Abstract
Introduction
This report presents a case of an exceptionally delayed distant recurrence of a choroidal melanoma, occurring 4 decades after the enucleation of the affected eye.
Case Presentation
In 1977, a 29-year-old man underwent enucleation for a choroidal melanoma. At the age of 68 years, he was diagnosed with advanced prostate cancer. Although the metastatic prostate cancer responded to treatment, a persistent lung lesion warranted further examination. A lung biopsy, somewhat surprisingly, confirmed the presence of melanoma metastasis, 4 decades after the enucleation. The cells were positive for Melan-A, while no BRAF mutation was identified. Two years later, new lesions appeared in the liver, and CT showed progression with multiple new sites. A liver biopsy revealed again melanoma recurrence, and its choroidal origin was verified by the presence of a GNA11 mutation. The patient underwent radiation therapy for the lung and liver lesions, followed by immunotherapy. However, the patient died 11 months after the recurrence in the liver. In this case report, the micrometastatic melanoma cells appear to have remained dormant for an extended period, before the patient’s treatment in 1977, but the reason for the late reactivation from the dormant state remains unclear.
Conclusion
The recurrence of a choroidal melanoma is substantiated by the histopathological and molecular analyses, including the finding of a GNA11 mutation. This case exemplifies a remarkably delayed distant recurrence of a choroidal melanoma, which manifested clinically 40 years following enucleation.
Keywords: Case report, Dormancy, GNA11, Late recurrence, Uveal melanoma
Introduction
Uveal melanoma (UM) is the second most common subtype of melanoma, accounting for about 5% of all melanoma cases, and is the most common intraocular malignancy in adults [1]. Follow-up studies of patients with primary UM have identified multiple additional malignancies. These malignancies occur either before, simultaneously with or in the years following the primary UM diagnosis [2]. Germline BAP1 mutations are rare, but they can cause UM, cutaneous melanoma as well as multiple malignancies in other organs (e.g., kidney, pancreas, breast, lung, meninges) [3]. Moreover, the different cancer treatment modalities may unfortunately cause a new primary malignancy, as documented for bilateral retinoblastoma after radiation therapy. A chemotherapy regimen that includes repeated sequences and involves multiple cytostatic drugs may thus as well increase the risk for a secondary cancer [4]. Distant metastasis following enucleation of an ocular melanoma may occur several years after the diagnosis and subsequent treatment of the primary tumour. The incidence curve for UM metastasis shows a bimodal distribution with the highest peak within the first 3 postoperative years, and a minor second top after about 10 years or even later [5]. Recurrences more than 15 years after surgery, despite primary enucleation, are very rare. Nevertheless, extremely late relapses are reported after 4 decades [6]. This phenomenon may be related to so far unknown mechanisms controlling the micrometastasis for long periods, probably involving immune surveillance and unknown mechanisms controlling regulating cancer cell dormancy. A decline in the general immune function with age may be another contributing factor in patients with late recurrences of UM [7].
Case Presentation
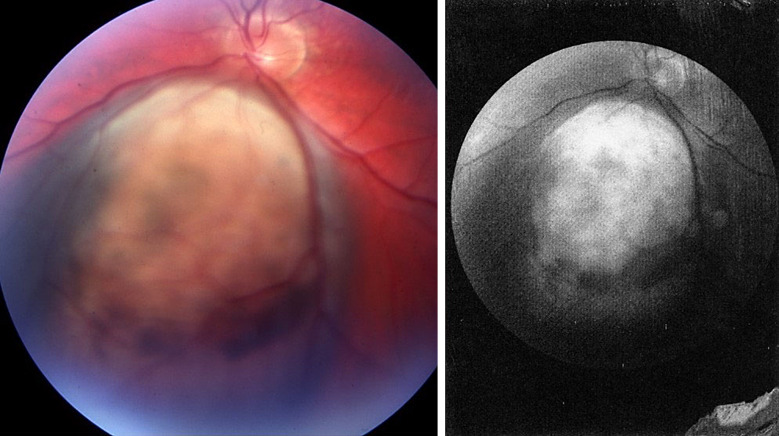
A 68-year-old man with excellent performance status presented to an evaluation at the Department of Ophthalmology at the Oslo University Hospital in October 2016. At that time, a residual metastatic melanoma lesion had been diagnosed in the lung. The medical history revealed that he was enucleated in August 1977, then 29 years old, due to an amelanotic juxtapapillary tumour in the left eye (shown in Fig. 1) shortly after debut of visual problems starting with flickering. The visual acuity in the affected eye was finger counting 2 m; visual field (a.m. Donders) was defect in the superior quadrants due to detachment inferior to the neoplasm. The cell-rich, amelanotic tumour measuring 5 mm in diameter was by histology classified as a Callender’s spindle B UM with distinct nucleoli in the tumour cells (shown in Fig. 2a, b). In the pathological report, no ingrowth in the sclera was seen. The X-ray examination of the lungs was normal. There were no problems with the prosthesis. He was controlled annually by an ophthalmologic specialist from the date of diagnosis.
Fig. 1.
Fundus photography shows an amelanotic tumour 7.5 × 9.0 mm close to the optic disk inferiorly with a circumscribed retinal detachment. Regrettably, the height of the tumour is not given in the primary eye journal, nor in the pathology report, but probably this is a T1aN0Mx tumour.
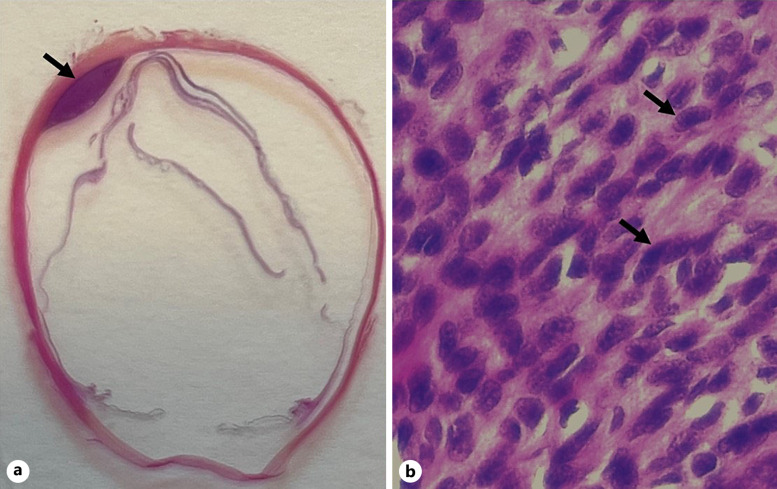
Fig. 2.
Haematoxylin and eosin (HE) staining of 4-μm-thick section of formalin-fixed paraffin-embedded enucleated eye with primary choroidal melanoma from the 68-year-old man. a Enucleated eye with an arrow pointing to the location of the choroidal primary tumour. b Histology shows cell-rich tumour tissue with sparse melanin content and cells classified as Callender’s type spindle B melanoma with an oval or spindle-shaped nucleus with distinct nucleoli. Black arrows are pointing to the distinct nucleoli in the tumour cells (HE, ×63).
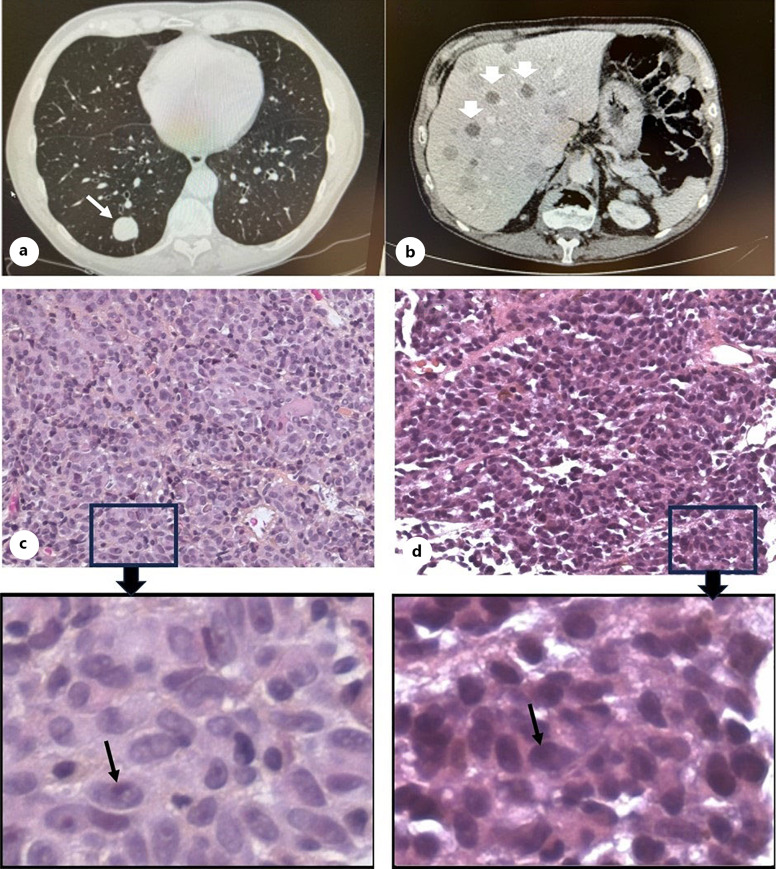
In 2016, the patient experienced problems with urination and the PSA titer had increased. A locally advanced prostate cancer was verified February 2016 with a biopsy. Infiltration in the seminal vesicles bilaterally and pelvic lymph nodes and skeletal metastases was documented. Moreover, there were radiological suspicion findings of a solitary nodule in the lower lobe of the right lung and a hypodense lesion in the spleen. The specimens from prostate showed two elements: an ordinary adenocarcinoma, Gleason score 4 + 4, and a component of an aggressive acinus small cell carcinoma, rT3bN1M1. Treatment of both carcinomas was initiated with an LHRH analogue to suppress testosterone (the intention was a lifelong chemical castration with goserelin) and six palliative PV courses (cisplatin and etoposide). Moreover, the staging revealed tumours in the stomach, spleen, lymph nodes, and the lungs in addition to a probable gastrointestinal stromal tumour. The metastatic manifestations responded markedly to antihormone therapy evaluated with CT after 3 months with a decrease in size of lymph nodes and sclerosing of skeletal metastases except for a solitary, sharply demarcated lesion in the lung in the posterior inferior lobe (shown in Fig. 3a). The lesion measured 2.3 × 2.5 × 2.5 cm. After completion of the chemotherapy and an observation without change in size, a lung biopsy was performed in August 2016. Histology of the solid tumour tissue obtained from the lung disclosed surprisingly a metastasis from a melanoma (shown in Fig. 3c). The tumour cells stained positive with Melan-A and S-100, showed some brownish cytoplasmic pigment, and had marked eosinophilic nucleoli (shown in online suppl. Fig. 1A, B; for all online suppl. material, see https://doi.org/10.1159/000541341). No mutation in the B-Raf-proto-oncogene (BRAF) gene, codon 600, was detected. The blood values and liver function tests were normal, including alkaline phosphatase (ALP) and lactate dehydrogenase.
Fig. 3.
a CT scans of lungs in 2016 show one sharply demarcated metastatic lesion in the posterior inferior lobe (white arrow). b CT scans of liver in July 2018 disclose multiple lesions (white arrowheads). Haematoxylin and eosin (HE) staining of metastatic tumour tissues from lung (c) and liver (d) are shown with low (×40) and higher magnification (box around area magnified from ×40). Melanoma spindle B cells with black arrows point to prominent nucleoli of the tumour cells, and pigmentation was only found in some areas.
We confirmed that his right eye was completely normal also in 2016 with corrected visual acuity 1.0 (+0.75–0.25 in 55°). IOP was measured to 17 mm Hg. Visual field with Donders test revealed normal extension. The prosthesis was cosmetically well adapted. The left orbit was unremarkable. The tumour tissue from 1977 was re-evaluated with new histological sections in 2017 confirming the previous diagnosis. BRAF gene examination disclosed again no mutation.
Evaluation at the Department of Dermatology (University in Oslo) did not reveal any suspicious cutaneous lesions. A nevus on the dorsal side in between the scapula revealed only a nevus tumour with some atypia. The lung lesion was considered to be a metastatic lesion, most probably originating from the eye melanoma enucleated in 1977. The ECOG performance status of the patient at this time point was 0, still excellent.
Additional genetic evaluations were performed. Both BRCA-1 and BRCA-2 tested negative in blood samples (germline mutations). Moreover, testing for the melanoma-associated genes CDKN2A, CDK4 exon 2, and BAP1 revealed no genetic abnormalities.
The follow-up of the lung lesion showed no changes, and the patient did not receive any further treatment for this melanoma lesion. In January 2018, a CT scan still showed unchanged metastases to the lung; however, several new lesions were detected in liver segment six. In July 2018, CT revealed progression with multiple lesions (shown in Fig. 3b). B-RAF-negative metastases (no mutation in BRAF gene, codon 600, Qiagen therascreen BRAF RGQ PCR Kit) were revealed in the liver by a needle biopsy. Pigmentation was only found in some spots, and the tumour cells showed prominent nucleoli; a similar spindle morphology/histopathology to the cells of the primary tumour in addition to some areas of epithelioid cells was confirmed by pathology (shown in Fig. 3d). They stained positively for Melan-A, variable positive for S-100, but negatively for AE1/AE3 and PSA (shown in online suppl. Fig. 1C–F). Thermo Fisher Oncomine Childhood Cancer Research Assay (OCCRA) was performed on the FFPE liver needle biopsy (assisted by personnel at OUS, Molecular Pathology, unit for solid tumours). This assay identified a mutation in GNA11 exon 4, c547 C>T transition in codon 183 (CGC), causing an arginine (R) to cysteine (C) replacement (R183C, p.Arg183Cys). Treatment with stereotactic radiation 10Gy × 5 to the lung and 15Gy × 3 to the liver was given in February–March 2018. Immunotherapy with anti-PD-1 monoclonal antibodies was initiated in May 2018 when progression with multiple metastases in both locations and peritoneal carcinomatosis was detected. In July 2018, a CT scan of the caput revealed brain metastases to the parietal regions. The findings on the patient’s right side dominated and resulted in visual symptoms, and palliative stereotactic radiation was administered. The patient, however, died shortly after the radiation in December 2018.
His medical familiar history revealed that his 10-year-older sister had excised a cutaneous melanoma without subsequent problems. His mother died of unknown reason when the patient was 5 years old, and his father died of lung cancer in his sixties.
Discussion
The most likely interpretation of this remarkable clinical case is a connection between the primary spindle cell melanoma with minimal atypia enucleated in 1977 and the melanoma lung metastasis in 2017 resistant to treatment. The other manifestation of simultaneous disseminations of an aggressive metastatic prostate cancer responded as expected. Histopathological specimens from both locations were examined and substantiated melanoma tumour. The long interval of 40 years is very exceptional, but has been reported before for an orbital recurrence [6].
A remarkable similarity between the histology in the primary eye tumour and the lung metastasis is described. Both biopsies confirmed a spindle cell melanoma with minimal atypia. The tumour cells contained little pigment and were relatively bland in appearance. These facts make it more acceptable to consider them as related. No skin, subungual, or mucosal melanomas were detected, and no problems were observed in the anophthalmic orbit. There was no evidence of an occult second melanoma at any time point. Moreover, the BAP1 and BRAF gene signatures were identical in the eye tumour and the metastatic lesions in the lung and the liver.
UMs are in general known as slow growing malignancies with a long doubling time, and the metastatic process is presumed to start years before a clinical diagnosis of the eye tumour is possible [8]. However, clinical metastases at distant sites detected at the time of the primary UM (synchronous metastases) are rare and found in 1–3% of all patients, only. In patients with very large UM, frequently combined with ciliary body involvement and extrascleral extension, exceptions from this rule may occur. Testing for micrometastases at diagnosis of UM has shown that melanoma cells are detected frequently outside the eye, in blood samples in 92% [9] and in bone marrow in 37% of all cases [10]. Regularly, a 10-year follow-up after treatment is considered adequate [2]. Micrometastatic disease of UM may be able to persist clinically dormant for years in different organs, even in the liver [11]. The concept of dormancy is often used to explain the phenomenon that tumour cells are present and are metabolic, but without any net growth, remaining asymptomatic and undetectable by normal means, sometimes over a long time period. Metastatic dissemination a long time after radical therapy with initial enucleation of small melanomas, T1aNOMx, is a rare but problematic clinical reality [10]. Our unique case presented here is a typical example.
The melanoma lung metastasis was detected in connection with the staging of the additional prostate cancer diagnosis in 2016. Its origin was revealed after a period of 6 months on an LHRH analogue to suppress testosterone and palliative cytostatic courses with a biopsy of the therapy-resistant lesion. The lung lesion did not change at all on CT scans in contrast to the other lesions. Nevertheless, 16 months later multiple lesions were seen on CT in the liver and examination of the specimen by histology confirmed again dissemination of melanoma cells in the liver. Eleven months later, the patients died, 16 months after the diagnosis of the lung metastasis in spite of attempts to treat. In the Small Fatal Choroidal Melanoma Study [12], a very different length from diagnosis of primary UM to diagnosis of dissemination was seen in this cohort. The survival time thereafter, however, was almost the same and very short, regardless of very different time intervals that had passed prior to distant metastasis. These findings may be explained by the general concept of cancer cell dormancy. The switch mechanisms that ultimately reactivate different cancers in individual patients are incompletely understood. Various mechanisms with effect on cellular cycles, angiogenesis, immunology, and microenvironment are considered to be operating [13]. In our case, it cannot be completely ruled out that a new malignancy and the initiated treatment of these metastases with chemotherapy and lung biopsy surgery may potentially have contributed to the progression of the UM metastasis.
Micrometastases at the time point of diagnosis of a UM have been verified in different organs in previous publications like in bone marrow [10], blood [9], liver [11] and also in postmortem tissues such as lungs, kidneys, myocardium, and bone marrow regardless of coexisting macrometastases [14] with different techniques. Metastatic UM outside the liver can sometimes be observed without rapid progression. When no hepatic affection is disclosed with imaging techniques, the survival of dissemination is substantially longer, 0.7 versus 3.3 years, respectively, as shown in our own micrometastasis study [10]. Involvement of the liver in over 90% of the patients, however, is usually detected first of all types of distant metastases and confirmed in many patients within 6 months after the UM diagnosis. The reason for this propensity is unknown. Moreover, the response of distant metastasis from UM to chemotherapy, immunotherapy, and surgery is in general very poor [5].
Micrometastases are believed to occur early and frequently in UM [9, 10]. In the present case, the obviously micrometastatic melanoma was controlled for a long time, probably involving tumour cell dormancy starting in 1977. The reasons for the end of the hibernation state 4 decades later, observed in 2018, are unknown. We can only speculate that an aggressive prostatic cancer with dissemination was enough to disturb a stabile homeostasis in the patient’s immune system, or that the treatment of this condition or that advanced age per se has weakened the immune system too much. In a recent report, a very late orbital recurrence of a choroidal melanoma 4 decades post-enucleation caused difficulty to contain the prosthesis [6]. The authors presumed that residual melanoma cells were clinically dormant in the orbit in this period. Our male patient and the female patient in this study were diagnosed at a much younger age than usually reported, 29 and 12 years, respectively. The mean age at diagnosis for UM materials is about 65 years. The main difference is an asymptomatic lung metastasis in our patient and a local symptomatic residue in the same-side orbit in the inferior-temporal quadrant in the female. It is worth noting that before enucleation in the female a fine needle aspiration biopsy was done and then a biopsy was extracted 2 days later through a scleral flap.
Genetic evaluation performed revealed no predisposition to cancer in our particular patient. Analysis of both BAP1 and BRAF revealed no somatic mutations in the primary eye tumour, nor later when repeated in the liver biopsy material. However, genetic analysis of GNAQ and GNA11, mutated in almost 80–90% of UMs, was performed on the liver metastasis. The analysis revealed a somatic GNA11 mutation, suggesting the recurrence was of choroidal melanoma origin. In cutaneous melanomas, a genetically and clinically different type of melanoma, somatic BRAF mutations are frequently observed (50%) and allow targeted treatment of dissemination for some time. Choroidal melanomas carry no V600E driver mutations in BRAF [15]. Checkpoint inhibitors are also less effective in uveal than cutaneous metastases. In our case, there was no clinical response documented at all. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material.
Conclusion
Our conclusion is that this case represents an extremely late distant recurrence of a choroidal melanoma, 4 decades after enucleation. The comparison between the metastatic and the primary tumour is performed, and the finding of GNA11 mutation in the metastasis substantiated this view. The mechanisms involved in cancer cell senescence are currently studied in many trials and will hopefully enable us to improve future treatment of UM patients.
Acknowledgments
We would like to express our sincere gratitude to Dr. Øystein Garred, MD, PhD, for his histopathological evaluation of the choroidal melanoma, as well as the lung and liver metastases. We are also grateful to Giang Huong Nguyen for the H&E staining of the lung and liver metastasis.
Statement of Ethics
This case report is part of a study approved by the Ethical Review Board: Regional Committee for Medical Research Ethics South East Norway, REK South East, Approval No. 595910. A written informed consent was obtained on October 15, 2022, from the patient’s wife (next-of-kin) for publication of the details of the medical case and accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
GNA11 mutation analysis was supported by a grant from the South-Eastern Norway Regional Health Authority (#2023061; Novel Prognostic and Predictive Markers in Uveal Melanoma – The PUMA Study).
Author Contributions
N.A.E. and J.G., conception and design; N.A.E., A.N., H.J., P.J., and J.G. collected and analysed data; A.N. and H.J. provided resources; N.A.E. and A.N. wrote the paper and prepared figures. All authors revised and approved the final version of the manuscript.
Funding Statement
GNA11 mutation analysis was supported by a grant from the South-Eastern Norway Regional Health Authority (#2023061; Novel Prognostic and Predictive Markers in Uveal Melanoma – The PUMA Study).
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary material files. Further enquiries can be directed to the corresponding author.
Supplementary Material.
Supplementary Material.
Supplementary Material.
References
- 1. Ghazawi FM, Darwich R, Le M, Rahme E, Zubarev A, Moreau L, et al. Uveal melanoma incidence trends in Canada: a national comprehensive population-based study. Br J Ophthalmol. 2019;103(12):1872–6. [DOI] [PubMed] [Google Scholar]
- 2. Robsahm TE, Falk RS, Eide NA. Additional malignancies and mortality in uveal melanoma: a 20-year follow-up of a Norwegian patient cohort. Acta Ophthalmol. 2023;101(6):696–704. [DOI] [PubMed] [Google Scholar]
- 3. Abdel-Rahman MH, Pilarski R, Cebulla CM, Massengill JB, Christopher BN, Boru G, et al. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet. 2011;48(12):856–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30(30):3734–45. [DOI] [PubMed] [Google Scholar]
- 5. Rantala ES, Hernberg M, Kivelä TT. Overall survival after treatment for metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 2019;29(6):561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menicacci C, Al-Jamal RT, De Francesco S, Barchitta M, Girolamo M, Di Maggio A, et al. Very late orbital recurrence of choroidal melanoma four decades post enucleation. Eur J Ophthalmol. 2022;32(4):Np88–93. [DOI] [PubMed] [Google Scholar]
- 7. Eide N, Faye RS, Høifødt HK, Sandvik L, Qvale GA, Faber R, et al. The results of stricter inclusion criteria in an immunomagnetic detection study of micrometastatic cells in bone marrow of uveal melanoma patients - relevance for dormancy. Pathol Oncol Res. 2019;25(1):255–62. [DOI] [PubMed] [Google Scholar]
- 8. Eskelin S, Pyrhönen S, Summanen P, Hahka-Kemppinen M, Kivelä T. Tumor doubling times in metastatic malignant melanoma of the uvea: tumor progression before and after treatment. Ophthalmology. 2000;107(8):1443–9. [DOI] [PubMed] [Google Scholar]
- 9. Tura A, Merz H, Reinsberg M, Lüke M, Jager MJ, Grisanti S, et al. Analysis of monosomy-3 in immunomagnetically isolated circulating melanoma cells in uveal melanoma patients. Pigment Cel Melanoma Res. 2016;29(5):583–9. [DOI] [PubMed] [Google Scholar]
- 10. Eide N, Faye RS, Høifødt HK, Sandstad B, Qvale G, Faber R, et al. Immunomagnetic detection of micrometastatic cells in bone marrow of uveal melanoma patients: a paradox. Acta Ophthalmol. 2015;93(1):59–66. [DOI] [PubMed] [Google Scholar]
- 11. Grossniklaus HE. Understanding uveal melanoma metastasis to the liver: the Zimmerman effect and the Zimmerman hypothesis. Ophthalmology. 2019;126(4):483–7. [DOI] [PubMed] [Google Scholar]
- 12. Jouhi S, Jager MJ, de Geus SJR, Desjardins L, Eide NA, Grange JD, et al. The small fatal choroidal melanoma study. A survey by the European ophthalmic oncology group. Am J Ophthalmol. 2019;202:100–8. [DOI] [PubMed] [Google Scholar]
- 13. Phan TG, Croucher PI. The dormant cancer cell life cycle. Nat Rev Cancer. 2020;20(7):398–411. [DOI] [PubMed] [Google Scholar]
- 14. Gill VT, Norrman E, Sabazade S, Karim A, Lardner E, Stålhammar G. Multiorgan involvement of dormant uveal melanoma micrometastases in postmortem tissue from patients without coexisting macrometastases. Am J Clin Pathol. 2023;160(2):164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smit KN, Jager MJ, de Klein A, Kiliҫ E. Uveal melanoma: towards a molecular understanding. Prog Retin Eye Res. 2020;75:100800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary material files. Further enquiries can be directed to the corresponding author.