Abstract
Introduction
Preclinical and clinical evidence has elucidated that cannabis-based medical formulations (CBMFs) may display anxiolytic, antidepressive, and neuroprotective properties. CBMFs are often considered as novel therapeutic anxiolytic agents that can be prescribed as pharmacotherapy for symptomatic domains in anxiety disorders (ADs). Our aim was to explore effectiveness and tolerability of enriched cannabidiol (CBD) oil extract formulations in adults with anxiety symptoms in an outpatient mental health program in Colombia during the COVID-19 pandemic.
Methods
We conducted an observational, retrospective, real-world evidence case series from electronic health records at Zerenia Clinic in Bogotá, Colombia between June 2021 and December 2022. Our convenience sample consisted of people searching for CBMFs for the treatment of anxiety symptoms. A cohort of 24 adults was prescribed with enriched CBD in the form of non-sterile oral liquids suspended in sesame seed oil extracts for DSM-5 unspecified anxiety disorder and followed throughout the first year of treatment. CBMFs were prepared by dissolving full-spectrum cannabis extracts in sesame seed oil to a standardized concentration of active ingredients which is CBD-enriched. The oil extract contained 100 mg/mL of CBD and less than 1.9 mg/mL of THC. Primary outcome measures established were the anxiety subscale in the Hospital Anxiety and Depression Scale (HADS-A), and the clinical global impression scale with regard to severity (CGI-S) and improvement (CGI-I) at baseline, 6 months, and 12 months during follow-up. Secondary outcome measures established were HADS depression subscale (HADS-D) and the Epworth Sleepiness Scale (ESS), respectively. Participants also completed the patient-reported outcome measures (PROMs) during each visit throughout the 12-month follow-up. PROMs documented both participantʼs subjective improvement experience and progressive adverse effects.
Results
After 6 months of treatment with sublingually administered enriched CBD oil extracts in a median dosage of 100 mg, more than half (54.17%) of the sample continued to report significant anxiety symptoms. After 12 months, only 37.50% persisted with significant anxiety symptoms with a median dose of 120 mg of enriched CBD oil extracts. Similar subjective improvements were reported with regard to sleep disturbances (SDs) as a secondary outcome. At baseline, less than half (46.83%) of the sample reported significant daytime sleepiness. After 6 months of enriched CBD oil extract treatment, less than one third (29.17%) continued to report SDs. At end point, a high proportion of the sample (87.50%) were considered to have normal daytime sleepiness. The cohort showed no clinically relevant depressive symptoms at baseline based on HADS-D scores; therefore, no improvement could be reported throughout the 12-month follow-up. Minimal gender differences with regard to HADS-D scores may be attributed to modifying effects of menopause-related symptoms. No significant adverse drug reactions or deaths were reported during the 12-month follow-up.
Conclusions
Further research should determine the long-term efficacy, safety, and appropriate dosages of enriched CBD oil extracts in treating specific ADs rather than broad and unspecified anxiety symptoms. The state of the art of CBMFs for ADs should be warranted by future randomized controlled trials. The next stage for cannabis research should be focused in performing head-to-head trials comparing enriched CBD extracts or capsules versus first-line treatments proven to be effective in ADs.
Keywords: Anxiety disorders, Sleep disturbances, Cannabidiol, Treatment, Cannabinoid treatment
Introduction
Anxiety, depressive symptoms, and sleep disturbances are the most prevalent clinical syndromes among participants admitted for a psychiatric evaluation in outpatient clinics worldwide [1–7]. Over the past decade, there is growing clinical evidence regarding efficacy, safety, and tolerability of cannabis-based medicinal formulations (CBMFs) to treat symptoms associated to a wide range of medical disorders such as chronic pain [8, 9], spasticity [10], and resistant epilepsy [11] among others. Moreover, a broad range of preclinical and clinical research in anxiety disorders (ADs) have pointed toward cannabidiol (CBD) as a novel therapeutic agent showing anxiolytic, antidepressive, and neuroprotective properties [12, 13]. Furthermore, sustained administration of CBD might trigger several multimodal neurobiological mechanisms which might result in expected therapeutic actions [14]: CBD administration in humans correlates with a sustained accumulation of the endocannabinoid anandamide [15], promotes partial agonism over 5-HT1A [15], a higher adenosine uptake regulation [16], and possible modulation of presynaptic and postsynaptic glutamatergic and GABAergic neurotransmission [15]. However, high-quality clinical data supporting these preclinical observations are still in its infancy. Available evidence is limited to a few studies of experimentally induced anxiety [17–20], a few observational studies on patients diagnosed with anxiety disorders [21, 22], and two recent clinical trials describing acute treatment and discontinuation of intermediate dosages of enriched CBD oral extracts (150 mg BID) in Brazilian health care workers (HCWs) during the COVID-19 pandemic [23, 24]. Authors reported significant response rates with regard to emotional exhaustion, anxiety and depressive symptoms. Nevertheless, most samples with anxious phenomenology will not be included in clinical trials mentioned above due to methodological issues. We should also keep in mind that despite the fact that anxious samples might be symptomatic and functionally impaired, diagnostic criteria and features may not be met for generalized anxiety disorder, panic disorder, or the phobias. In this troublesome scenario, unspecified anxiety disorders (UAD) has arisen from the fifth version of the diagnostic and statistical manual of mental disorders (DSM-5) as a helpful tool for clinicians when anxiety is transient, upcoming, or fading away. Thus, UAD should be considered for subtle and mild anxiety or phobia symptoms that do not meet criteria for any specific AD but are considered significant enough to cause distress. In other words, episodic anxiety-like symptoms that cause impairment, but there is not enough information to determine which specific type of AD or medical disorder might be responsible based on psychiatric and medical records. As mentioned above, unspecified anxiety symptoms were considered the rule rather than the exception during the COVID-19 pandemic. In fact, a high percentage of those who consulted to emergency rooms or specialized psychiatric outpatient treatments during the COVID-19 pandemic suffered from unspecified anxiety symptoms. Hence, we can argue that UAD was a prevalent diagnosis during this time.
The primary aim of this research effort was to explore long-term change from baseline to endpoint during a 12-month follow-up with regard to anxiety levels, sleep disturbances, and associated depressive symptoms in a convenient sample administered enriched CBD oil extracts sublingually. Our secondary aim was to report the rate adverse events occurring during the long-term CBD treatment follow-up as a subjective measure of participants’ safety.
Materials and Methods
Study Design
An observational, retrospective case series study from real-world evidence (RWE) was performed. A precise electronic health records (EHRs) of a sample searching for cannabis-based medical formulations (CBMFs) for the treatment of several psychiatric disorders has been documented at Zerenia Clinic in Bogotá, Colombia since 2021. Zerenia Clinic is an integrative health care facility that offers diverse line of pharmacological treatments for psychiatric patients, including adjuvant therapies with CBMFs. Zerenia clinic’s mental health program has focused thus far on ADs, sleep disturbances, major depressive disorders, bipolar disorders, and impulse control disorders. Both ADs and sleep disturbances are two important symptomatic psychiatric domains in which CBD has reported positive, significant therapeutic responses in available RWE [23, 24]. Therefore, we decided to explore long-term anxiolytic response and clinical global functioning in adults with broad-spectrum anxiety symptoms prescribed with enriched CBD oil extracts between June 2021 and December 2022.
Sample
Our sample consisted of 24 adults who had searched for psychiatric evaluation to receive CBMFs to treat unspecified anxiety symptoms in an outpatient mental health service. We selected a convenience sample with the following inclusion criteria: (1) men or women ≥18 years old, (2) DSM-5 criteria for UAD, (3) sufficient level of literacy (writing and reading), (4) being prescribed CBD oil extracts for the treatment of unspecified anxiety symptoms, and (5) having at least 3 different points of psychometric measurements during the 12-month follow-up. Exclusion criteria included (1) children and adolescents (<18 years old), (2) pregnant women, (3) history of bipolar disorder or psychotic symptoms, (4) a family history of adverse responses to the use of medical cannabis, (5) a history of cannabis use disorder, (6) epileptic participants, (7) participants with documented hematological, hepatic, or renal disease, and (8) the cohort of patients who were lost during the first year of follow-up. It is important to emphazise that convenience sampling is a non-probalistic method in which the units chosen for inclusion are the ones that are the most convenient for the researcher to access within the electronic health record. This may be the results of factors such as proximity to one another, availability at a specific moment, or interest in participating in the study.
Procedures and Intervention
All twenty-four subjects were prescribed CBMFs based on therapeutic algorithms available for anxiety outpatient treatment. Specifically, CBD in the form of non-sterile oral liquids suspended in sesame seed oil extracts. CBMFs were prepared by dissolving full-spectrum cannabis extracts in sesame seed oil to a standardized concentration of active ingredients which is CBD-enriched. The oil extract contained 100 mg/mL of CBD and less than 1.9 mg/mL of THC. All 24 patients were prescribed 0.1 mL of CBD-enriched oil extract sublingually twice per day. From there on, CBD was titrated upwards in a weekly fashion based on therapeutic response, tolerability, and/or reaching ceiling/therapeutic effect. While enriched CBD extract was added in 6 participants to an unchanged antidepressant regimen for anxiety, other 18 participants were focused in trying novel, monotherapeutic strategies such as CBMFs available for the treatment of UAD in Colombia. Specific CBMFs prescribed were fabricated and distributed to participants by Khiron Life Science Corp® in Colombia.
Clinical Assessments
A clinician-administered psychiatric evaluation and questionnaire with demographic, clinical records, psychometric evaluations as outcome measures were generated and saved in the EHRs managing system, Gomedisys®, as routine according to mental health guidelines inside the site. Additionally, the patient-reported outcome measure questionnaire was performed in order to measure long-term participants’ subjective experience of improvement and satisfaction as well as report of adverse effects (AEs) during each of the visits throughout the 12-month follow-up. From there on, data were extracted from Gomedisys® on to a research dashboard to develop our dataset. Available data were encrypted to keep adequate confidentiality and privacy. Beforehand, we had established three points of naturalistic assessment during a 12-month follow-up (at baseline [T1], 6 months [T2], and 12 months [T3], respectively). Baseline was determined as the clinical assessment on the day of established diagnosis and determined initiation of enriched CBD oil extract preparation. Patients were assessed by the same attending psychiatrist at three different predetermined points. As mentioned before, personal data (name, last name, or ID) was anonymized during data analyses. Informed consents were written, explained by our medical staff, and signed before the beginning of CBD-enriched oil extract therapies. It is important to explain that informed consent is included as part of needed paperwork for participants being included within the program and thus prescribed CBMFs at Zerenia clinic. This is also a requirement by Colombian legislation to tract CBMFs prescriptions from mental HCWs and other physicians.
Questionnaires
Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) is a self-assessment scale validated for the screening of symptoms associated to depression and anxiety in hospital or medical care settings [25].We decided to utilize cutoff points proposed by Rico et al. [26] in Colombian population (≥8 for anxiety and ≥9 for depression) attending intermediate complexity health care facilities such as Zerenia Clinics.
Epworth Sleepiness Scale
The Epworth Sleepiness Scale (ESS) is a self-administered questionnaire based on 8 simple questions about daytime sleepiness [27]. The ESS score (the sum of 8 item scores, 0–3) can range from 0 to 24. In general, cutoff points for the ESS scores can be interpreted as follows: (a) 0–5 lower normal daytime sleepiness, (b) 6–10 higher normal daytime sleepiness, (c) 11–12 mild excessive daytime sleepiness, (d) 13–15 moderate excessiveness daytime sleepiness, (e) 16–24 severe excessive daytime sleepiness [28].
Clinical Global Impression
The CGI is a psychometric scale that provides an assessment of the clinician’s view of the patient’s global functioning prior to and after initiating any therapeutic intervention in psychiatry. The CGI comprises two companions, and each with one-item measures evaluating the following: (1) severity of psychopathology from 1 to 7 and (2) change from the initiation of treatment on a similar seven-point scale [29].
Adverse Effects
Patient-reported outcome measure questionnaire was generated to collect mental health outcomes of interest and AEs in the sample involved in long-term treatment for UAD with enriched CBD oil extracts in Zerenia Clinic.
Statistical Analysis
Statistical analysis conducted using Stata v17.0 SE-Standard Edition (Stata Corp LLC, College Station, TX). Univariate and bivariate analyses were assessed; continuous variables were presented as mean and standard deviations (SD) or median and interquartile ranges (IQRs) as appropriate. Categorical variables were presented as frequencies and percentages. Group comparisons were analyzed by McNemar test. The Friedman test was used to determine whether there is a statistically significant difference between the median of psychometric scores in three different periods of follow-up in subjects prescribed with CBD. To determine which pairwise groups are significantly different following the Friedman test results, we performed the pairwise Wilcoxon Signed Rank and adjusted with Bonferroni correction. All probability values were two-tailed, the error alpha was = 0.05 and 95% for confidence interval.
Ethical Considerations
This is considered a RWE low-risk study according to current legislation for clinical research in Colombia. EHRs were revised following ethical considerations of the Declaration of Helsinki and Council for International Organizations of Medical Sciences. All the cohort provided written informed consent for the use of enriched CBD oil extract and thus authorization to utilize encrypted data of treatments outcomes for the purpose of scientific publications.
Results
Demographics
The main characteristics of the study cohort are outlined in Table 1. A total of 24 patients with UAD were enrolled and prescribed with enriched CBD oil extract for the long-term treatment of anxiety, associated depressive symptoms, and comorbid SDs. The median age for the entire sample was 56 (34.5–71) years, 12 were men (50.00%, median age 58 (36–71) years), and 12 women (50.00%, median age 56 (34.5–69) years). Enriched CBD 100 mg/mL oil extract was prescribed to 100% of the sample and systematically titrated throughout the 12-month follow-up.
Table 1.
Characteristics adults with UAD (n = 24) at baseline
| Male | Female | Total | p value | |
|---|---|---|---|---|
| Sample, n (%) | 12 (50.00) | 12 (50.00) | 24 (100) | |
| Age median (IQR) | 58 (36–71) | 56 (34.5–69) | 56 (34.5–71) | <0.001 |
| HADS-A median (IQR) | 10 (9.5–11.5) | 9 (7.5–10) | 9.5 (9–10.5) | <0.001 |
| HADS-D median (IQR) | 7 (7–7) | 7.5 (7–8) | 7 (7–8) | <0.001 |
| ESS median (IQR) | 4.5 (2.5–9.5) | 5 (1–8) | 4.5 (1.5–8.5) | <0.001 |
HADS-A, Hospital Anxiety and Depression Scale-Domain Anxiety; HADS-D, Hospital Anxiety and Depression Scale-Domain Depression; ESS, Epworth Sleepiness Scale; IQR, interquartile range.
Outcomes in HADS in Patients with UAD
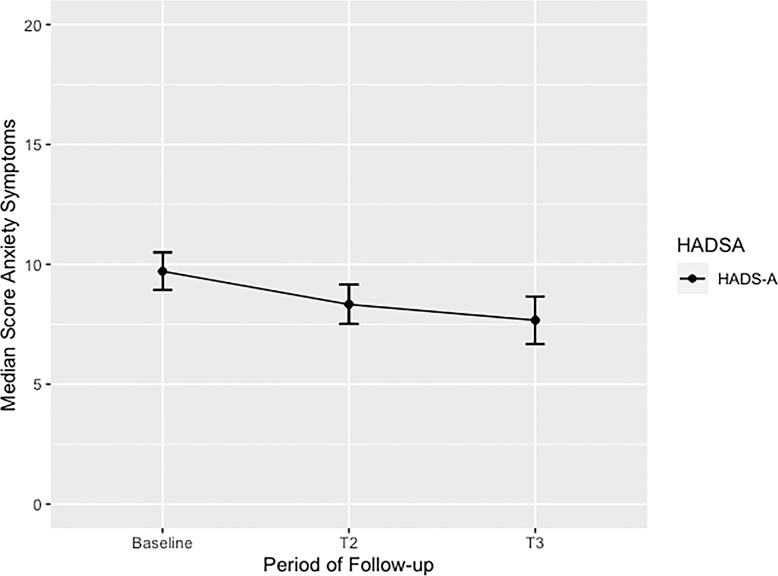
At baseline, the median of HADS-A was 9.5 (9–10.5), after 6 months, the median was 8 (7–9.5), and at the end point, after 12 months of treatment follow-up, the median was 7 (6–8.5), respectively (Fig. 1). We should remember predetermined cutoff points at 8 for HADS-A. After controlling for gender, the median overall HADS-A score at baseline in men was 10 (9–12) and in women, it was 9 (8–10). After a 6-month follow-up, the HADS-A median score for men was 7 (7–9) and for women was 8 (7–10). At the 12-month follow-up, the HADS-A median score was 6 (6–8) in men and 7 (7–9) in women.
Fig. 1.
Median scores of anxiety symptoms in adults with unspecified anxiety disorder (UAD) treated with enriched CBD oil extract during 12-month follow-up. This figure shows the overall median score of anxiety symptoms in adults with UAD treated with CBD during 12-month follow-up. A significant p value from baseline to end point (T3) was identified p < 0.008. HADS-A, Hospital Anxiety and Depression Scale-Domain Anxiety; T1, baseline; T2, 6-month follow-up; T3, 12-month follow-up.
At baseline, most of the cohort (87.5%) reported anxiety symptoms. After 6 months of continued treatment with enriched CBD oil extract, 54.17% of the sample continued to report significant anxiety symptoms. After 12 months of CBD maintenance treatment, only 37.5% of the cohort persisted with significant anxiety symptoms. We reported an overall reduction of 50% in anxiety symptoms during the long-term 12-month follow-up, and the median in the overall score of HADS-A reported was 9.5 considered to be persistent subtle to mild anxiety symptoms (9–10.5). After controlling for gender, male subgroup reported median overall HADS-A score of 10 (9–11.5) and females reported median overall HADS -A of 9 (7.5–10) respectively. This was also considered minimal to mild anxiety symptoms severity.
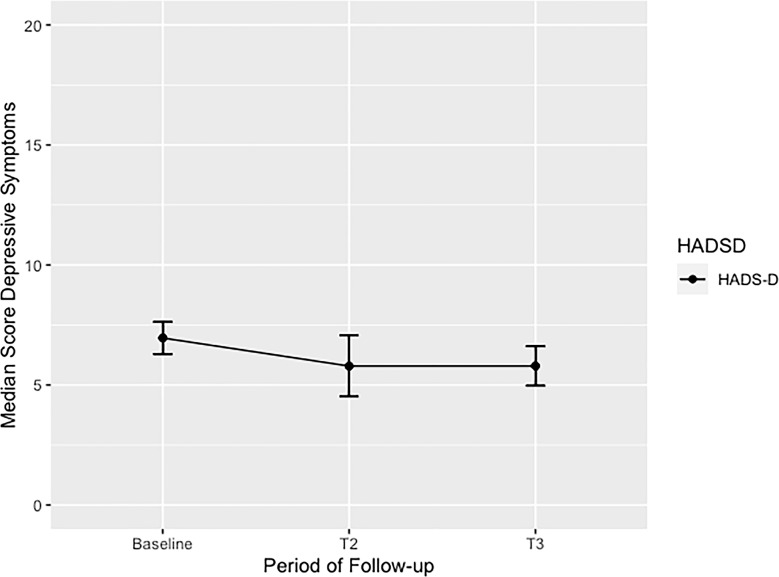
At baseline, the median score of HADS-D was 7 (7–8). At the 6-month follow-up, the median score reported was 5 (4–6), and at the 12-month follow-up, median score was 6 (4.5–7) (Fig. 2). At baseline, gender differences analysis reported the median HADS-D score in males 7 (7–7) and females 7 (7–8) to be quite similar. Following the same pattern, after 6 months of continued CBD treatment HADS-D median score reported in males was 5 (4–6), and in females 5 (4–7), with similar trends for mild improvement in depressive symptoms in both groups up until end point. Surprisingly, only 4.17% of the sample reported positive depressive symptoms at base line. At the 6-month follow-up, 12.50% of the samples were considered positive for depressive symptoms, and then at the end point, only 8.33% of the sample continued to report significant depressive symptoms. The median in the overall score of HADS-D was 7 (7–8). At the end, the median HADS-D score in men was 7 (7–7) and women was 7.5 (7–8). Beforehand, we had predetermined cutoff point of HADS-D was 9 based on local epidemiological studies [26].
Fig. 2.
Median scores of depressive symptoms in adults with unspecified anxiety disorder (UAD) treated with enriched CBD oil extract during 12-month follow-up. This figure shows the overall median score in adults of depressive symptoms with UAD treated with CBD during 12-month follow-up. A significant p value from baseline to end point (T3) was identified p < 0.013. HADS-D, Hospital Anxiety and Depression Scale-Domain Depression; T1, baseline; T2, 6-month follow-up; T3, 12-month follow-up.
Outcomes in ESS in Patients with UADs
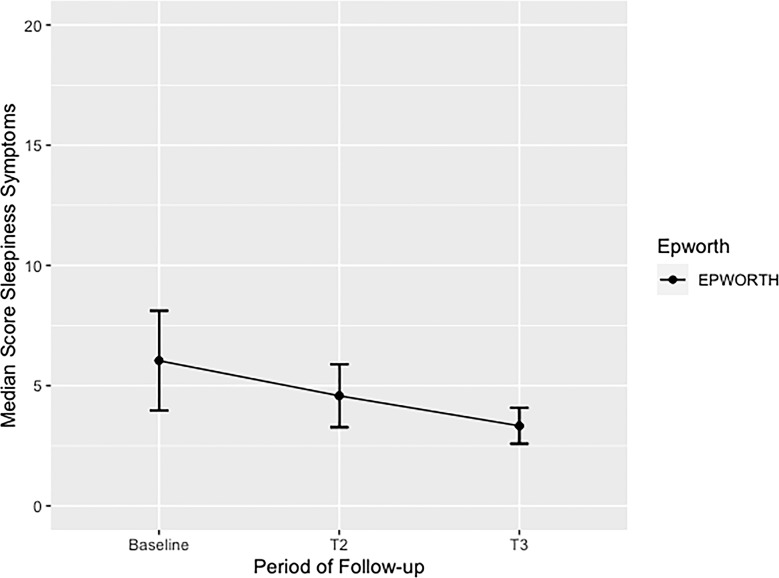
At baseline, the median ESS score in male was 4 (2–10), and in female was 5 (1–8). After 6 months of continued CBD treatment, male reported ESS median score 4 (2–5), and female reported ESS median score 5 (2–6). At end point, after 12 months of CBD maintenance treatment, similar ESS reduced median score in male 3 (2–3), and in female, 3 (2–5) were reported. At baseline, the overall median of ESS was 4.5 (1.5–8.5), at 6 months, it was 4 (2–6), and at 12 months, it was 3 (2–4.5) (Fig. 3). Thereby, at baseline, more than half or the sample (54.17%) had normal daytime sleepiness, almost a third (29.17%) had higher than normal daytime sleepiness, about 8.33% had mild excessive daytime sleepiness, 4.17% had moderate excessive daytime sleepiness, and 4.17% had severe excessive daytime sleepiness. After 6 months of continued CBD treatment, more than two-thirds of the sample (70.83%) had normal daytime sleepiness, about a fourth (25.00%) had higher than normal daytime sleepiness, and only 4.17% reported severe excessive daytime sleepiness. At the end point, a higher proportion (87.50%) were considered to have normal daytime sleepiness, and remaining patients (12.50%) had higher than normal daytime sleepiness.
Fig. 3.
Median scores of sleepiness symptoms in adults with unspecified anxiety disorder (UAD) treated with enriched CBD oil extract during 12-month follow-up. This figure shows the overall median score of sleepiness symptoms in adults with UAD treated with CBD during 12-month follow-up. A significant p value from baseline to end point (T3) was identified p < 0.035. ESS, Epworth Sleepiness Scale; T1, baseline; T2, 6-month follow-up; T3, 12-month follow-up.
Median Differences of Sleepiness, Anxiety, and Depressive Symptoms during the Follow-Up
There are no statistically significant differences reported in response time with regard to sleep-related symptoms [Q(2) = 3.67, p = 0.159] in the ESS. Conversely, for anxiety symptoms, statistically significant differences were reported [Q(2) = 11.80, p = 0.002] as well as with regard to depressive symptoms [Q(2) = 8.22, p = 0.016] in both HADS subscales (Table 2) The pairwise test with Wilcoxon signed rank and adjusted with Bonferroni correction reported median differences in overall score of HADS-A between measurements at baseline and 6 months. There are also median differences score between baseline and 12 months (Table 2). In contrast, the pairwise test with Wilcoxon signed rank and adjusted with Bonferroni correction reported median differences in overall score of HADS-D only between baseline and the 6-month and baseline with the 12-month end point measurement (Table 2).
Table 2.
Median differences of anxiety, depressive, sleepiness symptoms in adults with UAD during 12-month follow-up (n = 24)
| Q | df | p value | Baseline versus Q2 | Baseline versus Q3 | Q2 versus Q3 | |
|---|---|---|---|---|---|---|
| HADS-A | 11.8 | 2 | 0.002 | 0.019 | 0.008 | 0.139 |
| HADS-D | 8.2 | 2 | 0.016 | 0.026 | 0.013 | 0.454 |
| ESS | 3.7 | 2 | 0.159 | 0.029 | 0.035 | 0.136 |
| CGI-S | 32.8 | 2 | <0.001 | <0.001 | <0.001 | 0.010 |
HADS-A, Hospital Anxiety and Depression Scale-Domain Anxiety; HADS-D, Hospital Anxiety and Depression Scale-Domain Depression; ESS, Epworth Sleepiness Scale; Q, Statistic Test of Friedman; df, degree of freedom; Q2, 6-month follow-up; Q3, 12-month follow-up.
Outcomes in Clinical Global Impression-Severity in Anxiety Patients during Follow-Up
At baseline, the median score of CGI-S was 5 (IQR, 4–5). Men had a median score of 5 (IQR, 4–5) and women reported 5 (IQR, 4–5). At 6 months, the median score of CGI-S in the overall sample was reduced to 3 (IQR, 3–4). A parallel trend of reduction was reported of 3 (IQR, 3–4) in male and 3 (IQR, 3–4) in female. At 12 months, the median score for all the sample remained to be 3 (IQR, 2–3.5), but female showed a significant reduction during this period toward a 2 (IQR, 2–4). The median score had no change in men 3 (IQR, 2–3) (Table 2).
Median Differences of CGI-S in Patients with UAD during the Follow-Up
With regard to clinical global impression-severity (CGI-S), the results showed statistically significant differences Q(2) = 32.78, p < 0.001 (Table 2).
Median Differences between Doses of CBD Oil Extracts during Time Periods of Follow-Up
There are statistical differences among median of doses among CBD during the follow-up Q(2) = 33.68, p < 0.001 (Table 3). Patients started titrating in a very low median dose of enriched CBD oil extract of 20 mg. At 6-month follow-up, the median dose was 100 mg. At end point after a 12-month follow-up, the median dose was 120 mg, respectively.
Table 3.
Median (mg administered per day) of CBD at baseline and during 12-month follow-up
| Product | Q1 baseline (median, IQR) | Q2 (median, IQR) | Q3 (median, IQR) |
|---|---|---|---|
| CBD | 6 mg (6–18) | 100 mg (42.2–135) | 120 mg (90–150) |
AEs for Enriched CBD Oil Extracts in UADs
AEs are reported in Table 4. No deaths occurred or severe AEs were reported during the long-term follow-up. At the 12-month follow-up, 8/24 (33.33%) reported dry mouth, 8/24 (33.33%) reported dizziness, 8/24 (33.33%) reported somnolence, and 6/24 (25.00%) reported headaches. These AEs were frequent but were reported to be mild and transient in UAD.
Table 4.
Adverse effects in adults with UAD (n = 24) treated with CBD during the follow-up
| Adverse effect | Frequency (n) | Percentage (%) |
|---|---|---|
| Dry mouth | 8 | 33.33 |
| Dizziness | 8 | 33.33 |
| Somnolence | 8 | 33.33 |
| Headache | 6 | 25.00 |
| Orthostatic hypotension | 4 | 16.66 |
| Diarrhea | 2 | 8.33 |
| Fatigue | 2 | 8.33 |
| Tachycardia | 2 | 8.33 |
| Elevated liver function test | 1 | 4.17 |
| Cannabinoid hyperemesis syndrome | 1 | 4.17 |
Discussion
As far as we are aware, this is the first retrospective EHRs analysis to explore long-term treatment with enriched CBD oil extracts in a convenience sample with UAD in Colombia. Twenty-four adults considered to suffer from DSM-5 UAD and in search for CBMFs for their anxiety symptoms were prescribed enriched CBD oil extract and followed up to 12 months. Both anxiety symptoms and sleep problems improved during the first 6 months of CBD continuation treatment in a median dose of enriched CBD oil extract of 100 mg QD. Moreover, symptomatic recovery for both anxiety and associated sleep problems were maintained during the long-term treatment with CBD oil extracts regardless of age, sex, and severity of symptoms at enrollment. Median dosage for response at the 12-month end point was around 120 mg per day. In contrast, depressive symptoms did not show initial improvement with CBD-enriched oil extracts nor significant differences at any time of clinical evaluation during the 12-month follow-up. Based upon these findings, we have contributed to extend knowledge on long-term anxiolytic properties of continuous sublingual intermediate dosage administration of enriched CBD oil extracts after 12 months. CBD was reported to be safe, well tolerated, and effective. Furthermore, our data support long-term maintenance treatment with augmentation strategies for ADs and sleep-related problems with enriched CBD oil extract administration published elsewhere.
In the largest case series available, 72 patients were divided into whether they had anxiety or poor sleep quality as their primary medical concern. A capsule of CBD 25 mg was prescribed as add-on treatment [21]. About fourth-fifths (79.2%) of a sample of anxious patients reported rapid reductions in their anxiety scores and more than two-thirds of the sample (66.7%) of patients who reported sleep problems improved during the first month of enriched CBD treatment. Moreover, anxiolytic benefits from CBD prescriptions were sustained for all three measurements during a 12-week period. However sleep scores fluctuated over time. Consequently, CBD treatment showed higher and sustained reductions for anxiety symptoms rather than for related daytime sleepiness during a 3-month follow-up. It is important to highlight that the median dosage was four to six times lower than dosages used in our convenience sample. One can hypothesize that the upward titration of enriched CBD oil extract preparation allowed participants to achieve individualized and more effective dosage in time rather than taking an initial fixated oral low dose CBD assuring improved tolerability but reduced efficacy. Further research should also elucidate whether the sublingual route of administration is better than the oral route due to greater bioavailability of CBD throughout the treatment follow-up.
Berger et al. [22] prescribed CBD up to 800 mg/day in a group of 31 young adults with refractory AD. Each subject was administered a 99% purified CBD capsule formulation, and results were reported at week 12. In the same line, the overall anxiety severity and impairment scale (OASIS) decrease from 10.8 at baseline to 6.3 (p < 0.0001), and the CGI-S scale scores also improved (p = 0.0008). However, contrasting evidence with regard to early improvement of depressive symptoms with CBD treatment was reported (p < 0.0001). A similar trend has been reported by Sachedina et al. [24] from a registry in a sample of 7,362 patients treated with CBD and followed up to 24 months with improvements in both anxiety and depression. Moreover, the general anxiety disorder-7 item scale (GAD-7) and the patient health questionnaire-9 (PHQ-9) scores improved after 2 years of sustained CBD treatment (median difference of 5.2 [p < 0.001]; and 6.4 [p < 0.001], respectively). Unfortunately, median or fixated CBD dosage was not reported.
As mentioned before, a recent open label, randomized clinical trial in HCWs in Brazil replicated previous positive results of acute CBD treatment over anxiety symptoms. HCWs with significant levels of emotional exhaustion and burnout during the COVID-19 pandemic were prescribed CBD treatment (150 mg BID) plus standard care during a 3-month follow-up [23]. Significant anxiolytic responses were reported, and beneficial effects were maintained up to 1 month after treatment discontinuation. To date, most research on CBD anxiolytic response have reported dosage ranges between 10 mg/day up to 800 mg/day with a nonsignificant percentage of toxicity in samples with anxiety symptoms. Moreover, there are no lethality reports thus far associated with CBD treatment or overdose. In fact, evolving major medical complications or medical warnings associated with CBD acute prescription continue to be very limited [30]. Possible long-term risks remain unclear and to be unraveled by future research. Nevertheless, CBD seems to have a better risk profile related to dependence compared with agents such as benzodiazepines and opioids mediations [31]. Furthermore, CBD may have a benefit-risk profile compared to commonly prescribed medications in difficult-to-treat ADs and treatment-resistant depression such as atypical antipsychotics, anticonvulsants, and lithium [32]. In the same line, tricyclic antidepressants which are still prescribed thoroughly may also induce harmful metabolic syndrome and lethal arrhythmias in some vulnerable anxious patients [33]. In that sense, several ongoing clinical trials are on their way elsewhere with regard to the anxiolytic effects of CBD and other CBMFs [34–36]. Thereby, we consider our RWE preliminary results on long-term treatment for UAD continue to nurture and unravel available data with regard to CBD anxiolytic effects.
The mechanisms by which CBD decreases anxiety levels seem to be related to modulation of the limbic and paralimbic brain areas [34]. Previously, it has been suggested that the inhibition of fatty acid amide hydrolase enzyme mediated by CBD, increases the concentration of anandamide which impact on adenosine function, and may interact to some extent with 5-HT1A receptors [35]. Furthermore, the increased function of anandamide promotes slow-wave sleep which might explain improvement in sleep symptoms reported in our sample [36].
We should also highlight differences between short-term acute pharmacological interventions versus long-term maintenance treatments with CBD and other CBMFs. For example, Malaca et al. [37] described interindividual variability between the CBMFs dosage and its serum levels and between the different brands of CBMFs and serum metabolites levels. This might be explained by individual pharmacokinetics, variable pharmacodynamics, and/or symptomatic drug-response within the time of CBMFs exposure. Based on our preliminary results and considering recent findings by McCartney et al. [38], we consider CBMFs such as CBD-enriched oil extract can be prescribed once per day or even two or three times per week after reaching desired plasmatic concentrations and positive therapeutic response. It is known that CBMFs serum levels decrease slowly; therefore, it is not necessary for repetitive, continuous dosing during continuation and maintenance treatment. However, this might not be true for other psychiatric disorders such as chronic psychosis, other persistent sleep disorders, and continuous substance abuse among others. In fact, significant higher total daily dose of CBMFs are recommended for sustained and effective improvement to be achieved after acute treatment or to prevent withdrawal or craving for substance use disorders [39]. Recently, Anderson et al. [40] described minimal interaction between CBD and antidepressants in vivo (mirtazapine, sertraline, fluoxetine). Nevertheless, authors reported a potential for drug-drug interaction with citalopram and escitalopram. Fortunately, we did not report any type of drug interaction in our sample of anxious participants, despite a third of the sample being prescribed long-term antidepressants such as escitalopram and others before enrollment and after engaging in combined pharmacotherapy with CBD.
Caveats with regard to effect modification produced by menopause-related symptoms over both the subjective experience as well as in objective measures of improvement and severity of anxiety, depression, and sleep problems in female sample should be mentioned as a possible source of confounding bias. Dahlgren et al. [41] recently reported that 78.7% of perimenopausal and postmenopausal women searched for CBMFs to treat their sleep disturbances (67.4%) and mood/anxiety symptoms (46.1%). In the same line, Babyn et al. [42] have reported that a about a third (34%) of menopausal female in a community sample in AB, Canada were prescribed CBMFs for the treatment of sleep-related problems (65%) and anxiety symptoms (45%), respectively. While 74% of menopausal women reported improvements in associated psychiatric symptoms, vasomotor, and other perimenopausal/postmenopausal symptoms remained unaffected after CBMFs exposure. Previously, Mejia-Gomez et al. [43] reported scarce evidence over the impact of different cannabinoid treatment profiles on specific menopause-related psychiatric symptoms. Preclinical studies have also shown that estrogen levels and other changes might modulate anxiolytic effects by producing changes within the endocannabinoid system [44]. Authors have proven this theory on ovariectomized animals prescribed with CBMFs and reduced postsurgical anxiety [45]. It is also known that menopausal female have reported high expectations over prospective CBD treatment response prescribed for several menopausal-related symptoms such as hot flashes, headaches, sleep problems, depression, and anxiety levels [41–43]. Li et al. [46] reported higher expectations in menopausal women may trigger acute therapeutic responses involving placebo effect over menopausal-related symptoms that usually plateaus after week 12 of treatment. Moreover, it seems that hormonal treatments generate even higher expectations with protracted placebo effect up until week 24 compared to non-hormonal treatments such as enriched CBD oil extracts or other CBMFs. In that sense, both specific active comparators and subjective higher expectations can modify acute and continuation treatment CBD efficacy in females who also suffer menopause-related symptoms. Furthermore, positive expectation may produce additional augmentation of previously proven effective interventions during acute and continuation phase of specific interventions [47–49]. Hence, valence of expectations should be considered as a potential psychotherapeutic mechanism to boost long-term treatments. The extent to which multimodal interventions included for our convenience sample with UAD prescribed enriched CBD oil extracts in Zerenia Clinic contributed for sustained treatment response after the 12-month follow-up remains to be elucidated [50, 51]. In synthesis, while current research on the efficacy and long-term impact of specific CBMFs over menopause-related symptoms is absent, future research is warranted assessing benefits and risk of CBMFs during menopause over main outcome measures such as anxiety, depression, and sleep disturbances [52].
Limitations
This RWE study has several methodological limitations such as its retrospective nature in itself, lack of blinding, sample size, absence of control group, selection bias, lack of precise data of concurrent psychiatric medication prescriptions, and enhanced placebo effect due to a selected, convenience sample. Specifically, with regard to psychometric measures, we consider important to highlight the subjective nature of ESS in this sample. These methodological issues will also limit any possible causality derived from positive CBD therapeutic responses registered. However, gathered data are relevant as it elucidates a trend toward significant relief of anxiety symptoms with long-term CBD treatment exposure in broad and/or unspecified anxiety symptoms. Additionally, this research effort has benefited from structured and rigorous observational 12-month follow-up period, with predetermined objective psychometric outcomes measures for anxiety as primary outcome measures. Furthermore, continuous and systematic slow-dose enriched CBD oil extracts titration and constant monitoring of AEs and/or drug-drug interaction were performed throughout the CBD long-term treatment for every subject included in the sample. In fact, our associated leaders at Zerenia Clinics performed sequential psychoeducational sessions for all those included in our cohort with regard to CBMFs and treatment program to enhance tolerance to enriched CBD oil extracts. Our team was also able ensure adherence and compliance thought out the first year of clinical follow-up.
Conclusions
Anxiety levels, depressive symptoms, sleep-related problems, and other stress-related disorders have risen after the COVID-19 pandemic. Consequently, there seems to be a higher proportion of anxious cohorts getting involved in different types of psychotherapeutic and psychopharmacological treatments to reduce their anxiety levels. In fact, an important percentage is turning their heads to alternative therapeutic strategies such as CBD rather than first-line anxiolytic treatments available based upon usual standards of health care. In that sense, the international scientific community as well as health authorities worldwide should get actively involved in tailoring better regulations, administrative dynamics, and political approaches to include broad-spectrum CBMFs beyond enriched CBD oil extracts and capsules already available in several countries worldwide such as Colombia. Further research should also push forward on more sophisticated epidemiological methodologies, better control of confounding bias, larger samples, randomized clinical trials, and longer follow-ups. Specifically, psychiatric research focused on younger samples with anxiety, associated depressive symptoms, and sleep disturbances must be warranted. It is important to continue to determine efficacy of CBD treatments and other CBMFs available for specific ADs. Specific periods of the life cycle influenced by gender specific neuroendocrine functioning may also bring interesting and therapeutic niches for CBMFs. In the meantime, efforts should be focused in head-to-head comparisons with first-line treatments proven to be effective in ADs.
Acknowledgments
We express our gratitude to the patients and their families who consented to be involved in this observational. We also thank registered nurse Ivannia Rondon for her initial assistance with recruitment.
Statement of Ethics
This is considered a RWE low-risk study according to current legislation for clinical research in Colombia. EHRs were revised following ethical considerations of the Declaration of Helsinki and Council for International Organizations of Medical Sciences. This study protocol was reviewed and approved by the IRB of Zerenia Clinics with Approval No. CEZ-PI-003 at the date 06-30-2021. All patients provided written informed consent for the use of CBD prescription and authorization to utilize encrypted data with regard to treatments outcomes for the purpose of scientific publications.
Conflict of Interest Statement
J.F.G.-F.: full-time employee for Zerenia Clinic in Bogotá (Colombia). Zerenia is a center focused in cannabis-based medical formulations (CBMFs) treatments owned by Khiron Life Science Corp®. Khiron Life Science Corp® is responsible for the manufacturing processes of oil extracts cannabis-based magistral formulation used in this study. H.F.G.-B. and C.A.F.-P.: the authors declare no conflict of interest. C.E.N.: employee for Zerenia Clinic in Bogotá (Colombia). Zerenia is a center which specializes in medical cannabis treatments owned by Khiron Life Science Corp®. Khiron Life Science Corp® is responsible for the manufacturing processes of oil extracts cannabis-based magistral formulation used in this study. G.M.-S.: full-time employee. Scientific director for Khiron Life Science Corp® is responsible for the manufacturing processes of oil extracts cannabis-based magistral formulation used in this study.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
J.F.G.F.: conceptualization, review of the literature, writing and editing the original draft manuscript, approved the final draft manuscript, and funding acquisition. H.F.G.B.: review of the literature, formal analysis, methodology, and writing, review, and editing the manuscript. C.E.N.: review of the literature and writing, review, and editing the manuscript. C.A.F.P.: data curation and validation. G.M.S.: proofread of the manuscript, funding acquisition, and project administration.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Soehner AM, Harvey AG. Prevalence and functional consequences of severe insomnia symptoms in mood and anxiety disorders: results from a nationally representative sample. Sleep. 2012;35(10):1367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bei B, Asarnow LD, Krystal A, Edinger JD, Buysse DJ, Manber R. Treating insomnia in depression: insomnia related factors predict long-term depression trajectories. J Consult Clin Psychol. 2018;86(3):282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700–8. [DOI] [PubMed] [Google Scholar]
- 4. Cervena K, Matousek M, Prasko J, Brunovsky M, Paskova B. Sleep disturbances in patients treated for panic disorder. Sleep Med. 2005;6(2):149–53. [DOI] [PubMed] [Google Scholar]
- 5. Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry. 2007;68(2):254–60. [DOI] [PubMed] [Google Scholar]
- 6. Sun X, Liu B, Liu S, Wu DJH, Wang J, Qian Y, et al. Sleep disturbance and psychiatric disorders: a bidirectional mendelian randomisation study. Epidemiol Psychiatr Sci. 2022;31:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Pharmacological treatment of mental disorders in primary health care. World Health Organization; 2009; p. 1–82. [PubMed] [Google Scholar]
- 8. Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moreno-Sanz G, Madiedo A, Hernandez P, Kratz J, Aizpurua-Olaizola O, Brown MRD, et al. Sex-dependent prescription patterns and clinical outcomes associated with the use of two oral cannabis formulations in the multimodal management of chronic pain patients in Colombia. Front Pain Res. 2022;3:854795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navarro CE. Sistema endocannabinoide y el rol del cannabis medicinal en el tratamiento de la espasticidad: una revisión narrativa. Iatreia. 2022;37(1). [Google Scholar]
- 11. Navarro CE. Cannabis-based magistral formulation is highly effective as an adjuvant treatment in drug-resistant focal epilepsy in adult patients: an open-label prospective cohort study. Neurol Sci. 2023;44(1):297–304. [DOI] [PubMed] [Google Scholar]
- 12. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. García-Gutiérrez MS, Navarrete F, Gasparyan A, Austrich-Olivares A, Sala F, Manzanares J. Cannabidiol: a potential new alternative for the treatment of anxiety, depression, and psychotic disorders. Biomolecules. 2020;10(11):1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stollenwerk TM, Pollock S, Hillard CJ. Contribution of the adenosine 2A receptor to behavioral effects of tetrahydrocannabinol, cannabidiol and PECS-101. Molecules. 2021;26(17):5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bergamaschi MM, Queiroz RHC, Chagas MHN, de Oliveira DCG, De Martinis BS, Kapczinski F, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36(6):1219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zuardi AW, Rodrigues NP, Silva AL, Bernardo SA, Hallak JEC, Guimarães FS, et al. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol. 2017;8:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Linares IM, Zuardi AW, Pereira LC, Queiroz RH, Mechoulam R, Guimarães FS, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz J Psychiatry. 2019;41(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bloomfield MAP, Yamamori Y, Hindocha C, Jones APM, Yim JLL, Walker HR, et al. The acute effects of cannabidiol on emotional processing and anxiety: a neurocognitive imaging study. Psychopharmacol Berl. 2022;239(5):1539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shannon S, Lewis N, Lee H, Hughes S. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23(1):18-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berger M, Li E, Rice S, Davey CG, Ratheesh A, Adams S, et al. Cannabidiol for treatment-resistant anxiety disorders in young people: an open-label trial. J Clin Psychiatry. 2022;83(5):21m14130. [DOI] [PubMed] [Google Scholar]
- 23. Rapin L, Gamaoun R, El Hage C, Arboleda MF, Prosk E. Cannabidiol use and effectiveness: real-world evidence from a Canadian medical cannabis clinic. J Cannabis Res. 2021;3(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sachedina F, Chan C, Damji RS, de Sanctis OJ. Medical cannabis use in Canada and its impact on anxiety and depression: a retrospective study. Psychiatry Res. 2022;313:114573. [DOI] [PubMed] [Google Scholar]
- 25. Cassiani-Miranda CA, Scoppetta O, Cabanzo-Arenas DF. Validity of the hospital anxiety and depression scale (HADS) in primary care patients in Colombia. Gen Hosp Psychiatry. 2022;74:102–9. [DOI] [PubMed] [Google Scholar]
- 26. Rico J, Restrepo M, Molina M. Adaptación y validación de la escala hospitalaria de ansiedad y depresión (HAD) en una muestra de pacientes con cáncer del Instituto Nacional de Cancerología de Colombia. Avances en medición. 2005;3(1):73–86. [Google Scholar]
- 27. Gonçalves MT, Malafaia S, Moutinho Dos Santos J, Roth T, Marques DR. Epworth sleepiness scale: a meta-analytic study on the internal consistency. Sleep Med. 2023;109:261–9. [DOI] [PubMed] [Google Scholar]
- 28. Walker NA, Sunderram J, Zhang P, Lu SE, Scharf MT. Clinical utility of the Epworth sleepiness scale. Sleep Breath. 2020;24(4):1759–65. [DOI] [PubMed] [Google Scholar]
- 29. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry. 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 30. Oberbarnscheidt T, Miller NS. The impact of cannabidiol on psychiatric and medical conditions. J Clin Med Res. 2020;12(7):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kudrich C, Hurd YL, Salsitz E, Wang AL. Adjunctive management of opioid withdrawal with the nonopioid medication cannabidiol. Cannabis Cannabinoid Res. 2022;7(5):569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rhee TG, Mohamed S, Rosenheck RA. Antipsychotic prescriptions among adults with major depressive disorder in office-based outpatient settings: national trends from 2006 to 2015. J Clin Psychiatry. 2018;79(2):17m11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dorooshi G, Kermani R, Sabzghabaee A, Mansourian M, Eizadi-Mood N. Comparison of clinical manifestations of patients poisoned with tricyclic antidepressants alone or with benzodiazepine intoxication according to the dose of benzodiazepines. J Res Pharm Pract. 2022;11(2):59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crippa JAS, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FLS, Martin-Santos R, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25(1):121–30. [DOI] [PubMed] [Google Scholar]
- 35. Peng J, Fan M, An C, Ni F, Huang W, Luo J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin Pharmacol Toxicol. 2022;130(4):439–56. [DOI] [PubMed] [Google Scholar]
- 36. Murillo-Rodriguez E, Blanco-Centurion C, Sanchez C, Piomelli D, Shiromani PJ. Anandamide enhances extracellular levels of adenosine and induces sleep: an in vivo microdialysis study. Sleep. 2003;26(8):943–7. [DOI] [PubMed] [Google Scholar]
- 37. Malaca S, Gottardi M, Pigliasco F, Barco S, Cafaro A, Amadori E, et al. UHPLC-MS/MS analysis of cannabidiol and its metabolites in serum of patients with resistant epilepsy treated with CBD formulations. Pharmaceuticals. 2021;14(7):630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCartney D, Kevin RC, Suraev AS, Sahinovic A, Doohan PT, Bedoya-Pérez MA, et al. How long does a single oral dose of cannabidiol persist in plasma? Findings from three clinical trials. Drug Test Anal. 2023;15(3):334–44. [DOI] [PubMed] [Google Scholar]
- 39.Cannabidiol for the treatment of anxiety disorders: an 8-week pilot study. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT03549819 (accessed July 24, 2023).
- 40. Anderson LL, Doohan PT, Oldfield L, Kevin RC, Arnold JC, Berger M, et al. Citalopram and cannabidiol: in vitro and in vivo evidence of pharmacokinetic interactions relevant to the treatment of anxiety disorders in young people. J Clin Psychopharmacol. 2021;41(5):525–33. [DOI] [PubMed] [Google Scholar]
- 41. Dahlgren MK, El-Abboud C, Lambros AM, Sagar KA, Smith RT, Gruber SA. A survey of medical cannabis use during perimenopause and postmenopause. Menopause. 2022;29(9):1028–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Babyn K, Ross S, Makowsky M, Kiang T, Yuksel N. Cannabis use for menopause in women aged 35 and over: a cross-sectional survey on usage patterns and perceptions in Alberta, Canada. BMJ Open. 2023;13(6):e069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mejia-Gomez J, Phung N, Philippopoulos E, Murphy KE, Wolfman W. The impact of cannabis use on vasomotor symptoms, mood, insomnia and sexuality in perimenopausal and postmenopausal women: a systematic review. Climacteric. 2021;24(6):572–6. [DOI] [PubMed] [Google Scholar]
- 44. Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32(4):350–7. [DOI] [PubMed] [Google Scholar]
- 45. Saberivand A, Karimi I, Becker LA, Moghaddam A, Azizi-Mahmoodjigh S, Yousefi M, et al. The effects of Cannabis sativa L. seed (hempseed) in the ovariectomized rat model of menopause. Methods Find Exp Clin Pharmacol. 2010;32(7):467–73. [DOI] [PubMed] [Google Scholar]
- 46. Li L, Xu L, Wu J, Dong L, Lv Y, Zheng Q. Quantitative analysis of placebo response and factors associated with menopausal hot flashes. Menopause. 2017;24(8):932–7. [DOI] [PubMed] [Google Scholar]
- 47. Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3(70):70ra14. [DOI] [PubMed] [Google Scholar]
- 48. Schenk LA, Sprenger C, Geuter S, Büchel C. Expectation requires treatment to boost pain relief: an fMRI study. Pain. 2014;155(1):150–7. [DOI] [PubMed] [Google Scholar]
- 49. Kube T, Rief W. Are placebo and drug-specific effects additive? Questioning basic assumptions of double-blinded randomized clinical trials and presenting novel study designs. Drug Discov Today. 2017;22(4):729–35. [DOI] [PubMed] [Google Scholar]
- 50. Colloca L. The placebo effect in pain therapies. Annu Rev Pharmacol Toxicol. 2019;59:191–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chiffi D, Pietarinen AV, Grecucci A. Meaning and affect in the placebo effect. J Med Philos. 2021;46(3):313–29. [DOI] [PubMed] [Google Scholar]
- 52. National Academies of Sciences, Engineering, and Medicine . The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington (DC): National Academies Press; 2017. Vol. 15; p. 88–92. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.