Abstract
Creating a safe and effective vaccine against infection by the fungal pathogen Cryptococcus neoformans is an appealing option that complements the discovery of new small molecule antifungals. Recent animal studies have yielded promising results for a variety of vaccines that include live-attenuated and heat-killed whole-cell vaccines, as well as subunit vaccines formulated around recombinant proteins. Some of the recombinantly engineered cryptococcal mutants in the chitosan biosynthesis pathway are avirulent and very effective at conferring protective immunity. Mice vaccinated with these avirulent chitosan-deficient strains are protected from a lethal pulmonary infection with C. neoformans strain KN99. Heat-killed derivatives of the vaccination strains are likewise effective in a murine model of infection. The efficacy of these whole-cell vaccines, however, is dependent on a number of factors, including the inoculation dose, route of vaccination, frequency of vaccination, and the specific mouse strain used in the study. Here, we present detailed methods for identifying and optimizing various factors influencing vaccine potency and efficacy in various inbred mouse strains using a chitosan-deficient cda1Δcda2Δcda3Δ strain as a whole-cell vaccine candidate. This chapter describes the protocols for immunizing three different laboratory mouse strains with vaccination regimens that use intranasal, orotracheal, and subcutaneous vaccination routes after the animals were sedated using two different types of anesthesia.
Keywords: Cryptococcosis, Chitosan, Cell wall, Chitin deacetylase, Whole-cell vaccine, Attenuated vaccine, Vaccination dose, Vaccination schedule, Fungal virulence
1. Introduction
The human fungal pathogens Cryptococcus neoformans and Cryptococcus gattii are responsible for the infection known as cryptococcosis. While infections caused by C. neoformans are more common in immunocompromised hosts, infections by C. neoformans and C. gattii have also been reported in apparently healthy hosts [1–4]. This fungus is endemic worldwide and it is thought that the initial infection is caused by inhaling basidiospores or desiccated yeast cells. While the primary site of infection is the lung, yeast may spread to the brain and other organs, resulting in systemic infection and, if left untreated, the death of the infected host. According to estimates by Rajasingham et al. (2022), there are 152,000 cases of cryptococcal meningitis each year, and the illness results in 112,000 fatalities [5]. The World Health Organization has designated Cryptococcus as one of four fungal pathogens with unmet research needs, calling for increased research and development, as well as fresh public health intervention strategies [6]. The preferred anticryptococcal medication regimen is amphotericin B and 5-fluorocytosine. Unfortunately, this combination is extremely toxic and is not widely available in the developing world where the majority of reported cases occur [7, 8]. Fluconazole is frequently used in some areas as an alternative to amphotericin B. It is less effective and there have been reports of drug-resistant isolates emerging [9]. Echinocandins are effective against other fungal infections, but not against C. neoformans [10, 11]. As a result, it is critical to develop safe and effective treatments against cryptococcal infections. The development of vaccines against cryptococcosis would be an optimal strategy for preventing cryptococcal infections.
Various vaccine strategies have been developed for cryptococcal infections, including whole-cell vaccines, subunit vaccines, and glucan particles loaded with protein antigens [12–23]. In a murine infection model, both live and heat-killed forms of whole cells of either wild-type or avirulent recombinant cryptococcal strains have been effective. Multiple vaccination routes, including intradermal, intraperitoneal, subcutaneous, intravenous, intranasal, and intratracheal or orotracheal, were used in these studies. Multiple vaccination frequencies with varying doses of inoculum have been effective. Aside from the type of vaccine and its regimen for delivery, different inbred mouse lines demonstrated varying levels of vaccine effectiveness. A factor that also impacts testing vaccines in different mouse lines is that their inherent resistance to infection is not the same (Fig. 1). To reduce this effect, the number of cells of the virulent strain used for infection post-vaccination may be adjusted for the mouse line being used.
Fig. 1.
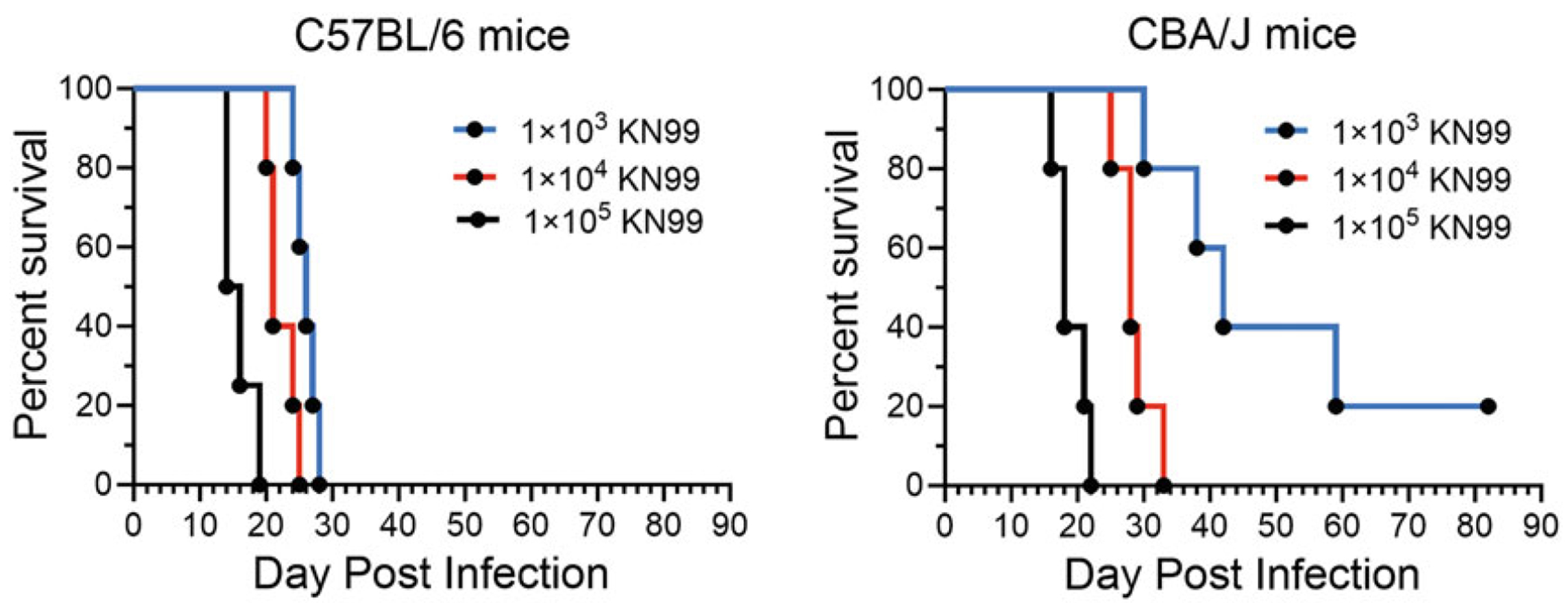
Effect of the number of colony forming units (CFUs) of KN99 used for infection on the survival of unvaccinated C57BL/6 and CBA/J mice. Groups of five mice were infected intranasally with 1 × 103, 1 × 104, or 1 × 105 cells (CFUs) of KN99. Mice were monitored for survival for 84 days.
In 1960, the first whole-cell vaccines against Cryptococcus infection were tested, using various strains, such as those attenuated for virulence, hypocapsular strains, and heat-killed cells. When challenged with a virulent strain, all show varying degrees of protection [24]. Immunization with wild-type yeast cells whose capsules have been stripped by gamma irradiation or enzymatic treatments also elicit varying degrees of protective immunity [25, 26]. A robust immunity against infection with a virulent strain of C. neoformans was achieved when strain H99 was engineered to produce murine INF-γ (H99γ) and used for vaccination. In this case, a 5 × 104 colony-forming unit (CFU) dose of live H99γ administered intranasally is effective in inducing long-lasting protective immunity in BALB/c mice [18]. Following the success of this whole-cell vaccine, several strains of Cryptococcus were engineered to delete genes associated with virulence. These strains were avirulent in mice, and when tested as vaccines, they demonstrated effective protective immunity against challenge with virulent progenitor strains [17, 20–22]. In all of these cases, either the intranasal or orotracheal route was employed for primary vaccination. Subsequent booster doses of the vaccine improved protective immunity in various inbred mouse strains when administered intranasally or subcutaneously. A single dose of 107 cells of the live Znf2oe strain is sufficient to induce protective immunity in A/J mice; however, a second intranasal booster dose is required for immunity when heat-killed cells are used for vaccination [22]. In CBA/J mice, intranasal vaccination with 5 × 105 live cells of a Cryptococcus mutant that lacks steryl glucosidase (sgl1Δ) elicits a robust protective immunity against a C. neoformans challenge [21]. However, in the case of another Cryptococcus mutant that lacks Fbp1, while two doses of vaccination with the fbp1Δ cells confer complete protection in BALB/c mice, it only confers partial protection in C57BL/6 J and A/J mouse strains [20]. Furthermore, it has been discovered that protective immunity is dose dependent: 5 × 107 cells provide complete protection, 2.5 × 107 and 1.0 × 107 cells provide partial protection, and 0.5 × 107 cells fail to induce protective immunity [27]. These tests have been carried out on BALB/c mice infected with the virulent H99 strain [27].
We have been characterizing the chitosan-deficient cryptococcal vaccine strain cda1Δcda2Δcda3Δ, which lacks three chitin deacetylases and produces no chitosan, and we have also observed dose-dependent protective immunity [17]. When delivered through intranasal inoculation, a minimum dose of 107 cda1Δcda2Δcda3Δ cells was required to induce protective immunity in CBA/J mice. While a single dose of 107 cda1Δcda2Δcda3Δ induced strong protective immunity in CBA/J and A/J mice, it was only partially effective in protecting C57BL/6 and BALB/c mice. In addition to cda1Δcda2Δcda3Δ, when used in its heat-killed form, the avirulent cda1Δcda2Δ strain, which has a reduced amount of chitosan, also induced robust protective immunity in CBA/J mice, but it was ineffective as a vaccine in C57BL/6 mice [19]. However, when a live culture of the cda1Δcda2Δ strain was used at a single dose of 107 cells, it was effective as a vaccine in C57BL/6 mice [19]. Additional findings clearly showed that when whole cells are used as a vaccine, several factors, including the number of cells per dose, the number of doses, and the route of vaccination, affect the potency and efficacy of the mutant strain as a vaccine. Furthermore, these variables have different effects on the nature of protective immunity in different inbred laboratory mouse strains. Therefore, using cda1Δcda2Δcda3Δ as an example, we will present a general method for determining the inoculum dose, mode of vaccination, and effect of these vaccination regimens on the protective immunity in different inbred laboratory mouse strains against a lethal pulmonary infection with the KN99 strain. KN99 is particularly virulent and was created by ten backcrosses to the extensively researched clinical strain H99 [28]. Over the course of several years at different institutions, we have developed two protocols that are equally effective at generating and testing the cda1Δcda2Δcda3Δ vaccine, but differ in some details. For example, we use different agents for anesthesia (ketamine/dexmedetomidine and isoflurane) and different routes to introduce Cryptococcus into the lungs (intranasal and orotracheal). We have been working principally with CBA/J mice at Washington University, St. Louis (WashU), and with BALB/c and C57BL/6 mice at University of Massachusetts Chan Medical School (UMASS). Working from a common set of materials, we note how the protocols differ by institution, although comparable vaccine efficacies are found.
2. Materials
2.1. Culturing of Cryptococcus Strains for Preparation of Live and Heat-Killed cda1Δcda2Δcda3Δ Vaccines and for Infection with KN99
Cryptococcus is a Biosafety Level 2 (BSL-2) organism. Personnel working in the BSL2 lab must have general training in handling pathogenic agents. It is necessary to have Institutional Biosafety Committee (IBC) approval to study Cryptococcus. As a BSL2 pathogen, work with Cryptococcus can be done on an open bench top as long as aerosols are not generated. But if the procedure involves the potential release of aerosols, working in a biosafety hood is preferable [29]. All culture-related materials are autoclaved prior to disposal. We recommend double distilled or Milli-Q water (18 megaohm) for making solutions and media.
YPD liquid medium: 10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose; for plates, add 20 g/L agar.
Sabouraud Dextrose Agar Emmons.
30 °C incubator for incubating culture plates.
Incubator for shaking cultures capable of maintaining 300 rpm and 30 °C.
14 mL polypropylene round-bottom tubes with cap for 4 mL cultures.
Wood applicators, 6 inches long, or inoculation loops to transfer cells.
2 mL cryogenic vials.
50% glycerol.
250 mL polycarbonate Erlenmeyer flasks with vented cap.
50 mL centrifuge tubes.
Centrifuge with a 1500 rpm (425 × g) minimum speed and rotor for 14 mL and 50 mL centrifuge tubes.
Dulbecco’s phosphate buffered saline (PBS), endotoxin-free.
Hemocytometer: Standard glass, or disposable hemocytometer or a TC20 automated cell counter and counting slides.
Light microscope to view hemocytometer.
Water or dry bath incubator at 70 °C to heat-kill Cryptococcus.
2.2. Vaccination of Mice with cda1Δcda2Δcda3Δ or Infection with KN99 Cell Suspensions
Approved Institutional Animal Care and Use Committee (IACUC) protocols.
Ear tags or other means of identification to aid in recording the weight of individual mice during a study.
Digital scale accurate to 0.1 g to measure the weight of a mouse.
Anesthesia (WashU): ketamine (8 mg/mL)/dexmedetomidine (0.05 mg/mL) mixture.
Atipamezole (200 μL) (0.25 mg/mL) to reverse effects of anesthesia.
Syringe (1 mL with 27G 0.4 mm × 13 mm hypodermic needle) for injections.
Veterinary eye ointment to counter eye dryness during anesthesia.
Anesthesia (UMASS Dept. of Animal Medicine): isoflurane, USP.
Anesthesia chamber for small rodents equipped with F/air carbon canister to trap isoflurane. Alternatively, anesthesia with isoflurane may be done in a chemical fume hood without an anesthesia chamber.
Glass jar with screw-on lid (height 4.5–5″, diameter 4.0–4.5″).
Absorbent pad (Nestlet pad, 2″ × 2″).
Intubation stand to suspend the mouse for intranasal or orotracheal inoculation.
Dental tape to suspend mouse on intubation stand.
100 μL pipettor and tips to administer 50 μL of cell suspension.
Vortex mixer for mixing the inoculum.
Heating pad for the postinoculation recovery of the animals.
0.3 mL insulin syringe, 29G × 1/2″ for subcutaneous injection of vaccine.
2.3. Determining Lung CFUs
Pen/Strep: 10,000 U/mL penicillin, 10,000 μg/mL streptomycin.
PBS Pen/Strep: Adjust PBS to 200 U/mL penicillin and 200 μg/mL streptomycin to homogenize lungs and make serial dilutions of homogenates to plate for CFUs.
Homogenizer with appropriate probes: PRO200 homogenizer connected to a 7 mm diameter PRO quick connect probe with a saw-tooth bottom or Omni TH tissue homogenizer with tip adaptor for 7 mm × 110 mm hard tissue probe.
CFU counting pen.
GraphPad Prism software to analyze data.
3. Methods
Evaluate the Vaccine‘s Efficacy in CBA/J Mice (Wash U).
3.1. Make Stocks of Cryptococcus Strains for Long-Term Storage
Strains of Cryptococcus are stored at −80 °C and serve as the primary source for starting cultures used to inoculate mice. Under sterile conditions, cells from the frozen stock may be scraped using either a sterile wood applicator or a sterile loop, then transferred to an YPD agar plate and streaked.
Add 4 mL of liquid YPD medium to a 14 mL culture tube.
Use a sterile wood applicator to transfer cells from agar medium to the culture tube. Briefly vortex to suspend cells.
Shake culture tube at approximately a 70° angle at 220 rpm and 30 °C for 48 h.
Combine 0.9 mL of culture with 0.9 mL 50% sterile glycerol and mix.
Store at −80 °C.
3.2. Preparing the Inoculum
The required strains of Cryptococcus are always kept at −80 °C as stocks in YPD medium containing 25% glycerol. Strains from the glycerol stocks KN99 (Lodge Lab Stock # JLCN 434) and cda1Δcda2Δcda3Δ (Lodge lab Stock # LBCN 632) are each streaked on YPD agar plates and incubated for 72 h at 30 °C.
In a 250 mL Erlenmeyer flask with a vented cap, inoculate a loop of yeast cells into 50 mL of YPD medium, as in Fig. 2. Incubate the flask at 30 °C while shaking at 300 rpm for 48 h. These growth conditions are critical to the vaccine‘s efficacy.
Transfer the 50 mL culture to a 50 mL falcon tube and centrifuge for 10 min at 3300 × g and 22 °C. Carefully decant the medium without disturbing the cell pellet. Sterilize the collected medium with 10% bleach to avoid disposing of live yeast cells into the sink.
Wash the cell pellet twice by suspending the cell pellet each time with 50 mL endotoxin free-PBS followed by centrifugation. Ensure that the cell pellet is completely resuspended in PBS with no visible cell clumps. After the second wash, resuspend the cell pellet in 20 mL of PBS.
By serial dilution, make a 1:200 dilution of the cda1Δcda2Δcda3Δ mutant. Using a hemocytometer, load 10 μL of the diluted cell suspension. Count the yeast cells in the four corner squares, each is 1 mm2 and has a volume of 0.1 mm3, to determine concentration. When counting cells, consider cells with 2–3 attached buds as one cell and cell clumps of 4–8 as two cells, especially in the case of the cda1Δcda2Δcda3Δ mutant.
Figure 2 depicts the preparation of an inoculum of cda1Δcda2Δcda3Δ for intranasal vaccination. For example, we counted an average of 52 cells per corner square in the 1:200 diluted sample. This results in a concentration of 1.04 × 108 cells/mL in the washed cell suspension of 20 mL. This starting yeast solution may be used to make various doses of inoculum. To make an inoculum with 2 × 106 cells/mL, add 100 μL of the cell suspension to 4.9 mL of PBS. In 50 μL of cell suspension, there will be 1 × 105 cells. Similarly, to make a suspension of 2 × 107 cells/mL, add 1 mL of the cell suspension to 4 mL of PBS. A 50 μL aliquot of this cell suspension will have 1 × 106 cells. To make a cell suspension with 2 × 108 cells/mL, centrifuge 10 mL of the initial cell suspension and resuspend the cell pellet in PBS to a final volume of 5 mL. In 50 μL of this cell suspension, there will be 1 × 107 cells. The cell count is confirmed by back plating the inoculum onto YPD. This is accomplished by spotting 50 μL of a 1:200,000 serially diluted cell suspension onto YPD. The plate was incubated at 30 °C for 3 days. There were 31 CFUs on average per spot in this case (Fig. 2E), resulting in a concentration of the cell suspension as 1.2 × 108 cells/mL, which is comparable to the count determined by counting the cells on the hemocytometer.
Fig. 2.

Steps taken to prepare cda1Δcda2Δcda3Δ cells for vaccination of mice. (a) The −80 °C stock is streaked out on a YPD agar plate and after 2–3 days at 30 °C is used as the inoculum for a flask containing YPD medium. (b) The flask is shaken for 2 days at 30 °C. (c) Cells are harvested by centrifugation and washed twice with phosphate buffered saline (PBS). The washed cells are suspended in 20 mL of endotoxin free PBS. An aliquot is diluted 1:200 and counted using a hemocytometer. Cell counts of squares outlined in red are averaged. The calculated cell density of the suspension is adjusted to 2 × 108 cells/mL. (d) Mice are vaccinated intranasally or orotracheally with 50 μL. (e) The inoculum is diluted and plated to confirm that the mice were inoculated with 1 × 107 cells (colony forming units [CFUs]). These steps are also used to prepare the KN99 inoculum to challenge vaccinated mice. Adjustments are made to the concentration of the inoculum for the mouse strain being challenged. The illustration was created with Biorender.com.
3.3. Intranasal Vaccination with a Live Vaccine Strain
Cryptococcus strains are kept at −80 °C as glycerol stocks in YPD containing 25% glycerol.
Strains from the glycerol stocks of cda1Δcda2Δcda3Δ (Lodge Lab Stock LBCN 632) and KN99 (Lodge Lab Stock JLCN 434) are streaked onto YPD plates and incubated for 72 h at 30 °C.
Use CBA/J mice (see Note 1) that are 4–6 weeks old (sex matched). After receiving the mice, allow at least 10 days for the mice to acclimate in the animal facility before beginning the experiment.
Place five mice in each cage.
Provide the standard diet and water ad libitum during the entire length of the experiment.
Prepare the inoculum of the vaccine strains on the day of vaccination by following the steps in Subheading 3.1, step 2. Use an inoculum dose of 1 × 107 cells/50 μL of PBS.
For one experiment, a minimum of two groups of mice with five animals each should be used, and the experiment must be repeated with an independent preparation of the inoculum. As a control, the first group should only receive PBS as a vaccine. The vaccine strain should be administered to the second group of mice.
For vaccination, sedate the animals with an intraperitoneal injection (200 μL) of a ketamine (8 mg/mL)/dexmedetomidine (0.05 mg/mL) mixture. Apply veterinary eye ointment to the animals’ eyes to keep them from drying out.
Tag each mouse with an ear tag and record its weight.
Suspend the animal by its top incisors at a height approximately twice the mouse’s body length (e.g., 20 cm) above the surface of the biosafety hood on a strong thin string (e.g., dental tape) secured to a fixed object (e.g., tied to two titration stands; Fig. 3).
Vortex the inoculum, then pipette 50 μL followed by slowly dripping it into the mouse’s nares (see Fig. 3). Allow the animal to hang for an additional 10 min.
Reverse the anesthesia by an intraperitoneal injection of atipamezole (200 μL) (0.25 mg/mL) to each mouse.
Transfer the animal to its cage with care and check on it until it is fully awake.
Monitor the mice and record their body weight twice daily until their weight has stabilized or begun to rise.
Allow the vaccinated animals to recover for 40 days (see Notes 2 and 3).
On the 41st day, challenge the animals intranasally with 5 × 104 cells (CFUs) of wild-type KN99 cells in 50 μL of PBS by repeating steps 9–13 above. Prepare the KN99 inoculum by following the steps outlined in Subheading 3.1, step 2. (see Note 4).
Monitor the mice twice daily and record their weight every day for 80 days after the post-challenge infection (DPI).
Euthanize the animals using CO2 asphyxiation followed by cervical dislocation when their body weight falls below 80% of their pre-challenge infection weight. Determine the fungal burden in the lungs, brain, spleen, kidney, and liver.
Place a harvested organ in a preweighed 8 mL flat base screw cap tube containing 2 mL of PBS, and keep the organs on ice. Record the weight of the tube containing the organ. Calculate the weight of the organ.
Homogenize the organs in a biosafety hood with a PRO200 homogenizer connected to a 7 mm diameter PRO quick connect generator homogenizer probe with a saw-tooth bottom.
In a 24-well non-tissue culture treated plate, serially dilute the homogenized samples in PBS and spot 50 μL of the sample in triplicate onto an YPD plate with a wide-orifice pipet tip. Incubate the plates at 30 °C for 48 h and count the CFU to determine the fungal burden. Express the fungal burden as CFUs/organ or CFUs/g of tissue.
Fig. 3.
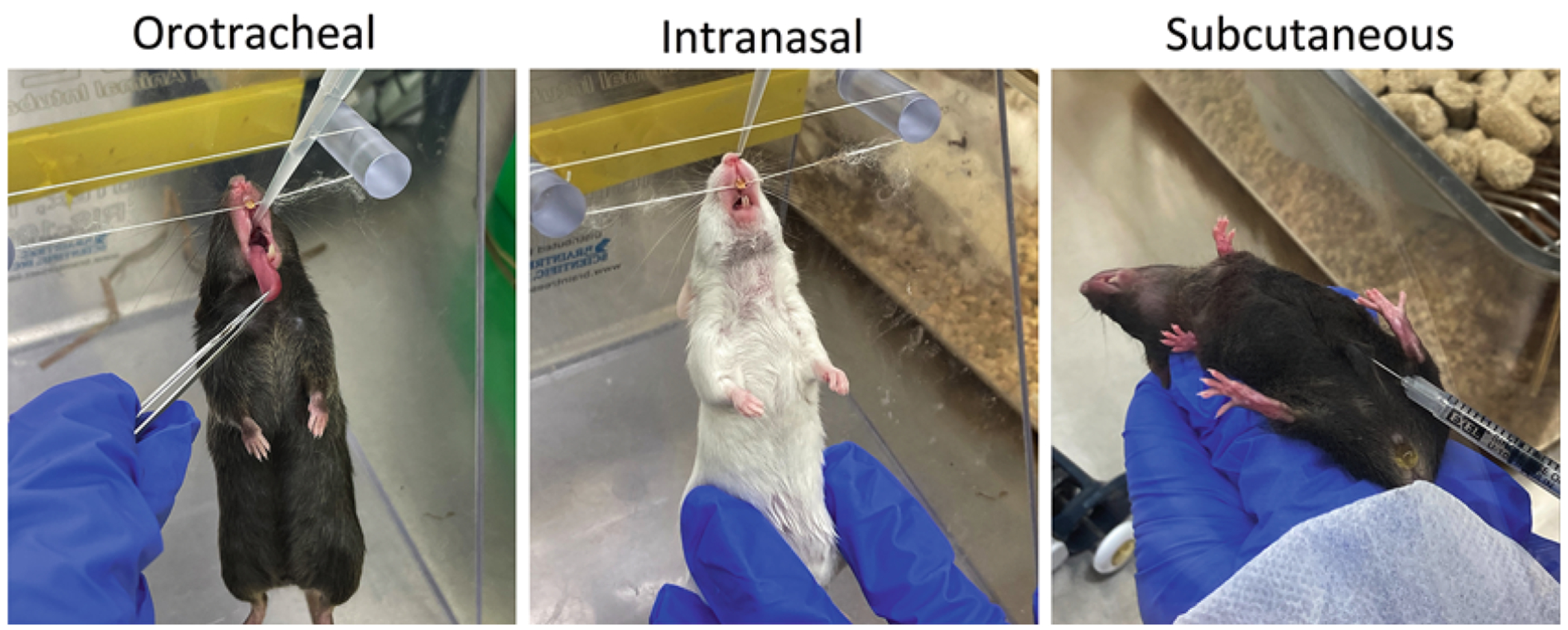
Administration routes in mice using the cda1Δcda2Δcda3Δ vaccine
3.4. Intranasal Vaccination with a Heat-Killed Preparation of cda1Δcda2Δcda3Δ
When determining the survival of mice vaccinated with heat-killed cda1Δcda2Δcda3Δ, use heat-killed KN99 grown in YPD as a control vaccine since it is ineffective at developing protective immunity. As an unvaccinated control, use PBS for inoculation. Grow the necessary yeast strains and prepare the inoculum as described in Subheading 3.1, step 2 to a final concentration of 2 × 108 cells (CFUs)/mL.
In a 70 °C dry bath, incubate the inoculum for 30 min.
Plate 100 μL of undiluted inoculum onto an YPD plate and incubate for 48 h at 30 °C to ensure that the incubation at 70 °C has killed all the yeast cells.
Perform intranasal vaccination as in Subheading 3.1, step 3.
-
After 40 days, follow the steps in Subheading 3.1, step 3. to infect the vaccinated mice with wild-type KN99 cells, monitoring for survival and fungal burden analysis at the end of the experiment.
Evaluating the cda1Δcda2Δcda3Δ Vaccine in BALB/c and C57BL/6 Mice (UMASS).
3.5. Preparation of Cryptococcus Cultures of cda1Δcda2Δcda3Δ for Vaccination
Prior to use in mouse studies, yeast cells from a − 80 °C stock are first grown on YPD agar medium to make a working stock plate, which may be stored at 4 °C for one month when sealed with a strip of parafilm. The stock plate is used to inoculate liquid YPD medium to generate live and heat-killed cda1Δcda2Δcda3Δ vaccines. Each mouse is vaccinated three times: the first vaccination is administered into the lungs, and the second and third vaccinations are by subcutaneous injection in the abdominal region (Fig. 3). Vaccinations are spaced at two-week intervals. The timeline for vaccination(s) and challenge is shown in Fig. 4.
Fig. 4.

Timeline to evaluate the efficacy of a vaccine in mice. The intranasal (IN) or orotracheal (OT) vaccination is done 40–42 days prior to challenge (Day 0). Two subsequent subcutaneous vaccinations of BALB/c and C57BL/6 mice are conducted at 28 days and 14 days before challenge. Following challenge, studies are typically ended 70–84 days (10–12 weeks) later. A study takes 16–18 weeks to complete
Without thawing the −80 °C glycerol stock, use a wood applicator to transfer the cells of the cda1Δcda2Δcda3Δ strain to an YPD agar plate. Spread cells over an area approximately 2.5 cm in diameter. Incubate the plate at 30 °C for 3 days. This stock plate is then stored at 4 °C using a strip of parafilm to seal the plate.
Add 25 mL of liquid YPD medium to a 250 mL culture flask.
To prepare the first vaccine, inoculate cells from the stock plate to the flask.
Shake the flask at 220 rpm and 30 °C for approximately 2 days.
Transfer the culture to a 50 mL centrifuge tube and spin at 425 × g for 5 min.
Suspend cells in 10 mL of PBS and centrifuge to wash the cells. Wash the cells twice.
Suspend cells in 10 mL of PBS.
Dilute an aliquot of the suspension 1:100 with PBS.
Count cells with a hemocytometer or a TC20 automated cell counter.
Adjust the concentration of cells to 2 × 108 cells/mL using PBS to administer 1 × 107 cells into the lungs. Make aliquots of the vaccine.
Measure CFUs. Dilute an aliquot of the vaccine 1:100,000 with PBS and spot 10 μL four times onto a Sabouraud agar plate for drip plating.
Count CFUs after 3 days of incubation at 30 °C and average the four counts.
For the second and third vaccinations, culturing is done in 14 mL tubes and 4 mL of YPD (as above in Subheading 3.1, step 2) with shaking at 30 °C for 2 days.
Collect cells by centrifugation and suspend in 4 mL of PBS.
Dilute the cell suspension 1:100 and count the cells.
Adjust the cell suspension to 2 × 107 cells/mL with PBS to administer 2 × 106 cells in 100 μL by subcutaneous injection of the skin at the midline of the abdomen of each mouse (Fig. 3).
3.6. Vaccination of Mice
For the first vaccination, the mice are anesthetized with isoflurane to administer the vaccine into the lungs. Steps for the orotracheal vaccination are listed first (steps 1–8) followed by steps for administering the second and third vaccinations using subcutaneous injection (steps 9–11). Vaccination of mice by the orotracheal, intranasal, and subcutaneous methods is depicted in Fig. 3.
Attach dental tape between posts of the intubation stand.
Moisten an absorbent pad by placing the pad over the opening of the isoflurane bottle and invert the bottle 2–3 times. Tape the pad to the lid of the glass jar. Place the mouse in the jar and hold the lid on the jar. Wait approximately 30–45 s for the mouse to stop moving and for the tail to drop.
Using the intubation stand, hang the mouse vertically on the dental tape by its incisors.
With a pair of tweezers pull the tongue out to one side.
Briefly vortex the 2 × 108 cells/mL suspension of cda1Δcda2Δcda3Δ.
Pipette 50 μL of suspended cells.
Insert the pipettor tip to the back of the throat and dispense the 50 μL. The mouse should gasp for air and inhale the vaccine.
Remove the mouse from the intubation stand and allow it to recover from the anesthesia. Place the mouse in a new cage.
Subcutaneous injections are done using a 0.3 mL insulin syringe. Briefly, vortex the 2 × 107 cells/mL suspension of cda1Δcda2Δcda3Δ.
Withdraw 100 μL of suspension into the syringe.
Hold the mouse securely with one hand and inject subcutaneously into the midline of the abdomen. Place the mouse in a new cage.
3.7. Preparation of Cryptococcus Cultures of KN99 for Challenge
Vaccinated mice are typically challenged with KN99 two weeks after the third vaccination. The amount of KN99 used is dependent on the mouse strain under study: C57BL/6 mice are challenged with 1 × 104 cells (CFUs), BALB/c mice with 2 × 104 cells (CFUs), and CBA/J mice with 5 × 104 cells (CFUs). The basis for this is empirical. The challenge doses have been adjusted so that infection with each unvaccinated strain of wild-type mouse is lethal within the third to fourth week of pulmonary infection.
Add 4 mL of liquid YPD medium to a 14 mL culture tube.
Use a sterile wood applicator to transfer cells from the stock plate to the culture tube. Briefly vortex to suspend cells.
Shake culture tube at approximately a 70° angle at 220 rpm and 30 °C for 18 h.
Collect cells by centrifugation for 5 min at 320 × g, suspend with 4 mL of PBS, and again centrifuge and suspend cells in 4 mL of PBS.
Dilute 1:100 and count cells (as in Subheading 3.1, step 2). Then dilute cells so that they are 20 times the amount (listed above for each mouse strain) per mL of PBS. For example, C57BL/6 mice are challenged with 50 μL of KN99 at a concentration of 2 × 105 cells/mL to deliver 1 × 104 cells into the lung.
Plate the inoculum used to infect the mice. Dilute 1:100, 1:200, or 1:500 depending on which inoculum is administered and spot 10 μL four times onto a Sabouraud dextrose agar plate for drip plating. Incubate for 2 days at 30 °C, count the CFUs for each drip, and determine the average. The CFUs and cell counts should be equivalent.
3.8. Infection of Mice with KN99
As in Subheading 3.2, step 2, the mice are anesthetized with isoflurane for orotracheal inoculation of the highly virulent strain KN99. Follow steps 1–4 listed in Subheading 3.2, step 2 to anesthetize and position the mouse on the intubation stand for inoculation.
Briefly vortex the suspension of KN99 prepared in Subheading 3.2, step 3.
Pipette 50 μL of suspended cells.
With the tongue drawn to one side, insert pipettor tip to the back of the throat and dispense the 50 μL. The mouse should gasp for air and inhale the KN99 cells.
Remove the mouse from the intubation stand and allow it to recover from the anesthesia.
3.9. Monitoring Mice Following Pulmonary Infection
Mice are monitored daily following infection, and vaccination studies are typically terminated 70–84 days postinfection. Unvaccinated wild-type mice succumb to infection during the third to fourth week postinfection. There are visual clues that indicate the health status of the infected mouse, including ruffled fur, hunched back, tip-toe walking, swollen cranium, and paralysis. Mice will begin to lose weight at approximately 2–3 weeks postinfection if unvaccinated. Weight loss of 70% from preinfection weight indicates a mouse is not likely to survive.
3.10. Euthanizing Mice
Protocols for euthanizing mice include asphyxiation with CO2, followed by bilateral thoracotomy, or an anesthetic overdose of isoflurane followed by heart puncture to remove approximately 1 mL of blood.
CO2 asphyxiation is performed according to institutional protocols.
Isoflurane on a small ball of absorbent material is placed in the bottom of a 50 mL centrifuge tube.
Place the head of the mouse in the opening of the tube. Breathing rate slows.
When the mouse does not respond to pinching of the hind foot, remove the mouse.
Use a 1 mL TB syringe with 25G × 5/8″ needle to remove blood from the heart. If collecting blood for plasma, coat the syringe by drawing up and expelling a 10,000 U/mL heparin solution. If collecting blood for serum, then omit coating the syringe with heparin and allow the blood to coagulate.
Remove approximately 1 mL of blood by heart puncture using the syringe.
Complete euthanasia according to institutional protocols.
3.11. Determining CFUs of Mouse Lungs and Other Organs
Cryptococcal CFUs of the lungs are measured following homogenization of the lungs. Dilutions are plated on Sabouraud dextrose agar and plates are incubated for 2–3 days at 30 °C. The fungal load of the lung may accumulate to >109 CFU/lung for C. neoformans strain KN99. The weight of the lung increases as the infection progresses. Lung weight may increase > fivefold for lungs with the higher fungal burdens. The cda1Δcda2Δcda3Δ vaccinated mice that survive 70 days following challenge with KN99 often clear the infection. Those that do not clear the infection also appear healthy, but may retain a fungal burden of up to 107 CFU/lung.
Make PBS with Pen/Strep by combining 490 mL of PBS with 10 mL of Pen/Strep 100× stock for final concentrations of 200 U/mL penicillin and 200 μg/mL streptomycin. Store at 4 °C.
Add 4 mL of PBS plus Pen/Strep to a 14 mL culture tube.
Remove the lungs from the euthanized mouse and place into the tube.
Homogenize lungs using the OMNI homogenizer for approximately 5–10 s in a Biosafety hood.
Make tenfold serial dilutions of the homogenate into PBS plus Pen/Strep. Store homogenates at 4 °C for 3–7 days in case they need to be replated.
Plate the serial dilutions on Sabouraud dextrose agar plates.
Incubate plates for 2 days at 30 °C and count CFUs.
3.12. Analysis of Data
GraphPad Prism Software generates Kaplan-Meier plots of survival data and will do pair-wise comparisons of survival curves to determine whether they are statistically different. To ensure rigor and reproducibility, each experiment is repeated at least one time and the total number of mice/group is at least 10. The organ CFU data of mice that, for example, were vaccinated and survived the lethal challenge with KN99 may also be analyzed using standard parametric and non-parametric statistical methods provided in the software package. Two studies were done that informed us during the development of a vaccination protocol: (1) We determined the dose of the live and heat-killed cda1Δcda2Δcda3Δ strain that generates protection (Fig. 5) and (2) we determined the amount of KN99 needed for each mouse line so that unvaccinated mice of each line succumbed to infection in 3–4 weeks (Fig. 1).
Fig. 5.
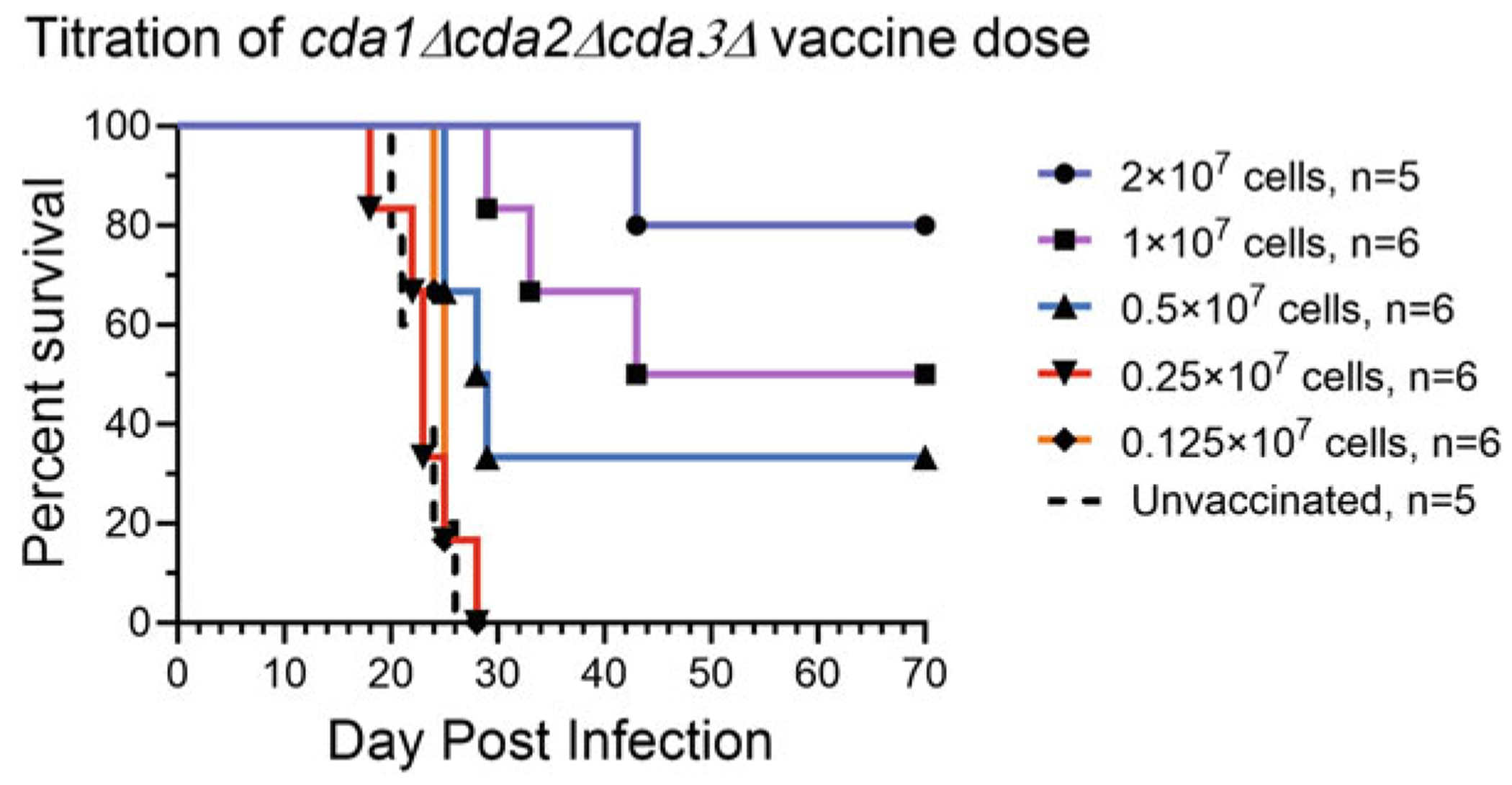
Effect of the number of cda1Δcda2Δcda3Δ cells (colony forming units [CFUs]) used for orotracheal vaccination of BALB/c mice on survival. BALB/c mice were divided into six groups of mice with 5–6 mice per group. Five groups received a single vaccination at the indicated dosage and the sixth group was unvaccinated. Six weeks following vaccination, mice were infected with 2 × 104 cells (CFUs) of strain KN99. Mice were monitored for 70 days.
In Fig. 1, groups of unvaccinated C57BL/6 and CBA/J mice (n = 5 mice per group) were challenged with 1 × 103, 1 × 104, or 1 × 105 cells (CFUs) of KN99. The CBA/J mice were more resistant to the infection than C57BL/6 mice. From studies like these, which were also done for BALB/c mice (not shown), we deduced challenge doses for each mouse line that would normalize each line’s susceptibility to KN99 infection. We decided on challenge doses of 1 × 104 cells (CFUs) for C57BL/6, 2 × 104 cells (CFUs) for BALB/c mice, and 5 × 104 cells (CFUs) for CBA/J mice so that mice succumb to infection in the same time frame (3–4 weeks) postinfection.
In Fig. 5, groups of BALB/c mice (n = 5 or 6 mice per group) were vaccinated with a series of twofold dilutions of cda1Δcda2Δcda3Δ cells (CFUs) that were cultured in YPD. Survival decreased with vaccine dose with the highest doses tested (2 × 107 CFU) generating the best protection. Vaccine doses of 0.25 × 107 CFU and 0.125 × 107 CFU did not protect. Heat-killed cda1Δcda2Δcda3Δ cells protect CBA/J mice given an intranasal vaccination of 1 × 107 cells, while 0.1 × 107 cells do not protect [17].
Acknowledgments
This work was supported by NIH grants AI125045 to JKL, CAS, and SML and AI172154 to SML. MMH was partially supported by NIH Training Grant T32 AI095213.
4 Notes
A single intranasal vaccination with 107 CFU of cda1Δcda2Δcda3Δ is effective in inducing robust protective immunity in 129 and A/J mice [17].
If the protective immunity induced by a single dose of vaccination is ineffective, more than one intranasal dose of vaccination may be tested. For example, in CBA/J mice, a single intranasal dose of chitosan-deficient cda1Δcda2Δ is sufficient to induce strong protective immunity, but in C57BL/6 mice, two intranasal doses of 107 CFU of cda1Δcda2Δ are required to induce robust protective immunity [19].
Different postvaccination times may be used to test vaccine efficacy. After a 10-day recovery period, a single intranasal dose of 107 CFU of the chitosan-deficient cda1Δcda2Δcda3Δ mutant induces robust protective immunity in CBA/J mice. A recovery period of 20 and 30 days was equally protective as a recovery period of 40 days.
A 1:1000 serial dilution is usually required to count a reasonable number of cells in 10 μL of wild-type KN99 grown in YPD.
References
- 1.Chau TT, Mai NH, Phu NH et al. (2010) A prospective descriptive study of cryptococcal meningitis in HIV uninfected patients in Vietnam - high prevalence of Cryptococcus neoformans var grubii in the absence of underlying disease. BMC Infect Dis 10:199. 10.1186/1471-2334-10-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Varma A, Diaz MR et al. (2008) Cryptococcus neoformans strains and infection in apparently immunocompetent patients. China Emerg Infect Dis 14(5):755–762. 10.3201/eid1405.071312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarvis JN, Harrison TS (2008) Pulmonary cryptococcosis. Semin Respir Crit Care Med 29(2):141–150. 10.1055/s-2008-1063853 [DOI] [PubMed] [Google Scholar]
- 4.Byrnes EJ 3rd, Li W, Lewit Y et al. (2010) Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the Northwest United States. PLoS Pathog 6(4):e1000850. 10.1371/journal.ppat.1000850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajasingham R, Govender NP, Jordan A et al. (2022) The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis 22(12):1748–1755. 10.1016/S1473-3099(22)00499-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO (2022) WHO. Fungal priority pathogens list to guide research, development and public health action. World Health Organization, Geneva [Google Scholar]
- 7.Johnson MD, Perfect JR (2010) Use of antifungal combination therapy: agents, order, and timing. Curr Fungal Infect Rep 4(2):87–95. 10.1007/s12281-010-0018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krysan DJ (2015) Toward improved anti-cryptococcal drugs: novel molecules and repurposed drugs. Fungal Genet Biol 78:93–98. 10.1016/j.fgb.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 9.Smith KD, Achan B, Huppler Hullsiek K et al. (2015) Increased antifungal drug resistance in Ugandan clinical isolates of Cryptococcus neoformans. Antimicrob Agents Chemother 59(12):7197–7204. 10.1128/AAC.01299-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartizal K, Gill CJ, Abruzzo GK et al. (1997) In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743, 872). Antimicrob Agents Chemother 41(11):2326–2332. 10.1128/AAC.41.11.2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abruzzo GK, Flattery AM, Gill CJ et al. (1997) Evaluation of the echinocandin antifungal MK-0991 (L-743, 872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother 41(11):2333–2338. 10.1128/AAC.41.11.2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturvedi AK, Wormley FL Jr (2013) Cryptococcus antigens and immune responses: implications for a vaccine. Expert Rev Vaccines 12(11):1261–1272. 10.1586/14760584.2013.840094 [DOI] [PubMed] [Google Scholar]
- 13.Levitz SM, Huang H, Ostroff GR et al. (2015) Exploiting fungal cell wall components in vaccines. Semin Immunopathol 37(2):199–207. 10.1007/s00281-014-0460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirza Z, Soto ER, Dikengil F et al. (2017) Beta-glucan particles as vaccine adjuvant carriers. Methods Mol Biol 1625:143–157. 10.1007/978-1-4939-7104-6_11 [DOI] [PubMed] [Google Scholar]
- 15.Hester MM, Lee CK, Abraham A et al. (2020) Protection of mice against experimental cryptococcosis using glucan particle-based vaccines containing novel recombinant antigens. Vaccine 38(3):620–626. 10.1016/j.vaccine.2019.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Specht CA, Homan EJ, Lee CK et al. (2021) Protection of mice against experimental cryptococcosis by synthesized peptides delivered in glucan particles. MBio 13(1):e0336721. 10.1128/mbio.03367-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Upadhya R, Lam WC, Maybruck B et al. (2016) Induction of protective immunity to cryptococcal infection in mice by a heat-killed, chitosan-deficient strain of Cryptococcus neoformans. mBio 7(3):e00547–e00516. 10.1128/mBio.00547-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wormley FL Jr, Perfect JR, Steele C et al. (2007) Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect Immun 75(3):1453–1462. 10.1128/IAI.00274-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upadhya R, Lam WC, Hole CR et al. (2021) Cryptococcus neoformans Cda1 and Cda2 coordinate deacetylation of chitin during infection to control fungal virulence. Cell Surf 7:100066. 10.1016/j.tcsw.2021.100066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masso-Silva J, Espinosa V, Liu TB et al. (2018) The F-box protein Fbp1 shapes the immunogenic potential of Cryptococcus neoformans. mBio 9(1):e01828–e01817. 10.1128/mBio.01828-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rella A, Mor V, Farnoud AM et al. (2015) Role of sterylglucosidase 1 (Sgl1) on the pathogenicity of Cryptococcus neoformans: potential applications for vaccine development. Front Microbiol 6:836. 10.3389/fmicb.2015.00836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhai B, Wozniak KL, Masso-Silva J et al. (2015) Development of protective inflammation and cell-mediated immunity against Cryptococcus neoformans after exposure to hyphal mutants. mBio 6(5):e01433–e01415. 10.1128/mBio.01433-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caballero Van Dyke MC, Wormley FL Jr (2018) A call to arms: quest for a cryptococcal vaccine. Trends Microbiol 26(5):436–446. 10.1016/j.tim.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louria DB (1960) Specific and non-specific immunity in experimental cryptococcosis in mice. J Exp Med 111(5):643–665. 10.1084/jem.111.5.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadebusch HH (1960) Specific degradation of Cryptococcus neoformans 3723 capsular polysaccharide by a microbial enzyme. III. Antibody stimulation by partially decapsulated cells. J Infect Dis 107:406–409. 10.1093/infdis/107.3.406 [DOI] [PubMed] [Google Scholar]
- 26.Fromtling RA, Kaplan AM, Shadomy HJ (1983) Immunization of mice with stable, acapsular, yeast-like mutants of Cryptococcus neoformans. Sabouraudia 21(2):113–119 [PubMed] [Google Scholar]
- 27.Wang Y, Wang K, Masso-Silva JA et al. (2019) A heat-killed Cryptococcus mutant strain induces host protection against multiple invasive mycoses in a murine vaccine model. mBio 10(6):e02145–e02119. 10.1128/mBio.02145-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen K, Cox GM, Wang P et al. (2003) Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and alpha isolates. Infect Immun 71(9):4831–4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthew J, Arduino WDA, Bailin H et al. (2020) Biosafety in Microbiological and Biomedical Laboratories, 6th edn. U.-S. Department of Health and Human Services Public Health Service, Centers for Disease Control and Prevention, National Institutes of Health, HHS Publication No. (CDC) 300859 [Google Scholar]


