Abstract
Forest expansion into savanna is a pervasive phenomenon in West and Central Africa, warranting comparative studies under diverse environmental conditions. We collected vegetation data from the woody and grassy components within 73 plots of 0.16 ha distributed along a successional gradient from humid savanna to forest in Central Africa. We associated spatially collocated edaphic parameters and fire frequency derived from remote sensing to investigate their combined influence on the vegetation. Soil texture was more influential in shaping savanna structure and species distribution than soil fertility, with clay-rich soils promoting higher grass productivity and fire frequency. Savanna featuring woody aboveground biomass surpassing 40 Mg ha−1 could escape the grass–fire feedback loop, by depressing grass biomass below 4 Mg ha−1. This thicker woody layer also favoured the establishment of fire-tolerant forest pioneers, which synergically contributed to the expansion of forests. Conversely, savannas below this fire suppression threshold sustained a balance between trees and grasses through the grass–fire feedback mechanism. This hysteresis loop, particularly pronounced on clayey soils, suggests that the contrast between grassy savanna and young forests might represent alternative ecosystem states, although savannas with low woody biomass remained vulnerable to forest edge encroachment.
Keywords: alternative ecosystem states, Central Africa, forest expansion, grass–fire feedback, savanna community, soil
1. Introduction
Woody encroachment and forest expansion into tropical savanna have been described as top drivers of biome transition [1]. In Central Africa, the interface between forest and savanna is the most dominant ecotone [2,3], where sizable tracts of savannas are juxtaposed with close canopy forest resulting in mosaic landscapes [3–6]. This situation has led some authors interpreting humid savannas and forests as alternative ecosystem states (AES) under the currently prevailing conditions [4–8]. Indeed, these regions have mean annual precipitation (MAP) exceeding 1400 mm year−1, which can support closed-canopy forests [9,10] and has favoured widespread forest expansion into savanna over recent decades [11–15]. Even though neither mosaic landscapes nor forest expansion are unambiguous clues of AES [8], the forest–savanna transition fringing the Congo Basin has been mapped as possible place of AES occurrence by most authors [6,14,16,17] and thereby deserves further scrutiny. Moreover, forest encroachment in these ‘zones of tension’ [18,19] comes with profound changes in function and composition of the initial savanna ecosystem [20–22]. This has generated considerable interest to ecologists as various key ecosystem functions (including functional diversity, nutrient cycling and hydrology), services (among which carbon sequestration) and production (livestock) depend on woody versus grassy proportions in a savanna [23–26].
The coexistence of forest and savanna under similar wet climates has triggered two hypotheses. The first one, compatible with AES existence refers to feedback between vegetation structure and disturbances, notably fires that prevent savannas from canopy closure by limiting the development of woody saplings [27–29]. Fire intensity and spread depend on the aboveground biomass (AGB) and flammability of a continuous heliophytic C4 grass layer [30–33]. Several studies have highlighted a level of grass AGB around which fire propagation swiftly increases (fire propagation threshold [30,31,33]). Within savanna landscapes, woody AGB has little influence on fire behaviour until the woody canopy cover becomes sufficient to strongly depress heliophytic flammable grasses below this fire propagation threshold. Furthermore, higher canopy can even eliminate fire from spreading within the understory (fire suppression threshold [7,34,35]). These two antagonistic feedback (i.e. grass–fire versus tree–shading [30,34,36–38]) are not only central to the ‘savanna conundrums’ (sensu [39]), but are also presented as source of possible vegetation switches between AES [4,7,35] (i.e. forests and open vegetation in the form of savanna or grassland) [40–42].
The second line of interpretation of forest–savanna mosaics refers to abiotic factors, notably those emanating from the underlaying substrate and deriving edaphic conditions. Some studies have emphasized spatial correlation between vegetation structure and soil variables (i.e. texture and/or nutrient availability) at landscape scale [17,43–46]. For example, extensive savanna tracts in the Bateke plateaus in Central Africa are found on poor edaphic conditions (low fertility) deriving from very sandy substrates [47–50], while adjacent geological substrates displaying soils of finer texture mainly harboured close canopy forests. Such observations have fostered caveats against interpreting contrasted forest–savanna mosaics as unequivocal evidence of AES and even led some authors to questioning the effectiveness of fire alone in stabilizing open ecosystem states as alternative to forest [32,51]. Both the ‘internal dynamics’ and ‘external forcing’ perspectives [8] concur that edaphic factors can interplay with fire disturbances to shape ecosystem characteristics. Understanding these interactions through vegetation metrics is crucial for enhancing resource management and conservation efforts in forest–savanna transition zones. The outcomes of interactions between savanna dynamics and soil properties are a priori uncertain and therefore deserve thorough investigation. On the one hand, productive soils are expected to enhance the growth of woody saplings and let them better benefit from fire return intervals to escape the fire trap, which further promotes canopy cover build-up [31,52]. But productive environments can also boost grass production [53,54] and are often associated with higher fire intensity and frequency [37,46,55–57]. These two simultaneous effects make non-trivial the influence of soil productivity gradients on the dynamical result of fire-mediated tree–grass interactions [46].
While most studies report forest-favouring dynamics, spatial analysis from remote sensing data shows that the progression of forest does not always consist of a regular advance [15–17,58,59]. Forest progression can be either fast, through the growth and coalescence of woody patches within savanna (also referred to as ‘nucleation’), which causes the edge to smoothen [16,55], or slow, through boundary expansion of existing forest [14,16]. Such observed spatial and temporal heterogeneity of forest encroachment modes likely overlap intrinsic site abiotic/biotic constraints liable to modulate the thresholds involved in the fire-mediated tree–grass feedback. Different studies [13,16,58,60,61] underlined the influence of the floristic composition of the ecotone and described a structurally and functionally distinct ecotonal tree community dominated by forest pioneers [58,59,62]. This ecotone was reported as spatially extensive and promoting woody encroachment by shading out grasses and attenuating the grass–fire feedback on trees. This systematic effect of the woody species composition of the ecotone is, however, challenged in some areas [63], where limited discontinuity in the floristic composition across a forest–savanna gradient is found.
Modelling is of course central to elucidate and disentangle such complex dynamics and substantial modelling efforts have logically stemmed from the savanna conundrum [64–66]. However, theoretical development in modelling has not yet been matched by sufficient empirical work considering the diversity of local biotic and abiotic conditions along with the variety of dynamics observed at forest–savanna interface. Notably, while the woody component of the savanna is quantified in different ways (stem counts, LAI, cover, etc. [39]), there are few existing sets of spatially collocated data of grass, trees and fire, making inter-study comparison and model calibration difficult. In addition, the locations of most of the studies describing African forest–savanna transition under MAP beyond 1200 mm (i.e. Lamto in Ivory Coast [67,68], Lopé in Gabon [13,69] or Kogyae in Ghana [63]) were found on oligotrophic sandy soils. Detailed field information on more productive edaphic conditions is therefore lacking.
The general objective of this study was to assess how the interaction between soil properties and other environmental variables modulates the grass–fire feedback and changes in woody structure and floristic composition amidst ongoing forest encroachment into savanna (which is overall swift but variable in space). We rely on a comprehensive dataset from spatially collocated remote sensing-based products and field measurements (73 field plots of 0.16 ha) collected in the forest–savanna mosaic of the Guineo–Congolian transitional area of Cameroon. Floristic composition of the woody layer, wood and grass AGB, together with the topsoil properties (<20 cm depth) were sampled within savanna, and recent colonizing forest (<10 years since forest gain) along a wide range of soil texture/fertility profiles. Data from Landsat-derived monitoring of fire frequency and forest age over 45 years (as per [15]) were associated to these data to address the following questions:
What is the influence of soil texture and fertility on grass biomass productivity and to what extent does the variability in the later influences fire frequency?
What are the limits of this grass–fire feedback control on tree development and associated shading effect on grasses?
How do plant functional types (forest pioneers versus savanna-specialist) contribute to the structure and composition of the woody layer across a forest–savanna transition?
How do soil and other environmental factors correlate with the main gradients of woody species composition?
2. Material and methods
(a). Study site
The study was conducted within the forest–savanna mosaics of the Guineo-Congolian transition zone [70] in the central region of Cameroon (figure 1a). The climate is equatorial of the Guinean type [73], with seasons alternating between dry (three dry months, mid-November to mid-March) and rainy. Average annual temperature is 25°C and mean annual rainfall is 1300 mm (between 1979 and 2019 [71]).
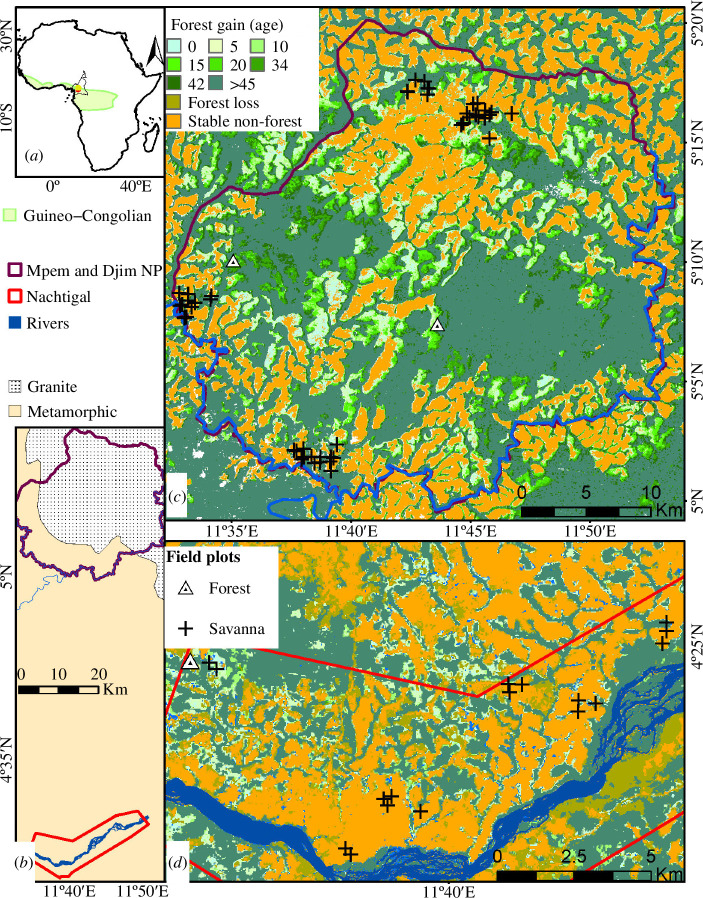
Figure 1.
Study area. (a) Location of the study sites within the Guineo-Congolian transitional area (light green) where no changes in mean annual rainfall were recorded between 1979 and 2019 [71]. (b) Geological substrates as modified from Gazel et al. [72] underlying the Mpem and Djim national park (purple polygon) and Nachtigal (red polygon). (c) and (d) landcover change dynamics since 1975 as modified from Sagang et al. [15] with the age in 2020 since forest gain (green palette); forest loss (brown) and stable savanna/open vegetation (non-forest; orange). The 0.16 ha sampling plots are displayed as triangles for recent (<10 years) forests and crosses for savanna.
Savanna fires has been decreasing in the study area over the last decade (−0.75%) [74], mainly attributed to a decrease in large-scale fires (>100 ha) which are more frequent between November and February. Fire ignition in the area is mainly maintained by herders to favour the regrowth of palatable grasses for cattle. The field data collection targeted two areas, located 60 km apart, with no detected difference in MAP [75] but with contrasted paces of forest expansion after 45 years (1975–2020) of monitoring from Landsat image archives [72]. The Nachtigal area (red polygon of figure 1b; elevations between 450 and 550 m above sea level) lays on a metamorphic substrate and displayed a forest expansion rate of ≈0.4% year−1 (figure 1d). The proximity of the Sanaga river (blue polygon; figure 1b), one of the main rivers in Central Africa [76], has made the Nachtigal area an attraction for small-scale agriculture (e.g. cocoa farms, staple crops and palm plantations [77]) resulting to an increase in human pressure on the vegetation [78,79]. The Mpem and Djim National Park (MDNP, purple polygon, elevations between 540 and 650 m above sea level; figure 1b) mostly lies on a granitic substrate (80%, central and northeastern) where a forest encroachment rate of ≈0.63% year−1 (figure 1c) was recorded, which is slightly lower than the ≈0.66% rates recorded for its metamorphic part (20%, southern and western). Human activities in MDNP are limited by the presence of the Mpem and Djim rivers bordering the park with several rapids (blue lines in figure 1c) in addition to the poor quality of the infrastructures (road, bridges). However, cattle grazing occurs there during the three- month dry season.
(b). Vegetation data
All our sampled locations were initially savanna in 1975 (figure 1c,d). Field campaigns took place between 2018 and 2022 (for details on the plot design, see [80]) where we established overall 73 plots of 0.16 ha (40 m × 40 m) each (59 in perpetuating savanna since 1975 and 14 in forest that transitioned in not more than 10 years). The minimum distance between plots was 100 m (median = 30 km) in savanna, and 500 m (median = 65 km) in young forests, to limit the effect of spatial autocorrelation [81,82]. The plots were distributed along a gradient of tree cover, ranging from open grassy savanna to savanna woodlands and recent forests (figure 1c,d). Forest age was determined from satellite image series [15]. Each of these plots were subdivided into four 20 m × 20 m quadrats from which the tree and grass layer was sampled. For the tree layers in the savanna plots, all woody individuals whose diameter (D) measured at 30 cm above the ground was at least 5 cm were identified and their diameter and total height (H) measured. The same census was done in forest plots for all woody individuals with a minimum D of 10 cm measured at breast height. A total of 2313 trees were sampled from which 76% were identified at species-level (143 woody species) and 24% at genus-level. In savanna plots, the AGB of individual trees (AGBWOOD, Mg ha−1) was computed by integrating D, H and the wood density of the individual using the allometric equation specific to savanna found in Colgan et al. [83]. For recent forest plots, AGBWOOD was estimated by integrating the previous parameters into the pantropical allometric equation found in Chave et al. [84]. Wood density values were extracted from the Dryad database [85]. The BIOMASS R package [86] was used for AGBWOOD estimation of individuals in forest plots. We later compiled information on the successional status for each woody species, namely savanna specialist, forest pioneers and old growth forest as referenced in local floras [87] and CoForTraits database [88].
Grass samples were collected after the growing season (November) within only 62 plots, after excluding those affected by fire at the time of sampling. For each of these plots, grass samples were clipped at the soil surface within 1 m² units placed at the centre of each 20 m × 20 m quadrat. Each fresh clipping was then oven dried to constant dry weight at 70°C. The grass layer was dominated by Hyparrhenia diplandra, Hyparrhenia smithiana, Imperata cylindrica and Panicum spp. The dry weight of the grass layer of a plot was translated to grass AGB (AGBGRASS) by averaging the dry weight of the clippings harvested from that plot. Chromolaena odorata, an invasive shrub described as favouring woody invasion of the savanna [14,20,61,89] occurred within less than 1% of our clipping units and was therefore not considered in further analyses.
(c). Soil data
Topsoil samples (0–20 cm depth) were collected at the centre of each plot after removing litter, with the help of a handheld soil auger. The soil sample was mixed and air-dried under ambient temperature. Samples were analysed at the laboratory of the International Institute of Tropical Agriculture (www.iita.org; ISO 17025) in Yaoundé. Soil texture was determined as the proportion (%) of clay, silt and sand using the hydrometer method [90]. Soil pH in water (pHwater) and in acid (pHKCl) were determined in a 1:2.5 (w/v) soil:water suspension. Soil fertility was characterized via the concentration of the main exchangeable cations (i.e. Ca+, Mg+, Na+ and K+) and cation exchange capacity (CEC) both determined using ammonium acetate method. The concentration of available phosphorus (BrayP, mg kg−1) was extracted using the Bray − 1 method [91]. Total P and N were determined from wet acid digestion [92] while organic carbon (C, %) was determined by chromic acid digestion [93]. Detailed analyses of soil minerals are reported by Libalah et al. [94].
(d). Environmental and ecological variables
For each plot, we determined the following set of environmental descriptors: (i) fire frequency index (FFI, fire year−1) which quantifies the yearly rate of fire occurrence between 2014 and 2018 based on 30 m resolution Landsat imagery [15]; (ii) the elevation from the digital elevation model (DEM) [95], and (iii) distance to the nearest forest edge (distance, m) which measures the minimum Euclidean distance to the edge of the forest as mapped by Sagang et al. [15].
(e). Statistical analyses
Principal component analysis (PCA) on the combination of soil and other environmental variables was used to reduce the dimensions, characterize the correlation structure and avoid collinearity issues during descriptive analysis. We focused on the key variables shaping soil texture and fertility gradients alongside fire frequency, to describe their influence on grassy and woody AGB. We used a 95th quantile nonlinear regression [96] implemented in the ‘quantreg’ package of R statistical software (v. 4.2.1) [97] to characterize the maximal effect to be expected from each of the selected variables on AGBWOOD and AGBGRASS. Whenever applicable, we identified the breakpoints at which maximum AGBGRASS was attained using ‘segmented’ package in R. We used a 50th quantile linear regression to describe the median effect on AGBWOOD and AGBGRASS of the selected variables in presence of possible effects from other variables, and tested whether the regression slopes were different from zero. Subsequently, significant differences between localities or geological substrates were tested using post-hoc paired comparisons from the Student’s t‐test. The spatial dynamics of woody savanna specialists and forest pioneers across a gradient of forest–savanna transition was indirectly assessed using plots data to quantify their contribution to species abundance and AGBWOOD within successive 20 m bins, starting from the forest edge and extending towards the interior of each ecosystem (up to 100 m). The main floristic patterns in woody species composition were identified by applying a non-symmetric correspondence analysis (NSCA) [98,99] to the plot (n = 73) × woody species (n = 143) cross-classification table. NSCA analyses the species abundance (number of individuals) in plots to identify compositional gradients by putting emphasis on abundant species and is thus robust against casual correspondences between plots with few individuals and rare species [100]. We focused on species’ scores along the most dominant axes of the NSCA to establish the gradients in woody species composition. We used the envfit function from the vegan R-package [101] to evaluate the linear correlation between the first two axes of the NSCA and soil and the key additional environmental variables.
3. Results
The PCA (electronic supplementary material, appendix S1) summarizing the correlations between soil properties and other environmental variables revealed two main gradients (58% of total variance explained; electronic supplementary material, appendix S2a). The first gradient (PCA 1 with 35% of variance explained) was driven by soil fertility variables (negative correlation with exchangeable cations, pH and silt content) and distinguished soils found on granite (mean scores on PCA 1 = 2.15; s.d. = 1) from those found on metamorphic substrate (mean scores on PCA 1 = −1.12; s.d. = 2.5). We further retained silt content and CEC as our descriptors of soil fertility considering that both parameters have significant contribution to PCA 1 and show loose intercorrelation. PCA 2 (23% of variance explained) was driven by soil textural variable and contrasted sandy versus clayey soil conditions. Even though the highest fractions of clay were found on granite in the MDNP, its southwestern part displayed metamorphic substrate and less-clayey soils, so that average PCA 2 scores of plot scores did not differ between both localities based on a post-hoc paired comparisons from the Student’s t‐test (electronic supplementary material, appendix S2; p‐value ≤ 0.05).
(a). Effects of soil on wood and grass–biomass interactions modulated by fire
The median trend (represented by solid black line in figure 2a-c) indicated an increase in AGBGRASS with higher soil texture and fertility, although the slopes were not significantly different from zero. Soil clay content drives the upper bound (represented by solid dotted line) of AGBGRASS (figure 2a); with highest values (>12 Mg ha−1) only reached on granitic substrates supporting clay-rich soils, and a peak found at very high clay levels (>40%). Conversely, AGBGRASS showed an overall decrease of its upper bound towards higher silt contents (figure 2b) and peaked at intermediate levels of CEC (between 5 and 10 cmol + kg−1; figure 2c).
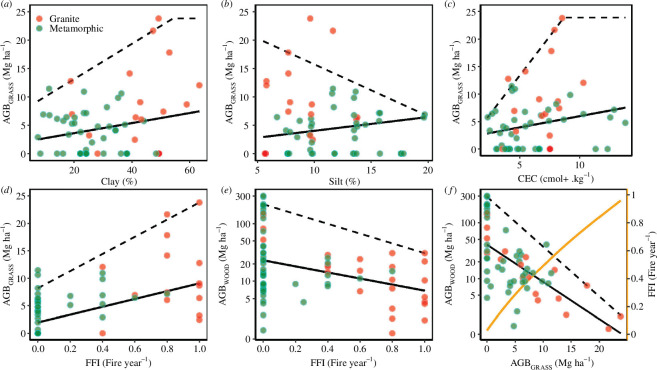
Figure 2.
Relationships between soil properties, FFI and AGB of grassy (AGBGRASS) and woody (AGBWOOD) layers. (a–d) Influence of soil texture (clay and silt content), fertility (CEC) and FFI on AGBGRASS. (e, f) Influence of FFI on the woody layer with its effects on the relationship between AGBWOOD and AGBGRASS. Colours denote plots located on granite (red) and metamorphic (green) substrates. Broken and unbroken lines, respectively, represent the 95th and 50th nonlinear piece-wise quantile regressions. The unbroken orange curve in (f) shows the mean FFI along the gradient of AGBGRASS.
We noticed a significant increase in the median and upper bound on AGBGRASS with increasing FFI (figure 2d). All plots exhibiting low FFI (<0.4 fire year−1) displayed lower AGBGRASS (<12 Mg ha−1). Conversely, the highest values of AGBGRASS (approaching 25 Mg ha−1) were recorded under FFI exceeding 0.6 fire year−1, where only two plots had AGBGRASS below 5 Mg ha−1. AGBWOOD decreased with FFI and all plots displaying non-null FFI had AGBWOOD values below 40 Mg ha−1 (figure 2e). Plots with no fire detection (FFI = 0) showed a wide range of AGBWOOD irrespective of the geological stratum. There was a general decreasing relationship between AGBWOOD and AGBGRASS (figure 2f), the latter limiting AGBWOOD below 40 Mg ha−1 once exceeding 5 Mg ha−1 of AGBGRASS.
(b). Patterns in woody species composition and structure along a forest–savanna gradient
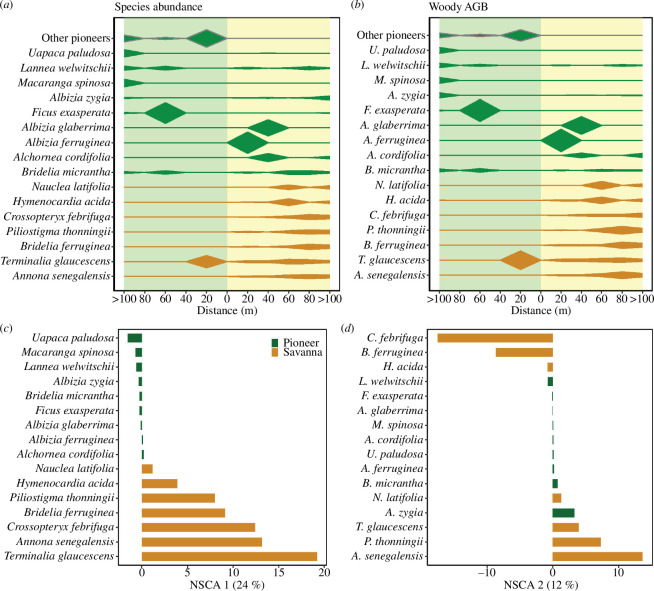
When tracking the contribution of woody species to the abundance and biomass across a forest−savanna mosaic, we notice the prevalence of forest pioneers including Alchornea cordifolia, Albizia ferruginea and Albizia glaberrima within plots in the first 20–60 m, as we move from the forest edge (distance to plot = 0) into the savanna (yellow background in figure 3). It should be noted that no flammable AGBGRASS was observed in plots located within this distance range. Terminalia glaucescens was the only savanna specialist which together with forest pioneers showed noticeable contribution in the species abundance and AGBWOOD within the first 40 m into the forest. Beyond ~60 m from both sides of the forest–savanna boundary, species composition was more specific to one of these two physiognomies except for forest pioneers Albizia zygia, A. cordifolia, Bridelia micranta and Lannea welwitschii whose abundances were still noticeable within savanna.
Figure 3.
Patterns in species composition and structure of the woody layer. (a,b) Contribution in species abundance and AGBWOOD of woody species based on their distance from the forest–savanna boundary (distance = 0) towards the young forest (light green background) or savanna (yellow background). The polygons represent the variation in (a) relative abundance and (b) AGBWOOD of savanna specialists (orange polygons) and forest pioneers (dark green polygons) from plots grouped within successive distance bins of 20 m. The size of polygons is proportional to the contribution of each species. (c–e) Species ordination along the first two axis from a NSCA. The proportion of total variance explained by each axis is given in parentheses. Orange and green bars are species scores, respectively, for savanna specialists and forest pioneers with the highest contribution to the axis.
The NSCA highlighted two dominant gradients of woody species composition (figure 3c–d) which together explained 39% of total variance. The first axis (NSCA 1, 24% of total variance) opposed savanna (positive end) with forest specialists (negative end, dominated among others by Uapaca paludosa, Macaranga spinosa and L. welwitschii) and reflects a gradient of floristic transition as forest encroaches over savanna.
The second axis (NSCA 2; 15% of total variance), highlighted two contrasted communities of savanna species. Annona senegalensis, Piliostigma thonningii and T. glaucescens dominated the positive end of NSCA 2, with a noticeable contribution of a forest pioneer A. zygia. The negative end was driven by Bridelia ferruginea, Crossopteryx febrifuga and Hymenocardia acida (figure 3d).
(c). Relationship between soil and other environmental factors with woody structure and species composition
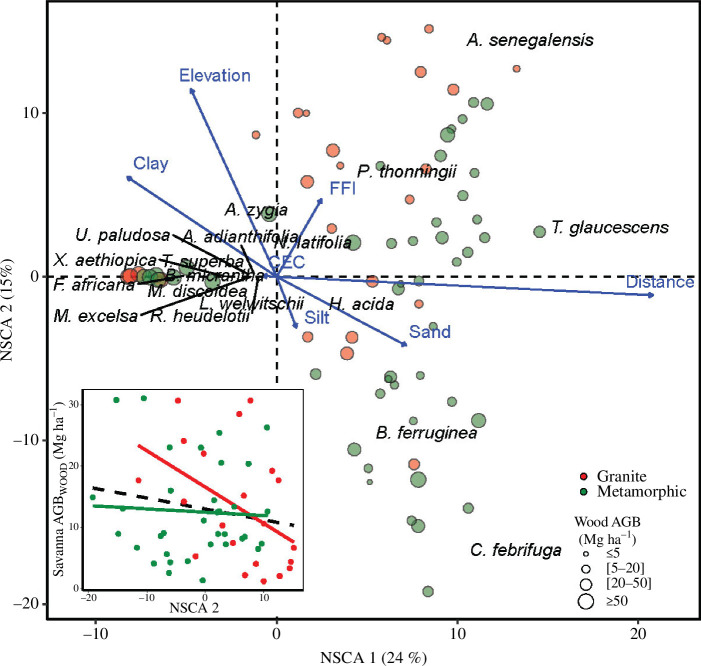
Variables related to soil texture exhibited stronger correlations with NSCA 1 as compared with soil fertility variables (figure 4). NSCA 1 showed a strong positive correlation with the distance to the forest edge (r = 0.6). NSCA 1 was also negatively correlated to clay content (r = −0.3) even though the clayey versus sandy plots gradient went obliquely to the NSCA axes. Clayey plots were either near the negative end of NSCA1 (dominated by forest pioneers) or not far away from the centre of the axis), while sandy sites displayed a dominance of savanna specialists (positive end). Nearly, all plots with AGBWOOD >40 Mg ha−1 had negative scores along NSCA 1 and were dominated by forest pioneers.
Figure 4.
Correlations between soil and other environmental variables over plots and the first and second axis of NSCA of woody species. Blue arrows represent the projection of soil and other environmental variables, with their lengths proportional to the correlation with the axes. Circles represent plots found on granite (red) and metamorphic (green) substrates with their sizes proportional to AGBWOOD. The inset shows linear regressions between AGBWOOD and NSCA 2 only for savanna plots (dotted line) over each geological substrate (granite = solid red, metamorphic = solid green).
The relationship between NSCA 2 and soil and environmental variables was more complex. NSCA 2 was positively correlated with elevation, clay content and FFI. Plots with high values of FFI showed a dominance of A. senegalensis and P. thonningii. Conversely, silt and sandy sites which showed negative correlation with NSCA 2 were dominated by B. ferruginea and C. febrifuga. When focusing on savanna vegetation, the dichotomy observed in species composition is also reflected in the structure, with AGBWOOD showing a decreasing relationship with NSCA 2 (solid dotted line in the inset of figure 4). This decrease was more pronounced and significant on granite (r = −0.5) as compared with metamorphic substrate (r = −0.1).
4. Discussion
The expansion of forest over savanna is of continental [12] and global significance [1]. This calls for a better understanding of the underlying modalities and mechanisms that drive tree–grass coexistence across diverse environmental conditions. Here, we analysed collocated data about grass, trees and fires in the humid part of the savanna biome. With respect to the questions posed at end of §1, we found (i) a strong influence of soil texture on grass production and fire frequency. (ii) Woody AGB remained low in areas with strong grass–fire feedback, while increasing woody AGB strengthened the tree shading effects on grasses. (iii) The structure and composition of the woody layer within the forest–savanna ecotone confirmed a rapid extension of the forest edge, with (iv) a strong influence of the distance to the forest boundary and soil texture.
(a). Soil modulation of the fire-mediated tree–grass interactions
A central, well-established concept in savanna ecology is the grass–fire feedback: high grass production during the rainy season favours both fire spatial propagation and severity during dry months [34,102,103]. Here, AGBGRASS increased with clay-content and reached its highest (up to 24 Mg ha−1) on granitic substrate displaying intermediate levels of soil fertility (CEC ranging between 5 and 10 cmol + kg−1). This appears consistent with statistical results indicating that grass biomass increases more directly with increasing clay content than it does in response to increasing soil nutrient availability [46]. Increasing AGBGRASS favours higher fire frequencies and intensity [57,104], with effects on woody plants that vary among species though always depressing the overall woody biomass. We document here evidence of a sharp nonlinear increase in observed FFI (based on Landsat) beyond a pivotal range of AGBGRASS between 3 and 5 Mg ha−1. AGBGRASS below 4 Mg ha−1 was mentioned as limiting for fire propagation and/or effectiveness in several situations within the savanna biome [36,37,55,105,106]. A lower yet similar threshold of 2.5 Mg ha−1 was observed as the minimal value to ensure fuel connectivity and fire spread from a more extensive study [30]. However, during field sampling, small-extent fires with low intensities, or fire scars on tree bark, could be observed within plots where AGBGRASS was below 4 Mg ha−1. These small-scale fire events were probably missed from remote sensing products due to limitations in both spatial and temporal resolution of Landsat image series [107]. Consequently, very low fire frequencies (FFI close to 0) were recorded for these areas. Beyond an AGBGRASS threshold of 4 Mg ha−1, FFI escalated to over ca 0.8 year−1 suggesting a burning regime approaching large scale annual fires [74]. The FFI proxy thus allowed us to sort out two kinds of scenarios: one characterized by large variance in AGBWOOD and low AGBGRASS, where fires were either absent or too small to be detected with Landsat-based FFI (FFI = 0), and a second scenario with non-null FFI values where AGBWOOD is consistently low (<40 Mg ha−1; figure 3b) with higher AGBGRASS (>5 Mg ha−1). Overall, we noticed a depressing effect of FFI on AGBWOOD from plot data (figure 2e). This finding aligns with the results from a broader survey in the area covering approximately 300 ha using satellite and air-borne Lidar data (see [15], and additional specific results in electronic supplementary material, appendix S3), which described a decreasing pattern of low AGBWOOD (<40 Mg ha−1 and median values of ca 20 Mg ha−1) for FFI > 0.6 fire year−1. FFI exceeding 0.4 year−1 systematically corresponded to low AGBWOOD (<40 Mg ha−1). This leaned towards the widespread idea that frequent fires of large extent, fostered by high grass standing crop can maintain AGBWOOD at very low levels in humid savannas [36,55,108], thereby keeping the grass–fire feedback effective. Conversely, situations associating intermediate fire frequency (0–0.4 fire year−1) and AGBWOOD values above 40 Mg ha−1 are barely observable in the studied savannas (electronic supplementary material, appendix S3). This is likely a consequence of rapid savanna transition towards close canopy forests in those areas, due to the strength of the tree–shade depressing feedback. In both cases, necessary conditions for hysteresis loops are met.
Our results from plot data confirmed the suppressing effect of AGBWOOD on AGBGRASS through leaf shading and/or root competition [109,110], since all our sampled situations with AGBWOOD above 40 Mg ha−1 corresponded to very low AGBGRASS (<4 Mg ha−1, figure 4c). The AGBWOOD versus AGBGRASS decreasing relationship appeared clearer in the most productive environments (as referenced by AGBGRASS > 10 Mg ha−1), mainly occurring on clayey soils. Thus, the two opposing positive feedback (grass–fire versus tree–shade), each prevail according to identified thresholds in grassy and woody state variables and currently prevail in distinct areas of our study site. The simultaneous occurrence of forests and very open grassy savannas is particularly striking on clayey soils and compatible with an interpretation of AES (yet of undefined levels of stability). The ecotone at the forest edge is a zone of sheer opposition between these two positive feedback loops, where vegetation may react to changes or variation in external forcings in the most direct and dramatic way.
(b). Structure and composition of the woody layer across a forest–savanna boundary
Over the last five decades, the study area experienced a massive encroachment of forests over savanna [15], and all our sampled locations (both savanna and recent forests plots) were initially savanna in 1975 (figure 1). We observed that the two tallest among frequent savanna species (i.e. T. glaucescens and H. acida) not only occurred on the savanna side at all distances to the edge but also had shares of AGBWOOD peaking in the most recent forests, closer to the forest boundary. Those two species neither regenerate nor survive for long in forest environments, and the observed individuals are of tall stature and have been overtopped by forest pioneers quite recently (because they were not found farther than 40 m from current edges). This is indicative not only of the past savanna state (also known from satellite series) but also of the favourable growth conditions close to forest edges as their AGBWOOD shares were higher than in neighbouring savanna. In these situations, a large portion of AGBWOOD was also accounted for by some forest pioneers (such as Albizia spp. and Alchornea spp.), which have already been reported as showing a certain degree of fire-tolerance and contributing to forest boundary movement within the forest–savanna ecotone [14,20,59,61,111]. This species succession was further reflected in the main floristic gradient (NSCA 1, figure 3) opposing forest pioneers with savanna specialists, which was correlated to sand/clay content unlike soil fertility. Forests pioneers were more dominant on clay-rich soils as soon as lower fire frequencies prevailed (indicating AGBGRASS depression by woody cover). This contrasts with sandy/silty soils where savanna community was of low woody AGB despite lower fire regimes (figure 4). This leads to sharper boundaries on clay soils and a more specialized composition of species than reported in some other sites experiencing similar climate and rainfall (MAP of 1300–1400 mm, dry season of 3–4 months). Notably, in Ghana, a set of ‘non-specialized’ woody species identified along a forest–savanna gradient on soils which were heavily sandy (>84%) and nutrient poor (CEC < 7.5 cmol dm−3) [63], led to the conclusion that a distinctive floristic and tree canopy cover gradients was absent in the forest savanna ecotone. Although AGBGRASS is not available in Ametsitsi et al. [63], it is likely to be quite low, with limited ‘fire sharpening’ effects on the floristic composition. We thus delineated two distinct situations of woody savanna structure and species composition that played a pivotal role in shaping the ecological dynamics of the ecotone.
(c). Implications for landscape-scale dynamics of the forest–savanna ecotone
The central and northeastern MDNP which is found on granite substate (dominated by clayey soils) displayed the highest range of grass production along with high fire frequency (mean FFI ~0.5 fire year−1 at plots scale). This is, however, concomitant to rapid afforestation (0.63% year−1 with respect to total area), principally due to the pervasive forest boundary expansion (figure 1). Although the overall fire regime seemed able to keep AGBWOOD under the threshold of 40 Mg ha−1 in the middle of large savanna patches, there were local fire-resistant/-attenuated situations, such as those close to forest boundaries (as already reported from Lopé, Gabon [13]), near small nucleation groves (as reported in littoral Congo [16]), or in savanna patches surrounded by forests [58,59]. Fire regimes in the MDNP are mainly maintained by herders who burn to promote palatable grasses for cattle. Fire ignition is, however, insufficient in some areas, which are often remote from core grazing areas and become rapidly colonized by forbs, vines and pioneer forest species as in Youta-Happi et al., Achoundong et al., Fandohan et al. and Deklerck et al. [14,61,89,112]. The southern and western MDNP found on metamorphic substrate (dominated by coarser soil texture) displayed lower Landsat-detected fires frequencies (mean FFI ~0.3 fire year−1 at plots scale over the stable savanna) and a slightly higher pace of forest encroachment (0.66% year−1). Forest expansion in this area mainly occurred along boundaries of preexisting forests (figure 1). We interpret it as consequence of loose fire ignition practices in a context of lesser fire propagation (due to lower AGBGRASS recorded on metamorphic substrate). Lastly, in the Nachtigal area where herding activities are coexist with agricultural practices [77], we noticed the slowest pace of afforestation (0.4%) [15], and an AGBGRASS similar to southern MDNP and FFI close to zero. Field inspections, however, confirmed that fire occurred in most of the areas, although most of them are too small to be detected by the Landsat FFI proxy. Our analysis presents high similarities in soils, AGBGRASS/AGBWOOD, and floristic woody composition between Nachtigal and southern MDNP, which suggests that contrasting observed encroachment rates result from differences in human practices. Forest edge expansion in Nachtigal could be hampered by an anthropogenic edge effect [69] involving casual tree removal or wood cutting in savanna close to agricultural areas (i.e. cocoa plantations, fallows). Moreover, in very similar humid savannas of Congo, it has been highlighted that subtle variation in human activities and fire management can either speed-up or slow-down the overall forest expansion trend [113].
5. Conclusion
Our results captured and quantified the positive feedback loop involving grass production, fire propagation and control of woody species composition in a context of humid savanna transitions and ample soil texture and fertility gradients. We here show how soil texture modulates the grass–fire feedback by allowing stronger grass production and easier fire propagation on clayey soils compared with sandy textures. We also provide quantitative thresholds about the negative feedback of woody biomass on grass biomass, a process which is scarcely documented in literature, and we highlight a threshold of ca 40 Mg ha−1 of woody biomass above which the grass–fire feedback loose effectiveness and vegetation rapidly transition to forest. Clayey soils displayed the most contrasted vegetation mosaics (grassy savannas versus recent forests), which can be seen as AES. However, the long-term maintenance of grassy savannas by the grass–fire hysteresis loop is only granted far from forest edges. This suggests that currently observable mosaics are transitory under the present conditions (climate, CO2 concentration, dominant anthropogenic fire regimes) since swift forest boundary movement was observed over the last four decades. Forest edges were sharp and floristically well characterized by advancing forest pioneers (e.g. Albizzia spp.) and overtopped savanna trees, yet were well developed (e.g. T. glaucescent, H. acida) and did not suggest any important role of generalist species liable to perpetuate in both savanna and forest contexts. At present, the largest share of existing literature about humid savannas has addressed sandy oligotrophic situations. Orienting further field studies and meta-analyses so as to thoroughly quantify vegetation properties and dynamics, while sampling the entire soil texture and fertility gradients in the forest–savanna transition zone, would provide a sound basis for modelling and anticipating the future of the extensive forest–savanna ecotone in the face of global change.
Acknowledgements
We thank Suzanne Aimée Nien Ngapout Nku, Pierre Cheben and Christine Doumbe Mbou from NHPC for their valuable support. We thank MDNP staff officers for their assistance during field data collection.
Contributor Information
Le Bienfaiteur Takougoum Sagang, Email: sagang.bienfaiteur@yahoo.fr.
Imma Tcheferi, Email: tchef.im199@gmail.com.
Pierre Ploton, Email: p.ploton@gmail.com.
Moses Libalah, Email: libalah_moses@yahoo.com.
Murielle Simo-Droissart, Email: murielle.simo@gmail.com.
Nelly Sirri, Email: sirrinelly@yahoo.com.
Gilles Dauby, Email: gildauby@gmail.com.
Eric Ngansop, Email: ericngansop_19@yahoo.fr.
Jean Pierre Bissek, Email: jpbiss@yahoo.fr.
Gislain I. I. Mofack, Email: mgislain2@yahoo.fr.
Donatien Zebaze, Email: zebaze2d@gmail.com.
Hugo Leblanc, Email: hugo.leblanc@ird.fr.
Fabrice Djonko, Email: djonkof@yahoo.com.
Bonaventure Sonké, Email: bsonke_1999@yahoo.com.
Nicolas Barbier, Email: nicolas.barbier@ird.fr.
Pierre Couteron, Email: pierre.couteron@ird.fr.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The dataset and codes used in this study are permanently accessible in this repository [114].
Supplementary material is available online [115].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
L.B.T.S.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, validation, visualization, writing—original draft, writing—review and editing; I.T.: data curation, methodology; P.P.: conceptualization, supervision, writing—original draft, writing—review and editing; M.L.: writing—review and editing; M.S.-D.: writing— review and editing; N.S.: data curation, writing—review and editing; G.D.: data curation; E.N.: data curation; J.P.B.: resources; G.I.I.M.: data curation; D.Z.: data curation; H.L.: data curation; F.D.: data curation; B.S.: data curation, methodology, supervision, writing—original draft, writing—review and editing; N.B.: conceptualization, data curation, investigation, methodology, supervision, validation, writing—original draft, writing—review and editing; P.C.: conceptualization, data curation, investigation, methodology, supervision, validation, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was financially supported by the Nachtigal Hydropower Company (NHPC, Contract no. C006C007-DES-2017) under the environmental impact study associated to the construction of a hydroelectric dam on the Sanaga river in the center region of Cameroon.This study is also part of the 3DForMod project (ANR-17-EGAS-0002-01) funded by the framework of the JPI FACCE ERA-GAS call under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 696356). L.B.T.S. benefited from the 'Allocations de recherche pour une thèse au Sud (ARTS)' grant programme to fund research stays in France.
References
- 1. Stevens N, Lehmann CER, Murphy BP, Durigan G. 2017. Savanna woody encroachment is widespread across three continents. Glob. Chang. Biol. 23, 235–244. ( 10.1111/gcb.13409) [DOI] [PubMed] [Google Scholar]
- 2. Bouvet A, Mermoz S, Le Toan T, Villard L, Mathieu R, Naidoo L, Asner GP. 2018. An above-ground biomass map of African savannahs and woodlands at 25 m resolution derived from ALOS PALSAR. Remote Sens. Environ. 206, 156–173. ( 10.1016/j.rse.2017.12.030) [DOI] [Google Scholar]
- 3. Torello-Raventos M, et al. 2013. On the delineation of tropical vegetation types with an emphasis on forest/savanna transitions. Plant Ecol. Divers. 6, 101–137. ( 10.1080/17550874.2012.762812) [DOI] [Google Scholar]
- 4. Pausas JG, Bond WJ. 2020. Alternative biome states in terrestrial ecosystems. Trends Plant Sci. 25, 250–263. ( 10.1016/j.tplants.2019.11.003) [DOI] [PubMed] [Google Scholar]
- 5. Higgins SI, Conradi T, Kruger LM, O’Hara RB, Slingsby JA. 2023. Limited climatic space for alternative ecosystem states in Africa. Science 380, 1038–1042. ( 10.1126/SCIENCE.ADD5190/SUPPL_FILE/SCIENCE.ADD5190_SM.PDF) [DOI] [PubMed] [Google Scholar]
- 6. Aleman JC, et al. 2020. Floristic evidence for alternative biome states in tropical Africa. Proc. Natl Acad. Sci. USA 117, 28183–28190. ( 10.1073/pnas.2011515117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Staver AC, Archibald S, Levin S. 2011. Tree cover in sub-Saharan Africa: rainfall and fire constrain forest and savanna as alternative stable states. Ecology 92, 1063–1072. ( 10.1890/10-1684.1) [DOI] [PubMed] [Google Scholar]
- 8. Higgins SI, Banerjee S, Baudena M, Bowman DMJS, Conradi T, Couteron P, Kruger LM, O’Hara RB, Williamson GJ. 2024. Reassessing the alternative ecosystem states proposition in the African savanna‐forest domain. New. Phytol. 243, 1660–1669. ( 10.1111/nph.19911) [DOI] [PubMed] [Google Scholar]
- 9. Sankaran M, et al. 2005. Determinants of woody cover in African savannas. Nature 438, 846–849. ( 10.1038/nature04070) [DOI] [PubMed] [Google Scholar]
- 10. Bond WJ, Woodward FI, Midgley GF. 2005. The global distribution of ecosystems in a world without fire. New Phytol. 165, 525–537. ( 10.1111/j.1469-8137.2004.01252.x) [DOI] [PubMed] [Google Scholar]
- 11. Aleman JC, Staver AC. 2018. Spatial patterns in the global distributions of savanna and forest. Glob. Ecol. Biogeogr. 27, 792–803. ( 10.1111/geb.12739) [DOI] [Google Scholar]
- 12. Mitchard ETA, Flintrop CM. 2013. Woody encroachment and forest degradation in sub-Saharan Africa’s woodlands and savannas 1982–2006. Phil. Trans. R. Soc. B 368, 20120406. ( 10.1098/rstb.2012.0406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuni-Sanchez A, et al. 2016. African savanna-forest boundary dynamics: a 20-year study. PLoS One 11, e0156934. ( 10.1371/journal.pone.0156934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Youta-Happi J, Bonvallot J, Hotyat M, Guillet B, Peltre P, Schwartz D, Servant M, Simonneaux V. 2003. Bilan de la dynamique du contact forêt-savane en quarante ans (1950–1990) dans la région du confluent du Mbam et du Kim, Centre-Cameroun. In Peuplements anciens et actuels des forêts tropicales, p. 380. Horizons IRD. ( 10.4000/books.irdeditions.1507). See https://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers10-05/010033344.pdf. [DOI] [Google Scholar]
- 15. Sagang LBT, Ploton P, Viennois G, Féret JB, Sonké B, Couteron P, Barbier N. 2022. Monitoring vegetation dynamics with open earth observation tools: the case of fire-modulated savanna to forest transitions in Central Africa. ISPRS J. Photogramm. Remote Sens. 188, 142–156. ( 10.1016/j.isprsjprs.2022.04.008) [DOI] [Google Scholar]
- 16. Favier C, De Namur C, Dubois M. 2004. Forest progression modes in littoral Congo, Central Atlantic Africa. J. Biogeogr. 31, 1445–1461. ( 10.1111/j.1365-2699.2004.01094.x) [DOI] [Google Scholar]
- 17. Favier C, Chave J, Fabing A, Schwartz D, Dubois MA. 2004. Modelling forest–savanna mosaic dynamics in man-influenced environments: effects of fire, climate and soil heterogeneity. Ecol. Modell. 171, 85–102. ( 10.1016/j.ecolmodel.2003.07.003) [DOI] [Google Scholar]
- 18. Veenendaal EM, et al. 2015. Structural, physiognomic and above-ground biomass variation in savanna–forest transition zones on three continents – how different are co-occurring savanna and forest formations? Biogeosciences 12, 2927–2951. ( 10.5194/bg-12-2927-2015) [DOI] [Google Scholar]
- 19. Odum E. 1971. Fundamentals of ecology. Philadelphia, PA: WB Saunders. [Google Scholar]
- 20. Oliveras I, Malhi Y. 2016. Many shades of green: the dynamic tropical forest-savannah transition zones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 15pp. ( 10.1098/rstb.2015.0308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Veldman JW, et al. 2015. Toward an old-growth concept for grasslands. Front. Ecol. Env. 13, 154–162. ( 10.1890/140270) [DOI] [Google Scholar]
- 22. Wieczorkowski JD, Lehmann CER. 2022. Encroachment diminishes herbaceous plant diversity in grassy ecosystems worldwide. Glob. Chang. Biol. 28, 5532–5546. ( 10.1111/gcb.16300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silveira FAO, et al. 2020. Myth‐busting tropical grassy biome restoration. Restor. Ecol. 28, 1067–1073. ( 10.1111/rec.13202) [DOI] [Google Scholar]
- 24. Parr CL, Lehmann CER, Bond WJ, Hoffmann WA, Andersen AN. 2014. Tropical grassy biomes: misunderstood, neglected, and under threat. Trends Ecol. Evol. 29, 205–213. ( 10.1016/j.tree.2014.02.004) [DOI] [PubMed] [Google Scholar]
- 25. Veldman JW, et al. 2014. Tyranny of trees in grassy biomes. Science 347, 484–485. ( 10.1126/science.347.6221.484) [DOI] [PubMed] [Google Scholar]
- 26. Lloyd J, Bird MI, Vellen L, Miranda AC, Veenendaal EM, Djagbletey G, Miranda HS, Cook G, Farquhar GD. 2008. Contributions of woody and herbaceous vegetation to tropical savanna ecosystem productivity: a quasi-global estimate. Tree Physiol. 28, 451–468. ( 10.1093/treephys/28.3.451) [DOI] [PubMed] [Google Scholar]
- 27. Bond WJ. 2008. What limits trees in C4 grasslands and savannas. Annu. Rev. Ecol. Evol. Syst. 39, 641–659. ( 10.1146/annurev.ecolsys.39.110707.173411) [DOI] [Google Scholar]
- 28. Hoffmann WA, Adasme R, Haridasan M, de Carvalho MT, Geiger EL, Pereira MAB, Gotsch SG, Franco AC. 2009. Tree topkill, not mortality, governs the dynamics of savanna-forest boundaries under frequent fire in central Brazil. Ecology 90, 1326–1337. ( 10.1890/08-0741.1) [DOI] [PubMed] [Google Scholar]
- 29. Swaine MD, Hawthorne WD, Orgle TK. 1992. The effects of fire exclusion on savanna vegetation at Kpong, Ghana. Biotropica 24, 166. ( 10.2307/2388670) [DOI] [Google Scholar]
- 30. Cardoso AW, et al. 2022. Quantifying the environmental limits to fire spread in grassy ecosystems. Proc. Natl Acad. Sci. USA 119, e2110364119. ( 10.1073/PNAS.2110364119/SUPPL_FILE/PNAS.2110364119.SAPP.PDF) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoffmann WA, Jaconis SY, Mckinley KL, Geiger EL, Gotsch SG, Franco AC. 2012. Fuels or microclimate? Understanding the drivers of fire feedbacks at savanna–forest boundaries. Austral Ecol. 37, 634–643. ( 10.1111/j.1442-9993.2011.02324.x) [DOI] [Google Scholar]
- 32. Scholes RJ, Archer SR. 1997. Tree-grass interactions in savannas. Annu. Rev. Ecol. Syst. 28, 517–544. ( 10.1146/annurev.ecolsys.28.1.517) [DOI] [Google Scholar]
- 33. Zupo T, Gorgone‐Barbosa E, Ninno Rissi M, Daibes LF. 2022. Experimental burns in an open savanna: greater fuel loads result in hotter fires. Austral. Ecol. 47, 1101–1112. ( 10.1111/aec.13202) [DOI] [Google Scholar]
- 34. Hoffmann WA, Geiger EL, Gotsch SG, Rossatto DR, Silva LCR, Lau OL, Haridasan M, Franco AC. 2012. Ecological thresholds at the savanna-forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecol. Lett. 15, 759–768. ( 10.1111/j.1461-0248.2012.01789.x) [DOI] [PubMed] [Google Scholar]
- 35. Staver AC, Archibald S, Levin SA. 2011. The global extent and determinants of savanna and forest as alternative biome states. Science 334, 230–232. ( 10.1126/SCIENCE.1210465/SUPPL_FILE/STAVERSOM.REVISION.1.PDF) [DOI] [PubMed] [Google Scholar]
- 36. Charles-Dominique T, Midgley GF, Tomlinson KW, Bond WJ. 2018. Steal the light: shade vs fire adapted vegetation in forest-savanna mosaics. New Phytol. 218, 1419–1429. ( 10.1111/nph.15117) [DOI] [PubMed] [Google Scholar]
- 37. Higgins SI, Bond WJ, Trollope WSW. 2000. Fire, resprouting and variability: a recipe for grass–tree coexistence in savanna. J. Ecol. 88, 213–229. ( 10.1046/j.1365-2745.2000.00435.x) [DOI] [Google Scholar]
- 38. Peterson DW, Reich PB. 2007. Fire frequency and tree canopy structure influence plant species diversity in a forest-grassland ecotone. Plant Ecol. 194, 5–16. ( 10.1007/s11258-007-9270-4) [DOI] [Google Scholar]
- 39. House JI, Archer S, Breshears DD, Scholes RJ, NCEAS Tree–Grass Interactions Participants . 2003. Conundrums in mixed woody–herbaceous plant systems. J. Biogeogr. 30, 1763–1777. ( 10.1046/j.1365-2699.2003.00873.x) [DOI] [Google Scholar]
- 40. Devine AP, McDonald RA, Quaife T, Maclean IMD. 2017. Determinants of woody encroachment and cover in African savannas. Oecologia 183, 939–951. ( 10.1007/s00442-017-3807-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Venter ZS, Cramer MD, Hawkins HJ. 2018. Drivers of woody plant encroachment over Africa. Nat. Commun. 9, 2272. ( 10.1038/s41467-018-04616-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilson JB, Agnew ADQ. 1992. Positive-feedback switches in plant communities. Adv. Ecol. Res. 23, 263–336. ( 10.1016/S0065-2504(08)60149-X) [DOI] [Google Scholar]
- 43. Lloyd J, et al. 2015. Edaphic, structural and physiological contrasts across Amazon Basin forest–savanna ecotones suggest a role for potassium as a key modulator of tropical woody vegetation structure and function. Biogeosciences 12, 6529–6571. ( 10.5194/bg-12-6529-2015) [DOI] [Google Scholar]
- 44. Cruz Ruggiero PG, Batalha MA, Pivello VR, Meirelles ST. 2002. Soil-vegetation relationships in Cerrado (Brazilian savanna) and semideciduous forest. Plant Ecol. 160, 1–16. ( 10.1023/A:1015819219386) [DOI] [Google Scholar]
- 45. Bond WJ. 2010. Do nutrient-poor soils inhibit development of forests? A nutrient stock analysis. Plant Soil 334, 47–60. ( 10.1007/s11104-010-0440-0) [DOI] [Google Scholar]
- 46. Staver AC, Botha J, Hedin L. 2017. Soils and fire jointly determine vegetation structure in an African savanna. New Phytol. 216, 1151–1160. ( 10.1111/nph.14738) [DOI] [PubMed] [Google Scholar]
- 47. Tricart J, Cailleux A. 1974. Le modelé des régions chaudes: forêts et savanes. Paris, France: Société d’édition d’enseignement supérieur. [Google Scholar]
- 48. Aubréville A. 1967. Bois et Forêts des Tropiques a 20 ans. Bois et Forêts des Tropiques 111, 2–3. ( 10.19182/BFT1967.111.A19020) [DOI] [Google Scholar]
- 49. Boulvert Y. 1990. Avancée ou recul de la forêt centrafricaine: changements climatiques, influence de l’homme et notamment des feux. In Paysages quaternaires de l’Afrique centrale atlantique (eds Lanfranchi R, Schwartz D), pp. 353–366. Paris, France: IRD Éditions. [Google Scholar]
- 50. Schwartz D, Dechamps R, Elenga H, Lanfranchi R, Mariotti AA, Vincens A. 1995. Les savanes du Congo: une végétation spécifique de l’holocène supérieur? Horizon IRD. See https://horizon.documentation.ird.fr/exl-doc/pleins_textes/pleins_textes_6/b_fdi_35-36/40555.pdf. [Google Scholar]
- 51. Murphy BP, Bowman DMJS. 2012. What controls the distribution of tropical forest and savanna? Ecol. Lett. 15, 748–758. ( 10.1111/j.1461-0248.2012.01771.x) [DOI] [PubMed] [Google Scholar]
- 52. Lehmann CER, Archibald SA, Hoffmann WA, Bond WJ. 2011. Deciphering the distribution of the savanna biome. New Phytol. 191, 197–209. ( 10.1111/j.1469-8137.2011.03689.x) [DOI] [PubMed] [Google Scholar]
- 53. Cramer M, Van CA, of EWJ. 2010. Growth of N2‐fixing African savanna Acacia species is constrained by below‐ground competition with grass. Wiley Online Library. 98, 156–167. ( 10.1111/j.1365-2745.2009.01594.x) [DOI] [Google Scholar]
- 54. Cech PG, Kuster T, Edwards PJ, Olde Venterink H. 2008. Effects of herbivory, fire and N2-fixation on nutrient limitation in a humid African Savanna. Ecosystems 11, 991–1004. ( 10.1007/s10021-008-9175-7) [DOI] [Google Scholar]
- 55. Accatino F, Wiegand K, Ward D, De Michele C. 2016. Trees, grass, and fire in humid savanna.the importance of life history traits and spatial processes. Ecol. Modell. 320, 135–144. ( 10.1016/j.ecolmodel.2015.09.014) [DOI] [Google Scholar]
- 56. Cardoso AW, et al. Grass species flammability, not biomass, drives changes in fire behavior at tropical forest-savanna transitions. Front. For. Glob. Change 1. ( 10.3389/ffgc.2018.00006) [DOI] [Google Scholar]
- 57. Wragg PD, Mielke T, Tilman D. 2018. Forbs, grasses, and grassland fire behaviour. J. Ecol. 106, 1983–2001. ( 10.1111/1365-2745.12980) [DOI] [Google Scholar]
- 58. Youta-Happi J, Hotyat M, Bonvallot J. 1993. La colonisation des savanes par la forêt à l’est du cameroun. Horizon IRD. See https://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers12-10/010023936.pdf. [Google Scholar]
- 59. Youta-Happi J, Bonvallot J. 1996. La disparition des savanes au entre Cameroun entre 1950 et 1990. Horizon IRD. See https://horizon.documentation.ird.fr/exl-doc/pleins_textes/pleins_textes_7/carton01/010007596.pdf. [Google Scholar]
- 60. Cardoso AW, et al. 2021. A distinct ecotonal tree community exists at central African forest–savanna transitions. J. Ecol. 109, 1170–1183. ( 10.1111/1365-2745.13549) [DOI] [Google Scholar]
- 61. Achoundong G, Youta-Happi J, Guillet B, Bonvallot J, Beyala KV. 1996. Formation et évolution des recrûs sur savanes. Horizon IRD. See https://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers12-10/010023936.pdf. [Google Scholar]
- 62. Letouzey R. 1968. Etude phytogéographique du cameroun. In Encyclopédie biologique, p. 511. Paris, France: Paul Lechevallier. [Google Scholar]
- 63. Ametsitsi GKD, et al. 2020. Fixed or mixed? Variation in tree functional types and vegetation structure in a forest-savanna ecotone in West Africa. J. Trop. Ecol. 36, 133–149. ( 10.1017/S0266467420000085) [DOI] [Google Scholar]
- 64. Hanan NP, Sea WB, Dangelmayr G, Govender N. 2008. . Do fires in savannas consume woody biomass? A comment on approaches to modeling savanna dynamics. 851–856. ( 10.1086/587527) [DOI] [PubMed]
- 65. Touboul JD, Staver AC, Levin SA. 2018. On the complex dynamics of savanna landscapes. Proc. Natl. Acad. Sci. USA 115, E1336–E1345. ( 10.1073/PNAS.1712356115/SUPPL_FILE/PNAS.201712356SI.PDF) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Djeumen IVY, Dumont Y, Doizy A, Couteron P. 2021. A minimalistic model of vegetation physiognomies in the savanna biome. Ecol. Modell. 440, 109381. ( 10.1016/j.ecolmodel.2020.109381) [DOI] [Google Scholar]
- 67. Abbadie L, Gignoux J, Roux X. 2006. Lamto: structure, functioning, and dynamics of a savanna ecosystem. NY: Springer. ( 10.1007/0-387-33857-8) [DOI] [Google Scholar]
- 68. Roland JC. 1962. Recherches écologiques dans la savane de lamto (Côte d’Ivoire): données préliminaires sur le cycle annuel de la végétation herbacée. In Laboratoire de biologie végétale de la faculté des sciences de paris, pp. 228–248. Paris, France: Revue d’Écologie (La Terre et La Vie). [Google Scholar]
- 69. Jeffery KJ, Korte L, Palla F, Walters G, White LJT, Abernethy KA. 2014. Fire management in a changing landscape: a case study from Lopé National Park, Gabon. PARKS 20, 39–52. ( 10.2305/IUCN.CH.2014.PARKS-20-1.KJJ.en) [DOI] [Google Scholar]
- 70. White F, Werger MJA. 1978. The guineo-congolian transition to southern africa. In Biogeography and ecology of southern africa (ed. Werger MJA), pp. 599–620, vol. 31. Dordrecht, The Netherlands: Springer. ( 10.1007/978-94-009-9951-0_14) [DOI] [Google Scholar]
- 71. Hersbach H, et al. 2020. The ERA5 global reanalysis. Quart. J. Royal Meteoro. Soc. 146, 1999–2049. ( 10.1002/qj.3803) [DOI] [Google Scholar]
- 72. Gazel J, Guiraudie C, Ribes GC, Cirotteau A, Leroy B. 1956. Carte géologique du cameroun. In Atlas du cameroun (eds Cirotteau A, Leroy B). Paris, France: ORSTOM. [Google Scholar]
- 73. Djoufack MV. 2011. Étude multi-échelles des précipitations et du couvert végétal au cameroun: analyses spatiales, tendances temporelles, facteurs climatiques et anthropiques de variabilité du NDVI. Thesis, Université de Bourgogne et Université de Yaoundé I. [Google Scholar]
- 74. Jiang Y, Zhou L, Raghavendra A. 2020. Observed changes in fire patterns and possible drivers over Central Africa. Environ. Res. Lett. 15, 0940b8. ( 10.1088/1748-9326/ab9db2) [DOI] [Google Scholar]
- 75. Karger DN, Wilson AM, Mahony C, Zimmermann NE, Jetz W. 2021. Global daily 1 km land surface precipitation based on cloud cover-informed downscaling. Sci. Data 8, 307. ( 10.1038/s41597-021-01084-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Harrison IJ, Brummett R, Stiassny MLJ. 2016. Congo river basin. In The wetland book (eds Finlayson C, Milton G, Prentice R, Davidson N), pp. 1–18. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 77. Alexandre C. 2013. Analyse de l’usage du sol de la région de bokito (mbam et inoubou, cameroun) à partir de données de télédétection et implications sur les systèmes de culture agroforestiers. Thesis, Université Montpellier III Paul-Valéry. [Google Scholar]
- 78. Gilbert M, Nicolas G, Cinardi G, Van Boeckel TP, Vanwambeke SO, Wint GRW, Robinson TP. 2018. Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Sci. Data 5, 180227. ( 10.1038/sdata.2018.227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. CIESIN . 2016. Documentation for the gridded population of the world, version 4 (GPWv4). Palisades, CA: NASA Socioeconomic Data and Applications Center (SEDAC). [Google Scholar]
- 80. Sagang LBT, et al. 2018. Using volume-weighted average wood specific gravity of trees reduces bias in aboveground biomass predictions from forest volume data. For. Ecol. Manage. 424, 519–528. ( 10.1016/j.foreco.2018.04.054) [DOI] [Google Scholar]
- 81. Gwenzi D, Lefsky MA. 2017. Spatial modeling of lidar-derived woody biomass estimates collected along transects in a heterogeneous savanna landscape. IEEE J. Sel. Top. Appl. Earth Observations Remote Sensing 10, 372–384. ( 10.1109/JSTARS.2016.2582148) [DOI] [Google Scholar]
- 82. Mutanga O, Rugege D. 2006. Integrating remote sensing and spatial statistics to model herbaceous biomass distribution in a tropical savanna. Int. J. Remote Sens. 27, 3499–3514. ( 10.1080/01431160600639735) [DOI] [Google Scholar]
- 83. Colgan MS, Asner GP, Swemmer T. 2013. Harvesting tree biomass at the stand level to assess the accuracy of field and airborne biomass estimation in savannas. Ecol. Appl. 23, 1170–1184. ( 10.1890/12-0922.1) [DOI] [PubMed] [Google Scholar]
- 84. Chave J, et al. 2014. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 20, 3177–3190. ( 10.1111/gcb.12629) [DOI] [PubMed] [Google Scholar]
- 85. Zanne AE, Lopez-Gonzalez G, Coomes DAet al. 2009. Data from: Towards a worldwide wood economics spectrum. Dryad Digital Repository. ( 10.5061/dryad.234) [DOI] [PubMed]
- 86. Réjou‐Méchain M, Tanguy A, Piponiot C, Chave J, Hérault B. 2017. Biomass : an r package for estimating above‐ground biomass and its uncertainty in tropical forests . Methods Ecol. Evol. 8, 1163–1167. ( 10.1111/2041-210X.12753) [DOI] [Google Scholar]
- 87. Arbonnier M. 2009. Arbres, arbustes et lianes d’Afrique de l’Ouest. See https://www.quae.com/produit/1554/9782759225484/arbres-arbustes-et-lianes-d-afrique-de-l-ouest.
- 88. Bénédet F, Doucet JL, Fayolle A, Gourlet-Fleury S, Vincke D. 2015. Cofortraits, African plant traits information database. In Cofortraits. See https://dataverse.cirad.fr/dataset.xhtml?persistentId=doi:10.18167/DVN1/Y2BIZK.
- 89. Fandohan AB, Oduor AMO, Sodé AI, Wu L, Cuni-sanchez A, Assédé E, Gouwakinnou GN. 2015. Modeling vulnerability of protected areas to invasion by Chromolaena odorata under current and future climates. Ecosyst. Health Sustain. 1, 1–12. ( 10.1890/EHS15-0003.1) [DOI] [Google Scholar]
- 90. Gavlak R, Horneck R, Miller RO. 2005. Potassium fixation test (incubation method). In Soil, plant and water reference methods for the western region (eds Gavlak R, Horneck DA, Miller RO), pp. 129–134. WREP. [Google Scholar]
- 91. Bray RH, Kurtz LT. 1945. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 59, 39–46. ( 10.1097/00010694-194501000-00006) [DOI] [Google Scholar]
- 92. Buondonno A, Rashad AA, Coppola E. 2008. Comparing tests for soil fertility. II. The hydrogen peroxide/sulfuric acid treatment as an alternative to the copper/selenium catalyzed digestion process for routine determination of soil nitrogen‐kjeldahl. Commun. Soil Sci. Plant Anal. 26, 1607–1619. ( 10.1080/00103629509369394) [DOI] [Google Scholar]
- 93. Heanes DL. 1984. Determination of total organic‐C in soils by an improved chromic acid digestion and spectrophotometric procedure. Commun. Soil Sci. Plant Anal. 15, 1191–1213. ( 10.1080/00103628409367551) [DOI] [Google Scholar]
- 94. Libalah MB, et al. 2020. Additive influences of soil and climate gradients drive tree community composition of Central African rain forests. J. Veg. Sci. 31, 1154–1167. ( 10.1111/jvs.12918) [DOI] [Google Scholar]
- 95. Airbus . 2020. Copernicus DEM copernicus digital elevation model validation report ( 10.5270/ESA-c5d3d65) [DOI]
- 96. Koenker R, Park BJ. 1996. An interior point algorithm for nonlinear quantile regression. J. Econom. 71, 265–283. ( 10.1016/0304-4076(96)84507-6) [DOI] [Google Scholar]
- 97. RStudio Team . 2016. RStudio: integrated development for R. Boston: RStudio, Inc. See http://www. rstudio. com. [Google Scholar]
- 98. Kroonenberg PM, Lombardo R. 1999. Nonsymmetric correspondence analysis: a tool for analysing contingency tables with a dependence structure. Multivariate Behav. Res. 34, 367–396. ( 10.1207/S15327906MBR3403_4) [DOI] [Google Scholar]
- 99. Pélissier R, Couteron P, Dray S, Sabatier D. 2003. Consistency between ordination techniques and diversity measurements: two strategies for species occurrence data. Ecology 84, 242–251. ( 10.1890/0012-9658(2003)084[0242:CBOTAD]2.0.CO;2) [DOI] [Google Scholar]
- 100. Couteron P, Pélissier R, Mapaga D, Molino JF, Teillier L. 2003. Drawing ecological insights from a management-oriented forest inventory in French Guiana. For. Ecol. Manage. 172, 89–108. ( 10.1016/S0378-1127(02)00310-9) [DOI] [Google Scholar]
- 101. Thioulouse J, Dray S, Dufour AB, Siberchicot A, Jombart T, Pavoine S. 2011. Multivariate analysis of ecological data with ade4. New York, NY: Springer. ( 10.1007/978-1-4939-8850-1) [DOI] [Google Scholar]
- 102. Cardoso AW, et al. 2018. Grass species flammability, not biomass, drives changes in fire behavior at tropical forest-savanna transitions. Front. For. Glob. Change. 1, 1–14. ( 10.3389/ffgc.2018.00006) [DOI] [Google Scholar]
- 103. Van Langevelde F, et al. 2003. Effects of fire and herbivory on the stability of savanna ecosystems. Ecology 84, 337–350. ( 10.1890/0012-9658(2003)084[0337:EOFAHO]2.0.CO;2) [DOI] [Google Scholar]
- 104. Jensen M, Michelsen A, Gashaw M. 2001. Responses in plant, soil inorganic and microbial nutrient pools to experimental fire, ash and biomass addition in a woodland savanna. Oecologia 128, 85–93. ( 10.1007/s004420000627) [DOI] [PubMed] [Google Scholar]
- 105. Cardoso AW, et al. 2020. The role of forest elephants in shaping tropical forest–savanna coexistence. Ecosystems 23, 602–616. ( 10.1007/s10021-019-00424-3) [DOI] [Google Scholar]
- 106. Trollope WSW. 1983. Control of bush encroachment with fire in the arid savannas of southeastern africa. Thesis, University of Natal, Pietermaritzburg. [Google Scholar]
- 107. Roteta E, Bastarrika A, Padilla M, Storm T, Chuvieco E. 2019. Development of a sentinel-2 burned area algorithm: generation of a small fire database for sub-Saharan Africa. Remote Sens. Environ. 222, 1–17. ( 10.1016/j.rse.2018.12.011) [DOI] [Google Scholar]
- 108. Devine AP, Stott I, McDonald RA, Maclean IMD. 2015. Woody cover in wet and dry African savannas after six decades of experimental fires. J. Ecol. 103, 473–478. ( 10.1111/1365-2745.12367) [DOI] [Google Scholar]
- 109. Le Roux X, Bariac T, Mariotti A. 1995. Spatial partitioning of the soil water resource between grass and shrub components in a West African humid savanna. Oecologia 104, 147–155. ( 10.1007/BF00328579) [DOI] [PubMed] [Google Scholar]
- 110. Menaut JC, Gignoux J, Prado C, Clobert J. 1990. Tree community dynamics in a humid savanna of the cote-d’Ivoire: modelling the effects of fire and competition with grass and neighbours. J. Biogeogr. 17, 471. ( 10.2307/2845379) [DOI] [Google Scholar]
- 111. Dantas V de L, Pausas JG. 2013. The lanky and the corky: fire‐escape strategies in savanna woody species. J. Ecol. 101, 1265–1272. ( 10.1111/1365-2745.12118) [DOI] [Google Scholar]
- 112. Deklerck V, De Mil T, Ilondea BA, Nsenga L, De Caluwé C, Van den Bulcke J, Van Acker J, Beeckman H, Hubau W. 2019. Rate of forest recovery after fire exclusion on anthropogenic savannas in the democratic republic of Congo. Biol. Conserv. 233, 118–130. ( 10.1016/j.biocon.2019.02.027) [DOI] [Google Scholar]
- 113. Walters GM. 2010. The land chief’s embers: ethnobotany of Batéké fire regimes, savanna vegetation and resource use in Gabon. Thesis, University College London. See http://eprints.ucl.ac.uk/20195. [Google Scholar]
- 114. Sagang LB. 2023. Soil-environmental interactions modulate forest expansion in humid savannas of Central Africa. Zenodo ( 10.5281/zenodo.8383918) [DOI] [PubMed]
- 115. Takougoum S, Bienfaiteur L, Tcheferi I, Ploton P, Libalah M, Simo-Droissart M, Sirri N. 2024. Supplementary material from: Interactions between soil and other environmental variables modulate forest expansion and ecotone dynamics in humid savannas of Central Africa. Figshare. ( 10.6084/m9.figshare.c.7486578) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset and codes used in this study are permanently accessible in this repository [114].
Supplementary material is available online [115].