J, et al. Methamphetamine Treatment Project Corporate Authors. Drug court treatment for methamphetamine dependence: treatment response and posttreatment outcomes. J Subst Abuse Treat 2008;34(2):242‐8. [DOI] [PubMed] [Google Scholar]
McCann 2012 {published data only}
- McCann DJ, Li SH. A novel, non binary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther 2012;18(5):414‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shearer 2001 {published data only}
- Shearer J, Wodak A, Mattick RP, Beek I, Lewis J, Hall W, Dolan K. Pilot randomized controlled study of dexamphetamine substitution for amphetamine dependence. Addiction 2001;96(9):1289‐96. [DOI] [PubMed] [Google Scholar]
Shearer 2010 {published data only}
- Shearer J, Shanahan M, Darke S, Rodgers C, Beek I, McKetin R, et al. A cost‐effectiveness analysis of modafinil therapy for psychostimulant dependence. Drug Alcohol Rev 2010;29(3):235‐42. [DOI] [PubMed] [Google Scholar]
Whinchell 2012 {published data only}
- Winchell C, Rappaport BA, Roca R, Rosebraugh CJ. Reanalysis of methamphetamine dependence treatment trial. CNS Neurosci Ther 2012;18(5):367‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Akhondzadeh L {published data only}
- Akhondzadeh. Slow‐release methylphenidate in the treatment of methamphetamine dependence. International Clinical Trials Registry Platform.
Franck J {published data only}
- Franck. Clinical trial of sustained release methylphenidate for attention‐deficit‐hyperactivity‐disorder (ADHD) in adult criminal offenders with amphetamine addiction. EU Clinical Trials Register.
Galloway GP a {published data only}
- Galloway GP. A dose ranging study of modafinil for methamphetamine dependence. ClinicalTrials.gov.
Galloway GP b {published data only}
- Galloway GP. A randomized, placebo‐controlled trial of modafinil for methamphetamine dependence. ClinicalTrials.gov.
Gorgon L {published data only}
- Gorgon L. Phase 2, double‐blind, placebo controlled trial of bupropion for methamphetamine dependence. ClinicalTrials.gov.
Heinzerling K {published data only}
- Heinzerling K. Study of medical treatment for methamphetamine addiction. ClinicalTrials.gov.
Ling W {published data only}
- Ling W. Methylphenidate to treat methamphetamine dependence. ClinicalTrials.gov.
Additional references
Amato 2005
- Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. The Journal of Substance Abuse Treatment 2005;28(4):321‐9. [DOI] [PubMed] [Google Scholar]
Baumann 2012
- Baumann MH, Ayestas MA Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue.. Neuropsychopharmacology 2012;37:1192‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutrel 2004
- Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness‐promoting medications. Sleep 2004;27:1181‐94. [DOI] [PubMed] [Google Scholar]
Brackins 2011
- Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract 2011;24(6):541‐50. [DOI] [PubMed] [Google Scholar]
Brensilver 2012
- Brensilver M, Heinzerling KG, Swanson AN, Shoptaw S. A retrospective analysis of two randomized trials of bupropion for methamphetamine dependence: suggested guidelines for treatment discontinuation/augmentation. Drug Alcohol Depend 2012;125(1‐2):169‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castells 2007
- Castells X, Casas M, Vidal X, Bosch R, Roncero C, Ramos‐Quiroga JA, et al. Efficacy of central nervous system stimulant treatment for cocaine dependence: a systematic review and meta‐analysis of randomized controlled clinical trials. Addiction 2007;102(12):1871‐87. [DOI] [PubMed] [Google Scholar]
Castells 2010
- Castells X, Casas M, Pérez‐Mañá C, Roncero C, Vidal X, Capellà D. Efficacy of Psychostimulant Drugs for Cocaine Dependence. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD007380.pub3] [DOI] [PubMed] [Google Scholar]
Chang 2007
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 2007;102:16‐32. [DOI] [PubMed] [Google Scholar]
Chen 2010
- Chen H, Wu J, Zhang J, Hashimoto K. Recent topics on pharmacotherapy for amphetamine‐type stimulants abuse and dependence. Current Drug Abuse Reviews 2010;3(4):222‐38. [DOI] [PubMed] [Google Scholar]
Colfax 2010
- Colfax G, Santos GM, Chu P, Vittinghoff E, Pluddemann A, Kumar S, et al. Amphetamine‐group substances and HIV. Lancet 2010;376:458‐74. [DOI] [PubMed] [Google Scholar]
De la Garza 2010
- Garza R 2nd, Zorick T, London ED, Newton TF. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine‐dependent volunteers. Drug and Alcohol Dependence 2010;106(2‐3):173‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Degenhardt 2010
- Degenhardt L, Bruno R, Topp L. Is ecstasy a drug of dependence?. Drug and Alcohol Dependence 2010;107:1‐10. [DOI] [PubMed] [Google Scholar]
Drobes 1999
- Drobes DJ, Thomas SE. Assessing craving for alcohol. Alcohol Research & Health 1999;23:179‐86. [PMC free article] [PubMed] [Google Scholar]
DSM IV TR
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenberg 2008
- Eisenberg MJ, Filion KB, Yavin D, Bélisle P, Mottillo S, Joseph L, et al. Pharmacotherapies for smoking cessation: a meta‐analysis of randomised controlled trials. Canadian Medical Association Journal 2008;179(2):135‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elkashef 2008 b
- Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacotherapy of methamphetamine addiction: an update. Substance Abuse 2008;29(3):31‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasner‐Edwards 2009
- Glasner‐Edwards S, Marinelli‐Casey P, Hillhouse M, Ang A, Mooney LJ, Rawson R, Methamphetamine Treatment Project Corporate Authors. Depression among methamphetamine users: association with outcomes from the Methamphetamine Treatment Project at 3‐year follow‐up. The Journal of Nervous and Mental Disease 2009;197(4):225‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasner‐Edwards 2010
- Glasner‐Edwards S, Mooney LJ, Marinelli‐Casey P, Hillhouse M, Ang A, Rawson RA, Methamphetamine Treatment Project Corporate Authors. Psychopathology in methamphetamine‐dependent adults 3 years after treatment. Drug and Alcohol Reviews 2010;29:12‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guy 1976
- Guy W. In: Clinical Global Impressions: ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute for Mental Health, 1976. [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32:50‐5. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herin 2010
- Herin DV, Rush CR, Grabowski J. Agonist‐like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Annals of the New York Academy of Sciences 2010;1178:76‐100. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Howell 2008
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochemical Pharmacology 2008;75(1):196‐217. [DOI] [PubMed] [Google Scholar]
Karila 2010
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Bristish Journal of Clinical Pharmacology 2010;69(6):578‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2005
- King GR, Ellinwood EH. Amphetamines and other stimulants. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG editor(s). Substance Abuse. A Comprehensive Textbook. 4th Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2005:207‐22. [Google Scholar]
Kirkpatrick 2012 a
- Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4‐methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology 2012;219:109‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkpatrick 2012 b
- Kirkpatrick MG, Gunderson EW, Johanson CE, Levin FR, Foltin RW, Hart CL. Comparison of intranasal methamphetamine and d‐amphetamine self‐administration by humans. Addiction 2012;107(4):783‐91. [DOI: 10.1111/j.1360-0443.2011.03706.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knapp 2007
- Knapp WP, Soares BG, Farrel M, Lima MS. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003023.pub2] [DOI] [PubMed] [Google Scholar]
Koob 2009
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropsychopharmacology 2009;56(1):18‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kosman 1968
- Kosman ME, Unna DR. Effects of chronic administration of the amphetamines and other stimulants on behavior. Clinical Pharmacology & Therapeutics 1968;9:240‐54. [DOI] [PubMed] [Google Scholar]
Lee 2008
- Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug and Alcohol Reviews 2008;27(3):309‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makris 2007
- Makris AP, Rush CR, Frederich RC, Taylor AC, Kelly TH. Behavioral and subjective effects of d‐amphetamine and modafinil in healthy adults. Experimental and Clinical Psychopharmacology 2007;15(2):123‐33. [DOI] [PubMed] [Google Scholar]
Martin 1971
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clinical Pharmacology & Therapeutics 1971;12(2):245‐58. [DOI] [PubMed] [Google Scholar]
McGregor 2008
- McGregor C, Srisurapanont M, Mitchell A, Wickes W, White JM. Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: a comparison of mirtazapine and modafinil with treatment as usual. The Journal of Substance Abuse Treatment 2008;35(3):334‐42. [DOI] [PubMed] [Google Scholar]
Moeller 2008
- Moeller FG, Schmitz JM, Herin D, Kjome KL. Use of stimulants to treat cocaine and methamphetamine abuse. Current Psychiatry Report 2008;10(5):385‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newton 2005
- Newton TF, Garza R, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacology Biochemistry and Behaviour 2005;82(1):90‐7. [DOI] [PubMed] [Google Scholar]
Newton 2006
- Newton TF, Roache JD, Garza R 2nd, Fong T, Wallace CL, Li SH, et al. Bupropion reduces methamphetamine‐induced subjective effects and cue‐induced craving. Neuropsychopharmacology 2006;31(7):1537‐44. [DOI] [PubMed] [Google Scholar]
NIDA 2006
- Methamphetamine: Abuse and Addiction. NIDA Research Report 2006; Vol. NIH Publication Number 06‐4210.
Pérez‐Mañá 2011
- Pérez‐Mañá C, Castells X, Vidal X, Casas M, Capellà D. Efficacy of indirect dopamine agonists for psychostimulant dependence: a systematic review and meta‐analysis of randomized controlled trials. The Journal of Substance Abuse Treatment 2011;40(2):109‐22. [DOI] [PubMed] [Google Scholar]
Robinson 1985
- Robinson JB. Stereoselectivity and isoenzyme selectivity of monoamine oxidase inhibitors. Enantiomers of amphetamine, N‐methylamphetamine and deprenyl. Biochemical Pharmacology 1985;34(23):4105‐8. [DOI] [PubMed] [Google Scholar]
Rossetti 1992
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. European Journal of Pharmacology 1992;221:227‐34. [DOI] [PubMed] [Google Scholar]
Salo 2011
- Salo R, Flower K, Kielstein A, Leamon MH, Nordahl TE, Galloway GP. Psychiatric comorbidity in methamphetamine dependence. Psychiatry Research 2011;186(2‐3):356‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shearer 2007
- Shearer J. Psychosocial approaches to psychostimulant dependence: a systematic review. The Journal of Substance Abuse Treatment 2007;32(1):41‐52. [DOI] [PubMed] [Google Scholar]
Shearer 2008
- Shearer J. The principles of agonist pharmacotherapy for psychostimulant dependence. Drug and Alcohol Reviews 2008;27(3):301‐8. [DOI] [PubMed] [Google Scholar]
Shoptaw 2009
- Shoptaw SJ, Kao U, Heinzerling K, Ling W. Treatment for amphetamine withdrawal. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003021.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Somoza 1995
- Somoza E, Dyrenforth S, Goldsmith J, Mezinskis J, Cohen M. In search of a universal drug craving scale. Proceedings of the Annual Meeting of the American Psychiatric Association, Miami, Florida. 1995.
Srisurapanont 2001
- Srisurapanont M, Jarusuraisin N, Kittirattanapaiboon P. Treatment for amphetamine dependence and abuse. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003022] [DOI] [PubMed] [Google Scholar]
UNODC 2012
- UNODC. World Drug Report 2012. United Nations Publication, Sales No. E.12.XI.1 2012.
WHO Technical Briefs 2011
- Patterns and consequences of the use of amphetamine‐type stimulants. http://www.who.int/hiv/pub/idu/ats_brief1.pdf. A ccessed 1 July 20 1 1 .
Wilens 2004
- Wilens TE. Attention‐deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship, subtypes at risk,and treatment issues. Psychiatric Clinics of North America 2004;27(2):283‐301. [DOI] [PubMed] [Google Scholar]
Xi 2008
- Xi ZX, Gardner EL. Hypothesis‐driven medication discovery for the treatment of psychostimulant addiction. Current Drug Abuse Reviews 2008;1(3):303‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zahniser 2009
- Zahniser NR, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions. Seminars in Cell & Developmental Biology 2009;20(4):411‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]


