Abstract
Introduction
Approximately 4% of the UK population experiences PTSD. Individuals must exhibit symptoms across four clusters to receive a diagnosis: intrusion, avoidance, altered reactivity, and altered mood. Evidence suggests that cannabinoid agonists such as nabilone and tetrahydrocannabinol (THC) may alleviate PTSD symptoms. We investigated the safety and effectiveness of THC-predominant cannabis flowers for inhalation to manage PTSD symptoms in a real-world setting.
Methods
We analysed data from the UK patient registry, T21. Validated questionnaires were used to collect PROMs for health-related quality of life (HRQoL), mood/anxiety, sleep, and PTSD-specific symptoms. Inclusion criteria were (i) a confirmed diagnosis of PTSD, (ii) completed PROMs questionnaires at baseline and at the 3-month follow-up, and (iii) received a prescription for a chemotype 1 (THC-predominant) cannabis flower.
Results
Fifty-eight patients were included, 34 of which also had PROMs recorded at 6 months. Most were males (65.5%) with an average age of 39.2 years who had previously used cannabis illicitly (95.6%). At 3 months, participants reported significant improvements in overall health, mood, and sleep quality (p < 0.001) but not in the proxy for HRQoL (p = 0.052). Similarly, participants reported substantial benefits in managing intrusion symptoms (p < 0.001), mood alterations (p < 0.001), and reactivity alterations (p = 0.002), which were sustained or further improved at 6 months. Participants did not report any side effects associated with CBMPs.
Conclusions
Inhalation of THC is well tolerated and useful for managing symptoms of PTSD in cannabis-experienced individuals. However, further research is needed to evaluate the long-term safety and outcomes of controlled inhalation of CBMP in patients naïve to cannabis.
Keywords: Post-traumatic stress disorder, Tetrahydrocannabinol, Cannabis flowers, Sleep, Real-world evidence
Introduction
Post-traumatic stress disorder (PTSD) is a persistent condition triggered by exposure to emotionally traumatic events, encompassing natural disasters, wartime experiences, or instances of sexual violence [1]. The global lifetime prevalence of PTSD is estimated at 3.9%, according to WHO World Mental Surveys, and notably peaks among veterans, reaching up to 20% in certain countries [2]. The economic burden of this disorder is substantial, accounting for an annual productivity loss of USD 3 billion in the USA alone [3]. The hallmarks of PTSD reside in four cognitive symptom clusters: (i) intrusive memories, flashbacks, and nightmares; (ii) avoidance of distressing triggers; (iii) intensified negative emotions encompassing guilt, anxiety, depression, impulsivity, and hyperarousal; and (iv) alterations in social behaviours across personal and interpersonal spheres [1]. Emerging preclinical evidence highlights the direct involvement of the endocannabinoid system in both the onset and severity of PTSD. Studies have firmly established that cannabinoid type-1 (CB1) receptors in the amygdala play a pivotal role in the hyper-consolidation of traumatic memories under stress-induced glucocorticoid influence [4]. Activation of these receptors by endogenous cannabinoids, namely, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), facilitates fear extinction in animal models [5]. Chronic stress has been correlated with decreased levels of anandamide and altered patterns of CB1 expression in the brain [6]. Furthermore, cannabinoids exhibit a dual effect by boosting activity in the ventromedial prefrontal cortex while suppressing neuronal activity in the amygdala – directly counteracting the deficits observed in PTSD [7]. Clinical studies corroborate these findings, demonstrating reduced circulating levels of endocannabinoids and heightened CB1 expression in key brain regions among individuals diagnosed with PTSD, including the amygdala, hippocampus, and the anterior cingulate cortex [8]. Notably, specific genetic variants leading to CB1 receptor overexpression have been linked to an increased susceptibility to PTSD and anxiety disorders in humans [9, 10].
Existing therapeutic approaches for PTSD primarily revolve around psychological therapies, including trauma-focused interventions and eye movement desensitization and reprocessing, alongside medication targeted at symptomatic relief. Nonetheless, these treatments exhibit a high dropout rate, with a significant portion of patients – ranging between 60 and 72 percent – reporting persistent symptoms that qualify as PTSD [11]. While selective serotonin reuptake inhibitors like sertraline and paroxetine hold approval for treating PTSD in the UK, their efficacy often yields suboptimal responses [12]. Consequently, polypharmacy and off-label use of medications are commonplace in managing PTSD, exemplified by the prevalent prescription rates of benzodiazepines, reaching up to 74% in some studies, despite contraindications and potential exacerbation of the condition [13]. The challenges in effectively treating PTSD underscore a critical unmet need for safer and more efficacious alternatives to address its symptoms and manage the disorder itself. Several small-scale clinical trials exploring pharmacological interventions involving oral CB1 agonists, such as Δ9-tetrahydrocannabinol (THC) or nabilone, in chronic PTSD patients have revealed promising outcomes. These interventions improved overall symptom severity and sleep quality, concomitant with reduced occurrences of nightmares and hyperarousal symptoms [14–16]. Real-world evidence also indicates heightened self-medication tendencies with cannabis among individuals grappling with PTSD [17]. Countries facilitating legal access to medicinal cannabis, such as Israel and Canada, promote its use for managing PTSD symptoms [18, 19]. However, comprehensive medical research assessing the efficacy of vaporizing cannabis flowers to alleviate PTSD symptoms typically encounters significant obstacles in recruiting participants and conducting adequately blinded randomized clinical trials [20, 21]. To help bridge this gap, our group and others are reporting real-world data associated with the clinical use of cannabis-based medicinal products (CBMPs) to manage PTSD symptoms, despite the limitations inherent to this type of clinical evidence such as the potential selection bias and the lack of a control group [22, 23]. The present investigation focuses on analysing clinical outcome measures reported by a cohort of civilian patients diagnosed with PTSD in the UK, specifically prescribed a chemotype 1 (THC-predominant) cannabis flos intended for inhalation.
Methods
Procedure
We examined patient-reported data collected prospectively between July 2020 and December 2022 to assess the clinical outcomes associated with the prescription of chemotype 1 (THC-predominant) cannabis flos in patients diagnosed with PTSD. Participants were enrolled in Project Twenty21 (T21), the UK’s first multi-centre registry for patients receiving treatment with CBMPs. Detailed information relating to T21 procedures is outlined elsewhere [24]. In brief, patients receiving CBPMs are entered into the registry and monitored for collection of patient-reported outcome measures (PROMs) as part of their standard of care. Participants provide consent to the collection of their medical history plus a series of symptomatic assessments based on validated self-report questionnaires. Participating physicians may prescribe from a product formulary that includes a wide range of cannabis oral extracts and flos of varying cannabidiol (CBD) and THC amounts, of which chemotype 1 flos remains the most prescribed CBMP. Patients were included in this analysis if they received a prescription for KHIRON HK 20/1, the most frequently prescribed chemotype 1 flos in T21, alone or in combination with other chemotype 1 flos. Additional inclusion criteria were that participants (i) had a confirmed diagnosis of PTSD and (ii) had completed the PTSD checklist for civilians (PCL-C) questionnaires both at the initial appointment (baseline) and, at least, at the subsequent 3-month follow-up. All clinics participating in T21 advise and warn patients that consuming illicit cannabis is forbidden and may result in suspension of services and patient discharge.
Drugs
KHIRON HK 20/1 is a chemotype 1 cannabis variety, which contains 20% (w/w) of Δ9-THC and less than 1% (w/w) of CBD in dried weight. This variety is also referred to by the breeder’s name, Hindu Kush, and is classified as an indica-type plant. indica/sativa terminology relates to structural and botanical features of the cannabis plant and, contrary to what commonly misconstrued, does not provide robust information on the chemical composition nor on the pharmacological characteristics of the flos. The batches of KHIRON HK 20/1 flos prescribed to T21 participants were produced in full compliance with good manufacturing practice requirements and to the standards established in the German monograph for cannabis flos, which specifies the content of THC and CBD as active pharmaceutical ingredients and limits the content of cannabinol (<1%) as a maker of degradation. Detailed information on the chemical composition of KHIRON HK 20/1 is provided as online supplementary information (for all online suppl. material, see https://doi.org/10.1159/000540978) (online suppl. Fig. 1). Additional chemotype 1 flos available to patients complied with the requirements for importation into the UK as final medicinal products or pharmaceutical intermediates for the manufacturing of bespoke medications. The UK’s Misuse of Drugs Regulations 2018 explicitly prohibits smoking cannabis and CBPMs. Therefore, an herbal vaporizer/inhaler is required for the therapeutic administration of cannabis flos. A dosing protocol was developed to guide T21 prescribers and cannabis-naïve participants through the process of personalizing cannabis administration based on the number, duration, and frequency of inhalations depending on the needs of each individual patient [25].
Patient-Reported Outcome Measures
PROMs questionnaires were completed by participants both at baseline (t = 0) and every 3 months at scheduled follow-ups. The following questionnaires were employed to capture outcome measures specific to PTSD but also related to general health, such as quality of life, mood/depression, and quality of sleep.
Measures of PTSD
Participants were asked to complete the PTSD checklist for civilians (PCL-C) [26]. The PCL-C is a 17-item self-report measure of the 17 DSM-IV symptoms of PTSD. The PCL-C has a variety of purposes, including screening individuals for PTSD, diagnosing PTSD, and/or monitoring symptom change during and after treatment. The PCL-C asks about symptoms in relation to “stressful experiences” and can be used with any population. The symptoms endorsed may not be specific to just one event, which can be helpful when assessing survivors who have symptoms due to multiple events. Furthermore, the 17 items within the PCL-C can be grouped into the four clusters of symptoms that characterize PTSD: items 1–5 refer to symptoms of intrusion, questions 6 and 7 capture symptoms of avoidance, questions 8–12 correspond to symptoms of altered mood, and finally questions 13–17 report symptoms of altered reactivity. Evidence suggests that a 5–10-point change represents reliable change (i.e., change not due to chance) and a 10–20-point change represents clinically significant change. Therefore, it is recommended to use 5 points as a threshold for determining whether an individual has responded to treatment and 10 points as a threshold for determining whether the improvement is clinically meaningful [26].
General Health Outcome Measures
Participants were asked to complete the EuroQol 5 Dimensions (EQ-5D-5L), a validated tool which is widely used to assess the quality of life of patients in many disease areas through evaluating the severity of each of the 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) [27]. Two measures of quality of life were considered: (i) the visual analogue scale (VAS) of general health (0–100) was interpreted as a patient-reported measure of general health and (ii) the sum of ratings for the five dimensions of the EuroQol (5–25) was interpreted as a patient-reported measure of HRQoL. Participants also completed the Patient Health Questionnaire (PHQ-9, scoring ranges 0–27), a reliable measure of depression severity composed of a 9-item, self-rated instrument previously validated in general populations, medical populations, and psychiatric samples [28]. Finally, sleep quality was assessed using four items adapted from the widely used Pittsburgh Sleep Quality Index (scoring ranges from 4 to 20) [29].
Data Analysis
Demographics are expressed either as a percentage or as the mean ± standard deviation. Results of PROMs analysis are represented in box and whisker graphs, which indicate upper and lower extreme values, median, upper quartile, and lower quartile. Post hoc analyses of outcome measures at three different time points were performed by Friedman repeated-measures analysis of variance (non-parametric). Pairwise comparisons (Durbin-Conover) were used to compare results from 3- and 6-month follow-ups to baseline. Post hoc analysis was considered statistically significant if p < 0.05.
Results
Participants
A total of 58 patients registered in T21 met the inclusion criteria, which required correct recording of PROMs questionnaires at the initial appointment (t = 0) and the 3-month follow-up (t = 3), along with receiving at least one prescription for KHIRON HK 20/1. Among them, 34 participants also reported PROMs at the 6-month follow-up (t = 6). Participants enrolled in T21 between August 2020 and April 2023. Demographics and clinical characteristics of the patient cohort are depicted in Table 1. Consistent with the overall patient population of T21, most participants were adult males (65.5%), with an average age of 39.2 ± 8.9 years old. Notably, only two participants were cannabis naïve before enrolling in T21, and a vast majority of patients (95.6%) had previously used cannabis illicitly. Among these, 3 out of 4 consumed cannabis to treat their PTSD symptoms (75.9%), and most participants reported administering CBPMs only once a day (65.5%).
Table 1.
Cohort demographics and cannabis consumption details
| Gender | Total | ||
|---|---|---|---|
| male | female | ||
| Participants | |||
| Sample, N (%) | 38 (65.5) | 20 (34.5) | 58 (100) |
| Age (mean±SD), years | 38.6 (8.4) | 40.2 (10.1) | 39.2 (8.9) |
| Previous experience with cannabis, N (%) | 37 (97.4) | 19 (95.0) | 56 (96.6) |
| Intention of treating their primary condition with cannabis, N (%) | 30 (79.9) | 14 (70.0) | 42 (75.9) |
| Frequency of use, N (%) | |||
| A few times a month | 1 (2.63) | 0 (0.00) | 1 (1.72) |
| A few times a week | 1 (2.63) | 1 (2.63) | 2 (3.45) |
| Once a day | 25 (65.8) | 13 (65.5) | 38 (65.5) |
| Multiple times a day | 4 (10.5) | 0 (0.00) | 4 (6.89) |
| Non-respondent | 7 (18.4) | 6 (30.0) | 13 (23.4) |
SD, standard deviation.
PTSD-Specific Outcome Measures
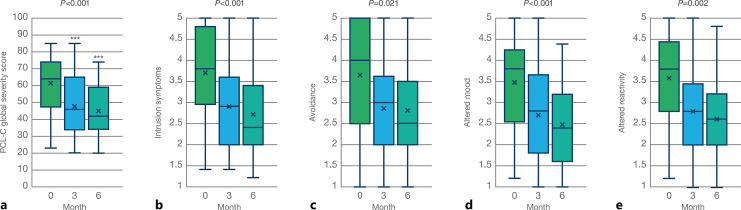
All participants satisfied the inclusion criterion of completing the Civilian version of the PTSD Checklist (PCL-C) before starting the treatment and at the 3-month follow-up. Significant changes were found in the global severity score of the PCL-C, as well as in the four different clusters of symptoms associated with PTSD, as represented in Figure 1. Figure 1a depicts a 13-point (21.1%) reduction in the PCL-C global symptom severity score after 3 months of treatment, from an average baseline value of 61.5 to a mean value of 48.5 (Fig. 1a; N = 58, T = 3.50; p < 0.001). This reduction became more robust after 6 months, with a 16.8-point decrease (27.3%) in the global PCL-C scoring to an average value of 44.7 (Fig. 1a; N = 34, T = 4.32; p < 0.001). When analysed by cluster of symptoms, treatment with chemotype 1 cannabis flowers also correlated with significant improvements: average scoring for questions 1–5 of the PCL-C, related to intrusive memories and re-experiencing the traumatic event, decreased from a baseline value of 3.7 to mean values of 2.9 and 2.7 at the 3- and 6-month follow-ups, respectively (Fig. 1b; χ2 = 14.2, p < 0.001). Figure 1c illustrates a modest but statistically significant reduction in symptoms of avoidance from an average baseline score of 3.6 to mean values of 2.85 and 2.80 at the 3- and 6-month follow-ups, respectively (χ2 = 7.76, p = 0.021). It is worth noticing that contrary to the rest of the clusters, which contain five questions each, the “avoidance” cluster includes only questions 6 and 7 of the PCL-C, which may account for the larger statistical deviation observed (Table 2). Symptoms of negative thoughts and emotional experiences showed a marked reduction, as indicated by the average score of questions 8–12 of the PCL-C (Fig. 1d; χ2 = 13.9, p < 0.001), from a baseline value of 3.48 to mean values of 2.72 and 2.48 at 3 and 6 months after starting the treatment with CBMP. Finally, controlled inhalation of chemotype-1 cannabis flowers correlated with a strong reduction in the average scoring for questions 13–17 of the PCL-C (Fig. 1e; χ2 = 12.7, p = 0.002), which refer to the increased state of physiological and psychological arousal often displayed by PTSD patients, from a baseline value of 3.58 to mean values of 2.77 and 2.62 at the 3- and 6-month follow-ups, respectively. Analysis of variance of average scoring for each individual question within the PCL-C at the beginning of treatment and subsequent 3- and 6-month follow-ups is represented in Table 2. Of note, the largest effect sizes were found for question 9: “Loss of interest in things that you used to enjoy?” (χ2 = 23.6, p < 0.001) and, particularly, question 13: “Trouble falling or staying asleep?” (χ2 = 27.0, p < 0.001). Additionally, questions 1, 3, and 10 also displayed larger statistical significance (χ2 > 15, p < 0.001) compared to the rest of PTSD-related symptoms in the PCL-C questionnaire. On the contrary, questions 8: “Trouble remembering important parts of a stressful experience from the past?” and 17: “Feeling jumpy or easily startled?” failed to display significant differences (p > 0.05).
Fig. 1.
PTSD-related outcome measures reported by participants at 3 months (N = 58) and 6 months (N = 34) after starting treatment with CBMP. a Global PTSD severity symptom score self-reported by patients measured as the sum of the 17 items of the PCL-C (17–85) (χ2 = 16.6, p < 0.001). b Symptoms of intrusion reported as the average of questions 1–5 of the PCL-C (χ2 = 14.2, p < 0.001). c Symptoms of avoidance reported as the average of questions 6 and 7 of the PCL-C (χ2 = 7.76, p = 0.021). d Negative alterations in cognition and mood reported as the average of questions 8–12 of the PCL-C (χ2 = 13.9, p < 0.001). e Negative alterations in reactivity/hyperarousal reported as the average of questions 13–17 of the PCL-C (χ2 = 12.7, p = 0.002).
Table 2.
ANOVA of the scoring for each individual symptom in the PCL-C questionnaire
| χ2 | p value | |
|---|---|---|
| Intrusion | ||
| 1. Repeated, disturbing memories, thoughts, or images of a stressful experience from the past? | 19.7 | <0.001 |
| 2. Repeated, disturbing dreams of a stressful experience from the past? | 11.9 | 0.003 |
| 3. Suddenly acting or feeling as if a stressful experience were happening again (as if you were reliving it)? | 16.0 | <0.001 |
| 4. Feeling very upset when something reminded you of a stressful experience from the past? | 11.1 | 0.004 |
| 5. Having physical reactions (e.g., heart pounding, trouble breathing, or sweating) when something reminded you of a stressful experience from the past? | 8.14 | 0.017 |
| Avoidance | ||
| 6. Avoid thinking about or talking about a stressful experience from the past or avoid having feelings related to it? | 8.59 | 0.014 |
| 7. Avoid activities or situations because they remind you of a stressful experience from the past? | 10.3 | 0.006 |
| Altered mood | ||
| 8. Trouble remembering important parts of a stressful experience from the past? | 1.43 | 0.49 |
| 9. Loss of interest in things that you used to enjoy? | 23.6 | <0.001 |
| 10. Feeling distant or cut off from other people? | 17.8 | <0.001 |
| 11. Feeling emotionally numb or being unable to have loving feelings for those close to you? | 10.7 | 0.005 |
| 12. Feeling as if your future will somehow be cut short? | 10.9 | 0.004 |
| Altered reactivity | ||
| 13. Trouble falling or staying asleep? | 27.0 | <0.001 |
| 14. Feeling irritable or having angry outbursts? | 10.9 | 0.004 |
| 15. Having difficulty concentrating? | 14.8 | <0.001 |
| 16. Being “super alert” or watchful on guard? | 8.22 | 0.016 |
| 17. Feeling jumpy or easily startled? | 3.38 | 0.185 |
ANOVA, analysis of variance.
General Health Outcome Measures
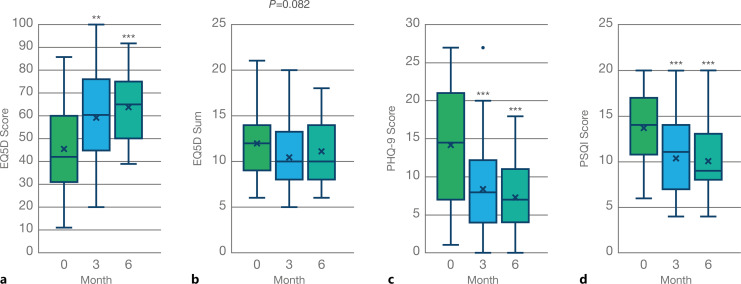
Inhalation of THC-predominant cannabis flowers was associated with a significant improvement in general health at the 3-month follow-up, as reflected in the change of scoring reported for the EQ-5D VAS (Fig. 2a, N = 58, T = 3.44, p = 0.001). This effect was further increased at the subsequent follow-up, 6 months after commencing CBMP treatment (Fig. 2a; N = 34, T = 4.40, p < 0.001). However, our findings did not show a statistically significant improvement in patient-reported HRQoL measured as the sum of ratings for the 5 dimensions of the EQ-5D either at the 3- or 6-month follow-up (Fig. 2b; T = 1.98, p = 0.052 and T = 1.98, p = 0.052, respectively). However, we found a significant improvement in general mood indicated by a reduction in the PHQ-9 questionnaire scoring (Fig. 2c; N = 58, T = 3.53, p < 0.001). This effect was maintained, although not further increased, at the 6-month follow-up (Fig. 2c; N = 34, T = 4.73; p < 0.001). Similarly, the inhalation of chemotype 1 cannabis flowers correlated with a patient-reported improvement in sleep quality after 3 months (Fig. 2d; N = 58, T = 4.34, p < 0.001), which was maintained but not further increased at the 6-month follow-up (Fig. 2d; N = 34; T = 4.77; p < 0.001).
Fig. 2.
General health outcome measures reported by participants at 3 months (N = 58) and 6 months (N = 34) after starting treatment with CBMP. a General health self-reported by patients measured as the average scoring of the visual analogue scale (0–100) of the EQ-5D questionnaire (χ2 = 16.8, p < 0.001). b Health-related quality of life reported as the sum of the five levels of the EQ-5D (χ2 = 5.0, p = 0.082). c Effect in general mood/anxiety captured with the PHQ-9 questionnaire (χ2 = 18.4, p < 0.001). d Effect in sleep quality reported by participants filling the PSQI (χ2 = 20.3, p < 0.001).
Adverse Side Effects
T21 participants were encouraged to report any adverse side effects they considered associated with CBPM treatment. Inhalation of THC-predominant flowers was generally found to be safe in this convenience cohort, as not a single adverse event (AE) was reported. However, it is plausible to assume that this lack of reporting may be largely because most patients in this cohort were already experienced cannabis consumers (96.5%) and likely failed to report any mild side effects that may have occurred. Notably, the 2 patients who were cannabis naïve at the beginning of the treatment, a male and a female, also did not report any AEs associated with the treatment with chemotype 1 cannabis flowers.
Discussion
PTSD ranks as the third most common mental health condition for which patients seek treatment with CBMPs in the UK, preceded only by generalized anxiety and ADHD. Our analysis of patient-reported clinical outcome measures revealed a temporal correlation between the initiation of medically supervised treatment with chemotype-1 cannabis dried flowers for inhalation and significant symptomatic improvement at 3- and 6-month follow-ups among individuals diagnosed with PTSD. The observed effect, marked by a 13-point reduction in the PCL-C global symptom severity score at 3 months and a 16.8-point reduction at 6 months, surpasses the 10-point threshold recognized for clinical meaningfulness. This finding aligns with prior research documenting reductions in both clinician-assessed and patient-reported PTSD symptoms with the use of cannabis and cannabinoids [17–20, 22, 23, 30–32]. Patients also reported significant enhancements in general health, mood, and sleep quality. Notably, we found no significant differences in health-related quality of life measured by the EQ-5D questionnaire, perhaps due to PTSD symptoms not being perceived as disabling within this scale [33]. However, improvements in general health, reported on a Likert scale (0–100), were marked at 3 months and further increased at 6 months. Additionally, scores on the PHQ-9 questionnaire showed substantial improvement, indicating a general psychological enhancement associated with treatment. Similar results were recently reported by our group in a larger cohort of T21 participants, where patients diagnosed with anxiety disorders reported an improvement in general mood significantly larger than participants diagnosed with chronic painful conditions [25]. Quality of sleep, assessed by the PSQI, showed robust improvements at both 3 and 6 months. This is consistent with the results obtained from the PCL-C questionnaire, where improvement in sleep was the most prominent effect among the 17 PTSD symptoms assessed. This effect on sleep quality adds to the existing evidence indicating a beneficial association between THC and sleep quality, insomnia, and the occurrence of nightmares in patients suffering from PTSD [34–36]. This finding highlights the relevance that activation of central CB1 receptors may have in the clinical management of this patient population, considering that sleep quality has been identified both as a vulnerability factor for developing PTSD and a landmark symptom that impacts overall outcomes [37]. Another significant effect reported in the PCL-C by patients was related to what researchers have termed “the restored self” [38]. Treatment with THC-predominant cannabis flowers facilitates the individual’s ability to engage in previously enjoyed activities, to socialize and to make meaningful connections, which were, according to our results, the main two drivers of the patient-reported improvement in mood alterations secondary to PTSD. Current evidence from both healthy individuals and PTSD patients supports the ability of THC to suppress anxiety and aversive memories [16]. The role of central CB1 receptors as key regulators of positive affective memory consolidation and emotional memory biases in humans has been previously documented [39]. In comparison with placebo, pharmacological blockade of CB1 induced a negative bias on a memory recognition task without producing a change in subjective mood [40]. Therefore, when administered as an adjuvant, chemotype 1 cannabis flowers may act as a memory-influencing drug, thus improving the effectiveness of behavioural and psychological interventions in PTSD patients [41, 42]. However, human studies exploring the beneficial effects of cannabinoids on human psychophysiological extinction learning have attained conflicting results, thus indicating that this potential clinical application of cannabis requires further study [43].
This report offers two key contributions to the existing literature. First, we established selection criteria to limit the intrinsic polypharmacology of cannabis flower, which often leads to complex interactions due to the presence of multiple active compounds [44]. Unlike other similar observational studies, all participants in our study received the same cannabis variety, KHIRON HK 20/1, either alone or in combination with other chemotype 1 flowers. Hindu Kush, a well-established cannabis strain with a stable genetic background and a well-characterized chemical and pharmacological profile, was chosen for this study [25]. Its availability from multiple manufacturers and its potential suitability for inclusion in national public healthcare access programs targeting a broader population make it an interesting candidate for further research. Second, while most observational research on inhaled cannabis in PTSD patients focuses on military and veteran populations, our data derive from a civilian cohort. The unique psychosocial and warfare-related experiences of military personnel may result in different outcomes in symptom severity and violent behaviour when using cannabis compared to civilians with PTSD [45].
Despite these potentially valuable clinical insights, our investigation has several significant limitations, which warrant a careful interpretation of these findings. Firstly, the study population is composed almost entirely (96.6%) of individuals who had previously tried cannabis and had a positive response, potentially introducing selection bias favouring reporting of benefits over side effects, compared to the general population [46]. The pre-selection of participants as cannabis responders underscores the importance for clinicians of questioning about cannabis self-medication in the initial assessment of PTSD in new patients. It remains unclear how these results would apply to cannabis-naive patients as the two naive participants in our study, while presenting similar results to the overall cohort, are not fully representative. A slightly lower proportion of participants who were current or previous cannabis users at baseline (88.9%) was reported in a PTSD cohort within the UK Medical Cannabis Registry, a single-centre patient registry run by Sapphire Clinic [23]. Sub-group analysis revealed that cannabis responders continued to report improved outcomes compared to baseline (p < 0.050). This is in agreement with our previous observations suggesting that patients who are cannabis users still find additional benefits from CBMPs beyond illicitly obtained cannabis and that these effects persist despite potential pharmacological tolerance [25]. Second, a key limitation of this study is the absence of a placebo control group, a factor that is often incompatible with real-world settings. The only randomized controlled trial conducted to date on the efficacy of inhaling cannabis flowers in PTSD patients suggests the presence of a significant placebo effect [20]. However, in that trial, participants treated with chemotype 1 flowers demonstrated a substantially greater improvement in PTSD symptom severity, as measured by the CAPS-5 scale (t = −6.19; p < 0.0001), compared to those receiving chemotype 2 (t = −2.57; p < 0.0143) or chemotype 3 cannabis flowers (t = −2.47; p < 0.0181). These findings indirectly support the pharmacological potential of CB1 modulation, as described in this report, although well-controlled, adequately powered studies will be necessary to determine whether inhaled cannabis effectively alleviates PTSD symptoms. A third limitation is that the dosing regimen utilized in our study remains unknown; however, it is noteworthy that most patients reported using cannabis only once a day, most likely at night-time before going to bed. This practice aligns with a concept previously characterized for the acute physiological effect following the inhalation of THC-predominant cannabis flowers termed “the sight of relief” [38]. However, data from T21 and the UK medical cannabis registry suggest that PTSD patients present an average daily consumption ranging between 0.5 and 1 g of dried cannabis flower, containing 100 and 200 mg of THC, respectively [23, 47]. This is coherent with “ad libitum” doses self-administered by military veterans diagnosed with PTSD over the course of 3 weeks in the only randomized controlled trial conducted with cannabis flowers [20]. Previous pharmacokinetic studies have revealed the existence of a “ceiling effect” for the pulmonary absorption of THC, suggesting that plasmatic levels achieved by inhaling either 100 or 200 mg of THC in flower format are indeed identical [48]. Therefore, it is essential to educate both patients and prescribers on the optimal dosing size and regime, particularly for heavy consumers, and consider spacing doses and reducing daily amounts to mitigate potential risks of addiction and related mental health issues in an already sensitive population. Further, some studies have reported short-term reductions in PTSD symptoms with THC-predominant cannabis flowers but no sustained long-term benefit despite increasing quantities of cannabis being administered, suggesting the appearance of tolerance [19]. Our data collection spans only 6 months, which underscores the need for further investigation into the long-term efficacy and safety of CBMPs in managing PTSD. Finally, a recurrent limitation when working with data stemming from the T21 patient registry is the low rate of reporting associated with AEs. While other cohorts of patients treated with CBMPs typically present an incidence of mild AEs associated with the treatment ranging from 20% to 30% [23, 49, 50], T21 participants failed to report any AEs. We interpret this result to indicate that cannabis responders will likely be less affected by potential side effects associated with treatment using THC-predominant cannabis flowers than cannabis-naïve individuals. However, precaution is advised when starting treatment with cannabis flowers, and a conservative dosing titration regime has been suggested to minimize the incidence of AEs in naïve patients, which could potentially lead to treatment discontinuation [25].
Conclusion
Results from this observational study suggest an association between treatment with THC-predominant cannabis flowers and symptomatic improvement for up to 6 months in a cohort of UK civilians diagnosed with PTSD. The treatment was safe and well tolerated and characterized by marked effects on quality of sleep, general mood, and severity of PTSD-associated symptoms. Despite previous exposure to cannabis, participants continued to report benefits after initiating treatment with THC-predominant cannabis flowers. Yet our results need to be interpreted carefully owing to the intrinsic limitations of the study design and data collection disclosed in the manuscript. However, considering the challenges of conducting properly designed and controlled clinical trials using cannabis flowers for inhalation, this study could inform current clinical practice with CBMPs so that both prescribing psychiatrists and patients suffering from PTSD could benefit from additional therapeutical options to tackle this debilitating condition.
Acknowledgments
The contribution of Sofia Antonopoulou and Alkyoni Athanasiou-Fragkouli is gratefully acknowledged.
Statement of Ethics
The work was conducted in accordance with the Declaration of Helsinki. At the time of enrolment, all T21 participants give written informed consent for their data to be used for research purposes. Based on the Medical Research Council decision tools, Research Ethics Committee review and approval is not required.
Conflict of Interest Statement
G.M.-S. is an employee of Khiron Life Sciences Corp, a global cannabis company that commercializes CBPMs. A.M. and W.S. are employed by Zerenia Clinics, a subsidiary of Khiron Life Sciences. No other Khiron employee or executive was involved in the design of the work; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors declare no other conflict of interest.
Funding Sources
This study received no external funding.
Author Contributions
Conceptualization: W.S. and G.M.-S.; methodology, writing – original draft preparation, visualization, and supervision: G.M.-S.; data curation: A.M. and G.M.-S.; and writing – review and editing: W.S., A.M., and G.M.-S. All authors have read and agreed to the published version of the manuscript.
Funding Statement
This study received no external funding.
Data Availability Statement
The data presented in this work are available on request from the corresponding author. The data are not publicly available due to privacy and ethical reasons.
Supplementary Material.
References
- 1. Pai A, Suris AM, North CS. Posttraumatic stress disorder in the DSM-5: controversy, change, and conceptual considerations. Behav Sci. 2017;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hill ML, Loflin M, Nichter B, Norman SB, Pietrzak RH. Prevalence of cannabis use, disorder, and medical card possession in U.S. military veterans: results from the 2019-2020 National Health and Resilience in Veterans Study. Addict Behav. 2021;120:106963. [DOI] [PubMed] [Google Scholar]
- 3. Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(Suppl 5):14866. Available from: https://www.psychiatrist.com/jcp/posttraumatic-stress-disorder-burden-individual-society [PubMed] [Google Scholar]
- 4. Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, Mcgaugh JL, et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. [cited 2023 Dec 11]; Available from: www.pnas.orgcgidoi10.1073pnas.0900835106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013;18(7):813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203(2):264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hill MN, Campolongo P, Yehuda R, Patel S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2018;43(1):80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry. 2013;18(9):1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Korem N, Duek O, Xu K, Harpaz-Rotem I, Pietrzak RH. Cannabinoid receptor 1 rs1049353 variant, childhood abuse, and the heterogeneity of PTSD symptoms: results from the National Health and Resilience in Veterans Study. Chronic Stress. 2021;5:247054702110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al Jowf GI, Ahmed ZT, Reijnders RA, de Nijs L, Eijssen LMT. To predict, prevent, and manage post-traumatic stress disorder (PTSD): a review of pathophysiology, treatment, and biomarkers. Int J Mol Sci. 2023;24(6):5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656–7. [DOI] [PubMed] [Google Scholar]
- 12. Marazziti D, Carmassi C, Cappellato G, Chiarantini I, Massoni L, Mucci F, et al. Novel pharmacological targets of post-traumatic stress disorders. Life. 2023;13(8):1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ehret M. Treatment of posttraumatic stress disorder: focus on pharmacotherapy. Ment Health Clin. 2019;9(6):373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fraser GA. The use of a synthetic cannabinoid in the management of treatment‐resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15(1):84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cameron C, Watson D, Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder–related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J Clin Psychopharmacol. 2014;34(5):559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raymundi AM, Da Silva TR, Sohn JMB, Bertoglio LJ, Stern CA. Effects of ∆ 9-tetrahydrocannabinol on aversive memories and anxiety: a review from human studies. BMC Psychiatry. 2020;20(1):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cougle JR, Bonn-Miller MO, Vujanovic AA, Zvolensky MJ, Hawkins KA. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25(3):554–8. [DOI] [PubMed] [Google Scholar]
- 18. Bonn-Miller MO, Moos RH, Boden MT, Long WR, Kimerling R, Trafton JA. The impact of posttraumatic stress disorder on cannabis quit success. Am J Drug Alcohol Abuse. 2015;41(4):339–44. [DOI] [PubMed] [Google Scholar]
- 19. LaFrance EM, Glodosky NC, Bonn-Miller M, Cuttler C. Short and long-term effects of cannabis on symptoms of post-traumatic stress disorder. J Affect Disord. 2020;274:298–304. [DOI] [PubMed] [Google Scholar]
- 20. Bonn-Miller MO, Sisley S, Riggs P, Yazar-Klosinski B, Wang JB, Loflin MJE, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walsh Z, Mitchell I, Crosby K, St. Pierre M, DeClerck D, Ong K, et al. A small clinical trial of vaporized cannabis for PTSD: suggestive results and directions for future study. Trials. 2023;24(1):578–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petersen M, Koller K, Straley C, Reed E. Effect of cannabis use on PTSD treatment outcomes in veterans. Ment Health Clin. 2021;11(4):238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pillai M, Erridge S, Bapir L, Nicholas M, Dalavaye N, Holvey C, et al. Assessment of clinical outcomes in patients with post-traumatic stress disorder: analysis from the UK Medical Cannabis Registry. Expert Rev Neurother. 22(11–12):1009–18. [DOI] [PubMed] [Google Scholar]
- 24. Sakal C, Lynskey M, Schlag AK, Nutt DJ. Developing a real-world evidence base for prescribed cannabis in the United Kingdom: preliminary findings from Project Twenty21. Psychopharmacol. 2021;239(5):1147–55. [DOI] [PubMed] [Google Scholar]
- 25. Moreno-Sanz G, Madiedo A, Lynskey M, Brown MRD. “Flower power”: controlled inhalation of THC-predominant cannabis flos improves health-related quality of life and symptoms of chronic pain and anxiety in eligible UK patients. Biomed. 2022;10(10):2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conybeare D, Behar E, Solomon A, Newman MG, Borkovec TD. The PTSD Checklist-Civilian Version: reliability, validity, and factor structure in a nonclinical sample. J Clin Psychol. 2012;68(6):699–713. [DOI] [PubMed] [Google Scholar]
- 27. Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. [Internet]. 2018;27(1):7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rancans E, Trapencieris M, Ivanovs R, Vrublevska J. Validity of the PHQ-9 and PHQ-2 to screen for depression in nationwide primary care population in Latvia. Ann Gen Psychiatry. 2018;17(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 30. Bonn-Miller MO, Brunstetter M, Simonian A, Loflin MJ, Vandrey R, Babson KA, et al. The long-term, prospective, therapeutic impact of cannabis on post-traumatic stress disorder. Cannabis Cannabinoid Res. 2022;7(2):214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Metrik J, Stevens AK, Gunn RL, Borsari B, Jackson KM. Cannabis use and posttraumatic stress disorder: prospective evidence from a longitudinal study of veterans. Psychol Med. 2022;52(3):446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greer GR, Grob CS, Halberstadt AL. PTSD symptom reports of patients evaluated for the New Mexico Medical Cannabis Program. J Psychoactive Drugs. 2014;46(1):73–7. [DOI] [PubMed] [Google Scholar]
- 33. Le QA, Doctor JN, Zoellner LA, Feeny NC. Minimal clinically important differences for the EQ-5D and QWB-SA in post-traumatic stress disorder (PTSD): results from a doubly randomized preference trial (DRPT). Health Qual Life Outcomes. 2013;11(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, open-label, pilot study of add-on oral Δ9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin Drug Investig. 2014;34(8):587–91. [DOI] [PubMed] [Google Scholar]
- 35. Roepke S, Schoofs N, Priebe K, Wülfing F, Schmahl C, Röhle R, et al. Treating nightmares in posttraumatic stress disorder with dronabinol: study protocol of a multicenter randomized controlled study (THC PTSD-trial). BMC Psychiatry. 2023;23(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sznitman SR, Meiri D, Amit BH, Rosenberg D, Greene T. Posttraumatic stress disorder, sleep and medical cannabis treatment: a daily diary study. J Anxiety Disord. 2022;92:102632. [DOI] [PubMed] [Google Scholar]
- 37. Lancel M, van Marle HJF, Van Veen MM, van Schagen AM. Disturbed sleep in PTSD: thinking beyond nightmares. Front Psychiatry. 2021;12:767760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lavie-Ajayi M, Shvartzman P. Restored self: a phenomenological study of pain relief by cannabis. Pain Med. 2019;20(11):2086–93. [DOI] [PubMed] [Google Scholar]
- 39. Horder J, Cowen PJ, Di Simplicio M, Browning M, Harmer CJ. Acute administration of the cannabinoid CB1 antagonist rimonabant impairs positive affective memory in healthy volunteers. Psychopharmacology. 2009;205(1):85–91. [DOI] [PubMed] [Google Scholar]
- 40. Horder J, Browning M, Di Simplicio M, Cowen PJ, Harmer CJ. Effects of 7 days of treatment with the cannabinoid type 1 receptor antagonist, rimonabant, on emotional processing. J Psychopharmacol. 2012;26(1):125–32. [DOI] [PubMed] [Google Scholar]
- 41. Ragnhildstveit A, Kaiyo M, Snyder MB, Jackson LK, Lopez A, Mayo C, et al. Cannabis-assisted psychotherapy for complex dissociative posttraumatic stress disorder: a case report. Front Psychiatry. 2023;14:1051542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marchetta E, Mancini GF, Morena M, Campolongo P. Enhancing psychological interventions for post-traumatic stress disorder (PTSD) treatment with memory influencing drugs. Curr Neuropharmacol. 2023;21(3):687–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ney LJ, Akosile W, Davey C, Pitcher L, Felmingham KL, Mayo LM, et al. Challenges and considerations for treating PTSD with medicinal cannabis: the Australian clinician’s perspective. Expert Rev Clin Pharmacol. 2023;16(11):1093–108. [DOI] [PubMed] [Google Scholar]
- 44. Naim-Feil E, Elkins AC, Malmberg MM, Ram D, Tran J, Spangenberg GC, et al. The cannabis plant as a complex system: interrelationships between cannabinoid compositions, morphological, physiological and phenological traits. Plants. 2023;12(3):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76(9):1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brown JD, Goodin AJ. Evidence in context: high risk of bias in medical cannabis and cannabinoid clinical trials dictates the need for cautious interpretation. Med Cannabis Cannabinoids. 2021;4(1):63–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schlag AK, Lynskey M, Fayaz A, Athanasiou-Fragkouli A, Brandner B, Haja B, et al. Characteristics of people seeking prescribed cannabinoids for the treatment of chronic pain: evidence from Project Twenty 21. Front Pain Res. 2022;3:891498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Van De Donk T, Niesters M, Kowal MA, Olofsen E, Dahan A, Van Velzen M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 2019;160(4):860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moreno-Sanz G, Madiedo A, Hernandez P, Kratz J, Aizpurua-Olaizola O, Brown MRD, et al. Sex-dependent prescription patterns and clinical outcomes associated with the use of two oral cannabis formulations in the multimodal management of chronic pain patients in Colombia. Front Pain Res. 2022;3:854795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aviram J, Lewitus GM, Vysotski Y, Berman P, Shapira A, Procaccia S, et al. Sex differences in medical cannabis-related adverse effects. Pain. 2022;163(5):975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this work are available on request from the corresponding author. The data are not publicly available due to privacy and ethical reasons.