Abstract
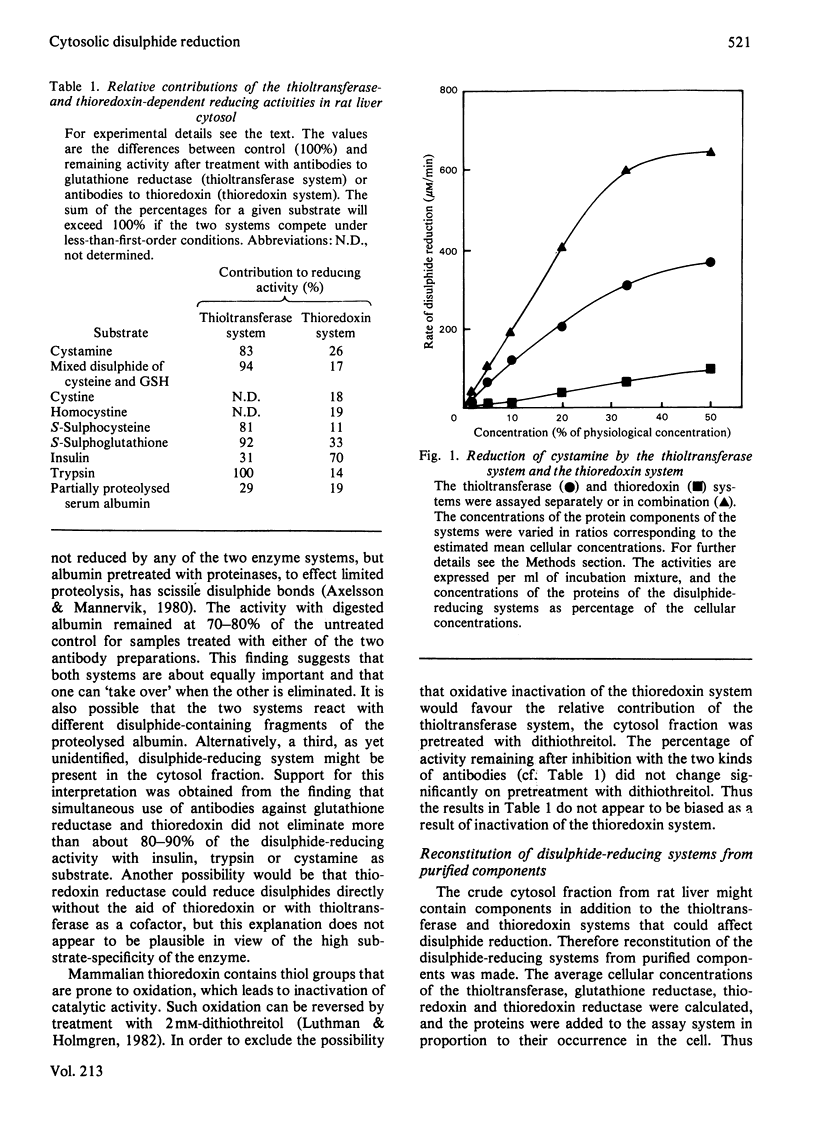
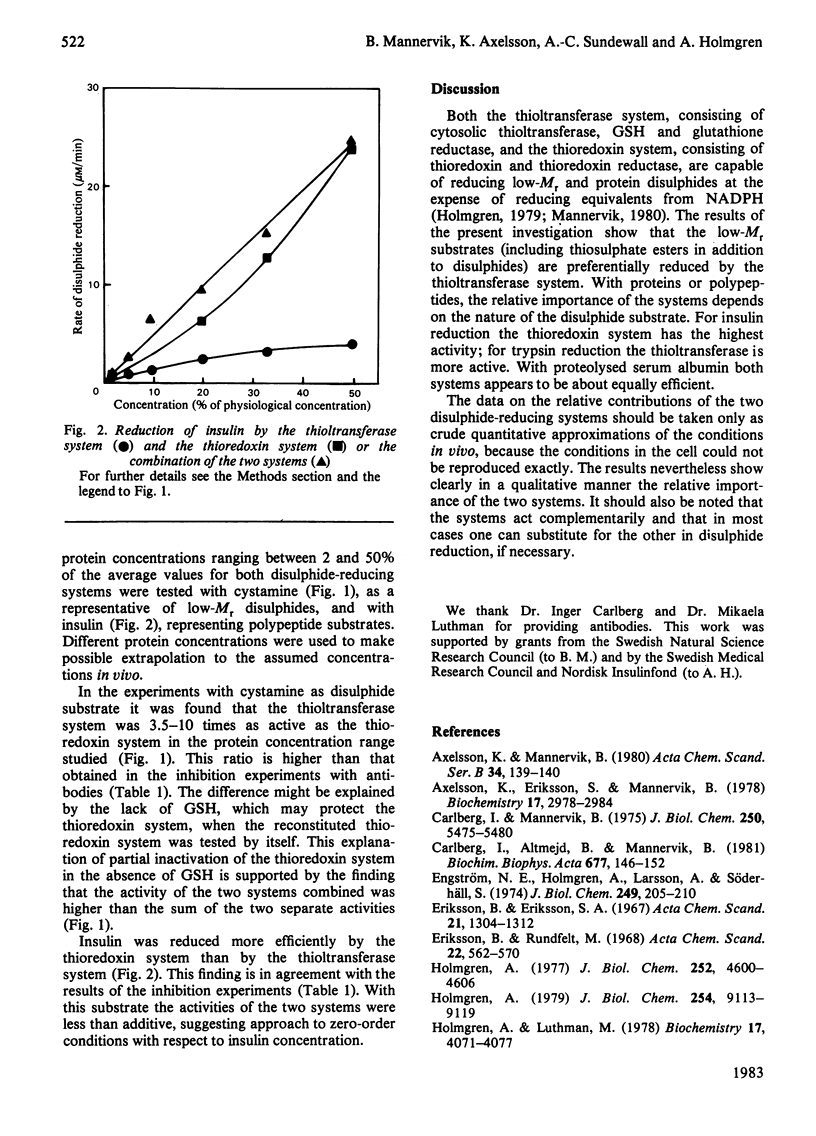
Two enzyme systems capable of reducing disulphide bonds both in low-Mr compounds and in polypeptides and proteins exist. One consists of thioltransferase in combination with reduced glutathione and glutathione reductase, and the second consists of thioredoxin in combination with thioredoxin reductase. Their relative effectiveness in catalysing disulphide reduction of various substrates in rat liver cytosol was evaluated in the present study. The thioltransferase-dependent system was found to be more efficient in reducing small molecules. Insulin was most effectively reduced by the thioredoxin system. Bovine trypsin was a better substrate for thioltransferase, and partially proteolysed bovine serum albumin was equally good for the two systems. Thus, in the case of protein disulphide bonds, the nature of the particular substrate used determines which of the two reducing systems is the more important.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson K., Eriksson S., Mannervik B. Purification and characterization of cytoplasmic thioltransferase (glutathione:disulfide oxidoreductase) from rat liver. Biochemistry. 1978 Jul 25;17(15):2978–2984. doi: 10.1021/bi00608a006. [DOI] [PubMed] [Google Scholar]
- Axelsson K., Mannervik B. A possible role of cytoplasmic thioltransferase in the intracellular degradation of disulfide-containing proteins. Acta Chem Scand B. 1980;34(2):139–140. doi: 10.3891/acta.chem.scand.34b-0139. [DOI] [PubMed] [Google Scholar]
- Carlberg I., Altmejd B., Mannervik B. Purification and immunological studies of glutathione reductase from rat liver. Evidence for an antigenic determinant at the nucleotide-binding domain of the enzyme. Biochim Biophys Acta. 1981 Sep 18;677(1):146–152. doi: 10.1016/0304-4165(81)90156-2. [DOI] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem. 1975 Jul 25;250(14):5475–5480. [PubMed] [Google Scholar]
- Engström N. E., Holmgren A., Larsson A., Söderhäll S. Isolation and characterization of calf liver thioredoxin. J Biol Chem. 1974 Jan 10;249(1):205–210. [PubMed] [Google Scholar]
- Eriksson B., Eriksson S. A. Synthesis and characterization of the L-cysteine-glutathione mixed disulfide. Acta Chem Scand. 1967;21(5):1304–1312. doi: 10.3891/acta.chem.scand.21-1304. [DOI] [PubMed] [Google Scholar]
- Eriksson B., Rundfelt M. Reductive decomposition of S-sulfoglutathione in rat liver. Acta Chem Scand. 1968;22(2):562–570. doi: 10.3891/acta.chem.scand.22-0562. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Bovine thioredoxin system. Purification of thioredoxin reductase from calf liver and thymus and studies of its function in disulfide reduction. J Biol Chem. 1977 Jul 10;252(13):4600–4606. [PubMed] [Google Scholar]
- Holmgren A., Luthman M. Tissue distrubution and subcellular localization of bovine thioredoxin determined by radioimmunoassay. Biochemistry. 1978 Sep 19;17(19):4071–4077. doi: 10.1021/bi00612a031. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Reduction of disulfides by thioredoxin. Exceptional reactivity of insulin and suggested functions of thioredoxin in mechanism of hormone action. J Biol Chem. 1979 Sep 25;254(18):9113–9119. [PubMed] [Google Scholar]
- Luthman M., Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982 Dec 21;21(26):6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Axelsson K., Larson K. Thioltransferase. Methods Enzymol. 1981;77:281–285. doi: 10.1016/s0076-6879(81)77038-1. [DOI] [PubMed] [Google Scholar]
- SEGEL I. H., JOHNSON M. J. Synthesis and characterization of sodium cysteine-S-sulfate monohydrate. Anal Biochem. 1963 Apr;5:330–337. doi: 10.1016/0003-2697(63)90085-x. [DOI] [PubMed] [Google Scholar]


