Abstract
Introduction
The pathophysiology of idiopathic intracranial hypertension (IIH) is not fully characterized, and less is known about its development in transgender patients. Several cases of IIH in transgender patients have been reported, but fewer cases have been published that identify a cerebrospinal fluid (CSF) leak as a complication of IIH in this population. These patients can serve as an important study population, as an association between exogenous testosterone use in karyotypical females and development of IIH may support a hormonally mediated mechanism of development of this disease.
Case Presentation
We describe the case of a 33-year-old obese (BMI: 30.58 kg/m2) female-to-male transgender patient on exogenous testosterone for 15 years who presented with 1 month of acute or chronic headache with profuse rhinorrhea. Fundoscopic exam revealed disk pallor and edema consistent with a Frisen grade 3 papilledema. Nasal secretion was positive for beta-2 transferrin, consistent with CSF. Computed tomography head demonstrated a 5-mm defect in the medial left middle cranial fossa, bilateral optic nerve prominence and tortuosity, and abnormal arachnoid granulations concerning for IIH. After a successful endoscopic endonasal repair of the left lateral sphenoid recess leak, our patient continued to report headaches, was started on acetazolamide, and noted improvement in symptoms.
Conclusion
The case described herein further supports the growing body of evidence that implicates a hormonal mechanism of action in the development of IIH. Importantly, it also addresses the need for increased study and conversation about rare neurologic diseases in transgender patients.
Keywords: Idiopathic intracranial hypertension, Cerebrospinal fluid, Cerebrospinal fluid leak, Female-to-male, Male-to-female, Hormone replacement therapy, Testosterone, Arachnoid granulations, Papilledema
Introduction
Idiopathic intracranial hypertension (IIH), also known as pseudotumor cerebri, is a disorder characterized by signs and symptoms of increased intracranial pressure without an isolated cause [1]. IIH is a clinical diagnosis with support drawn from patient history, semiology of headache, physical exam, fundoscopic exam, lumbar puncture, and neuroimaging findings. The most common presenting features of increased intracranial pressure are headache, vision changes, and pulsatile tinnitus. As in other causes of increased intracranial pressure (neoplasm, cerebral venous sinus thrombosis, intracranial hemorrhage, etc.), the headache semiology in IIH is positionally worse while supine and upon waking. Imaging and laboratory evaluation are helpful in the diagnosis of IIH, but no single finding is sensitive to rule out IIH in its absence. However, a good fundoscopic exam in these patients is critical to establishing a diagnosis, as it is exceedingly rare for patients to develop IIH without papilledema [2].
Although the symptoms of IIH are often similar to those seen in secondary causes of increased intracranial pressure, the pathophysiologic mechanism underlying its development is not fully understood. Even less is known about the development of IIH in transgender patients on exogenous testosterone. These patients can serve as an important study population, as an association between exogenous testosterone use in karyotypical females and development of IIH may support a hormonally mediated mechanism of development of this disease. Furthermore, cerebrospinal fluid (CSF) leak is a known complication of IIH, but has only been rarely described in the literature among transgender patients. As healthcare access for transgender patients continues to improve, so must physician recognition of important medical complications among this population. The case described herein addresses the need for increased study and conversation about the development of IIH and subsequent CSF leak in a vulnerable segment of our population.
Case Presentation
A 33-year-old obese (BMI: 30.58 kg/m2) female-to-male (FTM) transgender patient on exogenous testosterone 200 mg/mL every 2 weeks since 2009 presented with 1 month of acute or chronic headache with rhinorrhea. Our patient described chronic paroxysmal headaches with a pounding character, occasionally retro-orbital, worse on the left, worse in the morning, worse positionally while supine, and associated with photophobia and dizziness. Approximately 1 month prior to assessment, our patient developed sudden onset, 10/10 headache in the left temporal region with clear and profound rhinorrhea. The acute onset headache was different in character and more severe than our patient’s typical headache. Associated symptoms included new-onset blurry vision on the left, lightheadedness, subjective gait abnormality, nausea, and left ear fullness. He denied hearing whooshing sounds, heartbeat, or ringing in the ear. The pain, when severe, occurred throughout the entire day and frequently prevented him from working and sleeping. There was development of rhinorrhea onset at the same time as the acute headache. When leaning forward or in the lateral decubitus position, the rhinorrhea was present at the nares and would drench the pillow at night. When supine, the rhinorrhea would trickle down the back of the throat and cause gagging which would prevent restful sleep. Of note, our patient denied recent physical trauma, change in medication, recent nasal instrumentation, or usage of vitamin A containing supplements or skincare products.
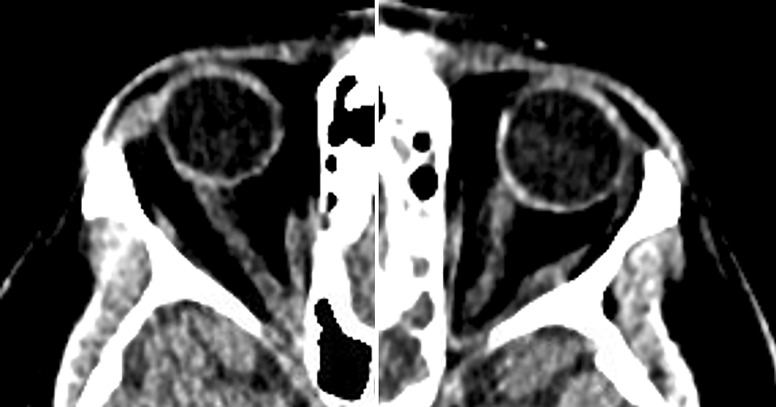
On physical exam, our patient was noted to have hyperemic and edematous nasal mucosa bilaterally, visual acuity of 20/25 bilaterally, and a fundoscopic examination notable for disk pallor and edema, consistent with a Frisen grade 3 papilledema. Nasal secretion was positive for beta-2 transferrin. His computed tomography (CT) head demonstrated a 5-mm defect in the medial left middle cranial fossa bordering the lateral recess of the left sphenoid sinus, a large quantity of material consistent with CSF and associated encephalocele in the left sphenoid sinus, bilateral optic nerve prominence and tortuosity, and abnormal arachnoid granulations (AGs) along the left middle cranial fossa floor (Fig. 1).
Fig. 1.
Pre-Op CT head – slight tortuosity of the optic nerves bilaterally and abnormal arachnoid granulations (AGs) along the left middle cranial fossa floor.
After an interdisciplinary discussion, otolaryngology and neurosurgery scheduled him for an endoscopic endonasal repair of the left lateral sphenoid recess leak and resection of the encephalocele with intraoperative lumbar puncture of fluorescein dye for visualization. An opening pressure of 5 cm of water was observed during intraoperative lumbar puncture in the right lateral decubitus position. The 5-mm defect was noted just lateral to the foramen rotundum in the sphenoid bone with associated encephalocele. The encephalocele was separated from the underlying brain and surrounding mucosa, which was disconnected at the margins of the bony defect and corrected. The procedure was tolerated well without complication, and follow-up head CT was only notable for postoperative changes.
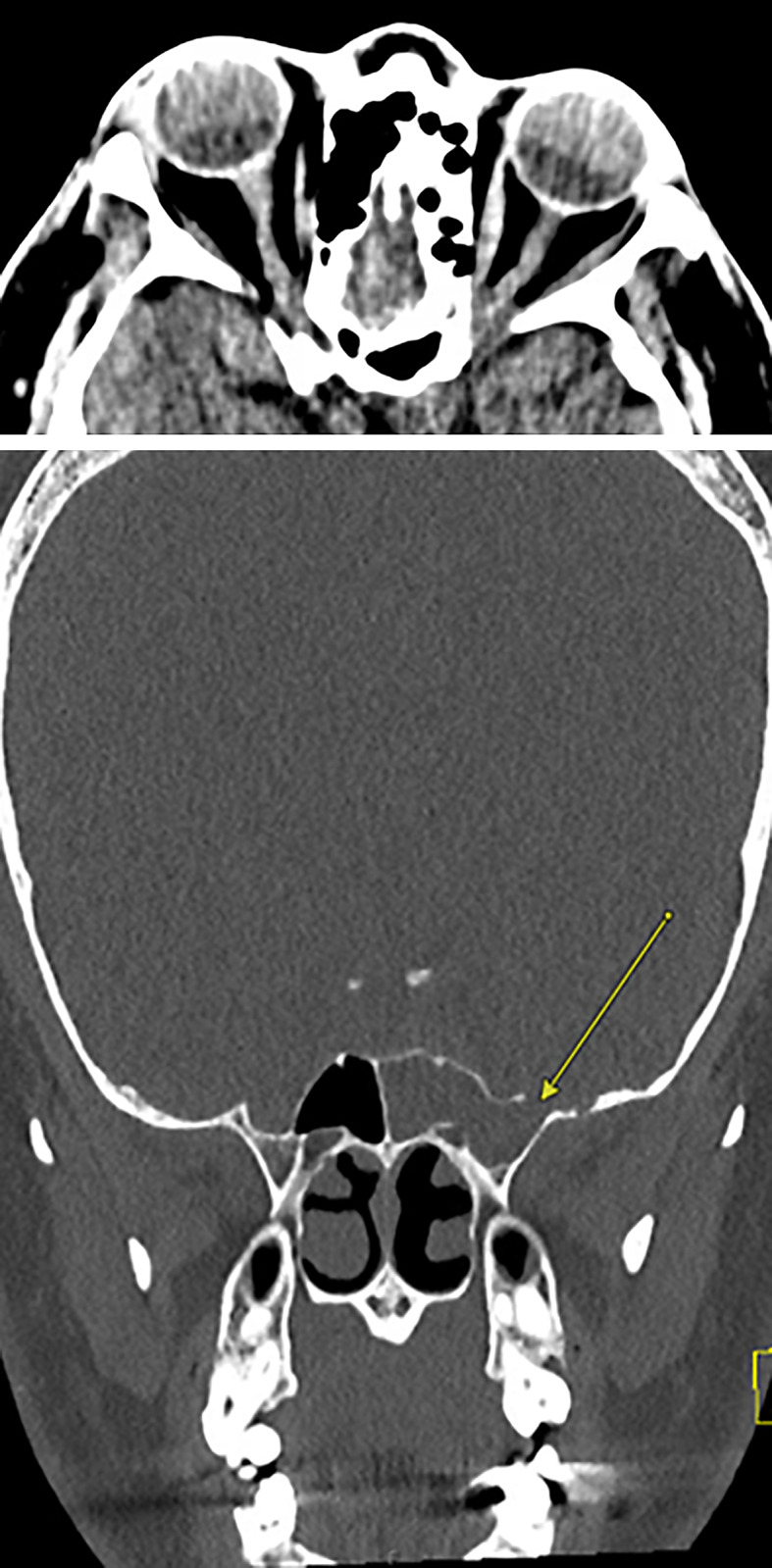
On subsequent 3-month follow-up visit, our patient reported resolution in his acute headaches and rhinorrhea but complained of reemergence of his chronic, intermittent left temporo-occipital and retro-orbital headache. Serum-free and total testosterone levels at 3-month follow-up visit were elevated to 296.0 ng/dL (ref. range 8.4–48.1 ng/dL) and 75.49 ng/dL (ref. range 0.77–9.30 ng/dL), and sex hormone binding globulin was low at 16.7 nmol/L (ref. range 24.6–122 nmol/L). After starting acetazolamide, our patient noted improvement of headache on follow-up. However, after discontinuing acetazolamide 7 months later, patient noted worsening symptoms and a repeat CT head demonstrated bilateral optic nerve prominence and tortuosity, increased conspicuity from prior imaging, and increased AGs in the right middle cranial fossa floor (Fig. 2).
Fig. 2.
Nine months post-Op CT head – bilateral optic nerve prominence and tortuosity, increased conspicuity from prior imaging, and increased arachnoid granulations (AGs) in the right middle cranial fossa floor.
Conclusion
The pathophysiology of IIH is not fully understood, and less is known about its development in FTM transgender patients. Given its nearly exclusive development in karyotypical females and close association with obesity and polycystic ovarian syndrome, a hormonally mediated mechanism of development in IIH has been suggested in the literature [3]. Independent of polycystic ovarian syndrome or BMI, hyperandrogenism in karyotypical females is associated with an earlier age of onset of IIH [4]. The association was made clear by O’Reilly et al. [5], who noted that karyotypical females with IIH have a particular pattern of androgen excess that targets the androgen-activating enzymes in the choroid plexus to stimulate production of CSF and lead to increased intracranial pressure.
FTM transgender patients can serve as an important study population, as an association between exogenous testosterone use in karyotypical females and development of IIH may support a hormonally mediated mechanism of development of this disease. Upon review of the literature, we found 14 cases that describe the development of IIH in FTM transgender patients [6–15]. Notably, each of these patients was on exogenous testosterone therapy prior to symptom onset, but differed in the overall length of testosterone therapy, ranging from 5 months to 6 years (Table 1). There have been comparatively few case reports that describe the development of IIH in male-to-female transgender patients on hormone replacement therapy [12, 13]. This disparity may indicate that karyotypical females are at an increased risk of developing IIH, despite gender identity, but that the mechanism is not mediated solely by estrogen or progesterone.
Table 1.
Pertinent characteristics of cases of transgender patients diagnosed with IIH
| Author, citation, year | BMI | Gender identity | Length of testosterone therapy prior to onset | Imaging findings | Treatment | Outcomes |
|---|---|---|---|---|---|---|
| Buchanan and Bedolla [6], 2017 | – | FTM | On testosterone but unclear length of therapy | Magnetic resonance imaging (MRI) brain was normal | LP and acetazolamide | Resolution of symptoms |
| Hornby et al. [7], 2017 | 27.9 | FTM | 5 months | Partially empty sella on MRI, normal magnetic resonance venogram (MRV) | Lumbo-peritoneal shunt | Resolution of symptoms |
| Kogachi et al. [8], 2019 | – | FTM | 50 weeks | MRV with mild narrowing of the left transverse sinus and partially occluded right transverse sinus | Endovascular stenting of left transverse | Resolution of symptoms |
| Mowl et al. [9], 2009 | 27.05 | FTM | On testosterone but unclear length of therapy | MRI with small Chiari I malformation | Reduce testosterone by 50 percent and start acetazolamide | Was able to titrate testosterone back to therapeutic goals |
| Park et al. [10], 2014 | Normal | FTM | On testosterone but unclear length of therapy | MRV with no evidence of CVST | Acetazolamide | Resolution of symptoms |
| Sheets et al. [11], 2007 | 29.8 | FTM | On testosterone but unclear length of therapy; discontinued 18 months prior to onset | CT angiogram with attention to venous phase was normal | Acetazolamide followed by optic nerve fenestration of the right eye | No improvement with acetazolamide but resolution of symptoms with surgery |
| Weinlander et al. [12], 2019 | 30.13 | FTM | On testosterone but unclear length of therapy; discontinued 1 year prior to onset | MRI and MRV were normal | Resumed testosterone therapy | Resolution of symptoms |
| Nguyen et al. [13], 2021 | 29.1 | FTM | 14 months | Partially empty sella, flattening of the posterior globes, enlarged optic nerve sheaths, distal tapering of the transverse sinuses | Acetazolamide | Resolution of symptoms |
| Nguyen et al. [13], 2021 | 35.0 | FTM | 2 years | MRI was normal | Acetazolamide without improvement; topiramate with improvement | Resolution of symptoms |
| Nguyen et al. [13], 2021 | 31.9 | FTM | 2 years | No MRI was done | Topiramate | Resolution of symptoms |
| Nguyen et al. [13], 2021 | 36.1 | FTM | 15 months | MRI with partially empty sella, enlarged optic nerve sheaths, distal tapering of the transverse sinuses | Lost to follow-up | Unknown |
| Nguyen et al. [13], 2021 | 44.0 | FTM | 15 months | MRI with partially empty sella | Acetazolamide increased to 500 twice daily, nortriptyline | Resolution of symptoms |
| Nayman et al. [14], 2021 | 25.8 | FTM | 4 years | CT head and CT angiography were normal | Acetazolamide and reduction in testosterone dose | Resolution of symptoms |
| Lin et al. [15], 2020 | – | FTM | 6 years | CT head with defect in the middle fossa lateral to the right foramen rotundum in a hyperpneumatized sphenoid sinus with extensive mottling of the skull base bilaterally including ovoid bony defects from arachnoid pits and aberrant granulations | Endonasal surgery by ENT and neurosurgery for CSF leak, lumbar drain, acetazolamide | CSF leak requiring repair |
In the present case, serum-free and total testosterone levels were elevated above normal limits at 3-month follow-up, which strengthens the case that the development of IIH in our patient may have been causally related to androgen excess. In addition, it adds to a growing body of evidence indicating that circulating androgens play an important role in the development of IIH in FTM patients on hormone replacement therapy, although the relationship has not been established in randomized controlled trials. Further studies are required to identify a causal relationship between testosterone excess and development of IIH in FTM transgender patients. Furthermore, the only other case report of a CSF leak in an FTM transgender patient with IIH was described in 2019 by Lin et al. [15]. The description of the case presented by Lin et al. [15] bears a number of similarities to our case, including a lengthy exogenous testosterone course, aberrant AG on imaging, and beta-2 transferrin-positive nasal secretions. That only 2 case reports of a CSF leak in an FTM transgender patient have been documented in the literature underscores the necessity for continued surveillance in this population and increased study about healthcare access and disparity in transgender patients. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000540259).
Statement of Ethics
Ethical approval is not required for this study in accordance with local or national guidelines. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received for this case report and publication.
Author Contributions
Isaac Smith acquired and analyzed the data and designed the conception of this work. Raissa Aoun reviewed this work and edited the intellectual content. Rebecca Lalchan provided final approval of this work.
Funding Statement
No funding was received for this case report and publication.
Data Availability Statement
All data generated or analyzed during this study are included in this article (and its supplementary material files). Further inquiries can be directed to the corresponding author.
Supplementary Material.
References
- 1. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mathew NT, Ravishankar K, Sanin LC. Coexistence of migraine and idiopathic intracranial hypertension without papilledema. Neurology. 1996;46(5):1226–30. [DOI] [PubMed] [Google Scholar]
- 3. Avisar I, Gaton DD, Dania H, Stiebel-Kalish H. The prevalence of polycystic ovary syndrome in women with idiopathic intracranial hypertension. Scientifica. 2012;2012:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klein A, Stern N, Osher E, Kliper E, Kesler A. Hyperandrogenism is associated with earlier age of onset of idiopathic intracranial hypertension in women. Curr Eye Res. 2013;38(9):972–6. [DOI] [PubMed] [Google Scholar]
- 5. O’Reilly MW, Westgate CS, Hornby C, Botfield H, Taylor AE, Markey K, et al. A unique androgen excess signature in idiopathic intracranial hypertension is linked to cerebrospinal fluid dynamics. JCI Insight. 2019;4(6):e125348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchanan I, Bedolla J. Idiopathic intracranial hypertension in a transgender male on hormone therapy. Arch Emerg Med Crit Care. 2017. [Google Scholar]
- 7. Hornby C, Mollan SP, Mitchell J, Markey KA, Yangou A, Wright BLC, et al. What do transgender patients teach us about idiopathic intracranial hypertension? Neuroophthalmology. 2017;41(6):326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kogachi K, Konstas A, Karanjia R, Sadun AA. Endovascular stenting in a transgender patient with idiopathic intracranial hypertension. J Neuro Ophthalmol. 2019;39(2):247–8. [DOI] [PubMed] [Google Scholar]
- 9. Mowl AD, Grogg JA, Klein J. Secondary pseudotumour cerebri in a patient undergoing sexual reassignment therapy. Clin Exp Optom. 2009;92(5):449–53. [DOI] [PubMed] [Google Scholar]
- 10. Park S, Cheng CP, Lim LT, Gerber D. Secondary intracranial hypertension from testosterone therapy in a transgender patient. Semin Ophthalmol. 2014;29(3):156–8. [DOI] [PubMed] [Google Scholar]
- 11. Sheets C, Peden M, Guy J. Idiopathic intracranial hypertension in a transgender man. J Neuro Ophthalmol. 2007;27(4):313–5. [DOI] [PubMed] [Google Scholar]
- 12. Weinlander E, Derani T, Cornblath WT, De Lott LB. Intracranial hypertension in transgender patients. J Neuro Ophthalmol. 2019;39(2):232–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nguyen HV, Gilbert AL, Fortin E, Vodopivec I, Torun N, Chwalisz BK, et al. Elevated intracranial pressure associated with exogenous hormonal therapy used for gender affirmation. J Neuro Ophthalmol. 2021;41(2):217–23. [DOI] [PubMed] [Google Scholar]
- 14. Nayman T, Hébert M, Ospina LH. Idiopathic intracranial hypertension in a pediatric transgender patient. Am J Ophthalmol Case Rep. 2021;24:101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin GC, Paz M, Porrmann JW, Scharf R, Benitez RP, Moshel YA. Spontaneous cerebrospinal fluid leak in a transgender man: is testosterone therapy a risk factor? JAMA Otolaryngol Head Neck Surg. 2020;146(10):973–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article (and its supplementary material files). Further inquiries can be directed to the corresponding author.