Abstract
Ocimum tenuiflorum, commonly known as Tulsi, is revered in Ayurveda for its extensive medicinal properties. However, there is a need to consolidate current knowledge on its phytochemical constituents and their pharmacological activities to identify potential areas for further research and drug development. This review aims to bridge this gap by providing a comprehensive analysis of the bioactive secondary metabolites found in O. tenuiflorum, such as rosmarinic acid, oleanolic acid, luteolin, ursolic acid, and limonene, and their associated therapeutic effects. The review will highlight the pharmacological importance of these metabolites, which exhibit antioxidant, neuroprotective, anticancer, and anti-inflammatory properties. Additionally, this study will explore the plant's wide range of beneficial qualities, including anti-inflammatory, antioxidant, anticholinergic, pain-relieving, antimicrobial, stress-reducing, antidiabetic, anticancer, liver-protective, ulcer-inhibiting, antifungal, and wound-healing attributes. Furthermore, this review focuses on the plant's potential in treating conditions such as asthma, persistent fever, tuberculosis, malaria, skin discoloration, itching, digestive issues, hemorrhoids, bone fractures, gout, urinary tract infection, and diabetes. By reviewing the current literature, the aim is to identify the gaps in the existing research and propose directions for future studies. This comprehensive review will serve as a valuable resource for researchers in the development and investigation of novel drugs derived from O. tenuiflorum.
Keywords: anticancer, antidiabetic, luteolin, Ocimum tenuiflorum, oleanolic acid, rosmarinic acid
1. Introduction
From ancient times, different parts of plants (roots, stems, leaves, flowers, seeds, and barks) have been used as medicine for the treatment of several ailments [1]. Since then, plants have served as a remarkable source of compounds of medicinal value. Many people in developing countries rely on medicinal plants because they are abundant and affordable and have no side effects [2]. One such sacred plant of high ethnomedicinal value is Ocimum tenuiflorum [3]. This aromatic medicinal herb belongs to the family Lamiaceae. In 1753, Linnaeus described the genus Ocimum including five species. However, these days, more than 50 species have been identified with potential therapeutic importance [3, 4]. The species of Ocimum are distributed all over the tropical and subtropical regions. Due to their therapeutic and economic importance, the cultivation of some species is undertaken [5]. This plant exhibits many medicinal properties such as antioxidant, antidiabetic, anti-inflammatory, anticancer, antinociceptive, antifertility, anthelmintic, cardioprotective, and antimicrobial [6]. Most species of Ocimum are used to treat disease and functional disorders such as diabetes, dysentery, hemorrhoids, diarrhea, constipation, coughs, tuberculosis, eye and ear complaints, stomach disorder, abdominal pains, headaches, febrile illness, malaise, soreness, fever, reducing swelling, and central nervous system disorder [4, 7, 8]. Among them, O. tenuiflorum is one of the most important species. Ocimum tenuiflorum is also known as Tulasi or Tulsi in Nepali and Hindi and Holy Basil in English. Ocimum tenuiflorum is mainly native to tropical and subtropical regions [9]. The name “Tulsi” is derived from Sanskrit, and it means “incomparable one” [10]. In Ayurveda, the aromatic medicinal plant Tulsi is often referred to as the “elixir of life” [11]. It is a branched perennial herb, which can grow up to 1 m tall, and possesses an aromatic odor, with some woody tissue at the stem bases. The leaves are broadly elliptical and pubescent on their surfaces, measuring 3–6 cm in length and 1–2.5 cm in width. The flowers are terminal and form slender racemes or panicles. The stems are four-angled, purplish, and hairy [12–14]. Because of its therapeutic value, the entire plant can be utilized for medical treatment, and it is often referred to as the “Queen of Plants” [10, 15]. This plant produces a variety of volatile oils, including terpenes, phenol, and aldehydes. Additionally, the plants are said to contain tannins, alkaloids, saponins, and glycosides [16]. Due to the presence of natural products, the plant possesses diverse biological activities such as cardioprotective, antidiabetic, antimicrobial, hepatoprotective, antifertility, antifungal, anticancer, stomachache, headaches, common colds, inflammation, analgesics, antiemetics, antipyretics, and stress reducers [17–19]. The primary objective of this review is to explore the chemistry of O. tenuiflorum, including the bioactivity of its metabolites and extracts in various solvent mediums. Traditionally, this plant has been employed for numerous therapeutic purposes in different countries as shown in Table 1, yet its chemical composition remains largely undocumented. The presence of secondary metabolites spanning different classes constitutes a pivotal factor contributing to the plant's substantial therapeutic significance. There exists an extensive body of the literature on the phytochemical and ethnomedical uses of O. tenuiflorum. Consequently, this article provides an in-depth review of the phytochemical constituents of O. tenuiflorum and their pharmacological activities. By systematically analyzing these metabolites and their therapeutic potential, the study highlights the plant's significance in modern pharmacology and its potential as a source of novel drug candidates.
Table 1.
Traditional uses of Ocimum tenuiflorum in different countries.
| Country | Local name | Used parts | Uses | References |
|---|---|---|---|---|
| Nepal | Tulasi | Leaves | Antioxidant | [15] |
| India | Tulsi | Leaves | Cough, stomachic, anthelmintic, alleviate muscular pain, joint pain, severe headache | [20, 21] |
| Saudi Arabia | Shajrat-az-zir | Leaves | Treat coughs, bronchitis | [22] |
| Bangladesh | Khalatulsi | Leaves | Insect sting, coughing, asthma, fiver | [23, 24] |
| Thailand | Kaphraodaeg | Leaf | Relieves nausea, stomachache, and flatulence, treats skin disease | [25–27] |
| Myanmar | Kala-pi-sein, pin-sein-net | Leaf, seed, root | Expectorant and stomachic, kidney diseases, diaphoretic. | [28] |
| Pakistan | Jungle booti | Leaves/whole plant | Appetizer, mosquito repellent, fodder, fever, cough, headache, diarrhea | [29] |
2. Methodology
To gather information on the ethnomedicinal uses, in vivo and in vitro biological activities, metabolites found in O. tenuiflorum, and the biological activities of its metabolites, we conducted a comprehensive search across various databases, including Google Scholar, Research gate, Web of Science, PubMed, SciFinder, Wiley Online Library, Science Direct, Springer, Taylor and Francis, Elsevier, Chemical Abstracts, and Scopus. Keywords such as antioxidants, antimicrobial, anti-inflammatory, anticancer, antistress, secondary metabolites, natural products, and phytochemicals of O. tenuiflorum (also known as O. sanctum) were used to explore the chemistry of the plant. We selected standard articles that provided sufficient pharmacological and ethnomedicinal insights. A total of 226 articles from 1987 to 2024 were reviewed to extract relevant information for the comprehensive evaluation of O. tenuiflorum pharmacological and phytochemical properties. These articles were chosen based on the biological activities of O. tenuiflorum and its available metabolites. The selection process also took into consideration a comparative study with similar species, as well as the identification of compounds using advanced analytical tools and advanced assays used to evaluate the biological activities.
3. Phytochemical Constituents
3.1. Flavonoids
Flavonoids are the most abundant phytochemicals with low-molecular-weight polyphenol structures [30, 31]. Ocimum tenuiflorum contains different kinds of flavonoids, which are responsible for the plant's therapeutic activity as mentioned in Table 2. Some flavonoids that are commonly present in O. tenuiflorum are luteolin, apigenin, eupalitin, xanthomicrol, genkwanin, demethylnobiletin, salvigenin, luteolin-7-O-glucuronide, isoorientin, orientin, galuteolin, apigenin-7-O-glucuronide, kaempferol, kaempferide, chrysoeriol, isosakuranetin, vitexin, isovitexin, quercetin, cirsimaritin, chrysoeriol, cirsilineol, isothymusin, molludistin, vicenin, luteolin-5-glucoside, esculin, robinetintrimethyl ether, and esculetin [32–36]. The structures of key bioactive flavonoids, which are particularly prominent in Ocimum species, are shown in Figure 1.
Table 2.
Biological activity of flavonoids present in Ocimum tenuiflorum.
| Secondary metabolites | Biological activities | Toxicology and side effect | References |
|---|---|---|---|
| Luteolin | • Antioxidant activity • Antibacterial activity • Anti-inflammatory activity • Anticancer activity • Antidiabetic activity • Antiasthmatic activity • Protect cardiomyocyte cells from LPS-induced apoptosis |
• DNA damage • Chromosome damage |
[37–40] |
|
| |||
| Eupalitin | • Antiproliferative against human colorectal tumor cells • Inhibition of the PC3 cell |
• Unknown | [41, 42] |
|
| |||
| Vitexin | • Anticonvulsant effects • Antidepressant effects • Antihypoxia/ischemia injury activity |
• Nausea • Headaches • Stomach upset • Skin reactions |
[43–46] |
|
| |||
| Quercetin | • Antioxidant activity • Anticancer activity • Anti-inflammatory activity |
• Kidney damage at high doses | [47–50] |
|
| |||
| Cirsilineol | • Anticancer properties • Antiplatelet agent |
• Unknown | [51, 52] |
|
| |||
| Apigenin | • Anticancer activity • Antioxidant activity • Reduce pulmonary hypertension • Enhance lipid metabolism |
• Diarrhea • Skin rashes • Itching • Swelling • Difficulty breathing |
[53–56] |
|
| |||
| Xanthomicrol | • Anticancer activities • Antifungal activities • Antioxidant activity |
• Unknown | [57, 58] |
|
| |||
| Orientin | • Antiviral activity against para 3 • Antibacterial activity • Vasodilatation effects • Antinociceptive effects |
• Unknown | [59–61] |
|
| |||
| Chrysoeriol | • Inhibit the induction of nitric oxide synthase by suppressing AP-1 activation • Antioxidant activity • Antimicrobial activity |
• Unknown | [62–64] |
|
| |||
| Esculin | • Anti-inflammatory activity • Antidiabetic activity • Antithrombotic activity • Antibacterial activity |
• Gastrointestinal effects • Neurologic effects • Risk of bleeding • Stomach upset • Muscle twitching • Weakness • Vomiting |
[65–69] |
|
| |||
| Esculetin | • Antitumor pharmacological activities against colorectal cancer, gastric cancer, prostate cancer, and breast cancer • Immunomodulatory activity • Antiatherosclerotic activity |
— | [65, 70–74] |
Figure 1.
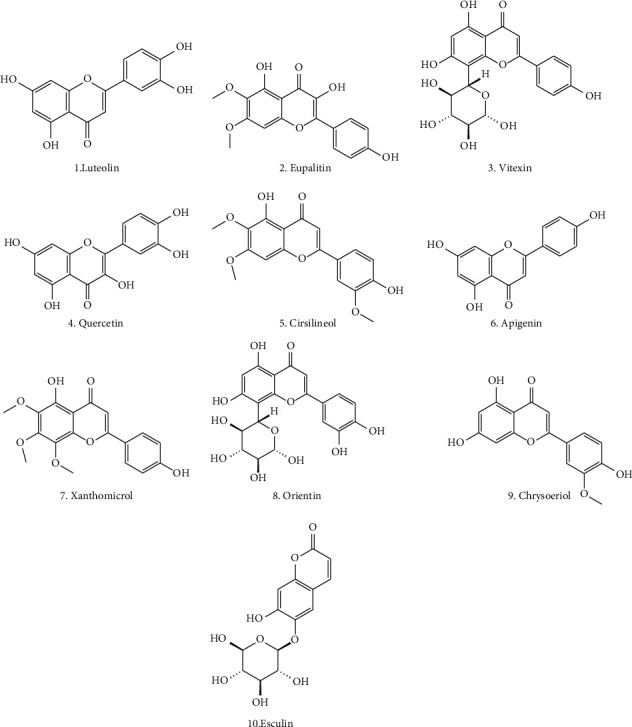
Some bioactive flavonoids from the Ocimum tenuiflorum.
3.2. Phenols and Phenolic Acids
Phenolic metabolites include phenols and phenolic acid. Phenols and phenolic acid are therapeutically important metabolites as they act as antioxidants and are responsible antisickling, antiosteoporotic, anticarcinogenic, and other bioactivity as mentioned in Table 3 [75, 76]. The shikimic acid and phenylpropanoid pathways serve as the synthetic routes for phenolic compounds [77]. Ocimum tenuiflorum is a well-known plant for its antioxidant properties due to the presence of several phenolic compounds. Few of them are rosmarinic acid, (E)-p-coumaroyl 4-O-β-D-glucoside, chlorogenic acid, caffeic acid, vanillin, methylisoeugenol, vanillic acid, sinapic acid, p-coumaric acid, 3-(3,4-dihydroxyphenyl) lactic acid, protocatechuic acid, 3,4-dimethoxycinnamic acid, p-hydroxybenzoic acid, ferulic acid, and bieugenol [33, 34, 78]. The structures of key bioactive phenolic metabolites, which are particularly prominent in Ocimum species, are shown in Figure 2.
Table 3.
Biological activity of phenol and phenolic acids present in Ocimum tenuiflorum.
| Secondary metabolites | Biological activities | Toxicology and side effect | References |
|---|---|---|---|
| Rosmarinic acid | • Antioxidant and DNA damage protection ability • Restore cognitive functions, anticancer |
• No significant toxic effects observed | [79–81] |
|
| |||
| Chlorogenic acid | • Anti-hepatitis B virus, regulation of carbohydrate and lipid metabolism • Protect liver and kidney • Protect the nervous system |
• Overdoses may cause anxiety, agitation, and irregular heartbeat | [82, 83] |
|
| |||
| Caffeic acid | • Antimicrobial activity • Antioxidant activity |
• Overdoses may cause fetal weight gain • Mild stomach upset at higher doses |
[84–86] |
|
| |||
| Vanillin | • Anticancer activity • Antioxidant activity • Protective effects against Huntington's disease • Antisickling agent • Antimicrobial activity |
• Mild headaches and allergic reactions | [87–91] |
|
| |||
| Sinapic acid | • Antiproliferative on colon cancer cells • Antioxidant activity • Antimicrobial activity |
• Unknown | [92–94] |
|
| |||
| p-Coumaric acid | • Antinecrotic and anticholestatic effects against liver injury • Antiamoebic activity • Hypopigmenting agent |
• Goitrogenic activity | [95–97] |
|
| |||
| Protocatechuic acid | • Antioxidant • Antibacterial • Antiviral (Control bird flu infection) • Anticancer • Antiosteoporotic • Analgesia • Antiwrinkle properties |
• Depletion of GSH in the liver and kidney • LD50 800 mg/kg |
[98–104] |
|
| |||
| Ferulic acid | • Antioxidant • Hepatoprotective • Anticarcinogenic • Antimicrobial, • Antiaging properties • Angiogenic agent |
• Unknown | [105–107] |
Figure 2.
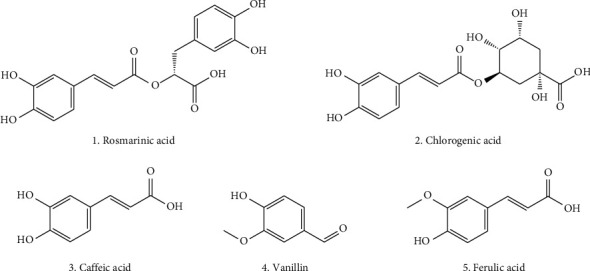
Some bioactive phenolics from the Ocimum tenuiflorum.
3.3. Triterpenoids and Steroids
Triterpenoids are important secondary metabolites present in animals and plants that possess immense pharmaceutical importance [108]. In living bodies, triterpenoids are considered the building block from which steroids are formed [109]. Steroids are well known for their biological activities related to growth-regulating activity in plants, and anti-inflammatory and immune-modulating properties [110, 111]. Small changes in the steroids may lead to significant biological changes. Ocimum tenuiflorum consists of multiple triterpenoids and steroids, which bear important therapeutic importance as shown in Table 4. Few of them are β-sitosterol, ursolic acid, trihydroxyursolic acid, β-sitosterol-3-Oβ-D-glucopyranoside, oleanolic acid (OA), stigmasterol, campesterol, ocimic acid, urs-12-en-3β,6β,20β-triol-28-oic acid, and 16-hydroxy-4,4,10,13-tetramethyl-17-(4-methyl-pentyl)-hexadecahydrocyclopenta [α] phenanthrene-3-one [36, 112–117]. The structures of key bioactive triterpenoids, which are particularly prominent in Ocimum species, are shown in Figure 3.
Table 4.
Biological activities, toxicology, and side effects of terpenoids present in Ocimum tenuiflorum.
| Secondary metabolites | Biological activities | Toxicology and side effect | References |
|---|---|---|---|
| β-Sitosterol | • Anxiolytic effects and sedative effects • Antibacterial activity • Anti-inflammatory • Antioxidant • Antidiabetic • Wound-healing effect |
• Mild effects observed such as nausea, indigestion, gas, diarrhea, or constipation • Pancreatitis |
[118–120] |
|
| |||
| Ursolic acid | • Anti-inflammatory property • Anticancer activity • Antibacterial • Antidiabetic • Neuroprotective activity • Herbicidal activity |
• Hepatotoxicity • Diarrhea • Nausea • Abdominal swelling • Trace amounts of blood in the urine |
[121, 122] |
|
| |||
| β-Sitosterol-3-Oβ-D glucopyranoside | • Potential as a leukemia treatment | — | [123] |
|
| |||
| Oleanolic acid | • Anticancer activity • Antimicrobial activity • Hepatoprotective effect • Antioxidant activities • Anti-hypertensive activity |
• Cholestatic liver injury • Fatigue • Nausea • Anorexia |
[124, 125] |
Figure 3.
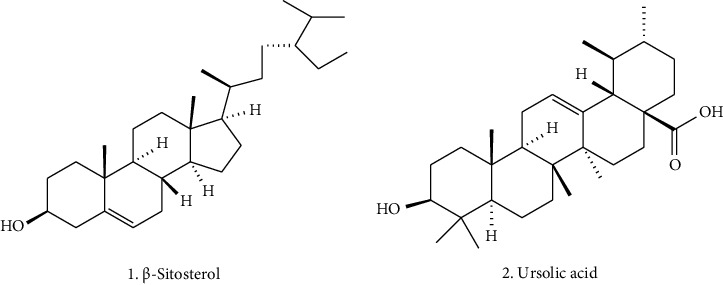
Some bioactive triterpenoids from the Ocimum tenuiflorum.
3.4. Monoterpenes
Monoterpenes are organic compounds present in the essential oils of plants, contributing to the plants' flavor and aroma, and play a significant role in various biological applications as shown in Table 5, particularly in the development and design of drugs [126, 127]. Some important monoterpenes reported in O. tenuiflorum are α-pinene, camphene, sabinene, β-pinene, 1,8-cineole, β-trans-ocimene, camphor, borneol, tricyclene, myrcene, phellandrene, terpinene, limonene, ocimene, terpinolene, sabinene hydrate, carene, fenchone, linalool, camphene hydrate, terpinen-4-ol, terpineol, estragole, and eugenol [18, 128–130]. The structures of key bioactive monoterpenes, which are particularly prominent in Ocimum species, are shown in Figure 4.
Table 5.
Biological activities, toxicology, and side effects of Monoterpenes present in Ocimum tenuiflorum.
| Secondary metabolites | Biological activities | Toxicology and side effect | References |
|---|---|---|---|
| α-Pinene | • Antibacterial activity • Antifungal activity • Antileishmania activity • Anti-inflammatory activity • Neuroprotective activity • Antiapoptotic activity • Antitumor activity, insecticidal activity |
• At 200 μg/mL, BEAS-2B cellular viability decreased • Respiratory and skin irritation |
[131–133] |
|
| |||
| Limonene | • Antibacterial activity • Antioxidant effect • Antidiabetic activity • Anti-inflammatory effect • Anticancer effect • Gastroprotective effect • Antistress effect |
• Skin and eye irritation | [134–140] |
|
| |||
| Estragole | • Anti-inflammatory • Antioxidant • Antibacterial activity |
• Genotoxic carcinogen • Hepatocellular adenoma |
[141, 142] |
|
| |||
| Eugenol | • Antioxidant • Antibacterial activity • Anti-inflammatory activity |
• LD50 value > 1930 mg·kg−1 in rodents • Excess use may cause vomiting, gastroenteritis, and systemic toxicity • May cause liver and kidney damage • Seizures • Coma • Bronchial irritation • Dizziness • Rapid breathing |
[143–147] |
|
| |||
| Terpineol | • Antioxidant activity • Anticancer activity • Anticonvulsant activity • Insecticidal activity • Antiulcer activity |
• Mild skin irritation or dermatologic allergic response • Eye irritation • Respiratory irritation • Skin irritation • Germ cell mutagenicity • Carcinogenicity, reproductive toxicity |
[148, 149] |
Figure 4.
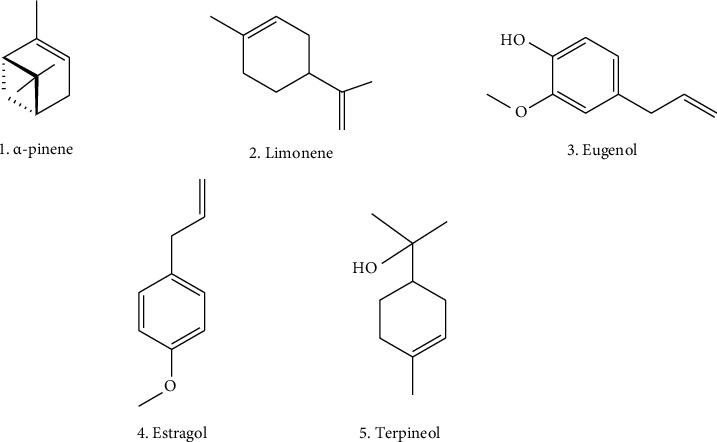
Some bioactive monoterpenes from the Ocimum tenuiflorum.
3.5. Sesquiterpenes
Sesquiterpenes are promising secondary metabolites with pharmaceutical importance. Ocimum tenuiflorum consists of a number of sesquiterpenes. Few of them are copaene, zingiberene, bourbonene, guaiene, bergamotene, sesquiphellandrene, farnesene, sesquisabinene, humulene, bicyclogermacrene, germacrene, bisabolene-(Z), δ-cadinene, α-bisabolene, amorphene, caryophyllene oxide, γ-muurolene, α-muurolene, α-cadinol, bourbonene, γ-cadinene, α-caryophyllene, β-caryophyllene, germacrene D, β-guaiene, α-longipinene, α-panasinsen, selina-6-en-4-ol, nerolidol, spathulenol, aromadendrene oxide, α-calacorene, 1-4-cadinadiene, β-bisabolene, alloaromadendrene, β-gurjunene, β-cubebene, β-elemene, and γ-eleneme [18, 128–130]. These metabolites are mostly found in the essential oils of the plant and possess multiple biological activities. More specifically, sesquiterpenes have shown pharmacological activities such as, antimicrobial, antifeedant, immunomodulatory, anti-inflammatory, antitumor, and antimalarial [150].
3.6. Esters, Aldehyde, and Ketone
Ester, aldehyde, and ketones are organic compounds containing different functional groups. Ocimum tenuiflorum consists of several esters, aldehydes, and ketones, which possess significant biological activities. Some of them are methyl isovalerate, ethyl isovalerate, pentanal, hexane-3-one, 4-methyl-4-hepten-3-one, and octyl ester [18, 114, 151, 152]. Some compounds belonging to this group possess pharmacological importance such as antioxidant, antibacterial, antifungal, and anticancer, but good literature is lacking on the reported compounds.
3.7. Other Secondary Metabolites
There are different classes of metabolites present in various parts of the O. tenuiflorum, ranging from aliphatic alcohol to complex compounds such as lumiflavine. These are included in another category of metabolites, and these metabolites exhibited crucial biological application as shown in Table 6. A few examples are sotolon, hexane-2-ol, benzene-1,2-dicarboxylic acids, benzeneacetic acid, lumiflavine (reported as lumiflavine), phytol, and 1,4-cyclohexadiene [114, 151, 152]. The structures of metabolites, which are crucial for biological applications, are presented in Figure 5.
Table 6.
Biological activities, toxicology, and side effects of metabolites present in Ocimum tenuiflorum.
| Secondary metabolites | Biological activities | Toxicology and side effect | References |
|---|---|---|---|
| Phytol | • Antiradical activity • Antibacterial activity • Antifungal activity • Antinociceptive activity |
• Decreased mitotic index, and increased DNA damage in the allium cepa test system at certain concentrations • Premature birth • Neonatal cardiovascular abnormalities • Reduced bone mineral density • Damage to lung tissue |
[153–158] |
|
| |||
| Benzeneacetic acid | • Antimicrobial activity | Unknown | [159] |
Figure 5.
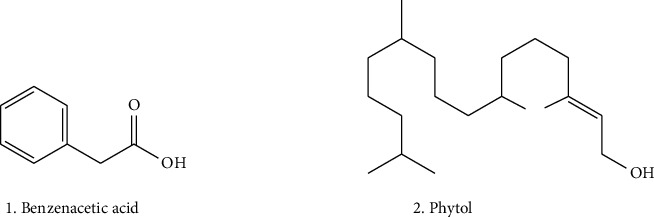
Some bioactive metabolites from the Ocimum tenuiflorum.
4. Biological Activities of Ocimum tenuiflorum
4.1. Antioxidant Activity
Ocimum tenuiflorum is a well-known potential source of antioxidants. Saravanan et al. evaluated the antioxidant property of O. tenuiflorum, i.e., Tulsi. The DPPH scavenging results indicated that at higher concentrations of 200–500 μg/mL, Tulsi exhibited strong antioxidant properties [160]. Similarly, Chaudhary et al. calculated the antioxidant properties of the sample in different solvent mediums by various methods [33]. In this study, n-butanol fraction was most effective in inhibiting DPPH radical, ABST radical, and phosphomolybdate, with EC50 values 3.91 ± 0.3 µg/mL, 1.6 ± 0.1 µg/mL, and 2.31 ± 0.1 µg/mL, respectively, whereas methanolic extract was most effective to inhibit the hydroxyl radical with an EC50 value of 5.30 ± 0.43 μg/mL [33]. Among the different species of the same genus, Agarwal reported that the ethyl acetate fraction of O. tenuiflorum has demonstrated a strong antioxidant capacity compared to the same solvent fraction of O. kilimandscharium [112]. Rindhe performed the antioxidant activity of Tulsi using two different methods, and among them Tulsi exhibited strong inhibition for DPPH and hydrogen peroxide. For DPPH at 100 μg/mL, Tulsi exhibited 80.19% inhibition, and at the same concentration for hydrogen peroxide, it exhibited 39.92% inhibition, which is stronger compared to ascorbic acid [161]. In addition, the in vivo analysis of O. tenuiflorum demonstrated it as a potential source of an antioxidant. Ramesh and Satakopan conducted the antioxidant activity of O. tenuiflorum against toxicity induced by Cadmium in rats [162]. Lipid peroxidation levels were shown to have significantly decreased following the oral treatment of O. tenuiflorum at doses of 100 and 200 mg/kg body weight, both before and after cadmium-induced toxicity, respectively. Lipid peroxidation levels had previously increased following the oral administration of 6.0 mg/kg body weight CdCl2. It also significantly increased the levels of catalase, reduced glutathione, glutathione peroxidase, superoxide dismutase, and vitamin C [161]. The mechanism behind the escalation of reduced glutathione is by diminishing the oxidative free radical by donating H. This increase in reduced glutathione helps to enhance the level of glutathione peroxidase in the liver [162]. Moreover, the oral administration of O. tenuiflorum before and after 10 mg/kg body weight increases the level of reduced glutathione, lowers the level of lipid peroxidation, and helps to alter the activities of serum glutamate, oxaloacetate, transaminase, and serum glutamate, pyruvate, and transaminase [162]. This was reported in Sharma et al.'s study on the toxicity induced by mercury in Swiss albino mice, which increased the levels of lipid peroxidation, serum glutamate, oxaloacetate, transaminase, serum glutamate, and pyruvate transaminase. The in vivo and in vitro antioxidant potential of O. tenuiflorum was ascribed to the presence of several bioactive phytochemicals.
The antioxidant property of O. tenuiflorum is due to the presence of metabolites such as caffeic acid, quercetin, luteolin, and eugenol. For reference, quercetin due to the presence of the hydroxyl group and its unique position can interact with various signal transduction pathways by either activating, inhibiting, upregulating, or downregulating numerous body molecules. This action helps enhance the body's antioxidant capacity and repair damage. Along with the mitochondrial electron transport chain, environmental factors can increase the production of reactive oxygen species (ROS). Quercetin regulates both enzyme-mediated and nonenzyme-dependent antioxidant defense systems, and the general process is shown in Figure 6. It also modulates the signal pathways such as NRFB, AMPK, and MAPK, which are influenced by ROS, to bolster the antioxidant defense system and maintain oxidative balance.
Figure 6.
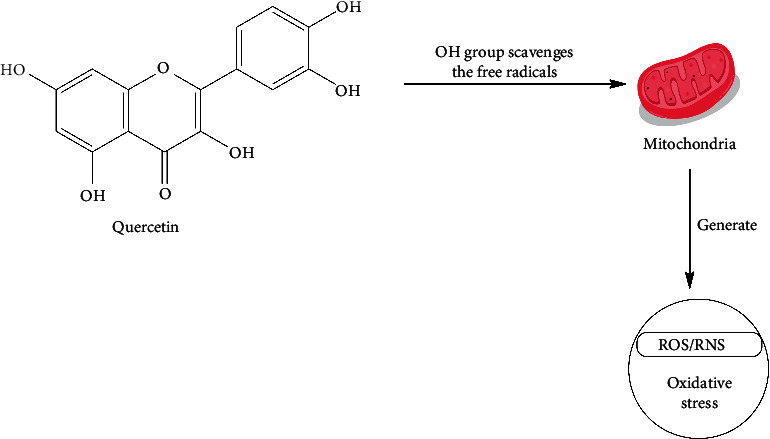
Function of quercetin as an antioxidant [163].
4.2. Antimicrobial Activity
Ocimum tenuiflorum has been extensively studied for its antimicrobial properties. The plant has demonstrated a strong inhibition against Gram-positive and Gram-negative bacteria. Dixit et al. studied the antibacterial activity of O. tenuiflorum at different concentrations of ethanolic, methanolic, and aqueous extracts against Bacillus subtilis. The results indicated that the methanolic extract was more effective compared to other solvent extracts as it showed inhibition of 2 and 5 mm at even 0.2 and 0.3 g/mL concentrations, respectively [164]. Similarly, Mahmood et al. reported the essential oil obtained from O. tenuiflorum showed strong inhibition against various Gram-negative and Gram-positive bacteria, including Escherichia coli, P. aeruginosa, Klebsiella sp., Proteus mirabilis, and S. aureus, with zones of inhibition measuring 15.4, 17.8, 20, 20, and 41.5 mm, respectively [165]. Ocimum tenuiflorum comprises antifungal agents to inhibit fungal pathogens. Sivareddy et al. conducted the antifungal activity of O. tenuiflorum leaf against Candida albicans. Both the ethyl acetate and ethanolic extract of the plant exhibited the same zone of inhibition and minimum inhibitory concentration (MIC) against the tested organism, i.e., 13 mm and 2000 μg/mL, respectively [166]. Piras et al. also conducted the antifungal activity of essential oil of two species of Ocimum, O. basilicum and O. tenuiflorum. Ocimum tenuiflorum essential oil was found to be the most effective against the tested species of C. albicans, C. tropicalis, C. krusei, C. guilliermondii, C. parapsilosis, Cryptococcus neoformans, T. mentagrophytes, Trichophyton rubrum, T. verrucosum, Microsporum canis, M. gypseum, and Epidermophyton floccosum. For Candida spp., Cr. neoformans, and dermatophytes, the MICs and minimum lethal concentrations (MLCs) were, respectively, 0.16 and 0.64 μg/mL, 0.32, and 0.32–0.64 μg/mL, 1.25–2.5 and 0.64 μg/mL. Eugenol and methyl eugenol, two metabolites of O. tenuiflorum that showed high antifungal activity against the aforementioned species, are responsible for the plant's potent antifungal action [129].
Another study was conducted by Balakumar et al. against clinically isolated dermatophyte fungi and observed that the alcoholic and aqueous extract and fractions illustrated strong antifungal activity [167]. The bioactive compounds isolated from O. tenuiflorum, particularly flavonoids, alkaloids, and essential oils, have antimicrobial properties. Metabolites such as eugenol, estragole, ursolic acid, and ferulic acid are well documented for their antimicrobial activity. Eugenol exerts its antibacterial activity through several mechanisms as shown in Figure 7. It penetrates bacterial cell membranes, particularly in Gram-negative bacteria, causing structural alterations that lead to the leakage of intracellular components and ultimately cell death. Additionally, eugenol inhibits crucial bacterial enzymes, such as proteases and membrane-bound ATPases, disrupting essential metabolic processes. Furthermore, it induces oxidative stress by generating ROS, which damage cellular components, including DNA, proteins, and lipids [168, 169].
Figure 7.
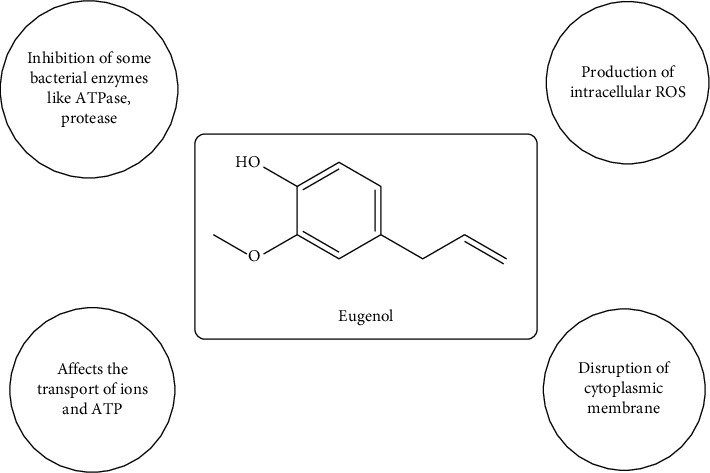
Mechanism of eugenol as an antibacterial agent.
4.3. Antidiabetic Activity
Secondary metabolites found in O. tenuiflorum were reported to inhibit the α-glucosidase enzyme, which is a key enzyme responsible for catalyzing carbohydrate digestion. For the treatment of type 2 diabetes, α-glucosidase inhibitors are used. These drugs impede the absorption of carbohydrates [170]. Leaves of O. tenuiflorum are well known to lower blood glucose levels. Sethi et al. studied the antidiabetic activity of leaves of O. tenuiflorum and reported that chewing the leaves 2-g/kg body weight for the subsequent 30 days led to lower blood glucose levels in the tested group [171]. Rao et al. also conducted a comparative study of ethanolic extract of O. tenuiflorum with glibenclamide; with regular administration of the ethanolic extract, the level of blood glucose reduced abruptly in the hyperglycemic rats. When the ethanolic extract was administered, the results were comparable to those obtained with standard drugs. There was a 51.5% reduction in blood glucose levels on the third day and a 52% reduction in fasting blood glucose levels on the tenth day with the use of standard drugs. Similarly, there was a 50% reduction in blood glucose levels on the third day, and a 45% reduction in fasting blood glucose levels on the tenth day with the use of the ethanolic extract on the alloxan induced diabetes rats [172]. Mousavi, Salleh, and Murugaiyah discovered the interesting results on the in vitro analysis of α-amylase and α-glucosidase inhibition activity. The results indicated that ethyl acetate–butanol and ethanol–water fractions of O. tenuiflorum leaves exhibited less IC50 values of 0.59 ± 0.03 mg/mL and 1.45 ± 0.04 mg/mL, respectively, for α-amylase. The values were 0.05 ± 0.00 mg/mL and 0.10 ± 0.00 mg/mL, respectively, for α-glucosidase. The values for standard acarbose are IC50 1.54 ± 0.21 mg/mL and 0.36 ± 0.21 mg/mL for α-amylase and α-glucosidase, respectively [173]. Parasuraman et al. reported the hydroalcoholic extract of O. tenuiflorum demonstrated significant antidiabetic and anti-hyperlipidemic effects in diabetic rats induced by STZ and NIC when administered at doses of 250 and 500 mg/kg body weight. It reduced the glucose levels from 229.80 ± 10.00 to 129.00 ± 13.20 [174].
The antidiabetic activity of O. tenuiflorum may be attributed to the presence of metabolites such as oleanolic acid, ursolic acid, and rosmarinic acid, both of which possess strong antidiabetic properties [174, 175]. For reference, OA shown in Figure 8 helps to improve the body's response to insulin and supports the health of pancreatic β-cells, which are crucial for insulin production. It also inhibits enzymes such as α-amylase and α-glucosidase that play a key role in maintaining balanced blood sugar levels. Additionally, OA activates antioxidant pathways, reducing oxidative stress, and blocks inflammatory pathways, both of which are important in managing diabetes and preventing complications [176, 177].
Figure 8.
Structure of oleanolic acid.
4.4. Antifertility Activity
Ocimum tenuiflorum is well known for antifertility activity. Mankapure, Mankapure, and Sohani conducted a study on albino rats with doze 400 mg of Tulsi leaves per 100 g of body weight daily for 72 days, which showed a reversible reduction in the testis weight and significant derangements in the histoarchitecture of the testis and epididymis in tested rats [178]. Sethi et al. observed that a doze of 2 g of O. tenuiflorum leaves for 30 days resulted in a notable decline in the sperm count, a reduction in follicle-stimulating hormone, and a rise in serum testosterone levels [179]. Similarly, Ahmed et al. concluded that the administration of 250 mg/kg body weight of the benzene extract of O. tenuiflorum for 48 days resulted in a lessening of total sperm count, sperm motility, forward velocity, and decreased content of fructose in the caudal plasma of epididymis [180]. This antifertility activity of O. tenuiflorum can be attributed to the presence of phytochemicals such as OA and ursolic acid, which are known for antifertility properties [181, 182]. Srinivasulu and Changamma also suggested that ursolic acid acts as an antifertility agent and the study summarized that when the O. tenuiflorum leaf extract was administered to rats, it led to a significant decrease in the sperm count and spermatozoa motility by modulating testosterone levels [183].
4.5. Anti-Inflammatory Activity
Inflammation occurs when infectious microorganisms invade, reside in tissues, or circulate in the blood and may be triggered by processes such as tissue injury, cell death, cancer, ischemia, and degeneration [184]. The O. tenuiflorum as an anti-inflammatory agent has been practiced for a long time. Mirje, Zaman, and Ramabhimaiah found that O. tenuiflorum has a superior anti-inflammatory activity compared to the standard anti-inflammatory drug indomethacin in a carrageenan-induced rat paw edema, with administration improving its anti-inflammatory profile [185]. This property may be due to the dual inhibitory property of O. tenuiflorum against cyclooxygenase and lipoxygenase [185]. Kaur had synthesized iron nanoparticles using O. tenuiflorum to study the anti-inflammatory activity and found that at 100 mg/mL concentration of iron nanoparticles synthesized at 25°C and 0.1 M molarity, the anti-inflammatory activity was maximum of 118.25 [186]. Godhwani, Godhwani, and Vyas observed that the methanol extract and aqueous suspension of O. tenuiflorum effectively inhibited inflammation in rats, comparable to the response observed with sodium salicylate with the concentration of 500 mg/kg for prior and 300 mg/kg for later, respectively [187]. Kewlani et al. compared the anti-inflammatory activity of O. tenuiflorum and Azadirachta indica. In this study, albino rats were injected with formalin to induce inflammation. The samples were administered orally with distilled water, resulting in a remarkable reduction in edema compared to the control group in rats [188]. Similarly, Sharma et al. compared the anti-inflammatory activity of different Ocimum species: O. basilicum L., O. gratissimum L., and O. tenuiflorum L. To evaluate the anti-inflammatory activity, a protein denaturation assay, which is used to induce tissue inflammation, was performed. The results showed that the acetone, methanol, and ethanol extracts of the three Ocimum species significantly protected bovine serum albumin against protein denaturation. The ethanolic extract exhibited the most anti-inflammatory activity, while the water extract of jungle Tulsi and green Tulsi showed the least protection of bovine serum albumin against denaturation [189]. Besides these, essential oil obtained from O. tenuiflorum also exhibited strong anti-inflammatory activity by inhibiting the MMP-9 expression in lipopolysaccharide-induced inflammatory cells as per Manaharan et al. [190].
The bioactive compounds isolated from O.tenuiflorum, particularly rosmarinic acid, eugenol, ursolic acid, apigenin, and luteolin are well documented for their anti-inflammatory activity. Rosmarinic acid (Figure 9) has become well known for its potent anti-inflammatory properties, as supported by numerous studies. It works by suppressing the production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukins (IL-1β, IL-6). By inhibiting these cytokines, rosmarinic acid helps reduce inflammation in various inflammatory disease models, such as arthritis and colitis [191].
Figure 9.
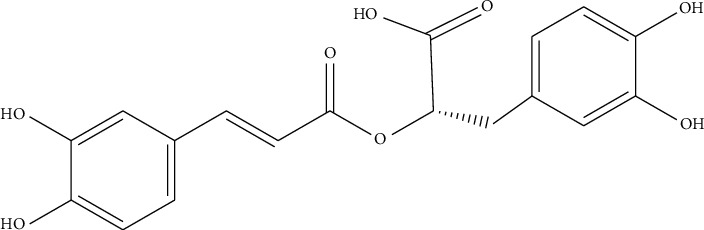
Structure of rosmarinic acid.
4.6. Antistress Activity
Stress can manifest differently in individuals, and it represents a physiological response that prepares an organism for any action [192]. For relief and freedom from stress, O. tenuiflorum is the best medicinal plant. Multiple studies have been conducted to elucidate the antistress properties of O. tenuiflorum, and the results consistently indicate a robust antistress effect. Richard et al. explored the antistress property of O. tenuiflorum in the chronic variable stress (CVS) model. It demonstrated a concentration-dependent decrease in the cortisol level, i.e., 89% inhibition at 100 μg/mL and 50% inhibition at 6.25 μg/mL. Additionally, the O. sanctum-administrated rat's weight increased remarkably compared to the CVS group. This effect on the body weight was attributed to the antistress activity of O. tenuiflorum [193]. Similarly, Mohan et al. conducted an in vivo swim endurance study on mice, with an extract of O. tenuiflorum escalated the swimming time in the tested sample and lessened the stress-induced increase in immobility time. This result suggested the antistress property of the tested sample [194]. On top of that, Saxena et al. reported the effect of O. tenuiflorum to manage stress without causing any side effect [195]. Gupta et al. explored the bioactive compound of O. tenuiflorum or the antistress activity and investigated that Ocimumoside A, Ocimumoside B, and 4-allyl-1-O-β-D-glucopyranosyl-2-hydroxybenzene are responsible for the antistress property [196].
4.7. Anticancer Activity
Due to the presence of secondary metabolites such as flavonoids, sterols, esters, and acyl lipids, plants can be considered a source of anticancer agents [197]. Multiple investigations were carried out to explore the anticancer activity of O. tenuiflorum. Boonyanugomol et al. conducted a study on the anticancer activity of O.tenuiflorum essential oil against a gastric cancer cell line. They used MTT assays and cell migration and invasion assays to assess cell viability and inhibit metastasis. The results indicated that the viability of AGS cells decreased with an IC50 of 163.42 μg/mL when treated with O.tenuiflorum essential oil. Furthermore, this treatment induced cellular changes, including cell shrinkage, chromatin condensation, and fragmentation, which are commonly recognized as structural characteristics of apoptotic cell death [198]. Karthikeyan et al. also reported that the ethanolic extract of O. tenuiflorum treatment caused a significant reduction in the tumor volume in inoculated sarcoma—180 cells. Along with this, the lifespan of tested animals also increased by 73% for the aqueous extract and 118% for ethanolic extract treatment. These results indicate that for the reduction of tumor development, the ethanolic extract seems to be more effective compared to the aqueous extract [199]. Indrayudha and Hapsari also compared the cytotoxic activity of two plant species: Cinnamomum burmannii and O. tenuiflorum Linn against T47D cancer cells. For the cell viability, an MTT assay was conducted. From the cytotoxicity tests, it was concluded that O. tenuiflorum is more effective compared to Ci. burmannii with IC50 values of 266.43 and 456.01 μg/mL, respectively [200]. Similarly, Lam, Neda, and Mohd Salleh also conducted the anticancer activity of O. tenuiflorum leaves, against human breast cancer cell lines and human fibroblast cell lines, and suggested a remarkable decrease in viability in MCF-7 cells when treated with variable concentrations of the methanolic extract with an IC50 of less than 100 μg/mL [201].
The anticancer mechanism of compounds isolated from this plant may be due to different actions such as the inhibition of the signaling pathway, cell cycle arrest, modulation of autophagy, transcription regulation, membrane disruption, and suppression of metabolic enzyme, which is depicted in Figure 10 [202–204].
Figure 10.
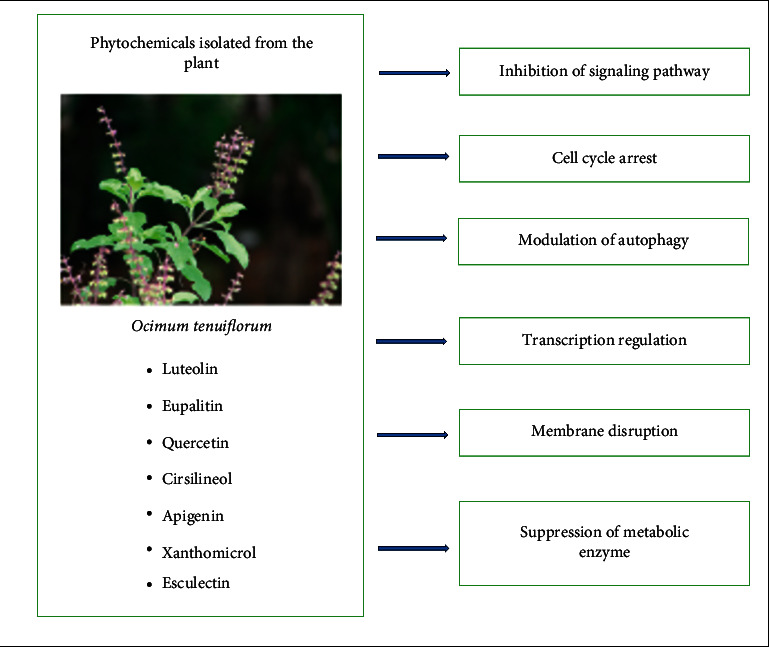
Mechanism of anticancer action of compounds isolated from Ocimum tenuiflorum.
For reference, luteolin is a potent bioactive compound that works synergistically with anticancer drugs to inhibit cancer progression. It is effectively used in treating different cancers as shown in Figure 11, including colon, breast, prostate, and liver cancers by inducing apoptosis, arresting the cell cycle, and inhibiting metastasis and angiogenesis. The strength of its anticancer effects comes from its oxidative properties and its ability to interact with multiple targets and signaling pathways in tumor cells, enhancing its overall efficacy [205, 206].
Figure 11.
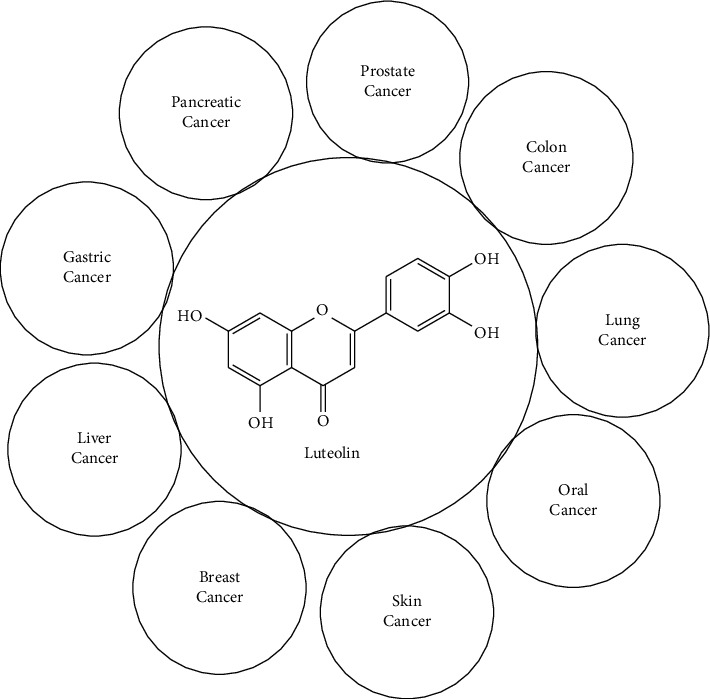
Anticancer activity of luteolin.
4.8. Other Biological Activities
Ocimum tenuiflorum has been studied for its multiple biological purposes. It has been reported as a significant wound-healing agent. This test was conducted using incision, excision, and dead space wounds in rats [207]. Extracts and oil obtained from O. tenuiflorum were reported to have remarkable analgesic and antipyretic properties [187, 208, 209]. It is also effectively combated against heavy metals, anti-TB drugs, gastric ulcerations, reducing hepatocarcinogenesis, and improving hepatic metabolism [9]. Additionally, the extract containing metabolites such as OA, ursolic acid, rosmarinic acid, eugenol, carvacrol, linalool, and β-caryophyllene of O. tenuiflorum inhibit COX-2, which is responsible for the inflammation and pain [210]. On the other hand, rosmarinic acid present in O. tenuiflorum is reported to be responsible for antiaging activities of the O. tenuiflorum [211]. In bovine subclinical mastitis, the aqueous extract of the O. tenuiflorum demonstrated immunotherapeutic potential through intramammary infusion, enhancing the phagocytic activity and phagocytic index, reducing total bacterial count, and increasing neutrophil and lymphocyte counts [212]. This plant has been tested for anticonvulsant efficacy, and its ethanol and chloroform extracts from the stem, leaf, and stem callus are potent in suppressing trans corneal electroshock-induced tonic convulsions, comparable to the standard drug phenytoin [213]. Eugenol, an important constituent of O. tenuiflorum acts as a strong anthelmintic agent with an ED50 of 62.1 μg/mL, which makes plant essential oil effective in the anthelmintic activity [214]. The plant was investigated to explore antithyroid properties and the effects of the O. tenuiflorum leaf extract on serum triiodothyronine, and thyroxine showed significant decreases in serum T4 concentrations and no changes in T3 and the ratio between T3 and T4. This suggested that O. tenuiflorum exhibited antithyroid properties [215]. Ocimum tenuiflorum has been shown to protect against toxicants such as industrial chemicals, pesticides, and pharmaceuticals, preventing liver, kidney, and brain injury. It also protects against the harmful effects caused by acetaminophen, meloxicam, chlorpyrifos, butyl p-hydroxybenzoic acid, copper sulfate, and antitubercular drugs [216–222]. Besides these, O. tenuiflorum has been reported to possess properties such as aldose reductase inhibitor, antispasmodic, adaptogenic, cardioprotective, diaphoretic, immune-modulating, anti-inflammatory, antibacterial, antiviral, antifungal, antipyretic, antidiuretic, antidiabetic, antimalarial, and hypolipidemic properties [223–226]. All these properties of O. tenuiflorum are due to metabolites present in the plant. Therefore, it is supposed to be an elixir of life.
5. Conclusion
Ocimum tenuiflorum has long been a cornerstone of traditional medicine, valued for its wide range of therapeutic uses. Even today, its antibacterial, antiviral, antifungal, antipyretic, antidiuretic, antidiabetic, and antimalarial properties are well recognized, largely due to its rich content of metabolites such as flavonoids, phenolics, and terpenoids. Given its potential as a source of antidiabetic, anticancer, antimicrobial, and antioxidant agents, further research into these bioactive compounds is crucial. This includes exploring their design and development, bioavailability, toxicity, and effectiveness, both in their natural state and as derivatives. Ocimum tenuiflorum holds promise as a valuable source of bioactive metabolites, offering the potential for the development of novel therapeutic agents in the future.
Acknowledgments
The authors acknowledge the Center for Applied and Natural Sciences for providing a platform to conduct this work. They are also thankful to all the researchers and other individuals who have made significant contributions, insights, and suggestions that helped us improve the quality of our work. Additionally, it is important to mention that grammar checking and editing were done using artificial intelligence (AI) software Grammarly.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
K.B., R.B., R.D.P., B.P., and H.D.B. have conducted a comprehensive literature review and data collection, data presentation, and manuscript writing. K.B., B.P., and H.D.B. were involved in creating tables and figures. Additionally, R.P., B.P., and H.D.B. have done the editing, quality checks, content verification, and overall review. All authors agreed to the final version of the manuscript.
Funding
No funding was obtained for this study.
References
- 1.Walia A., Kumar N., Singh R., et al. Bioactive Compounds in Ficus Fruits, Their Bioactivities, and Associated Health Benefits: A Review. Journal of Food Quality . 2022 April;2022:1–19. doi: 10.1155/2022/6597092. [DOI] [Google Scholar]
- 2.Adebayo S. A., Amoo S. O., Mokgehle S. N., Aremu A. O. Ethnomedicinal Uses, Biological Activities, Phytochemistry and Conservation of African Ginger (Siphonochilus aethiopicus): A Commercially Important and Endangered Medicinal Plant. Journal of Ethnopharmacology . 2021 February;266 doi: 10.1016/j.jep.2020.113459. [DOI] [PubMed] [Google Scholar]
- 3.Bast F., Rani P., Meena D. Chloroplast DNA Phylogeography of Holy Basil (Ocimum tenuiflorum) in Indian Subcontinent. The Scientific World Journal . 2014 January;2014:1–6. doi: 10.1155/2014/847482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali H., Nguta J., Musila F., Ole-Mapenay I., Matara D., Mailu J. Evaluation of Antimicrobial Activity, Cytotoxicity, and Phytochemical Composition of Ocimum Americanum L. (Lamiaceae) Evidence-based Complementary and Alternative Medicine . 2022 March;2022:1–11. doi: 10.1155/2022/6484578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Costa A. S., Arrigoni-Blank M. D. F., de Carvalho Filho J. L. S., et al. Chemical Diversity in Basil (Ocimum sp.) Germplasm. The Scientific World Journal . 2015 January;2015(1):p. e352638. doi: 10.1155/2015/352638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Islam A. K. M. M., Kato-Noguchi H. Phytotoxic Activity of Ocimum tenuiflorum Extracts on Germination and Seedling Growth of Different Plant Species. The Scientific World Journal . 2014 June;2014:1–8. doi: 10.1155/2014/676242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adela Alemu M., Andargie Y., Sisay W., et al. Antidiarrheal Effect of 80% Methanol Extract and Fractions of the Leaves of Ocimum lamiifolium in Swiss Albino Mice. Evidence-based Complementary and Alternative Medicine . 2022 May;2022:1–9. doi: 10.1155/2022/6838295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medeiros Venancio A., Ferreira-da-Silva F. W., da Silva-Alves K. S., et al. Essential Oil of Ocimum basilicum L. and (−)-Linalool Blocks the Excitability of Rat Sciatic Nerve. Evidence-Based Complementary and Alternative Medicine . 2016 June;2016(1):p. e9012605. doi: 10.1155/2016/9012605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baliga M. S., Shivashankara A. R., Azmidah A., Sunitha V., Palatty P. L. Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease . Amsterdam, Netherlands: Elsevier; 2013. Gastrointestinal and Hepatoprotective Effects of Ocimum sanctum L. Syn (Holy Basil or Tulsi): Validation of the Ethnomedicinal Observation; pp. 325–335. [Google Scholar]
- 10.Singh V., Amdekar S., Verma O. Ocimum sanctum (Tulsi): Bio-Pharmacological Activities. WebmedCentral Pharmacology . 2010;1(10):p. WMC001046. [Google Scholar]
- 11.Jamshidi N., Cohen M. M. The Clinical Efficacy and Safety of Tulsi in Humans: A Systematic Review of the Literature. Evidence-Based Complementary and Alternative Medicine: eCAM . 2017 March;2017(1):p. e9217567. doi: 10.1155/2017/9217567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datiles M. J., Acevedo-Rodríguez P. Ocimum tenuiflorum (Holy Basil) CABI Compendium . 2014 August;:p. 110287. [Google Scholar]
- 13.NParks, Ocimum tenuiflorum. https://www.nparks.gov.sg/florafaunaweb/flora/2/2/2275 .
- 14. Ocimum tenuiflorum L. | Species. India Biodiversity Portal . https://indiabiodiversity.org/species/show/33205 . [Google Scholar]
- 15.Pathak I., Niraula M. Assessment of Total Phenolic, Flavonoid Content and Antioxidant Activity of Ocimum sanctum Linn. Journal of Nepal Chemical Society . 2019 December;40:30–35. doi: 10.3126/jncs.v40i0.27275. [DOI] [Google Scholar]
- 16.Joseph B., Nair V. M. Ethanopharmacological and Phytochemical Aspects of Ocimum sanctum Linn- The Elixir of Life. Journal of Pharmaceutical Research International . 2013 March;:273–292. [Google Scholar]
- 17.Selvam K., Rajinikanth R., Govarthanan M., Paul A., Selvankumar T., Sengottaiyan A. Antioxidant Potential and Secondary Metabolites in Ocimum sanctum L. at Various Habitats. Journal of Medicinal Plants Research . 2013;7(12):706–712. [Google Scholar]
- 18.Yamani H. A., Pang E. C., Mantri N., Deighton M. A. Antimicrobial Activity of Tulsi (Ocimum tenuiflorum) Essential Oil and Their Major Constituents Against Three Species of Bacteria. Frontiers in Microbiology . 2016;7 doi: 10.3389/fmicb.2016.00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samanta S., Das N. K., Ghosh A. Precious Phytomedicinal Components of Tulsi (Ocimum sanctum Linn.) and Their Therapeutic Importance. International Journal of Current Research . 2021;13(10):19119–19141. [Google Scholar]
- 20.Gogoi P., Nath N. Indigenous Knowledge of Ethnomedicinal Plants by the Assamese Community in Dibrugarh District, Assam, India. Journal of Threatened Taxa . 2021 April;13(5):18297–18312. doi: 10.11609/jott.6772.13.5.18297-18312. [DOI] [Google Scholar]
- 21.Uritu C. M., Mihai C. T., Stanciu G.-D., et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Research and Management . 2018 May;2018:1–44. doi: 10.1155/2018/7801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghaleb Dailah H. The Ethnomedicinal Evidences Pertaining to Traditional Medicinal Herbs Used in the Treatment of Respiratory Illnesses and Disorders in Saudi Arabia: A Review. Saudi Journal of Biological Sciences . 2022 September;29(9) doi: 10.1016/j.sjbs.2022.103386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul A. K., Alam M. J., Alam A. H. M. J. A Preliminary Survey of Ethno-Medicinal Plants Used by the Chakma Community of Rangamati and Khagrachari Hill District, Bangladesh. Arabian Journal of Medicinal and Aromatic Plants . 2019 June;5(2):1–22. [Google Scholar]
- 24.Kadir M. F., Bin Sayeed M. S., Setu N. I., Mostafa A., Mia M. M. K. Ethnopharmacological Survey of Medicinal Plants Used by Traditional Health Practitioners in Thanchi, Bandarban Hill Tracts, Bangladesh. Journal of Ethnopharmacology . 2014 August;155(1):495–508. doi: 10.1016/j.jep.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 25.JIRCAS: Ocimum tenuiflorum: Local Vegetables of Thailand: Color Illustrated. https://www.jircas.go.jp/project/value_addition/Vegetables/079.html .
- 26.Pikulthong V., Bhirompan S., Dechkla M., et al. Local Herbs for Pain Relief in the Area of Tumbon Khao Hin Son, Chachoengsao, Thailand. Biodiversitas Journal of Biological Diversity . 2022 October;23(10) doi: 10.13057/biodiv/d231007. [DOI] [Google Scholar]
- 27.Maneenoon K., Khuniad C., Teanuan Y., et al. Ethnomedicinal Plants Used by Traditional Healers in Phatthalung Province, Peninsular Thailand. Journal of Ethnobiology and Ethnomedicine . 2015 May;11(1):p. 43. doi: 10.1186/s13002-015-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFilipps R. A., Krupnick G. A. The Medicinal Plants of Myanmar. PhytoKeys . 2018 June;102:1–341. doi: 10.3897/phytokeys.102.24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zahoor M., Yousaf Z., Aqsa T., et al. An Ethnopharmacological Evaluation of Navapind and Shahpur Virkanin District Sheikupura, Pakistan for Their Herbal Medicines. Journal of Ethnobiology and Ethnomedicine . 2017 May;13(1):p. 27. doi: 10.1186/s13002-017-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernández-Rodríguez P., Baquero L. P., Larrota H. R. Flavonoids: Potential Therapeutic Agents by Their Antioxidant Capacity. In: Campos M. R. S., editor. Bioactive Compounds . Sawston, UK: Woodhead Publishing; 2019. pp. 265–288. [Google Scholar]
- 31.Roy A., Khan A., Ahmad I., et al. Flavonoids a Bioactive Compound From Medicinal Plants and Its Therapeutic Applications. BioMed Research International . 2022 June;2022:p. e5445291. doi: 10.1155/2022/5445291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venuprasad M. P., Kandikattu H. K., Razack S., Amruta N., Khanum F. Chemical Composition of Ocimum sanctum by LC-ESI–MS/MS Analysis and Its Protective Effects against Smoke Induced Lung and Neuronal Tissue Damage in Rats. Biomedicine and Pharmacotherapy . 2017 July;91:1–12. doi: 10.1016/j.biopha.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhary A., Sharma S., Mittal A., Gupta S., Dua A. Phytochemical and Antioxidant Profiling of Ocimum sanctum. Journal of Food Science and Technology . 2020 October;57(10):3852–3863. doi: 10.1007/s13197-020-04417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dharsono H. D. A., Putri S. A., Kurnia D., Dudi D., Satari M. H. Ocimum Species: A Review on Chemical Constituents and Antibacterial Activity. Molecules . 2022 September;27(19):p. 6350. doi: 10.3390/molecules27196350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mousavi L., Salleh R. M., Murugaiyah V. Phytochemical and Bioactive Compounds Identification of Ocimum tenuiflorum Leaves of Methanol Extract and Its Fraction With an Anti-Diabetic Potential. International Journal of Food Properties . 2018 January;21(1):2390–2399. doi: 10.1080/10942912.2018.1508161. [DOI] [Google Scholar]
- 36.Baliga M. S., Jimmy R., Thilakchand K. R., et al. Ocimum sanctum L (Holy Basil or Tulsi) and Its Phytochemicals in the Prevention and Treatment of Cancer. Nutrition and Cancer . 2013 January;65(sup1):26–35. doi: 10.1080/01635581.2013.785010. [DOI] [PubMed] [Google Scholar]
- 37.Punia Bangar S., Kajla P., Chaudhary V., Sharma N., Ozogul F. Luteolin: A Flavone With Myriads of Bioactivities and Food Applications. Food Bioscience . 2023 April;52 doi: 10.1016/j.fbio.2023.102366. [DOI] [Google Scholar]
- 38.Caporali S., De Stefano A., Calabrese C., et al. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients . 2022 March;14(6):p. 1155. doi: 10.3390/nu14061155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z., Gao S., Bu Y., Zheng X. Luteolin Protects Cardiomyocytes Cells Against Lipopolysaccharide-Induced Apoptosis and Inflammatory Damage by Modulating Nlrp3. Yonsei Medical Journal . 2022 March;63(3):220–228. doi: 10.3349/ymj.2022.63.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X., He X., Chen S., et al. The Genotoxicity Potential of Luteolin Is Enhanced by CYP1A1 and CYP1A2 in Human Lymphoblastoid TK6 Cells. Toxicology Letters . 2021 June;344:58–68. doi: 10.1016/j.toxlet.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghalib R. M., Mehdi S. H., Hashim R., et al. Eupalitin From Asparagus falcatus (Linn.) Has Anti-Cancer Activity and Induces Activation of Caspases 3/7 in Human Colorectal Tumor Cells. Journal of Medicinal Plants Research . 2013 May;7(20):1401–1405. [Google Scholar]
- 42.Kaleem S., Siddiqui S., Siddiqui H. H., et al. Eupalitin Induces Apoptosis in Prostate Carcinoma Cells Through ROS Generation and Increase of Caspase-3 Activity. Cell Biology International . 2016;40(2):196–203. doi: 10.1002/cbin.10552. [DOI] [PubMed] [Google Scholar]
- 43.Abbasi E., Nassiri-Asl M., Shafeei M., Sheikhi M. Neuroprotective Effects of Vitexin, a Flavonoid, on Pentylenetetrazole-Induced Seizure in Rats. Chemical Biology and Drug Design . 2012;80(2):274–278. doi: 10.1111/j.1747-0285.2012.01400.x. [DOI] [PubMed] [Google Scholar]
- 44.Can Ö. D., Demir Özkay Ü., Üçel U. İ. Anti-Depressant-Like Effect of Vitexin in BALB/c Mice and Evidence for the Involvement of Monoaminergic Mechanisms. European Journal of Pharmacology . 2013 January;699(1-3):250–257. doi: 10.1016/j.ejphar.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Min J.-W., Hu J.-J., He M., et al. Vitexin Reduces Hypoxia–Ischemia Neonatal Brain Injury by the Inhibition of HIF-1alpha in a Rat Pup Model. Neuropharmacology . 2015 December;99:38–50. doi: 10.1016/j.neuropharm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Vitexin: Benefits, Safety, and Uses in Dietary Supplements. https://www.digicomply.com/dietary-supplements-database/vitexin .
- 47.Xu D., Hu M.-J., Wang Y.-Q., Cui Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules . 2019 March;24(6):p. 1123. doi: 10.3390/molecules24061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou H., Ye H., Kamaraj R., Zhang T., Zhang J., Pavek P. A Review on Pharmacological Activities and Synergistic Effect of Quercetin With Small Molecule Agents. Phytomedicine . 2021 November;92 doi: 10.1016/j.phymed.2021.153736. [DOI] [PubMed] [Google Scholar]
- 49.Wang G., Wang Y., Yao L., et al. Pharmacological Activity of Quercetin: An Updated Review. Evidence-Based Complementary and Alternative Medicine . 2022 December;2022:1–12. doi: 10.1155/2022/3997190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quercetin: Uses and Risks. https://www.webmd.com/vitamins-and-supplements/quercetin-uses-and-risks .
- 51.Pathak G., Singh S., Kumari P., et al. Cirsilineol Inhibits Proliferation of Lung Squamous Cell Carcinoma by Inducing ROS Mediated Apoptosis. Food and Chemical Toxicology . 2020 September;143 doi: 10.1016/j.fct.2020.111550. [DOI] [PubMed] [Google Scholar]
- 52.Kim G. O., Heo J. B., Park D. H., Song G. Y., Bae J.-S. Antiplatelet Aggregation Properties of Cirsilineol: A Novel Inhibitor of Blood Coagulation Factor Xa. Pharmaceuticals . 2023 April;16(4):p. 588. doi: 10.3390/ph16040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madunić J., Madunić I. V., Gajski G., Popić J., Garaj-Vrhovac V. Apigenin: A Dietary Flavonoid With Diverse Anticancer Properties. Cancer Letters . 2018 January;413:11–22. doi: 10.1016/j.canlet.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 54.Chen P., Chen F., Guo Z., Lei J., Zhou B. Recent Advancement in Bioeffect, Metabolism, Stability, and Delivery Systems of Apigenin, a Natural Flavonoid Compound: Challenges and Perspectives. Frontiers in Nutrition . 2023;10 doi: 10.3389/fnut.2023.1221227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Recent Insights into the Biological Functions of Apigenin - PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7415933/ [DOI] [PMC free article] [PubMed]
- 56.Apigenin Supplement Side Effects - Statcare. https://statcarewalkin.com/info/apigenin-supplement-side-effects.html .
- 57.Nieddu M., Pollastro F., Caria P., Salamone S., Rosa A. Xanthomicrol Activity in Cancer HeLa Cells: Comparison With Other Natural Methoxylated Flavones. Molecules . 2023 January;28(2):p. 558. doi: 10.3390/molecules28020558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fattahi M., Cusido R. M., Khojasteh A., Bonfill M., Palazon J. Xanthomicrol: A Comprehensive Review of Its Chemistry, Distribution, Biosynthesis and Pharmacological Activity. Mini-Reviews in Medicinal Chemistry . 2014;14(9):725–733. doi: 10.2174/1389557514666140820122818. [DOI] [PubMed] [Google Scholar]
- 59.Li Y.-L., Ma S.-C., Yang Y.-T., Ye S.-M., But P. P.-H. Antiviral Activities of Flavonoids and Organic Acid From Trollius chinensis Bunge. Journal of Ethnopharmacology . 2002 March;79(3):365–368. doi: 10.1016/s0378-8741(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 60.Ali H., Dixit S. In Vitro Antimicrobial Activity of Flavanoids of Ocimum sanctum With Synergistic Effect of Their Combined Form. Asian Pacific Journal of Tropical Disease . 2012 January;2:S396–S398. doi: 10.1016/s2222-1808(12)60189-3. [DOI] [Google Scholar]
- 61.Lam K. Y., Ling A. P. K., Koh R. Y., Wong Y. P., Say Y. H. A Review on Medicinal Properties of Orientin. Advances in Pharmacological Sciences . 2016 May;2016:1–9. doi: 10.1155/2016/4104595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi D. Y., Lee J. Y., Kim M. R., Woo E. R., Kim Y. G., Kang K. W. Chrysoeriol Potently Inhibits the Induction of Nitric Oxide Synthase by Blocking AP-1 Activation. Journal of Biomedical Science . 2005 December;12(6):949–959. doi: 10.1007/s11373-005-9028-8. [DOI] [PubMed] [Google Scholar]
- 63.Tofighi Z., Alipour F., Hadavinia H., Abdollahi M., Hadjiakhoondi A., Yassa N. Effective Antidiabetic and Antioxidant Fractions of Otostegia Persica Extract and Their Constituents. Pharmaceutical Biology . 2014 August;52(8):961–966. doi: 10.3109/13880209.2013.874463. [DOI] [PubMed] [Google Scholar]
- 64.Bashyal P., Parajuli P., Pandey R. P., Sohng J. K. Microbial Biosynthesis of Antibacterial Chrysoeriol in Recombinant Escherichia coli and Bioactivity Assessment. Catalysts . 2019 Feb.9(2):p. 112. doi: 10.3390/catal9020112. [DOI] [Google Scholar]
- 65.Cai T., Cai B. Pharmacological Activities of Esculin and Esculetin: A Review. Medicine (Baltimore) . 2023 October;102(40):p. e35306. doi: 10.1097/md.0000000000035306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang K. S., Lee W., Jung Y., et al. Protective Effect of Esculin on Streptozotocin-Induced Diabetic Renal Damage in Mice. Journal of Agricultural and Food Chemistry . 2014 March;62(9):2069–2076. doi: 10.1021/jf403840c. [DOI] [PubMed] [Google Scholar]
- 67.Mokdad-Bzeouich I., Mustapha N., Chaabane F., et al. Oligomerization of Esculin Improves Its Antibacterial Activity and Modulates Antibiotic Resistance. Journal of Antibiotics . 2015 March;68(3):148–152. doi: 10.1038/ja.2014.127. [DOI] [PubMed] [Google Scholar]
- 68.Horse Chestnut: Overview, Uses, Side Effects, Precautions, Interactions, Dosing and Reviews. https://www.webmd.com/vitamins/ai/ingredientmono-1055/horse-chestnut .
- 69.Plumlee K. H. Plants. In: Plumlee K. H., editor. Clinical Veterinary Toxicology . Saint Louis: Mosby; 2004. pp. 337–442. [Google Scholar]
- 70.Choi Y. J., Lee C. M., Park S.-H., Nam M. J. Esculetin Induces Cell Cycle Arrest and Apoptosis in Human Colon Cancer LoVo Cells. Environmental Toxicology . 2019;34(10):1129–1136. doi: 10.1002/tox.22815. [DOI] [PubMed] [Google Scholar]
- 71.Wang G., Lu M., Yao Y., Wang J., Li J. Esculetin Exerts Antitumor Effect on Human Gastric Cancer Cells Through IGF-1/PI3K/Akt Signaling Pathway. European Journal of Pharmacology . 2017 November;814:207–215. doi: 10.1016/j.ejphar.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 72.Chang H. T., Chou C. T., Lin Y. S., et al. Esculetin, a Natural Coumarin Compound, Evokes Ca(2+) Movement and Activation of Ca(2+)-Associated Mitochondrial Apoptotic Pathways That Involved Cell Cycle Arrest in ZR-75-1 Human Breast Cancer Cells. Tumor Biology . 2016 April;37(4):4665–4678. doi: 10.1007/s13277-015-4286-1. [DOI] [PubMed] [Google Scholar]
- 73.Turkekul K., Colpan R. D., Baykul T., Ozdemir M. D., Erdogan S. Esculetin Inhibits the Survival of Human Prostate Cancer Cells by Inducing Apoptosis and Arresting the Cell Cycle. Journal of Cancer Prevention . 2018 March;23(1):10–17. doi: 10.15430/jcp.2018.23.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Q.-H., Qin S.-W., Jiang J.-G. Improvement Effects of Esculetin on the Formation and Development of Atherosclerosis. Biomedicine and Pharmacotherapy . 2022 June;150 doi: 10.1016/j.biopha.2022.113001. [DOI] [PubMed] [Google Scholar]
- 75.Saranraj P., Behera S. S., Ray R. C. Traditional Foods from Tropical Root and Tuber Crops: Innovations and Challenges. In: Galanakis C. M., editor. Innovations in Traditional Foods . Sawston, UK: Woodhead Publishing; 2019. pp. 159–191. [Google Scholar]
- 76.Lin D., Xiao M., Zhao J., et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules . 2016 October;21(10):p. 1374. doi: 10.3390/molecules21101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de la Rosa L. A., Moreno-Escamilla J. O., Rodrigo-García J., Alvarez-Parrilla E. Postharvest Physiology and Biochemistry of Fruits and Vegetables . Sawston, UK: Woodhead Publishing; 2019. Phenolic Compounds; pp. 253–271. [Google Scholar]
- 78.Flegkas A., Milosević Ifantis T., Barda C., Samara P., Tsitsilonis O., Skaltsa H. Antiproliferative Activity of (−)-Rabdosiin Isolated From Ocimum sanctum L. Medicines . 2019 March;6(1):p. 37. doi: 10.3390/medicines6010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sevgi K., Tepe B., Sarikurkcu C. Antioxidant and DNA Damage Protection Potentials of Selected Phenolic Acids. Food and Chemical Toxicology . 2015 March;77:12–21. doi: 10.1016/j.fct.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 80.Noor S., Mohammad T., Rub M. A., et al. Biomedical Features and Therapeutic Potential of Rosmarinic Acid. Archives of Pharmacal Research . 2022;45(4):205–228. doi: 10.1007/s12272-022-01378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jang Y.-G., Hwang K.-A., Choi K.-C. Rosmarinic Acid, a Component of Rosemary Tea, Induced the Cell Cycle Arrest and Apoptosis Through Modulation of HDAC2 Expression in Prostate Cancer Cell Lines. Nutrients . 2018 November;10(11):p. 1784. doi: 10.3390/nu10111784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zuo J., Tang W., Xu Y. Anti-Hepatitis B Virus Activity of Chlorogenic Acid and Its Related Compounds. In: Preedy V. R., editor. Coffee in Health and Disease Prevention . San Diego: Academic Press; 2015. pp. 607–613. [Google Scholar]
- 83.Wang L., Pan X., Jiang L., et al. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Frontiers in Nutrition . 2022 June;9 doi: 10.3389/fnut.2022.943911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khan F., Bamunuarachchi N. I., Tabassum N., Kim Y.-M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs Toward Microbial Pathogens. Journal of Agricultural and Food Chemistry . 2021 March;69(10):2979–3004. doi: 10.1021/acs.jafc.0c07579. [DOI] [PubMed] [Google Scholar]
- 85.Gülçin I. Antioxidant Activity of Caffeic Acid (3,4-Dihydroxycinnamic Acid) Toxicology . 2006 January;217(2–3):213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y., Qiu S., Wang L., et al. Reproductive and Developmental Toxicity Study of Caffeic Acid in Mice. Food and Chemical Toxicology . 2019 January;123:106–112. doi: 10.1016/j.fct.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 87.Ramadoss D. P., Sivalingam N. Vanillin Extracted From Proso and Barnyard Millets Induce Apoptotic Cell Death in HT-29 Human Colon Cancer Cell Line. Nutrition and Cancer . 2020;72(8):1422–1437. doi: 10.1080/01635581.2019.1672763. [DOI] [PubMed] [Google Scholar]
- 88.Tai A., Sawano T., Yazama F., Ito H. Evaluation of Antioxidant Activity of Vanillin by Using Multiple Antioxidant Assays. Biochimica et Biophysica Acta (BBA) - General Subjects . 2011 Feb.1810(2):170–177. doi: 10.1016/j.bbagen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 89.Gupta S., Sharma B. Pharmacological Benefits of Agomelatine and Vanillin in Experimental Model of Huntington’s Disease. Pharmacology Biochemistry and Behavior . 2014 July;122:122–135. doi: 10.1016/j.pbb.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 90.Abraham D. J., Mehanna A. S., Wireko F. C., Whitney J., Thomas R. P., Orringer E. P. Vanillin, a Potential Agent for the Treatment of Sickle Cell Anemia. Blood . 1991 March;77(6):1334–1341. doi: 10.1182/blood.v77.6.1334.1334. [DOI] [PubMed] [Google Scholar]
- 91.Ngarmsak M., Delaquis P., Toivonen P., Ngarmsak T., Ooraikul B., Mazza G. Antimicrobial Activity of Vanillin Against Spoilage Microorganisms in Stored Fresh-Cut Mangoes. Journal of Food Protection . 2006 July;69(7):1724–1727. doi: 10.4315/0362-028x-69.7.1724. [DOI] [PubMed] [Google Scholar]
- 92.Taştemur Ş., Hacısüleyman L., Karataş Ö., Yulak F., Ataseven H. Anticancer Activity of Sinapic Acid by Inducing Apoptosis in HT-29 Human Colon Cancer Cell Line. Canadian Journal of Physiology and Pharmacology . 2023 July;101(7):361–368. doi: 10.1139/cjpp-2022-0523. [DOI] [PubMed] [Google Scholar]
- 93.Cos P., Rajan P., Vedernikova I., et al. In Vitro Antioxidant Profile of Phenolic Acid Derivatives. Free Radical Research . 2002 January;36(6):711–716. doi: 10.1080/10715760290029182. [DOI] [PubMed] [Google Scholar]
- 94.Gurbuzer A. Investigation of In Vitro Antimicrobial Activities of Some Hydroxybenzoic and Hydroxycinnamic Acids Commonly Found in Medicinal and Aromatic Plants. International Journal of Plant Based Pharmaceuticals . 2021 June;1(1):42–47. doi: 10.62313/ijpbp.2021.3. [DOI] [Google Scholar]
- 95.Aldaba-Muruato L. R., Ventura-Juárez J., Perez-Hernandez A. M., et al. Therapeutic Perspectives of P-Coumaric Acid: Anti-Necrotic, Anti-Cholestatic and Anti-Amoebic Activities. World Academy of Sciences Journal . 2021 September;3(5):47–48. doi: 10.3892/wasj.2021.118. [DOI] [Google Scholar]
- 96.Pei K., Ou J., Huang J., Ou S. p-Coumaric Acid and Its Conjugates: Dietary Sources, Pharmacokinetic Properties and Biological Activities. Journal of the Science of Food and Agriculture . 2016;96(9):2952–2962. doi: 10.1002/jsfa.7578. [DOI] [PubMed] [Google Scholar]
- 97.Khelifi-Touhami F., Taha R. A., Badary O. A., Lezzar A., Hamada F. M. A. Goitrogenic Activity of P-Coumaric Acid in Rats. Journal of Biochemical and Molecular Toxicology . 2003;17(6):324–328. doi: 10.1002/jbt.10094. [DOI] [PubMed] [Google Scholar]
- 98.Li X., Wang X., Chen D., Chen S. Antioxidant Activity and Mechanism of Protocatechuic Acid In Vitro. Functional Foods in Health and Disease . 2011 July;1(7):p. 232. doi: 10.31989/ffhd.v1i7.127. [DOI] [Google Scholar]
- 99.Wu M., Tian L., Fu J., et al. Antibacterial Mechanism of Protocatechuic Acid Against Yersinia Enterocolitica and Its Application in Pork. Food Control . 2022 March;133 doi: 10.1016/j.foodcont.2021.108573. [DOI] [Google Scholar]
- 100.Xie Z., Guo Z., Wang Y., Lei J., Yu J. Protocatechuic Acid Inhibits the Growth of Ovarian Cancer Cells by Inducing Apoptosis and Autophagy. Phytotherapy Research . 2018 November;32(11):2256–2263. doi: 10.1002/ptr.6163. [DOI] [PubMed] [Google Scholar]
- 101.Jang S.-A., Song H. S., Kwon J. E., et al. Protocatechuic Acid Attenuates Trabecular Bone Loss in Ovariectomized Mice. Oxidative Medicine and Cellular Longevity . 2018 July;2018(1):p. e7280342. doi: 10.1155/2018/7280342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dikmen D. Y., Okcay Y., Arslan R., Bektas N. Cannabinoid System Involves in the Analgesic Effect of Protocatechuic Acid. DARU Journal of Pharmaceutical Sciences . 2019 July;27(2):605–612. doi: 10.1007/s40199-019-00288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin S., Cho S. H., Park D., Jung E. Anti-Skin Aging Properties of Protocatechuic Acid In Vitro and In Vivo. Journal of Cosmetic Dermatology . 2020 April;19(4):977–984. doi: 10.1111/jocd.13086. [DOI] [PubMed] [Google Scholar]
- 104.Kakkar S., Bais S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacology . 2014 March;2014:1–9. doi: 10.1155/2014/952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar N., Pruthi V. Potential Applications of Ferulic Acid From Natural Sources. Biotechnology Reports . 2014 December;4:86–93. doi: 10.1016/j.btre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin C.-M., Chiu J.-H., Wu I.-H., Wang B.-W., Pan C.-M., Chen Y.-H. Ferulic Acid Augments Angiogenesis via VEGF, PDGF and HIF-1α. The Journal of Nutritional Biochemistry . 2010 July;21(7):627–633. doi: 10.1016/j.jnutbio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 107.Borges A., Ferreira C., Saavedra M. J., Simões M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microbial Drug Resistance . 2013 August;19(4):256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 108.D’yakonov V. A., Dzhemileva L. U., Dzhemilev U. M. Studies in Natural Products Chemistry . Vol. 54. Amsterdam, Netherlands: Elsevier; 2017. Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors; pp. 21–86. [DOI] [Google Scholar]
- 109.Seigler D. S. Triterpenes and Steroids. In: Seigler D. S., editor. Plant Secondary Metabolism . Boston, MA: Springer US; 1998. pp. 427–455. [Google Scholar]
- 110.Zhou L., Wang J., Wang K., et al. Studies in Natural Products Chemistry . Vol. 37. Amsterdam, Netherlands: Elsevier; 2012. Secondary Metabolites With Antinematodal Activity From Higher Plants; pp. 67–114. [DOI] [Google Scholar]
- 111.Ericson-Neilsen W., Kaye A. D. Steroids: Pharmacology, Complications, and Practice Delivery Issues. The Ochsner Journal . 2014;14(2):203–207. [PMC free article] [PubMed] [Google Scholar]
- 112.Agarwal K., Singh D. K., Jyotshna J., et al. Antioxidative Potential of Two Chemically Characterized Ocimum (Tulsi) Species Extracts. Biomedical Research and Therapy . 2017 September;4(9):1574–1590. doi: 10.15419/bmrat.v4i9.366. [DOI] [Google Scholar]
- 113.Le X. T., Nguyen H. T., Nguyen T. V., et al. Ocimum sanctum Linn. Extract Improves Cognitive Deficits in Olfactory Bulbectomized Mice via the Enhancement of Central Cholinergic Systems and VEGF Expression. Evidence-Based Complementary and Alternative Medicine . 2021 July;2021:1–12. doi: 10.1155/2021/6627648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Joshi S., Karna A. K. Analysis of Phytoconstiuents and Cytotoxic Activities of Different Parts of Ocimum sanctum. International Journal of Applied Sciences and Biotechnology . 2013 September;1(3):137–144. doi: 10.3126/ijasbt.v1i3.8609. [DOI] [Google Scholar]
- 115.Suzuki A., Shirota O., Mori K., et al. Leishmanicidal Active Constituents From Nepalese Medicinal Plant Tulsi (Ocimum sanctum L.) Chemical and Pharmaceutical Bulletin . 2009;57(3):245–251. doi: 10.1248/cpb.57.245. [DOI] [PubMed] [Google Scholar]
- 116.Ahmad M. Z., Ali M., Mir S. R. Anti-diabetic Activity of Ocimum sanctum L. Roots and Isolation of New Phytoconstituents Using Two-Dimensional Nuclear Magnetic Resonance Spectroscopy. Journal of Pharmacognosy and Phytotherapy . 2012 October;4(6):75–85. [Google Scholar]
- 117.Patil R., Patil R., Ahirwar B., Ahirwar D. Isolation and Characterization of Anti-Diabetic Component (Bioactivity—Guided Fractionation) From Ocimum sanctum L. (Lamiaceae) Aerial Part. Asian Pacific Journal of Tropical Medicine . 2011 April;4(4):278–282. doi: 10.1016/s1995-7645(11)60086-2. [DOI] [PubMed] [Google Scholar]
- 118.Babu S., Jayaraman S. An Update on β-Sitosterol: A Potential Herbal Nutraceutical for Diabetic Management. Biomedicine and Pharmacotherapy . 2020 November;131 doi: 10.1016/j.biopha.2020.110702. [DOI] [PubMed] [Google Scholar]
- 119.Beta-Sitosterol: Overview, Uses, Side Effects, Precautions, Interactions, Dosing and Reviews. https://www.webmd.com/vitamins/ai/ingredientmono-939/beta-sitosterol .
- 120.Lorenze A., Hsueh W., Nasr J. Beta-Sitosterol-Induced Acute Pancreatitis: A Case Report and Review of the Literature. Cureus . 2020;12(3):p. e7407. doi: 10.7759/cureus.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mlala S., Oyedeji A. O., Gondwe M., Oyedeji O. O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules . 2019 July;24(15):p. 2751. doi: 10.3390/molecules24152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ursolic Acid Benefits + Food Sources and Side Effects. SelfDecode Supplements . 2019 https://supplements.selfdecode.com/blog/ursolic-acid/ [Google Scholar]
- 123.Sarkar S., Pal A., Chouni A., Paul S. A Novel Compound β-Sitosterol-3-O-β-D-glucoside Isolated From Azadirachta indica Effectively Induces Apoptosis in Leukemic Cells by Targeting G0/G1 Populations. Indian Journal of Biochemistry and Biophysics . 2020 Feb.57:27–32. [Google Scholar]
- 124.Ayeleso T. B., Matumba M. G., Mukwevho E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules . 2017 November;22(11):p. 1915. doi: 10.3390/molecules22111915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu Y.-F., Wan X.-L., Xu Y., Liu J. Repeated Oral Administration of Oleanolic Acid Produces Cholestatic Liver Injury in Mice. Molecules . 2013 March;18(3):3060–3071. doi: 10.3390/molecules18033060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zielińska-Błajet M., Feder-Kubis J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. International Journal of Molecular Sciences . 2020 September;21(19):p. 7078. doi: 10.3390/ijms21197078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loza-Tavera H. Monoterpenes in Essential Oils. Biosynthesis and Properties. Advances in Experimental Medicine and Biology . 1999;464:49–62. doi: 10.1007/978-1-4615-4729-7_5. [DOI] [PubMed] [Google Scholar]
- 128.Salvi P., Kumar G., Gandass N., et al. Antimicrobial Potential of Essential Oils From Aromatic Plant Ocimum sp.; A Comparative Biochemical Profiling and In-Silico Analysis. Agronomy . 2022 March;12(3):p. 627. doi: 10.3390/agronomy12030627. [DOI] [Google Scholar]
- 129.Hussain A. I., Chatha S. A. S., Kamal G. M., Ali M. A., Hanif M. A., Lazhari M. I. Chemical Composition and Biological Activities of Essential Oil and Extracts from Ocimum sanctum. International Journal of Food Properties . 2017 July;20(7):1569–1581. doi: 10.1080/10942912.2016.1214145. [DOI] [Google Scholar]
- 130.Piras A., Gonçalves M. J., Alves J., et al. Ocimum tenuiflorum L. and Ocimum basilicum L., Two Spices of Lamiaceae Family With Bioactive Essential Oils. Industrial Crops and Products . 2018 March;113:89–97. doi: 10.1016/j.indcrop.2018.01.024. [DOI] [Google Scholar]
- 131.Allenspach M., Steuer C. α-Pinene: A Never-Ending Story. Phytochemistry . 2021 October;190 doi: 10.1016/j.phytochem.2021.112857. [DOI] [PubMed] [Google Scholar]
- 132.Khan F., Kwapiszewska K., Zhang Y., et al. Toxicological Responses of α-Pinene-Derived Secondary Organic Aerosol and Its Molecular Tracers in Human Lung Cell Lines. Chemical Research in Toxicology . 2021 March;34(3):817–832. doi: 10.1021/acs.chemrestox.0c00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Program N. T. NTP Technical Report on the Toxicity Studies of α-Pinene (CASRN 80-56-8) Administered by Inhalation to F344/N Rats and B6C3F1/N Mice . Durham, NC: National Toxicology Program; 2016. Introduction. [Google Scholar]
- 134.Han Y., Sun Z., Chen W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene Against Listeria Monocytogenes. Molecules . 2019 December;25(1):p. 33. doi: 10.3390/molecules25010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murali R., Karthikeyan A., Saravanan R. Protective Effects of D-Limonene on Lipid Peroxidation and Antioxidant Enzymes in Streptozotocin-Induced Diabetic Rats. Basic and Clinical Pharmacology and Toxicology . 2013 March;112(3):175–181. doi: 10.1111/bcpt.12010. [DOI] [PubMed] [Google Scholar]
- 136.Rufino A. T., Ribeiro M., Sousa C., et al. Evaluation of the Anti-Inflammatory, Anti-Catabolic and Pro-Anabolic Effects of E-Caryophyllene, Myrcene and Limonene in a Cell Model of Osteoarthritis. European Journal of Pharmacology . 2015 March;750:141–150. doi: 10.1016/j.ejphar.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 137.Bishayee A., Rabi T. D-Limonene Sensitizes Docetaxel-Induced Cytotoxicity in Human Prostate Cancer Cells: Generation of Reactive Oxygen Species and Induction of Apoptosis. Journal of Carcinogenesis . 2009 May;8(1):p. 9. doi: 10.4103/1477-3163.51368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Souza M. C., Vieira A. J., Beserra F. P., Pellizzon C. H., Nóbrega R. H., Rozza A. L. Gastroprotective Effect of Limonene in Rats: Influence on Oxidative Stress, Inflammation and Gene Expression. Phytomedicine . 2019 Feb.53:37–42. doi: 10.1016/j.phymed.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 139.d’Alessio P. A., Bisson J.-F., Béné M. C. Anti-Stress Effects of D-Limonene and Its Metabolite Perillyl Alcohol. Rejuvenation Research . 2014 April;17(2):145–149. doi: 10.1089/rej.2013.1515. [DOI] [PubMed] [Google Scholar]
- 140.Gad S. E., Hakkinen P. J. Limonene. In: Wexler P., editor. Encyclopedia of Toxicology . 2nd. New York: Elsevier; 2005. pp. 720–725. [Google Scholar]
- 141.Mahendra M. Y., Purba R. A., Dadi T. B., Pertiwi H. Estragole: A Review of Its Pharmacology, Effect on Animal Health and Performance, Toxicology, and Market Regulatory Issues. Iraqi Journal of Veterinary Sciences . 2023 April;37(2):537–546. doi: 10.33899/ijvs.2022.135092.2445. [DOI] [Google Scholar]
- 142.Bristol D. W. NTP 3-Month Toxicity Studies of Estragole (CAS No. 140-67-0) Administered by Gavage to F344/N Rats and B6C3F1 Mice. Toxicity Report Series . 2011 January;(82):1–111. [PubMed] [Google Scholar]
- 143.Pérez-Rosés R., Risco E., Vila R., Peñalver P., Cañigueral S. Biological and Nonbiological Antioxidant Activity of Some Essential Oils. Journal of Agricultural and Food Chemistry . 2016 June;64(23):4716–4724. doi: 10.1021/acs.jafc.6b00986. [DOI] [PubMed] [Google Scholar]
- 144.Elbestawy M. K. M., El-Sherbiny G. M., Moghannem S. A. Antibacterial, Antibiofilm and Anti-Inflammatory Activities of Eugenol Clove Essential Oil against Resistant Helicobacter pylori. Molecules . 2023 January;28(6):p. 2448. doi: 10.3390/molecules28062448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury . Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Eugenol (Clove Oil) [PubMed] [Google Scholar]
- 146. EUGENOL | CAMEO Chemicals . Washington, DC: NOAA; https://cameochemicals.noaa.gov/chemical/20399 . [Google Scholar]
- 147.Ataei M., Maghsoudi A. S., Hassani S. Eugenol. In: Wexler P., editor. Encyclopedia of Toxicology . 4th. Oxford: Academic Press; 2024. pp. 513–517. [Google Scholar]
- 148.Khaleel C., Tabanca N., Buchbauer G. α-Terpineol, a Natural Monoterpene: A Review of Its Biological Properties. Open Chemistry . 2018 January;16(1):349–361. doi: 10.1515/chem-2018-0040. [DOI] [Google Scholar]
- 149.What Is Terpineol? Benefits, Uses, and Where to Find It. Leafwell. . https://leafwell.com/blog/terpineol . [Google Scholar]
- 150.Gong D.-Y., Chen X.-Y., Guo S.-X., Wang B.-C., Li B. Recent Advances and New Insights in Biosynthesis of Dendrobine and Sesquiterpenes. Applied Microbiology and Biotechnology . 2021 September;105(18):6597–6606. doi: 10.1007/s00253-021-11534-1. [DOI] [PubMed] [Google Scholar]
- 151.Manonmani R., Kaviya K. Phytochemical Screening and Gc–Ms Analysis of Ocimum tenuiflorum L. Leaves. Indian Journal of Applied Research . 2019;9(8) [Google Scholar]
- 152.Selvaraju R., Sakuntala P., Jaleeli K. A. Study on Different Parts of Ocimum tenuiflorum Plant Using GC-MS. XRD and UV-Visible Methods . 2020;63(1):90–94. [Google Scholar]
- 153.Pejin B., Savic A., Sokovic M., et al. Further In Vitro Evaluation of Antiradical and Antimicrobial Activities of Phytol. Natural Product Research . 2014 March;28(6):372–376. doi: 10.1080/14786419.2013.869692. [DOI] [PubMed] [Google Scholar]
- 154.Ghaneian M. T., Ehrampoush M. H., Jebali A., Hekmatimoghaddam S., Mahmoudi M. Antimicrobial Activity, Toxicity and Stability of Phytol as a Novel Surface Disinfectant. Environmental Health Engineering and Management Journal . 2 [Google Scholar]
- 155.Santos C., Salvadori M., Mota V., et al. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neuroscience Journal . 2013 June;2013:1–9. doi: 10.1155/2013/949452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Phytol - an Overview | ScienceDirect Topics. https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/phytol .
- 157.Schwotzer D., Gigliotti A., Irshad H., Dye W., McDonald J. Phytol, Not Propylene Glycol, Causes Severe Pulmonary Injury After Inhalation Dosing in Sprague-Dawley Rats. Inhalation Toxicology . 2021 January;33(1):33–40. doi: 10.1080/08958378.2020.1867260. [DOI] [PubMed] [Google Scholar]
- 158.What Is Phytol? Benefits, Uses, and Risks. Leafwell. . https://leafwell.com/blog/phytol . [Google Scholar]
- 159.Kim Y., Cho J.-Y., Kuk J.-H., et al. Identification and Antimicrobial Activity of Phenylacetic Acid Produced by Bacillus Licheniformis Isolated From Fermented Soybean, Chungkook-Jang. Current Microbiology . 2004 April;48(4):312–317. doi: 10.1007/s00284-003-4193-3. [DOI] [PubMed] [Google Scholar]
- 160.Saravanan R., Ramamurthy J. Evaluation of Antioxidant Activity of Ocimum sanctum-An In Vitro Study. International Journal of Dentistry and Oral Science . 2021 November;:5001–5005. doi: 10.19070/2377-8075-21001007. [DOI] [Google Scholar]
- 161.Rindhe P. S. In-Vitro Antioxidant Activity of Ocimum sanctum Linn. International Journal of Technical Research and Applications . 2018;6(3):47–54. [Google Scholar]
- 162.Ramesh B., Satakopan V. N. Antioxidant Activities of Hydroalcoholic Extract of Ocimum sanctum Against Cadmium Induced Toxicity in Rats. Indian Journal of Clinical Biochemistry . 2010 July;25(3):307–310. doi: 10.1007/s12291-010-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Deepika, Maurya P. K. Health Benefits of Quercetin in Age-Related Diseases. Molecules . 2022 January;27(8):p. 2498. doi: 10.3390/molecules27082498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dixit A., Gulati B., Sharma G., Bhatia G., Priya R., Bhattacharya S. Evaluation of Phytochemical and Antimicrobial Activity of Ocimum spp. Integr. Food Nutr. Metab. . 2021;8(1) doi: 10.15761/ifnm.1000299. [DOI] [Google Scholar]
- 165.Mahmood K., Yaqoob U., Bajwa R. Antibacterial Activity of Essential Oil of Ocimum sanctum L. Mycopath . 2008;6(1&2):63–65. [Google Scholar]
- 166.Sivareddy B., Reginald B., Sireesha D., Samatha M., Reddy Kh., Subrahamanyam G. Antifungal Activity of Solvent Extracts of Piper Betle and Ocimum sanctum Linn on Candida albicans: An In Vitro Comparative Study. Journal of Oral and Maxillofacial Pathology . 2019;23(3):p. 333. doi: 10.4103/jomfp.jomfp_167_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Balakumar S., Rajan S., Thirunalasundari T., Jeeva S. Antifungal Activity of Ocimum sanctum Linn. (Lamiaceae) on Clinically Isolated Dermatophytic Fungi. Asian Pacific Journal of Tropical Medicine . 2011 August;4(8):654–657. doi: 10.1016/s1995-7645(11)60166-1. [DOI] [PubMed] [Google Scholar]
- 168.Ulanowska M., Olas B. Biological Properties and Prospects for the Application of Eugenol—A Review. International Journal of Molecular Sciences . 2021 April;22(7):p. 3671. doi: 10.3390/ijms22073671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bai X., Li X., Liu X., et al. Antibacterial Effect of Eugenol on Shigella Flexneri and Its Mechanism. Foods . 2022 January;11(17):p. 2565. doi: 10.3390/foods11172565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van de Laar F. A., Lucassen P. L., Akkermans R. P., van de Lisdonk E. H., Rutten G. E., van Weel C. α-Glucosidase Inhibitors for Patients With Type 2 Diabetes: Results From a Cochrane Systematic Review and Meta-Analysis. Diabetes Care . 2005 January;28(1):154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 171.Sethi J., Sood S., Seth S., Talwar A. Evaluation of Hypoglycemic and Antioxidant Effect of Ocimum sanctum. Indian Journal of Clinical Biochemistry . 2004 July;19(2):152–155. doi: 10.1007/bf02894276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.R.S A., Y V., Ch S., et al. Anti Diabetic Effect of Ethanolic Extract of Leaves of Ocimum sanctum in Alloxan Induced Diabetes in Rats. International Journal of Basic and Clinical Pharmacology . 2013;2(5):613–616. doi: 10.5455/2319-2003.ijbcp20131018. [DOI] [Google Scholar]
- 173.Mousavi L., Salleh R. M., Murugaiyah V. Antidiabetic and In Vitro Enzyme Inhibition Studies of Methanol Extract of Ocimum tenuiflorum Linn Leaves and Its Fractions. Tropical Life Sciences Research . 2020 April;31(1):141–158. doi: 10.21315/tlsr2020.31.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Parasuraman S., Balamurugan S., Christapher P. V., et al. Evaluation of Antidiabetic and Antihyperlipidemic Effects of Hydroalcoholic Extract of Leaves of Ocimum tenuiflorum (Lamiaceae) and Prediction of Biological Activity of Its Phytoconstituents. Pharmacognosy Research . 2015;7(2):156–165. doi: 10.4103/0974-8490.151457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Errichiello F., D’Amato M., Gambuti A., et al. Oleanolic Acid: A Promising Antidiabetic Metabolite Detected in Aglianico Grape Pomace. Journal of Functional Foods . 2023 May;104 doi: 10.1016/j.jff.2023.105548. [DOI] [Google Scholar]
- 176.Castellano J. M., Guinda A., Delgado T., Rada M., Cayuela J. A. Biochemical Basis of the Antidiabetic Activity of Oleanolic Acid and Related Pentacyclic Triterpenes. Diabetes . 2013 May;62(6):1791–1799. doi: 10.2337/db12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang X., Li Y.-L., Wu H., et al. Antidiabetic Effect of Oleanolic Acid: A Promising Use of a Traditional Pharmacological Agent. Phytotherapy Research . 2011 July;25(7):1031–1040. doi: 10.1002/ptr.3385. [DOI] [PubMed] [Google Scholar]
- 178.Mankapure V. A., Mankapure A. G., Sohani P. V. Male Antifertility Effect of Ocimum sanctum Linn.: A Study in Albino Rat. Journal of Endocrinology and Reproduction . 2013 December;:123–132. [Google Scholar]
- 179.Sethi J., Yadav M., Sood S., Dahiya K., Singh V. Effect of Tulsi (Ocimum sanctum Linn.) on Sperm Count and Reproductive Hormones in Male Albino Rabbits. International Journal of Ayurveda Research . 2010;1(4):208–210. doi: 10.4103/0974-7788.76782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ahmed M., Ahamed R. N., Aladakatti R. H., Ghosesawar M. G. Reversible Anti-Fertility Effect of Benzene Extract of Ocimum sanctum Leaves on Sperm Parameters and Fructose Content in Rats. Journal of Basic and Clinical Physiology and Pharmacology . 2002;13(1):51–60. doi: 10.1515/jbcpp.2002.13.1.51. [DOI] [PubMed] [Google Scholar]
- 181.Ampofo-Yeboah A., Lambrechts H., D B., Hiten N., E A.-G. Analysis of Oleanolic Acid and Ursolic Acid, Potential Antifertility Agents in Moringa (Moringa oleifera) Seed. Journal of Agricultural Science and Technology A . 2013 January;3:989–999. [Google Scholar]
- 182.Anandjiwala S., Kalola J., Rajani M. Quantification of Eugenol, Luteolin, Ursolic Acid, and Oleanolic Acid in Black (Krishna Tulasi) and Green (Sri Tulasi) Varieties of Ocimum sanctum Linn. Using High-Performance Thin-Layer Chromatography. Journal of AOAC International . 2006;89(6):1467–1474. doi: 10.1093/jaoac/89.6.1467. [DOI] [PubMed] [Google Scholar]
- 183.Srinivasulu K., Changamma C. A Study on the Effect of Ocimum sanctum (Linn.) Leaf Extract and Ursolic Acid on Spermatogenesis in Male Rats. Indian Journal of Pharmaceutical Sciences . 2017 March;79(01):158–163. doi: 10.4172/pharmaceutical-sciences.1000213. [DOI] [Google Scholar]
- 184.Azab A., Nassar A., Azab A. N. Anti-Inflammatory Activity of Natural Products. Molecules . 2016 October;21(10):p. 1321. doi: 10.3390/molecules21101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mirje M., Zaman S., Ramabhimaiah S. Evaluation of the Anti-Inflammatory Activity of Ocimum sanctum Linn (Tulsi) in Albino Rats. International Journal of Current Microbiology and Applied Sciences . 2014;3(1):198–205. [Google Scholar]
- 186.Kaur M. Impact of Response Surface Methodology–Optimized Synthesis Parameters on In Vitro Anti-Inflammatory Activity of Iron Nanoparticles Synthesized Using Ocimum tenuiflorum Linn. BioNanoScience . 2020 March;10(1):1–10. doi: 10.1007/s12668-019-00681-5. [DOI] [Google Scholar]
- 187.Godhwani S., Godhwani J. L., Vyas D. S. Ocimum sanctum: An Experimental Study Evaluating Its Anti-Inflammatory, Analgesic and Antipyretic Activity in Animals. Journal of Ethnopharmacology . 1987 November;21(2):153–163. doi: 10.1016/0378-8741(87)90125-5. [DOI] [PubMed] [Google Scholar]
- 188.Kewlani A., Bhalerao S., Bhide H., Deshpande T., Tilak A., Dharkar N. Comparison of Anti-Inflammatory Efficacy of Ocimum sanctum, Azadirachta Indica and Their Combination With Aspirin in Chemically Induced Acute Inflammation. Biomedical and Pharmacology Journal . 2021 September;14(3):1509–1518. doi: 10.13005/bpj/2252. [DOI] [Google Scholar]
- 189.Sharma A. D., Kaur I., Angish S., Thakur A., Sania S., Singh A. Comparative Phytochemistry, Antioxidant, Antidiabetic, and Anti-Inflammatory Activities of Traditionally Used Ocimum basilicum L. Ocimum gratissimum L., and Ocimum tenuiflorum L. BioTechnologia . 2022 June;103(2):131–142. doi: 10.5114/bta.2022.116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Manaharan T., Thirugnanasampandan R., Jayakumar R., Ramya G., Ramnath G., Kanthimathi M. S. Antimetastatic and Anti-Inflammatory Potentials of Essential Oil From Edible Ocimum sanctum Leaves. The Scientific World Journal . 2014 November;2014:1–5. doi: 10.1155/2014/239508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Luo C., Zou L., Sun H., et al. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Frontiers in Pharmacology . 2020 Feb.11:p. 153. doi: 10.3389/fphar.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vignesh M., Priya V. V., Gayathri R. Antistress Activity of Methanolic Extract of Ficus Benghalensis. Drug Invention Today . 2019;12(6):p. 1281. [Google Scholar]
- 193.Jothie Richard E., Illuri R., Bethapudi B., et al. Anti-Stress Activity of Ocimum sanctum: Possible Effects on Hypothalamic-Pituitary-Adrenal Axis. Phytotherapy Research . 2016 May;30(5):805–814. doi: 10.1002/ptr.5584. [DOI] [PubMed] [Google Scholar]
- 194.Mohan M. G. C., Murugan S. K., Bethapudi B., Purusothaman D., Mundkinajeddu D., D’Souza P. Ocimum tenuiflorum Extract (HOLIXERTM): Possible Effects on Hypothalamic–Pituitary–Adrenal (HPA) axis in Modulating Stress. PLoS One . 2023 May;18(5):p. e0285012. doi: 10.1371/journal.pone.0285012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saxena R. C., Singh R., Kumar P., et al. Efficacy of an Extract of Ocimum tenuiflorum (OciBest) in the Management of General Stress: A Double-Blind, Placebo-Controlled Study. Evidence-based Complementary and Alternative Medicine . 2012;2012:1–7. doi: 10.1155/2012/894509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gupta P., Yadav D. K., Siripurapu K. B., Palit G., Maurya R. Constituents of Ocimum sanctum With Antistress Activity. Journal of Natural Products . 2007 September;70(9):1410–1416. doi: 10.1021/np0700164. [DOI] [PubMed] [Google Scholar]
- 197.AlGhalban F. M., Khan A. A., Khattak M. N. K. Comparative Anticancer Activities of Ficus carica and Ficus salicifolia Latex in MDA-MB-231 Cells. Saudi Journal of Biological Sciences . 2021 June;28(6):3225–3234. doi: 10.1016/j.sjbs.2021.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Boonyanugomol W., Rukseree K., Prapatpong P., et al. An In Vitro Anti-Cancer Activity of Ocimum tenuiflorum Essential Oil by Inducing Apoptosis in Human Gastric Cancer Cell Line. Medicina . 2021 July;57(8):p. 784. doi: 10.3390/medicina57080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Karthikeyan K., Gunasekaran P., Ramamurthy N., Govindasamy S. Anticancer Activity of Ocimum sanctum. Pharmaceutical Biology . 1999 January;37(4):285–290. doi: 10.1076/phbi.37.4.285.5801. [DOI] [Google Scholar]
- 200.Indrayudha P., Hapsari H. S. Cytotoxic Activity of Combined Ethanolic Extract of Cinnamomum burmannii and Ocimum tenuiflorum Linn Against T47D Cancer Cells. Archivos Venezolanos de Farmacología y Terapéutica . 2021;40(2) [Google Scholar]
- 201.Lam S. N., Neda G. D., Mohd Salleh R. The Anticancer Effect of Ocimum tenuiflorum Leaves. Food Research . 2017 November;2(2):154–162. doi: 10.26656/fr.2017.2(2).251. [DOI] [Google Scholar]
- 202.Çetinkaya M., Baran Y. Therapeutic Potential of Luteolin on Cancer. Vaccines . 2023 Feb.11(3):p. 554. doi: 10.3390/vaccines11030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lotfi N., Yousefi Z., Golabi M., et al. The Potential Anti-Cancer Effects of Quercetin on Blood, Prostate and Lung Cancers: An Update. Frontiers in Immunology . 2023 Feb.14 doi: 10.3389/fimmu.2023.1077531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yan X., Qi M., Li P., Zhan Y., Shao H. Apigenin in Cancer Therapy: Anti-Cancer Effects and Mechanisms of Action. Cell and Bioscience . 2017;7(1) doi: 10.1186/s13578-017-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Prasher P., Sharma M., Singh S. K., et al. Luteolin: A Flavonoid With a Multifaceted Anticancer Potential. Cancer Cell International . 2022 December;22(1):p. 386. doi: 10.1186/s12935-022-02808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rauf A., Wilairatana P., Joshi P. B., et al. Revisiting Luteolin: An Updated Review on Its Anticancer Potential. Heliyon . 2024 March;10(5) doi: 10.1016/j.heliyon.2024.e26701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shetty S., Udupa S., Udupa L. Evaluation of Antioxidant and Wound Healing Effects of Alcoholic and Aqueous Extract of Ocimum sanctum Linn in Rats. Evidence-Based Complementary and Alternative Medicine . 2008;5(1):95–101. doi: 10.1093/ecam/nem004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Umamageswari A., Kudagi B. L. Anti-Inflammatory and Analgesic Properties of Ocimum sanctum: A Comparative Study Using Animal Models. International Journal of Basic and Clinical Pharmacology . 2015;4(5):981–986. doi: 10.18203/2319-2003.ijbcp20150878. [DOI] [Google Scholar]
- 209.Singh S., Majumdar D. K. Anti-Inflammatory and Antipyretic Activities of Ocimum sanctum Fixed Oil. International Journal of Pharmacognosy . 1995 January;33(4):288–292. doi: 10.3109/13880209509065380. [DOI] [Google Scholar]
- 210.Arora R., Malhotra P., Sharma A., Haniadka R., Yashawanth H. S., Baliga M. S. Medicinal Efficacy of Indian Herbal Remedies for the Treatment of Arthritis. In: Watson R. R., Preedy V. R., editors. Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases . San Diego: Academic Press; 2013. pp. 601–617. [Google Scholar]
- 211.Chaiyana W., Anuchapreeda S., Punyoyai C., et al. Ocimum sanctum Linn. As a Natural Source of Skin Anti-Ageing Compounds. Industrial Crops and Products . 2019 January;127:217–224. doi: 10.1016/j.indcrop.2018.10.081. [DOI] [Google Scholar]
- 212.Mukherjee R., Dash P. K., Ram G. C. Immunotherapeutic Potential of Ocimum sanctum (L) in Bovine Subclinical Mastitis. Research in Veterinary Science . 2005 August;79(1):37–43. doi: 10.1016/j.rvsc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 213.Jaggi R. K., Madaan R., Singh B. Anticonvulsant Potential of Holy Basil, Ocimum sanctum Linn., and Its Cultures. Indian Journal of Experimental Biology . 2003 November;41(11):1329–1333. [PubMed] [Google Scholar]
- 214.Asha M. K., Prashanth D., Murali B., Padmaja R., Amit A. Anthelmintic Activity of Essential Oil of Ocimum sanctum and Eugenol. Fitoterapia . 2001 August;72(6):669–670. doi: 10.1016/s0367-326x(01)00270-2. [DOI] [PubMed] [Google Scholar]
- 215.Panda S., Kar A. Ocimum sanctum Leaf Extract in the Regulation of Thyroid Function in the Male Mouse. Pharmacological Research . 1998 August;38(2):107–110. doi: 10.1006/phrs.1998.0338. [DOI] [PubMed] [Google Scholar]
- 216.Cohen M. M. Tulsi—Ocimum sanctum: A Herb for All Reasons. Journal of Ayurveda and Integrative Medicine . 2014;5(4):251–259. doi: 10.4103/0975-9476.146554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Makwana M., Rathore H. Prevention of Hepatorenal Toxicity of Acetaminophen With Ocimum sanctum in Mice. International Journal of Pharmacy and Technology . 2011 January;3:1385–1396. [Google Scholar]
- 218.Kurkure N., Mahaprabhu R., Jangir B. L., Bhandarkar A., Rahangadale S. Ameliorative Effect of Ocimum sanctum on Meloxicam Induced Toxicity in Wistar Rats. Toxicology International . 2011 July;18(2):130–136. doi: 10.4103/0971-6580.84265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shukla P., Tabassum S., Khanna A. Role of Ocimum sanctum as a Genoprotective Agent on Chlorpyrifos-Induced Genotoxicity. Toxicology International . 2011;18(1):9–13. doi: 10.4103/0971-6580.75845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shah K., Verma R. J. Protection Against Butyl P-Hydroxybenzoic Acid Induced Oxidative Stress by Ocimum sanctum Extract in Mice Liver. Acta Poloniae Pharmaceutica . 2012;69(5):865–870. [PubMed] [Google Scholar]
- 221.Shyamala A. C., Devaki T. Studies on Peroxidation in Rats Ingesting Copper Sulphate and Effect of Subsequent Treatment With Ocimum sanctum. Journal of Clinical Biochemistry and Nutrition . 1996;20(2):113–119. doi: 10.3164/jcbn.20.113. [DOI] [Google Scholar]
- 222.Ubaid R. S., Anantrao K. M., Jaju J. B., Mateenuddin M. Effect of Ocimum sanctum (OS) Leaf Extract on Hepatotoxicity Induced by Antitubercular Drugs in Rats. Indian Journal of Physiology and Pharmacology . 2003 October;47(4):465–470. [PubMed] [Google Scholar]
- 223.Halder N., Joshi S., Gupta S. K. Lens Aldose Reductase Inhibiting Potential of Some Indigenous Plants. Journal of Ethnopharmacology . 2003 May;86(1):113–116. doi: 10.1016/s0378-8741(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 224.Pattanayak P., Behera P., Das D., Panda S. K. Ocimum sanctum Linn. A Reservoir Plant for Therapeutic Applications: An Overview. Pharmacognosy Reviews . 2010 January;4(7):95–105. doi: 10.4103/0973-7847.65323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Prakash P., Gupta N. Therapeutic Uses of Ocimum sanctum Linn (Tulsi) With a Note on Eugenol and Its Pharmacological Actions: A Short Review. Indian Journal of Physiology and Pharmacology . 2005 April;49(2):125–131. [PubMed] [Google Scholar]
- 226.Mohan L., Amberkar M. V., Kumari M. Ocimum sanctum Linn (TULSI) - An Overview. International Journal of Pharmaceutical Sciences Review and Research . 2011 March;7(1):51–53. [Google Scholar]


