Abstract
Introduction
Neuropsychiatric symptoms (NPS) may be disruptive and problematic for patients with Alzheimer’s disease (AD) and for their caregivers. Cannabidiol (CBD) may be a safer alternative. The objective was to evaluate whether CBD-rich oil was effective, and safe in adults with NPS secondary to AD.
Methods
An open-label, prospective cohort, single-center study in patients with AD onset after the age of 65 with untreated NPS. A CBD-rich oil was administrated 0.1 mL sublingually every 8–12 h, up-titrated weekly. The primary outcome was to establish a reduction in the NPI-Q severity score of >30% at 12 weeks compared with the baseline. A p value of <0.05 was statistically significant.
Results
Between July 2020 and July 2023, 59 (93.5%) patients completed ≥3 months of follow-up. The patients were under treatment for a mean of 23.2 months, the median dose of CBD was 111 mg/day. The median NPI-Q severity and caregiver’s distress scores at baseline were 24 and 29, respectively. At 3 months, the median NPI-Q severity score shifted to 12 (p < 0.001) and 14 (p < 0.001), respectively. The proportion of patients who achieved a reduction in the NPI-Q severity score of >30% was 94.9%, while a reduction of >50% was achieved by 54.2%. The improvement was maintained for up to 24 months.
Conclusion
This study shows that CBD-rich oil is an effective and safe therapy for treating NPS in AD patients, while also reducing the caregivers’ distress.
Keywords: Alzheimer disease, Behavioral symptoms, Cannabidiol, Cannabis, Dementia
Plain Language Summary
There is a need for an alternative treatment to significantly improve NPS in AD and decrease the caregiver’s stress as well as the financial burden resulting from polypharmacy and institutionalization. Any promising treatment should be safe and reduce the risk of adverse effects. This study evaluated the efficacy of a CBD-rich oil in treating NPS in a cohort of 59 patients with AD over a follow-up of more than 1 year, with a specific focus on its impact on caregiver burden. The study showed a significant reduction in the NPI-Q severity and caregiver’s distress scores after 3 months of intervention, and sustained for up to 24 months of follow-up. Notably, the effectiveness was independent of age, sex, years with AD, type of acetylcholinesterase inhibitors, and NPI-Q severity score before CBD treatment. A low CBD dose and a slow dose titration improve tolerance. These results may indicate that alleviating NPS in people with AD facilitates daily caregiving and improves caregivers’ emotional and physical distress.
Introduction
Neuropsychiatric symptoms (NPSs) usually accompany progressive cognitive and functional decline in Alzheimer’s disease (AD). These symptoms may be disruptive and problematic for caregivers in particular because they limit daily care such as feeding and personal hygiene. NPS contribute to patients’ overall morbidity and polypharmacy. Assessing and treating NPI in patients with AD is critical in order to provide high-quality homecare or nursing care. A meta-analysis has suggested that acetylcholinesterase inhibitors (AChEIs) may improve NPS on Neuropsychiatric Inventory Questionnaire (NPI-Q) compared with placebo (SMD −0.12; 95% CI: −0.23 to −0.02; I2 = 67%); but this was not the case for memantine (SMD −0.12; 95% CI: −0.27 to 0.03; I2 = 75%) [1]. This improvement is particularly seen in apathy and psychotic symptoms, which may be related to the higher degree of cholinergic denervation in the prefrontal-limbic circuit observed in patients with AD [2, 3]. The use of atypical antipsychotics and antidepressants has been suggested at the low doses [4], but comorbidities must also be considered for this population. The use of antipsychotics is associated with a high risk of stroke (hazard ratio [HR] 1.57 [95% CI: 1.28–1.92] at 30 days), heart failure (HR 1.95 [95% CI: 1.59–2.40] at 30 days), myocardial infarction (HR 1.61 [95% CI: 1.15–2.26] at 30 days), fractures (HR 1.49 [95% CI: 1.22–1.83] at 30 days), and death [4–7], but the use of antidepressants appears to be safe [8]. The aforementioned risks have driven the research for pharmacological alternatives, and drugs that target the endocannabinoid system have gained interest because of their behavioral effect as well as their ability to modulate neuroinflammation and oxidative stress [9]. Dronabinol has been evaluated previously as an alternative to treat NPS in AD in different studies with relative small samples [10–15], and cannabidiol (CBD) has recently been evaluated with promising results [16]. Taking this into account, it is important to increase the evidence about the usefulness of CBD in older patients with AD because it might be safer considering its pharmacological evidence as a multi-target drug that may treat several symptoms [17]. The aim of this study was to evaluate whether CBD-rich oil was effective, safe, and well-tolerated in adults with NPS secondary to AD.
Materials and Methods
Study Design and Population
An open-label, prospective cohort, single-center study was conducted at Clínica Zerenia (a reference center specializing in medical cannabis therapy) in Bogotá, Colombia. The inclusion criteria were adult patients with AD onset after the age of 65 with a Global Deterioration Scale (GDS) of >4 and with NPS not treated with antidepressants, antipsychotics, or benzodiazepine drugs for at least 1 month before enrollment. All patients should use AChEIs such as donepezil, galantamine, or rivastigmine at full doses because some NPS are sensitive to these drugs [1, 3]. Memantine use was unremarkable [1, 18]. Each patient had to have at least 3 months of follow-up to be included in the final analysis. The exclusion criteria were patients with dementia other than AD, patients being treated for NPS, patients without caregivers to provide accurate information, and individuals with baseline cardiac, liver, renal, or hematological laboratory abnormalities. This study was approved by the Institutional Review Board; the research was completed in accordance with the Declaration of Helsinki as revised in 2013. Patients provided written informed consent to participate and to use a cannabis-based magistral formulation (CBMF). This study is reported in accordance to the Strengthening the reporting of observational studies in epidemiology (STROBE) checklist for cohort studies (online suppl. material; for all online suppl. material, see https://doi.org/10.1159/000541364) [19].
Procedures and Intervention
Before initiating CBD-rich oil therapy, patients and caregivers had a first consultation to answer the Neuropsychiatric Inventory Questionnaire (NPI-Q) [20]. This is a retrospective (1 month) caregiver informant questionnaire covering 12 NPS domains such as delusions, hallucinations, agitation/aggression, dysphoria/depression, anxiety, euphoria/elation, apathy/indifference, disinhibition, irritability/lability, aberrant motor behaviors, night-time behavioral disturbances, and appetite/eating disturbances. Caregivers were asked to report severity symptoms using a three-point scale and the caregiver’s distress using a six-point scale. The total severity score adds up to 36 points, and the total distress score adds up to 60 points. After this period, patients received the CBMF, which was prepared by diluting cannabis extracts, using sesame oil and ethanol as excipients, with added sucralose and flavoring agents. The concentration of CBMF was 100 mg/mL CBD and THC <1.9 mg/mL, and it was produced by Khiron Life Science Corp® [21]. Patients were given 0.1 mL CBD-rich oil sublingually every 8–12 h, up-titrated weekly based on symptoms response and tolerability [22]. CBD dose titration was stopped when the patient exhibited a symptoms improvement, and further dosing would no longer yield benefits. The maximum theoretical dose considered was 50 mg/kg/day as reported in a previous study in adult patients [21]. The CBD dose was administered after food intake to facilitate sublingual absorption. However, if a patient ingested it immediately, this was not considered a missed dose. This sublingual administration was done to avoid the entero-hepatic circulation of CBD and its first-pass metabolism, which eliminates it by 85% [22].
Every month, each patient had a neurological consultation to evaluate the adherence of the CBD-rich oil, to determine the tolerance, to collect information about patients’ NPS, to weigh the patient, and to inquire about any adverse-drug reactions (ADRs). A complete blood count, liver function test (transaminases, bilirubin, gamma-glutamyl transferase, alkaline phosphatase), and kidney function test (serum creatinine, blood urea nitrogen) were performed before initiating CBD therapy and during follow-up. If any severe ADR or laboratory test abnormality was detected, the intervention was to be discontinued.
Outcomes
The primary outcome was to establish the effectiveness of CBD-rich oil, defined as a reduction in the NPI-Q severity score by >30% at 12 weeks compared with the baseline (response rate). The secondary outcomes were a reduction in the NPI-Q severity score by >50% at 12 weeks, a reduction in the NPI-Q caregiver’s distress score by >30% and 50% at 12 weeks, and a proportion of subjects experiencing any ADR.
Statistical Analysis
tThe study sample size was not predetermined; the entire cohort of patients recruited since study initiation was considered. Descriptive statistics (percentage, median, interquartile range [IQR], mean, standard deviation [SD]) were applied to age, age at AD diagnosis, years with AD, sex, type of AChEIs, duration of CBD-rich oil treatment, NPI-Q severity and caregiver scores, CBD dosage, patients’ weight, and ADR. The null hypothesis of normality was verified with the Kolmogorov-Smirnov test. Quantitative variables were compared using the paired Student’s t test or the nonparametric Wilcoxon test, as appropriate. The independence between qualitative variables was evaluated using the McNemar test. The correlation between the dose of CBD-rich oil and the previous NPI-Q severity score was analyzed with Spearman’s correlation coefficient. A multiple logistic regression analysis was performed to investigate the association between the response rate and CBD dose, with adjustments for age, sex, years with AD, type of AChEIs, and NPI-Q severity score before CBD treatment. A p value of <0.05 was considered statistically significant. The data were obtained from the clinical record system Gomedisys® and organized in a Microsoft Excel-Office 365® sheet. The database was analyzed with R software version 4.4.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Role of the Funding Source
In Colombia, medical cannabis is an off-label treatment, so it is an out-of-pocket expense. To avoid a conflict of interest, Khiron Life Science Corp® was not allowed to sponsor the CBMF treatment and did not deliver CBMF to patients. Each patient agreed to pay for their treatment. Clínica Zerenia had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or in the decision to submit the paper for publication.
Results
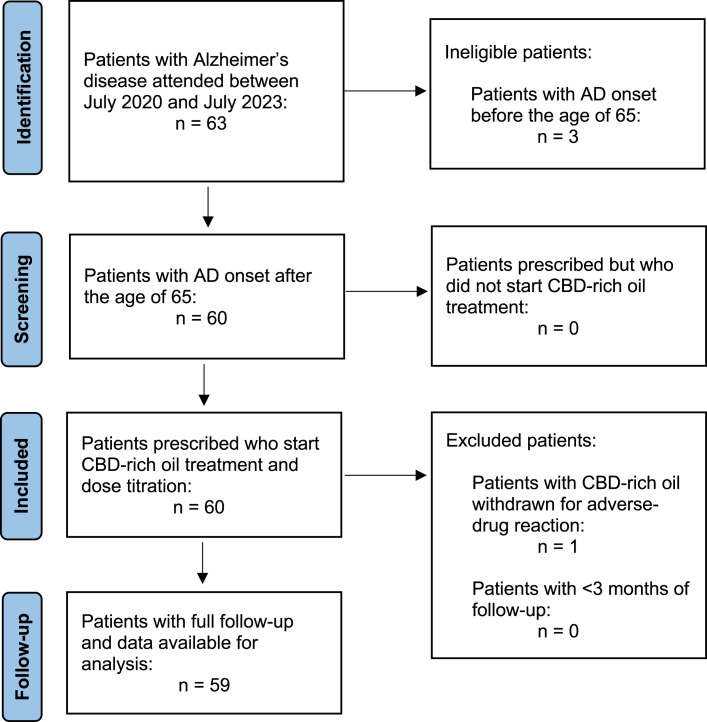
Between July 2020 and July 2023, 63 patients were evaluated at our medical cannabis clinic. Of them, 60 (95.2%) patients had AD onset after the age of 65, all of them opted for CBD-rich oil treatment (60 patients could pay for CBMF). Fifty-nine (93.5%) patients completed ≥3 months of follow-up, 55 patients completed ≥6 months, and 51 subjects had ≥12 months of follow-up (Fig. 1). Table 1 shows patients’ baseline clinical characteristics. Their mean age was 78.9 years (SD 7.6) and had been living with AD for a median of 6 years (IQR 5–7). The median GDS score was 6 (IQR 5–7). Twenty-nine patients (29 of 59; 49.2%) were treated with memantine in combination with an AChEI. Thirty patients (30 of 59; 50.8%) used rivastigmine, and 29 of 59 (49.2%) were treated with donepezil. None of the patients were treated with galantamine. The median NPI-Q severity and caregiver’s distress scores at baseline was 24 (IQR 23–26) and 29 (IQR 26–33), respectively.
Fig. 1.
STROBE flow diagram.
Table 1.
Baseline characteristics of patients with Alzheimer’s disease treated with CBD-rich oil
| Variable | Number (%) | Mean (SD) | Median (IQR) |
|---|---|---|---|
| Male | 15 (25.4) | ||
| Female | 44 (74.6) | ||
| Age, years | 78.9 (7.6) | 79 (73–84) | |
| Age at AD diagnosis, years | 72.1 (6.6) | 71 (68–77) | |
| Years with AD | 6.7 (2.4) | 6 (5–7) | |
| Global Deterioration Scale | 6.1 (0.8) | 6 (5–7) | |
| Weight, kg | 74.7 (11.2) | 76 (65–84.5) | |
| Patients treated with memantine | 29 (49.2) | ||
| Patients treated with donepezil | 29 (49.2) | ||
| Patients treated with galantamine | 0 (0) | ||
| Patients treated with rivastigmine | 30 (50.8) | ||
| Patients treated with memantine plus AChEI | 29 (49.2) | ||
| NPI-Q severity score without CBD | 24.2 (2.6) | 24 (23–26) | |
| NPI-Q caregiver’s distress score without CBD | 28.9 (6.0) | 29 (26–33) |
AChEI, Acetylcholinesterase inhibitor; AD, Alzheimer’s disease; CBD, cannabidiol; IQR, interquartile range; NPI-Q, Neuropsychiatric Inventory Questionnaire; SD, standard deviation.
Effectiveness Outcomes
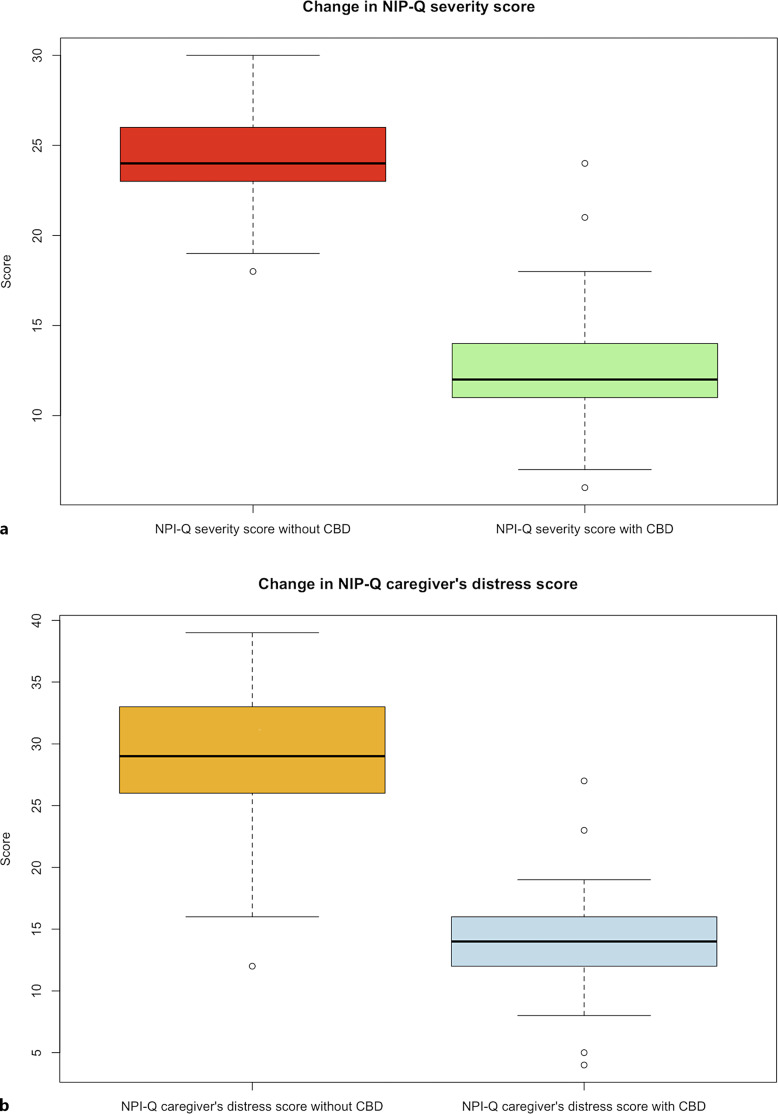
The patients were being treated with CBMF for a mean time of 23.2 months (SD 9.97). The median daily dose of CBD was 111 mg (IQR 79–144.5), and that of CBD per kilogram of weight was 1.49 mg (IQR 0.99–1.96). The maximum CBD dose was 196 mg daily (3.92 mg/kg/day). In the male subgroup, the median daily dose of CBD was 112 mg (IQR 84.5–136.5), and in the female subgroup was 108 mg (IQR 78.25–150.25) (median difference between subgroups, p = 0.9792). At 3 months of treatment, the median NPI-Q severity score was 12 (IQR 11–14) (median difference, p < 0.001) (Fig. 2a); and the median NPI-Q caregiver’s distress score was 14 (IQR 12–16) (median difference, p < 0.001) (Fig. 2b). The proportion of patients who achieved a reduction in the NPI-Q severity score of >30% (response rate) was 94.9% (56 of 59); and a reduction of >50% occurred in 54.2% of patients (33 of 59). A decrease in the NPI-Q caregiver’s distress score of >30% was documented in 94.9% of subjects (56 of 59); 86.4% (51 of 59) had a reduction in the NPI-Q caregiver’s distress score of >50% (Table 2).
Fig. 2.
NPI-Q scores before and after cannabidiol therapy. a Red and green boxplots show the median NPI-Q severity score at baseline of 24 (IQR 23–26) and its reduction after CBD-rich oil treatment to a median of 12 (IQR 11–14), respectively (p < 0.001). b Orange and blue boxplots show the median NPI-Q caregiver’s distress score at baseline of 29 (IQR 26–33) and its reduction after CBD-rich oil treatment to a median of 14 (IQR 12–16), respectively (p < 0.001).
Table 2.
Outcomes of patients with Alzheimer’s disease who achieve the response rates at 12 weeks after treatment with CBD-rich oil
| Outcome | Number (%) | Mean (SD) | Median (IQR) |
|---|---|---|---|
| NPI-Q severity score | 12.5 (3.2) | 12 (11–14) | |
| NPI-Q severity score >30% | 56/59 (94.9) | ||
| NPI-Q severity score >50% | 33/59 (54.2) | ||
| NPI-Q caregiver’s distress score | 13.8 (3.7) | 14 (12–16) | |
| NPI-Q caregiver’s distress score >30% | 56/59 (94.9) | ||
| NPI-Q caregiver’s distress score >50% | 51/59 (86.4) |
CBD, cannabidiol; IQR, interquartile range; NPI-Q, Neuropsychiatric Inventory Questionnaire; SD, standard deviation.
In patients who completed ≥6 months of follow-up, 94.6% (53 of 56) had a sustained reduction in the NPI-Q severity score of >30%, and 55.4% (31 of 56) had a reduction of >50%. The reduction in the NPI-Q caregiver’s distress score of >30% was achieved in 94.6% (53 of 56) of subjects, and a reduction of >50% was observed in 87.5% (49 of 56). The majority of patients have a sustained response at 12, 18 and 24 months (Table 3).
Table 3.
Proportion of patients with Alzheimer’s disease with a sustained response at 6, 12, 18, and 24 months after treatment with CBD-rich oil
| Outcome | At 6 months (%) | At 12 months (%) | At 18 months (%) | At 24 months (%) |
|---|---|---|---|---|
| NPI-Q severity score >30% | 53/56 (94.6) | 49/51 (96.1) | 37/39 (94.9) | 30/32 (93.8) |
| NPI-Q severity score >50% | 31/56 (55.4) | 27/51 (52.9) | 23/39 (59) | 17/32 (53.1) |
| NPI-Q caregiver’s distress score >30% | 53/56 (94.6) | 49/51 (96.1) | 37/39 (94.9) | 30/32 (93.8) |
| NPI-Q caregiver’s distress score >50% | 49/56 (87.5) | 46/51 (90.2) | 35/39 (89.7) | 28/32 (87.5) |
CBD, cannabidiol; NPI-Q, Neuropsychiatric Inventory Questionnaire.
Considering each domain of the NPI-Q severity score, the CBD treatment had a positive result in each of them (Table 4). The doses of CBMF and previous NPI-Q severity scores did not correlate at 12 weeks (Spearman’s coefficient = 0.234702; p = 0.07357) and did not correlate with previous NPI-Q caregiver’s distress scores either (Spearman’s coefficient = −0.03335825; p = 0.8015). No variable was associated with the response rate to CBMF at 12 weeks, as per the logistic regression analysis.
Table 4.
Domains of neuropsychiatric inventory questionnaire severity score at baseline and at 12 weeks after treatment with CBD-rich oil
| Domain NPI-Q severity | Median at baseline (IQR) | Median at 12 weeks (IQR) | Median differencea |
|---|---|---|---|
| Delusions | 2 (1–3) | 1 (0–2) | p <0.001 |
| Hallucinations | 2 (1–3) | 1 (0–2) | p <0.001 |
| Agitation/aggression | 2 (1–3) | 1 (0–2) | p <0.001 |
| Depression/dysphoria | 2 (1–2) | 1 (0–1) | p <0.001 |
| Anxiety | 2 (1–3) | 1 (0–2) | p <0.001 |
| Elation/euphoria | 2 (1–3) | 1 (0–2) | p <0.001 |
| Apathy/indifference | 2 (1–3) | 1 (0–2) | p <0.001 |
| Disinhibition | 2 (1–3) | 1 (0–2) | p <0.001 |
| Irritability/lability | 2 (1–3) | 1 (0–2) | p <0.001 |
| Motor disturbance | 2 (1–2.5) | 1 (0–2) | p <0.001 |
| Nightime behaviors | 2 (1–3) | 1 (0–2) | p <0.001 |
| Appetite/eating | 2 (1–3) | 1 (0–2) | p <0.001 |
CBD, cannabidiol; IQR, interquartile range; NPI-Q, Neuropsychiatric Inventory Questionnaire.
aWilcoxon test.
Safety Outcomes
The only patient with ADR was a 75-year-old man living with AD for 11 years and a GDS of 7. He has a dose of 2.05 mg/Kg/day of CBD (119 mg in total), and during the dose-titration phase he developed somnolence. Because the patient had an initial improvement in the NPI severity score (20 points before treatment) the family decided to continue with the CBMF and wait for the patient to tolerate it. After 8 weeks of treatment and no improvement of somnolence, the CBD-rich oil was suspended. None of the patients showed any abnormalities in laboratory tests during the follow-up (Table 5).
Table 5.
Laboratory results for the 51 patients who had ≥12 months of follow-up with CBD-rich oil treatment
| Laboratory test | Mean at baseline | Mean at 12 months |
|---|---|---|
| Neutrophils, cells/μL | 4,150 | 4,010 |
| Lymphocytes, cells/μL | 3,810 | 3,770 |
| Hemoglobin, g/dL | 12.7 | 12.5 |
| Hematocrit, % | 33.8 | 32.7 |
| Platelets, cells/μL | 251,000 | 285,000 |
| Alanine aminotransferase, U/L | 37.5 | 34.1 |
| Aspartate aminotransferase, U/L | 26.7 | 27.1 |
| Total bilirubin, mg/dL | 0.99 | 0.98 |
| Gamma-glutamyl transferase, U/L | 32.2 | 31.8 |
| Alkaline phosphatase, U/L | 88.2 | 89.6 |
| Serum creatinine, mg/dL | 1.02 | 1.1 |
| Blood urea nitrogen, mg/dL | 20.4 | 21.7 |
CBD, cannabidiol; g/dL, gram per deciliter; mg/dL, milligram per deciliter; µL, microliter; U/L, units per liter.
Discussion
This study shows that treatment with CBD-rich oil is effective and safe for patients with NPS secondary to AD at a median daily dose of 111 mg, independent of sex, age, time with AD, type of AChEIs, and NPI-Q severity score before CBD treatment. The improvement was maintained over time. The reduction in the NPI-Q severity score of >30% was evident in the majority of sample (94.9%), as well as the reduction of >50% (54.2%). Each of the 12 domains of the scale was positively impacted. Also, the median NPI-Q caregiver’s distress score was reduced significantly and the decrease in NPI-Q caregiver’s distress score of >50% was observed in 86.4% of the cases, which suggest that the caregiver benefits significantly from the intervention. The last is an important factor that may impact the patient’s daily care and influence admission to a long-term care facility [4, 23]. The dose of CBD was not high (median of 1.49 mg/kg/day), and it was tolerated by almost all patients who initiated the intervention, except one male-patient who experienced somnolence not complicated by any other symptoms. This case was resolved completely after CBMF suspension. The low incidence of ADR may be due to the slow dose titration of CBD, and the exclusion of patients with baseline cardiac, liver, renal, or hematological laboratory abnormalities; as well as the exclusion of patients using antidepressants, antipsychotics, or benzodiazepine therapy. Therefore, patients with multi-comorbidity were not included and the drug-drug interaction was mitigated.
To the best of our knowledge, this is the first study that evaluated the use of CBD as a treatment in NPS in AD with a sample greater than 50 patients and with a follow-up of more than 1 year. A previously published study was a double-blind, placebo-controlled trial evaluating CBD in 8 patients and placebo in 7 patients, with an adherence to treatment evaluated at 6 weeks [16]. The mean age was similar (77.91 years), but the CBD doses were far superior (200 mg and titrated up to 600 mg/day). Regarding safety, the incidence of ADR was higher (dizziness 63%, falls 25%) compared to our study, which may be explained by the high doses of CBD used. Similar to our study, the NPI rating scale was reduced in comparison to placebo (−29.86 vs. −10.14 points) for hallucinations, anxiety, agitation, apathy/indifference, and irritability/lability. However, in addition to these domains, our study shows improvement in depression/dysphoria, elation/euphoria, disinhibition, motor disturbance, night-time behaviors, and appetite/eating domains. Our study is different from the study of Hermush et al. [24] where they evaluated the effect of a CBD-rich oil in a sample of 60 patients, but after the randomization and allocation, 40 patients received CBD and 20 patients received placebo. Another methodological difference is that they included patients over 60 years of age with any type of dementia (not only AD). After 16 weeks of follow-up, they found a statistically significant difference in the proportion of patients who had a Cohen-Mansfield Agitation Inventory score reduction of ≥4 points (60% vs. 30%) for CBD and control groups, respectively (p = 0.03). In other studies, dronabinol has been evaluated to treat NPS in AD [10–15]. These studies are characterized because the sample was between 2 and 40 patients, and the follow-up lasted only for a short time (2–6 weeks). Aggressiveness and nocturnal motor activity were improved, but other symptoms were not evaluated as in this study.
As cognitive decline and NPS progress, people with Alzheimer’s go from being vulnerable to being completely dependent on another person for daily care. Informal caregiving can be devastating to physical and mental health due to the additional burden and stress associated with caring for people with dementia. Chi et al. [25] estimated that 22.3% of informal caregivers of people with dementia reported emotional difficulties, compared to 9.5% of informal caregivers of older adults without dementia. The lack of effective treatments for NPS has led caregivers to explore alternative interventions, including medical cannabis [26]. To our knowledge, the effect of CBD on caregiver burden in AD has not been evaluated previously. This study showed a significant reduction in the NPI-Q caregiver’s distress score from 29 points to 14 points after 12 weeks of intervention. This may indicate that reducing NPS in people with AD facilitates daily caregiving and improves caregivers’ emotional and physical distress. The possibility of controlling NPS in terms of agitation, anxiety, and insomnia are the main reasons why caregivers may consider using CBD-rich oil as part of patients’ treatment [27, 28].
The mechanisms through which CBD may be beneficial in NPS secondary to AD are not fully understood. CBD modulates the CB1 and CB2 endocannabinoid receptors and other orphan G-protein-coupled receptors such as Transient Potential Receptor Vanilloid-1 (TRPV1), the G Protein-Coupled Receptor-55 (GPR55), and the Equilibrative Nucleoside Transporter-1 (ENT-1) [9, 22]. The TRPV1 is involved in the regulation of affect, memory, and appetite by modulating AMPA receptor function; the GPR55 down-regulates pro-inflammatory pathways and calcium influx into mitochondria; and the ENT-1 potentiates anti-inflammatory pathways through adenosine function. CBD has also been described as an N-methyl-d-aspartate antagonist, which has a similar function to memantine [21]. The CB2 activation has been related to the removal of amyloid-beta plaques from human AD tissues [29]. On the other hand, CBD has been related to a reduction in the activity of glycogen synthase kinase-3 beta (GSK-3B), a tau kinase, resulting a lower tau hyperphosphorylation [30, 31]. These effects may modify the progression of AD because amyloid-beta accumulation contributes to the dysregulation of calcium homeostasis and excessive activation of the N-methyl-d-aspartate receptor, inducing excitotoxicity and neuronal death [32].
Animal models with CB1 receptor knockout mice were observed to have anxiety, depression, and aggressive behavior [33–35]. Other observations include a significant decrease in cannabinoid receptor binding in the cerebellum, cerebral cortex, limbic system, hypothalamus, and hippocampus [36]. The endocannabinoid system may improve dopamine neurotransmission, modulate the release of norepinephrine, and activate the serotonin system (binding with 5-HT1A receptor), which are molecules related to agitation, aggression, depression, psychosis, and apathy in AD patients [9]. The activation of the 5-HT1A receptor has been associated with antidepressant and anxiolytic effects [37]; also, CBD may increase the availability of tryptophan, increasing serotonin production [38]. Similarly, the analgesic effect of cannabinoids and the improvement in insomnia may reduce the level of anxiety, aggressiveness, and agitation in AD patients.
Limitations
This study has several limitations. The small sample size is explained by (1) the significant number of patients with AD being treated with antipsychotic or antidepressant drugs, which was an exclusion criterion; and (2) CBD treatment in Colombia is expensive and not covered by the healthcare system for the adult population, so fewer patients might be able to afford it; this explained the loss to follow-up after the first year of treatment. Another limitation is that the study has a lack of blinding and a lack of a placebo-control group, but it was controlled by selecting a quasi-experimental design (pre-post intervention). Although CBD has an affinity for multiple receptors that modulate the neurotransmission in the physiopathology of NPS, it may not be ruled out that the placebo effect explains the effectiveness observed in patients, which may be due to regression to the mean or psychosocial response to an intervention [24, 39].
This study is benefited from a structured and rigorous observation period with a continuous slow dose titration and ADR monitoring to ensure tolerance. Another point in favor is the restrictive inclusion criteria which allow conclusions to be drawn only for the population with NPS secondary to AD.
Conclusion
There is a need for an alternative treatment to significantly improve NPS in AD and decrease the caregiver’s stress as well as the financial burden resulting from polypharmacy and institutionalization. Any promising treatment should be safe and reduce the risk of adverse effects. This study shows that CBMF (high-CBD/low-THC 50:1) is an effective and safe therapy to treat NPS in AD patients while also reducing caregivers’ distress. This effectiveness was independent of age, sex, years with AD, type of AChEIs, and NPI-Q severity score before CBD treatment. A low CBD dose and a slow dose titration improve tolerance. Further information derived from randomized controlled clinical trials is needed to confirm the findings presented in this article.
Acknowledgments
The authors thank patients and their families who agreed to participate in this study.
Statement of Ethics
This study was reviewed and approved by Clínica Zerenia Review Board, Approval No. (012–2021). Patients provided written informed consent to participate and to use a CBMF. Written informed consent was obtained from the patient reported in Safety Outcomes paragraph for publication of the details of his medical case.
Conflict of Interest Statement
Cristian E. Navarro worked at Clínica Zerenia in Bogotá (Colombia) which is a reference center specializing in medical cannabis therapy owned by Khiron Life Science Corp®; Khiron Life Science Corp® manufactures the CBMF used in this study. Juan C. Pérez has nothing to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. E.S.E Hospital Emiro Quintero Cañizares was the source of funding for the article publication charges.
Author Contributions
C.E.N: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing – original draft, and writing-review and editing. J.C.P: formal analysis, investigation, supervision, validation, writing – original draft, and writing – review and editing.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. E.S.E Hospital Emiro Quintero Cañizares was the source of funding for the article publication charges.
Data Availability Statement
Due to reasons of patient data sensitivity, the data cannot be found openly available; however, the dataset will be anonymized and made available from the authors upon reasonable request from qualified investigators.
Supplementary Material.
References
- 1. Wang J, Yu JT, Wang HF, Meng XF, Wang C, Tan CC, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86(1):101–9. [DOI] [PubMed] [Google Scholar]
- 2. Sultzer D, Mahler M, Mandelkern M, Cummings JL, Van Gorp WG, Hinkin CH, et al. The relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1995;7(4):476–84. [DOI] [PubMed] [Google Scholar]
- 3. Kaufer D. Beyond the cholinergic hypothesis: the effect of metrifonate and other cholinesterase inhibitors on neuropsychiatric symptoms in Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9(Suppl 2):8–14. [DOI] [PubMed] [Google Scholar]
- 4. Lopera F, Custodio N, Rico-Restrepo M, Allegri RF, Barrientos JD, Garcia Batres E, et al. A task force for diagnosis and treatment of people with Alzheimer’s disease in Latin America. Front Neurol. 2023;14:1198869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herrmann N, Lanctôt KL. Do atypical antipsychotics csause stroke? CNS Drugs. 2005;19(2):91–103. [DOI] [PubMed] [Google Scholar]
- 6. Magierski R, Sobow T, Schwertner E, Religa D. Pharmacotherapy of behavioral and psychological symptoms of dementia: state of the art and future progress. Front Pharmacol. 2020;11:1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mok PLH, Carr MJ, Guthrie B, Morales DR, Sheikh A, Elliott RA, et al. Multiple adverse outcomes associated with antipsychotic use in people with dementia: population based matched cohort study. BMJ. 2024;385:e076268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seitz DP, Adunuri N, Gill SS, Gruneir A, Herrmann N, Rochon P. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011(2):CD008191. [DOI] [PubMed] [Google Scholar]
- 9. Liu CS, Chau SA, Ruthirakuhan M, Lanctôt KL, Herrmann N. Cannabinoids for the treatment of agitation and aggression in Alzheimer’s disease. CNS Drugs. 2015;29(8):615–23. [DOI] [PubMed] [Google Scholar]
- 10. Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 1997;12(9):913–9. [PubMed] [Google Scholar]
- 11. Walther S, Mahlberg R, Eichmann U, Kunz D. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacol Berl. 2006;185(4):524–8. [DOI] [PubMed] [Google Scholar]
- 12. Mahlberg R, Walther S. Actigraphy in agitated patients with dementia. Monitoring treatment outcomes. Z Gerontol Geriatr. 2007;40(3):178–84. [DOI] [PubMed] [Google Scholar]
- 13. Walther S, Schüpbach B, Seifritz E, Homan P, Strik W. Randomized, controlled crossover trial of dronabinol, 2.5 mg, for agitation in 2 patients with dementia. J Clin Psychopharmacol. 2011;31(2):256–8. [DOI] [PubMed] [Google Scholar]
- 14. Woodward MR, Harper DG, Stolyar A, Forester BP, Ellison JM. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. 2014;22(4):415–9. [DOI] [PubMed] [Google Scholar]
- 15. Palmieri B, Vadalà M. Oral THC:CBD cannabis extract in main symptoms of Alzheimer disease: agitation and weight loss. Clin Ter. 2023;174(1):53–60. [DOI] [PubMed] [Google Scholar]
- 16. Velayudhan L, Dugonjic M, Pisani S, Harborow L, Aarsland D, Bassett P, et al. Cannabidiol for behavior symptoms in Alzheimer’s disease (CANBiS-AD): a randomized, double-blind, placebo-controlled trial. Int Psychogeriatr. 2024:1–3. [DOI] [PubMed] [Google Scholar]
- 17. Ahmed A, van der Marck M, van den Elsen G, Olde Rikkert M. Cannabinoids in late‐onset Alzheimer’s disease. Clin Pharmacol Ther. 2015;97(6):597–606. [DOI] [PubMed] [Google Scholar]
- 18. Ballard C, Thomas A, Gerry S, Yu LM, Aarsland D, Merritt C, et al. A double-blind randomized placebo-controlled withdrawal trial comparing memantine and antipsychotics for the long-term treatment of function and neuropsychiatric symptoms in people with Alzheimer’s disease (MAIN-AD). J Am Med Dir Assoc. 2015;16(4):316–22. [DOI] [PubMed] [Google Scholar]
- 19. Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–9. [DOI] [PubMed] [Google Scholar]
- 21. Navarro CE. Cannabis-based magistral formulation is highly effective as an adjuvant treatment in drug-resistant focal epilepsy in adult patients: an open-label prospective cohort study. Neurol Sci. 2023;44(1):297–304. [DOI] [PubMed] [Google Scholar]
- 22. Navarro CE. Endocannabinoid system and the role of medical cannabis in treating spasticity: a narrative review. Iatreia. 2024;31(1):55–70. [Google Scholar]
- 23. Outen JD, Burhanullah MH, Vandrey R, Amjad H, Harper DG, Patrick RE, et al. Cannabinoids for agitation in Alzheimer’s disease. Am J Geriatr Psychiatry. 2021;29(12):1253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hermush V, Ore L, Stern N, Mizrahi N, Fried M, Krivoshey M, et al. Effects of rich cannabidiol oil on behavioral disturbances in patients with dementia: a placebo controlled randomized clinical trial. Front Med. 2022;9:951889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chi W, Graf E, Hughes L. Older adults with dementia and their caregivers: key indicators from the national health and aging trends study. US Department of Health and Human Services; 2019. p. 1–54 [cited 2024 Apr 25] Available from: https://aspe.hhs.gov/reports/community-dwelling-older-adults-dementia-their-caregivers-key-indicators-national-health-aging [Google Scholar]
- 26. Kaskie B, Bobitt J, Herrera J, Bhagianadh D, Segal-Gidan F, Brummel-Smith K, et al. Cannabis use among persons with dementia and their caregivers: lighting up an emerging issue for clinical gerontologists. Clin Gerontol. 2021;44(1):42–52. [DOI] [PubMed] [Google Scholar]
- 27. Leszko M. Use of cannabidiol oil by caregivers: a focus on Alzheimer’s disease. Medicinal usage of cannabis and cannabinoids Elsevier; 2023. p. 129–34. [Google Scholar]
- 28. Kłosińska U, Leszko M. CBD oil as a miracle drug: a thematic analysis of caregivers’ attitudes and practices towards cannabidiol in dementia treatment. J Drug Issues. 2024;54(1):38–56. [Google Scholar]
- 29. Tolón RM, Núñez E, Pazos MR, Benito C, Castillo AI, Martínez-Orgado JA, et al. The activation of cannabinoid CB2 receptors stimulates in situ and in vitro beta-amyloid removal by human macrophages. Brain Res. 2009;1283:148–54. [DOI] [PubMed] [Google Scholar]
- 30. Esposito G, De Filippis D, Carnuccio R, Izzo AA, Iuvone T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J Mol Med. 2006;84(3):253–8. [DOI] [PubMed] [Google Scholar]
- 31. Aso E, Juvés S, Maldonado R, Ferrer I. CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AβPP/PS1 mice. J Alzheimers Dis. 2013;35(4):847–58. [DOI] [PubMed] [Google Scholar]
- 32. Smith IF, Green KN, LaFerla FM. Calcium dysregulation in Alzheimer’s disease: recent advances gained from genetically modified animals. Cell Calcium. 2005;38(3–4):427–37. [DOI] [PubMed] [Google Scholar]
- 33. Urigüen L, Pérez-Rial S, Ledent C, Palomo T, Manzanares J. Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology. 2004;46(7):966–73. [DOI] [PubMed] [Google Scholar]
- 34. Steiner MA, Wanisch K, Monory K, Marsicano G, Borroni E, Bächli H, et al. Impaired cannabinoid receptor type 1 signaling interferes with stress-coping behavior in mice. Pharmacogenomics J. 2008;8(3):196–208. [DOI] [PubMed] [Google Scholar]
- 35. Aso E, Ozaita A, Valdizán EM, Ledent C, Pazos Á, Maldonado R, et al. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J Neurochem. 2008;105(2):565–72. [DOI] [PubMed] [Google Scholar]
- 36. Berrendero F, Romero J, Garcıa-Gil L, Suarez I, De la Cruz P, Ramos JA, et al. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim Biophys Acta. 1998;1407(3):205–14. [DOI] [PubMed] [Google Scholar]
- 37. De Gregorio D, McLaughlin RJ, Posa L, Ochoa-Sanchez R, Enns J, Lopez-Canul M, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160(1):136–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jenny M, Schröcksnadel S, Überall F, Fuchs D. The potential role of cannabinoids in modulating serotonergic signaling by their influence on tryptophan metabolism. Pharmaceuticals. 2010;3(8):2647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosenberg PB, Drye LT, Porsteinsson AP, Pollock BG, Devanand DP, Frangakis C, et al. Change in agitation in Alzheimer’s disease in the placebo arm of a nine-week controlled trial. Int Psychogeriatr. 2015;27(12):2059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to reasons of patient data sensitivity, the data cannot be found openly available; however, the dataset will be anonymized and made available from the authors upon reasonable request from qualified investigators.