Abstract
Objectives
To investigate the effectiveness of case finding of patients at risk of developing chronic obstructive pulmonary disease, whether the method is suitable for use in general practice, how patients should be selected, and the time required.
Design
Cross sectional study.
Setting
Two semirural general practices in the Netherlands.
Participants
651 smokers aged 35 to 70 years.
Main outcome measures
Short standardised questionnaire on bronchial symptoms for current smokers, lung function with a spirometer, and the quality of the spirometric curve.
Results
Of the 201 smokers not taking drugs for a pulmonary condition, 169 produced an acceptable curve (fulfilling American Thoracic Society criteria). Of these, 30 (18%, 95% confidence interval 12% to 24%) had a forced expiratory volume in one second (FEV1) <80% of predicted. When smokers were preselected on the basis of chronic cough, the proportion with an FEV1 <80% of predicted increased to 27% (17 of 64; 12% to 38%). Chronic cough was a better predictor of airflow obstruction than other symptoms, such as wheeze and dyspnoea. The presence of two symptoms was a slightly better predictor than cough only (odds ratio 3.02 (1.37 to 6.64) v 2.50 (1.14 to 5.52)). Age was also a good predictor of obstruction; smokers over 60 with cough had a 48% chance of having an obstruction. The mean time needed for spirometry was four minutes. Detecting one smoker with an FEV1 <80% of predicted cost €5 to €10.
Conclusions
Trained practice assistants could check all patients who smoke for chronic obstructive pulmonary disease at little cost to the practice. Cough and age are the most important predictors of the disease. By testing one smoker a day, an average practice could identify one patient at risk a week.
What is already known on this topic
The number of patients with chronic obstructive pulmonary disease continues to increase
Screening a practice population for the disease is not feasible
Smoking is a known risk factor for patients developing the disease
What this study adds
Case finding of chronic obstructive pulmonary disease by examining smokers is effective and can be implemented in general practice
Cough and age are good predictors of the disease in smokers
Practice assistants can measure lung function at low cost
Introduction
Chronic obstructive pulmonary disease is a major health problem, and the number of patients with the disease is increasing.1–5 About 10% of the general population show signs of the disease, and 26% of patients aged 45 years or over have indications of chronic airflow obstruction.1,3 Only about a quarter to a half of these patients are known to their doctors.1,4 The decrease in lung function is gradual. The disease is usually diagnosed late because patients may adapt to the condition or doctors may not notice the symptoms.4,6–8 By then lung function is often poor, sometimes <50% of normal.9,10 Patients may also not see the doctor until the disease is advanced.11
At least 95% of people who develop the disease are smokers, and their lung function decreases faster than that of non-smokers.4,10,12 The most effective intervention is to stop smoking, preferably early.12,13 Smokers who quit will not recover lost lung function, but the rate of decline may revert to that of a non-smoker.10 Stopping smoking at an early stage improves the prognosis, regardless of how many attempts it takes to quit.10,12–14 Depending on the number of cigarettes smoked per day, only 24-47% of smokers develop airflow obstruction.10 Smokers at risk of developing a smoking related disease are more motivated to stop smoking than those not at risk.14
Treatment with anti-inflammatory drugs at an early stage of the disease might improve the prognosis.2 Inhaled corticosteroids can improve lung function to a certain extent, although the long term effects have been disappointing in chronic obstructive pulmonary disease.15–18
Forced expiratory volume in one second (FEV1) is a reliable and valid method for assessing lung function.4,19,20 Several spirometers are available for use in general practice. Screening the general population for respiratory symptoms and lung function is effective but not feasible in the daily routine of a general practice.21 Selecting patients on the basis of risk factors should identify those at risk of developing chronic obstructive pulmonary disease.
We investigated the predictive value of bronchial symptoms for airflow obstruction in current smokers. As airflow obstruction is an essential feature of chronic obstructive pulmonary disease, patients with obstruction can be considered at risk of developing the disease. Repeated measurements of FEV1 and measurement of reversibility after bronchodilation would establish the diagnosis, but this was beyond the scope of our study.
Patients with the disease are usually over 40, and the prevalence of the disease increases with age. We therefore investigated whether selecting for age could further increase the probability of detecting smokers with airflow obstruction. We also assessed the feasibility and efficiency of case finding of patients with chronic obstructive pulmonary disease in general practice by time and cost.
Methods
Patient selection
We recruited patients from two semirural practices in the south east of the Netherlands. Each practice had two general practitioners consisting of a total population of around 10 400. The practices were representative of other practices for patients with chronic obstructive pulmonary disease.
We randomly selected patients between 35 and 70 years of age who visited their doctor. We invited them to complete a form about smoking and whether they were taking drugs for a pulmonary condition. We asked smokers who were not taking pulmonary drugs (corroborated by the doctor's register) to complete another short questionnaire on chronic respiratory symptoms, smoking behaviour, allergy, asthma, and family history (box). We also recorded height.
Questions asked of smokers before spirometry
How many cigarettes do you smoke a day?
Do you have or have you had any allergies? If so, to what?
Have you ever had asthma or bronchitis?
Have you started to get tired more quickly in the past few years?
Have you been short of breath more often in the past few years?
Have you coughed more in the past few years?
Have you started to wheeze in the past few years?
Are there any lung diseases in your family?
Assessment of lung function
We assessed lung function with a spirometer that displayed and printed out the flow volume curve.22 The curves were classified according to the criteria of the American Thoracic Society.23 Curves meeting these criteria were “good.”23 “Acceptable” curves were those where the first part of the spirometric curve concurred with the criteria, allowing accurate assessment of FEV1. If accurate assessment was not possible the curves were classified as “unacceptable” (table 1). The spirometer was calibrated before and once during the study. The patients had to produce at least three acceptable curves; we chose the one with the highest sum of FEV1 and forced vital capacity. For analysis we used the FEV1 as a percentage of the predicted value, standardised for age, height, and sex.23 We considered the FEV1 abnormal if it was below 80% of the value predicted by the guidelines of the Dutch College of General Practitioners and the World Health Organization.24,25 Practice assistants with at least three years' experience assessed the curves. Before the study the assistants were trained by a laboratory assistant qualified in lung function in two sessions lasting four hours each. The first session comprised instruction and training in the use of spirometry, the second evaluation and refresher training. The performance of the practice assistants during the study was evaluated and corrected when needed by JMCL. Patients with an FEV1 <80% of predicted were considered to have undiagnosed bronchial obstruction and were advised to see their doctor.
Table 1.
Quality of curves produced by spirometry in 201 smokers according to criteria of American Thoracic Society20
| Quality of curve
|
No (%) of patients (n=201)
|
No (%) of patients with FEV1 <80% (n=34)
|
|---|---|---|
| Unacceptable | 32 (16) | 4 (12) |
| Acceptable | 79 (39) | 11 (32) |
| Good | 90 (45) | 19 (56) |
FEV1=forced expiratory volume in one second.
Power calculation
Data collected previously by our group showed that about 10% of the general population have airflow obstruction.1 An α of 5% and a power of 80%, and a minimum detectable relevant difference of 10% in lung function, require at least 78 patients who smoke, whereas a detectable difference of 7.5% requires 166 patients who smoke. A difference of less than 7.5% in lung function is difficult to detect because of the many sources of variability in lung function (for example, variability during the day). Assuming (on the basis of national data) that 33% of the study population smoke, we needed to investigate at least 498 people who were not taking pulmonary drugs.
Analysis
We used both univariate and multivariate analyses. We performed logistic regression using both a stepwise forward and a backward procedure (forward procedure not shown). We also used multilevel analysis to assess the intraclass correlation coefficient to detect possible differences between the two practices in the quality of the spirometry.
Results
Patients' characteristics
We excluded 16 of 683 eligible patients because of insufficient data, and 16 refused to participate. Of the 651 patients remaining, 229 (35%) smoked, and 28 (4%) were excluded for taking pulmonary drugs. Overall, 201 patients completed the questionnaires and underwent spirometry.
Women comprised 62% of the group (404 women). The sex of smokers and non-smokers was similar: 38% (94) of the men smoked compared with 34% (137) of the women. The mean age of patients was 46.7 (SD 7.7) years.
Outcome
The two practices did not differ in the quality of testing (tests good in 85% v 83%; intraclass correlation coefficient=0). Of the 169 patients with an acceptable or good spirometric curve, 30 (18%, 95% confidence interval 12% to 24%) had an FEV1 <80% of predicted (table 1).
Of 64 patients who reported chronic cough, 17 (27%) had an FEV1 <80% of predicted, indicating bronchial obstruction; the negative predictive value for bronchial obstruction was 87% (table 2). Chronic cough was significantly related to lung function (odds ratio 2.50, 1.14 to 5.52). Wheeze, dyspnoea, and tiredness were not related to FEV1 <80% of predicted (table 2).
Table 2.
Bronchial obstruction in smokers with forced expiratory volume in one second (FEV1) <80% of predicted within different groups of symptoms
| Symptom
|
No of patients reporting symptom
|
No (%, 95% CI) of patients with FEV1 <80%
|
Odds ratio* (95% CI)
|
Negative predictive value (%)
|
|---|---|---|---|---|
| Family history: | ||||
| Asthma | 33 | 6 (18, 7 to 35) | 1.03 (0.38 to 2.77) | 82 |
| Allergy | 28 | 6 (21, 8 to 41) | 1.35 (0.49 to 3.70) | 83 |
| Symptom: | ||||
| Tiredness | 90 | 19 (21, 13 to 31) | 1.77 (0.77 to 4.06) | 87 |
| Chronic wheeze | 44 | 12 (27, 15 to 43) | 2.15 (0.94 to 4.88) | 85 |
| Chronic dyspnoea | 79 | 19 (24, 15 to 35) | 2.19 (0.98 to 4.90) | 87 |
| Chronic cough | 64 | 17 (27, 16 to 39) | 2.50 (1.14 to 5.52) | 87 |
| No of symptoms: | ||||
| 1 | 105 | 23 (22, 14 to 31) | 2.28 (0.93 to 5.60) | 89 |
| 2 | 59 | 17 (29, 18 to 42) | 3.02 (1.37 to 6.64) | 88 |
| 3 | 23 | 8 (35, 16 to 57) | 3.01 (1.17 to 7.70) | 85 |
Estimates of common odds ratio by Mantel-Haenszel test.
Overall, 105 patients had one symptom (chronic cough, dyspnoea, or wheeze), of whom 23 (22%) had an FEV1 <80% of predicted (table 2). Fifty nine had two symptoms, of whom 17 (29%) had an FEV1 <80% of predicted. Twenty three patients had all three symptoms, of whom eight (35%) had an FEV1 <80% of predicted.
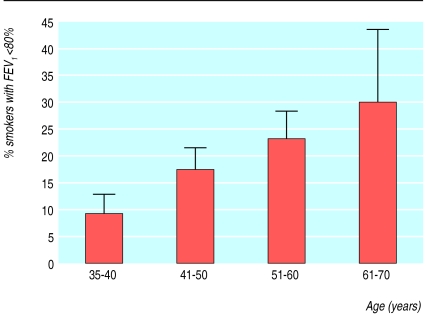
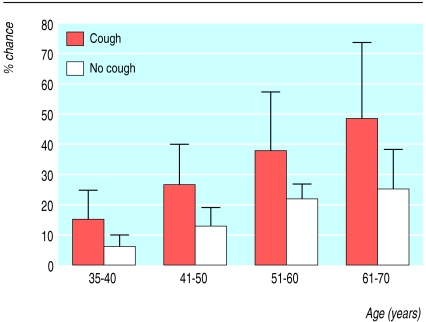
The 35-40 year age group (24% of the total group) had the lowest percentage (of patients with an FEV1 <80% of predicted (fig 1). Of the 127 patients aged over 40 who had spirometry, 26 (21%) had an FEV1 <80% of predicted. When cough was present there was an increased chance of detecting patients with obstruction with increasing age (fig 2). Smokers with cough who were older than 60 had a 48% chance of having bronchial obstruction (fig 2).
Figure 1.
Percentage of smokers with forced expiratory volume in one second <80% of predicted by age group
Figure 2.
Chance of having bronchial obstruction with increasing age in smokers with or without cough. Bars are 95% confidence intervals
Time and costs
One lung function assessment took a mean time of 4 (SD 1.1) minutes. Thirty patients with an FEV1 <80% of predicted were detected. Therefore detecting one patient with an FEV1 <80% of predicted would take about 23 (21 to 26) minutes (table 3). If only smokers with chronic cough were tested, detecting one patient would take 16 (14 to 18) minutes. If patients with more than one symptom were tested, detecting one patient would take 14 (12 to 16) minutes.
Table 3.
Time (minutes) needed for detecting one patient with a forced expiratory volume in one second <80% of predicted for selected groups of smokers in general practice
| Group | Time for detecting patient (95% CI) |
|---|---|
| All smokers | 23 (21 to 26) |
| Smokers with cough | 16 (14 to 18) |
| Smokers with 2 symptoms (cough, dyspnoea, or wheeze) | 14 (12 to 16) |
| Smokers with 3 symptoms (cough, dyspnoea, and wheeze) | 12 (10 to 14) |
The costs of performing spirometry include the time of the practice assistants and the costs of the equipment. At an average salary of €13 (£7.90, $11.60) per hour, one minute of assessment by a practice assistant would cost €0.22. The cost of detecting one smoker with an FEV1 <80% of predicted would thus be between €2.64 and €3.96. A spirometer of the type used in our study costs €1275, but a range of machines of varying prices and quality is available. A qualified assistant trained the practice assistants in two sessions. In the Netherlands, these courses cost €64. Therefore the direct costs of detecting one smoker with obstruction would be €5 to €10, depending on the number investigated.
Discussion
Efficient case finding
Case finding of patients with chronic obstructive pulmonary disease in general practice is a more realistic approach than screening. Since smoking is the most important risk factor for developing the disease and smoking cessation is the most effective intervention at any stage of the disease, the first step should be to select smokers. In our study one patient at risk of developing chronic obstructive pulmonary disease was found for every six smokers tested. The positive predictive value of cough for airflow obstruction among smokers was 27%. Therefore for every smoker with cough found to be at risk, about four smokers with cough had to be tested. The positive predictive value of at least two of the symptoms of cough, dyspnoea, or wheeze was only slightly higher (29%), so there is only little additive value in asking about symptoms other than cough. In patients with all three symptoms the prevalence was 35%; among these patients, for every smoker found to be at risk, three smokers had to be tested.
We needed a mean of 4.1 minutes to assess one patient and could detect one patient with an FEV1 <80% of predicted in a group of smokers in 12 to 23 minutes, depending on the selection criteria used. We recommend testing all smokers who have chronic cough. It would then take about 15 minutes of the practice assistant's time to find one patient at risk of developing chronic obstructive pulmonary disease. It would be more time efficient to test only smokers with all three symptoms, in which case finding one patient would take about 12 minutes, but more smokers with airflow obstruction would not be detected (in this case 22 out of 30).
In our survey, 35% of a random sample of patients visiting their doctor smoked. Of the women, 34% smoked, compared with 38% of the men. This is comparable to the average smoking rates in Holland.26 Some recruitment bias may have been present because more women than men visit their doctors. Also, more men currently smoke. Recruitment bias can be regarded as a potential disadvantage of case finding, as the method can be expected to detect a lower proportion of men with airflow obstruction.
A low FEV1 is an essential feature for a diagnosis of chronic obstructive pulmonary disease, although certainly not the only one. Patients with a low FEV1 have not yet been diagnosed as having chronic obstructive pulmonary disease, but they are at a high risk of developing it. To find out which of these patients will ultimately be diagnosed as having the disease, the FEV1 has to be measured repeatedly. In addition, forced vital capacity has to be determined and reversibility after bronchodilation has to be assessed. This was beyond the scope of our study; we aimed to find patients at a high risk of developing the disease. Therefore we assessed the most suitable and efficient examination method for general practice, using practice assistants without directly involving the doctor. Assessing reversibility after salbutamol takes 10-15 minutes and after ipratropium bromide 45 minutes. Moreover, the doctor has to be involved in assessing reversibility (which should be done at least under the supervision of the doctor). Previous studies in the general population have shown that patients have no objections to spirometry but do often object to taking drugs for the assessment of reversibility.21 Therefore a two step procedure seems logical and efficient: the practice assistant would be responsible for case finding of patients at a high risk of developing chronic obstructive pulmonary disease (including the assessment of lung function if appropriate) and the doctor would make the diagnosis and instigate treatment.
The quality of the spirometric curve depends on the device used and the patient's cooperation as well as on the practice assistants' performance.27 Of the spirometric curves we obtained, 84% were of sufficient quality to determine FEV1 according to the criteria of the American Thoracic Society.20 FEV1 can be measured in general practice by a practice assistant, after brief instruction and training.28
Limitations of the study
Mainly for logistical reasons, our study included only two practices. We aimed to test case finding under real life circumstances over a period long enough to allow the build up of some routine. To this end, the practices kept up this case finding procedure for about six months, operating as much as possible in natural circumstances to imitate real daily practice as closely as possible.
Using only two practices might have hampered the external validity of the study, but this is unlikely as the doctors did not have any special interest either at present or in the past in detecting asthma or chronic obstructive pulmonary disease, nor had they any other research programmes running. Hence, these practices were neither more nor less likely than other practices to have cases of undetected asthma or chronic obstructive pulmonary disease.
Preventive measures
If no effective remedial measures are available, there is no sense in screening or case finding. We believe, however, that such a measure is available. Stopping smoking reverses the development of chronic obstructive pulmonary disease. That smokers are probably more motivated to stop smoking when they know that they are at risk of developing a chronic lung disease is illustrated by a study of a longitudinal cohort in which patients who continued to smoke had a much steeper decline in lung function than those who stopped smoking, whereas smokers who stopped smoking still had a steeper decline than never smokers.13 The lung health study confirmed that smoking cessation could reverse the steep decline in lung function.12 Further follow up showed that attempts to quit smoking could prevent loss of lung function, especially in patients with mild disease.29 Follow up also resulted in fewer respiratory symptoms after prolonged abstinence.30 It is difficult to get smokers to quit. However, smokers are more motivated to stop smoking if they realise that their respiratory problems are caused by smoking and that they are at risk of developing chronic obstructive pulmonary disease or other smoking related diseases.14,31
Screening or case finding?
Detecting one smoker aged between 35 and 70 years with an FEV1 <80% of predicted took an average of 23 minutes in our survey. Increasing the detection rate by further selection on cough can reduce the time to about 15 minutes per patient detected. By testing one smoker with cough a day, one or two patients at risk would be found every week. All these patients are at high risk of developing chronic obstructive pulmonary disease. They should be carefully followed and should at least be encouraged to give up smoking. It seems likely that patients who are clearly at risk of the disease are more likely to stop smoking.14,31
Considering that chronic obstructive pulmonary disease is one of the main diseases in the Western world and that it is estimated to become the third leading cause of death in the next decade (while mortality from cardiovascular diseases seems to be declining), smoking cessation will become even more important. Because only half, at most, of the patients with an obstructive airflow disease are known to their doctor, the first step should be to detect patients at an early stage and to offer them an effective intervention—namely, to stop smoking at a time when they are motivated to do so.1,4 Our study shows that this first step can be done in an effective and efficient way by case finding in general practice. Although screening the general population is possible,1,11,21,31 implementing screening in primary care is rather difficult,6,16 and case finding is likely to be a more suitable approach in this setting.
The feasibility of case finding depends on several factors: the way patients are selected, the time needed to perform the assessment, the availability of skilled staff, the quality of the assessment, the availability of a suitable spirometer, the doctor's knowledge, a suitable room, and a cooperative patient. We have shown that case finding for chronic obstructive pulmonary disease is a suitable method in general practice. It can be implemented by practice assistants after a brief training course.
Footnotes
Funding: Research Institute ExTra, Maastricht University.
Competing interests: None declared.
References
- 1.Tirimanna PRS, Schayck CP van, Otter JJ den, Weel C van, Herwaarden CL van, Boom G van den, et al. Prevalence of asthma and COPD in general practice in 1992: has it changed since 1977? Br J Gen Pract. 1996;46:277–281. [PMC free article] [PubMed] [Google Scholar]
- 2.Dompeling E, Schayck CP van, Grunsven PM van, Herwaarden CL van, Akkermans R, Molema J, et al. Slowing the deterioration of asthma and chronic obstructive pulmonary disease observed during bronchodilator therapy by adding inhaled corticosteroids. Ann Intern Med. 1993;118:770–778. doi: 10.7326/0003-4819-118-10-199305150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Renwick DS, Conolly MJ. Prevalence and treatment of chronic airways obstruction in adults over the age of 45. Thorax. 1996;51:164–168. doi: 10.1136/thx.51.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siafakis NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, et al. ERS-consensus statement. Optimal assessment and management of chronic obstructive pulmonary disease (COPD) Eur Respir J. 1995;8:1398–1420. doi: 10.1183/09031936.95.08081398. [DOI] [PubMed] [Google Scholar]
- 5.Thiadens HA, Bock GH de, Dekker FW, Huysman JAN, Houwelingen JC van, Springer MP, et al. Identifying asthma and chronic obstructive pulmonary disease in patients with persistent cough presenting to general practitioners: descriptive study. BMJ. 1998;316:1286–1290. doi: 10.1136/bmj.316.7140.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boom G van den, Rutten-Mölken MPMH van, Tirimanna PRS, Schayck CP van, Folgering H, Weel C van. Association between health-related quality of life and consultation for respiratory symptoms: results from the DIMCA programme. Eur Respir J. 1998;11:67–72. doi: 10.1183/09031936.98.11010067. [DOI] [PubMed] [Google Scholar]
- 7.Burdon JWG. Chronic lung disease and the perception of breathlessness: a clinical perspective. Eur Respir J. 1994;7:1342–1349. doi: 10.1183/09031936.94.07071342. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ. Poorly perceived asthma. Thorax. 1992;47:408–409. doi: 10.1136/thx.47.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thoracic Society of Australia and New Zealand. Guidelines for the management of chronic obstructive pulmonary disease. Mod Med Aust. 1995;(Jul):132–146. [Google Scholar]
- 10.Fletcher C, Peto R, Tinker C. The natural history of chronic airflow obstruction. BMJ. 1977;i:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schayck CP van, Heijden FMMA van der, Boom G van den, Tirimanna PRS, Herwaarden CLA van. Underdiagnosis of asthma: is the doctor or the patient to blame? The DIMCA project. Thorax. 2000;55:562–565. doi: 10.1136/thorax.55.7.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anthonissen NR, Conett JE, Kiley JP, Altose MD, Bailey WC, Buist AS. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the lung health study. JAMA. 1995;273:1497–1505. [PubMed] [Google Scholar]
- 13.Xu X, Dockery DW, Ware JH, Speizer FE, Ferris BG. Effects of cigarette smoking on rate of loss of pulmonary function in adults: a longitudinal assessment. Am Rev Respir Dis. 1992;146:1345–1348. doi: 10.1164/ajrccm/146.5_Pt_1.1345. [DOI] [PubMed] [Google Scholar]
- 14.Humerfelt S, Eide GE, Kvale G, Aarø LE, Gulsvik A. Effectiveness of postal smoking cessation advice: a randomised controlled trial in young men with reduced FEV1 and asbestos exposure. Eur Respir J. 1998;11:284–290. doi: 10.1183/09031936.98.11020284. [DOI] [PubMed] [Google Scholar]
- 15.Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multi centre randomized placebo controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. Lancet. 1998;351:773–780. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- 16.Boom G van den, Rutten-Mölken MPMH van, Molema J, Tirimanna PRS, Weel C van, Schayck CP van. The cost-effectiveness of early treatment with fluticasone propionate 250 μg twice daily in subjects with obstructive airway disease, detected by a two stage screening program. Am J Respir Crit Care Med. 2001;164:2057–2066. doi: 10.1164/ajrccm.164.11.2003151. [DOI] [PubMed] [Google Scholar]
- 17.Vestbo J, Sørensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353:1819–1823. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- 18.Burge PS, Calverley PMA, Jones PW, Spencer S. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quanjer PhH, Tammeling GJ, Cotes JE, Pedersen O, Peslin R, Yernault J. Lung volumes and forced ventilatory flows. Official statement of the European Respiratory Society. Eur Respir J. 1993;6(suppl 16):5–40. doi: 10.1183/09041950.005s1693. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 21.Boom G van den, Schayck CP van, Rutten-Mölken MPMH van, Tirimanna PRS, Otter JJ den, Grunsven PM van, et al. Active detection of chronic obstructive pulmonary disease and asthma in the general population. Am J Respir Crit Care Med. 1998;158:1730–1738. doi: 10.1164/ajrccm.158.6.9709003. [DOI] [PubMed] [Google Scholar]
- 22.Crapo RO, Jensen RL. Test report Micro Medical Limited 3300 Spirometer. Salt Lake City, UT: LDS Hospital; 1998. [Google Scholar]
- 23.American Thoracic Society. Standardization of spirometry—1987 update. Am Rev Respir Dis. 1987;136:1285–1298. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 24.Geijer RMM, Schayck CP van, Weel C van, Sachs APE, Zwan AAC van der, Bottema BJAM, et al. Treatment of COPD in general practice. Huisarts Wetenschap. 1997;40:430–432. [Google Scholar]
- 25.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 26.NIPO survey on smoking among adults. The Hague, the Netherlands: Foundation for Public Health and Smoking (STIVORO); 1996. [Google Scholar]
- 27.Otter JJ den, Knitel M, Akkermans RPM, Schayck CP van, Folgering HTM, Weel C van. Spirometry in general practice: the performance of practice assistants scored by lung function technicians. Br J Gen Pract. 1997;47:41–42. [PMC free article] [PubMed] [Google Scholar]
- 28.Schermer TRJ, Folgering HTM, Bottema BJAM, Jacobs JE, Schayck CP van, Weel C van. The value of spirometry for primary care: asthma and COPD. Primary Care Respir J. 2000;9:48–52. doi: 10.1038/pcrj.2000.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray RP, Anthonisen NR, Connett JE, Wise RA, Lindgren PG, Greene PG, et al. Effects of multiple attempts to quit smoking and relapses to smoking on pulmonary function. J Clin Epidemiol. 1998;51:1317–1326. doi: 10.1016/s0895-4356(98)00120-6. [DOI] [PubMed] [Google Scholar]
- 30.Kanner RE, Connett JE, Williams DE, Buist AS. Effects of randomised assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the lung health study. Am J Med. 1999;106:410–416. doi: 10.1016/s0002-9343(99)00056-x. [DOI] [PubMed] [Google Scholar]
- 31.Zielinsky J, Bednarek M. Early detection of COPD in a high-risk population using spirometric screening. Chest. 2001;119:731–736. doi: 10.1378/chest.119.3.731. [DOI] [PubMed] [Google Scholar]