Abstract
The genus Diospyros has gained significant attention in the scientific community owing to its diverse bioactivities ascribed to specific bioactive constituents present in different species of this plant. Phytochemicals like flavonoids, terpenoids, and xanthones have been reported to be present in other Diospyros species responsible for their pharmacological properties. These compounds are well known for their diverse potent therapeutic potentials, such as antimicrobial, antioxidant, anti‐inflammatory, and anticancer properties. This review enlightens the details of the Genus Diospyros, ranging from an overview of its species to an in‐depth analysis of phytochemistry, ethnopharmacology, and their potential as anticancer agents. Different species, including Diospyros lotus, Diospyros kaki, Diospyros maritima, Diospyros mespiliformis, and Diospyros tricolor, presented with an enormous range of anticancer activities against human cancer cell cultures. Moreover, this review highlights the results of various in vitro (antiproliferative, cytotoxic effects against), in vivo (inhibition of tumor, apoptosis), and in silico (GLU234, GLU278, and LYS158 protein residues) studies, elucidating its preclinical anticancer potential. The anticancer potential displays inhibition of cellular proliferation, induction of apoptosis, and mitigation of angiogenesis. Furthermore, this review may elaborate the use of traditional knowledge, modern research, and potential therapeutic applications in the field of anticancer ethnopharmacology. As the modern‐day research approaches novel alternatives to combat diseases like cancer, the Genus Diospyros may emerge as a promising avenue with the potential to yield innovative and effective therapeutic agents.
Keywords: angiogenesis, anticancer agents, apoptosis, cellular proliferation, Diospyros, ethnopharmacology, in silico studies, in vitro studies, in vivo studies
The Genus Diospyros has garnered significant scientific interest due to its diverse bioactivities, attributed to various phytochemicals like flavonoids, terpenoids, and xanthones. Several species, including D. lotus, D. kaki, and D. tricolor, exhibit promising anti‐cancer properties through in vitro and in vivo studies, demonstrating inhibition of proliferation, induction of apoptosis, and mitigation of angiogenesis. Integrating traditional knowledge with modern research, Diospyros shows potential as a source for novel and effective anti‐cancer therapeutics in ethno‐pharmacology.
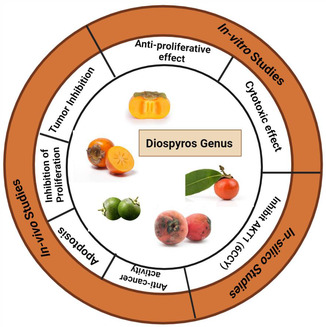
1. INTRODUCTION
Cancer is a complicated disease that results in uncontrolled tumor cell proliferation due to signaling failure of oncogenic expressions. Since tumors can begin in any organ, the resulting malignancies are highly diverse (Anusewicz et al., 2020). In 2020, cancer was anticipated to result in over 10 million fatalities and 19.3 million new diagnoses (Sung et al., 2021). Breast cancer accounts for approximately 11.7% of all new cancer diagnoses in women annually (Bray et al., 2018). Until now, efforts have been made to create effective methods for diagnosing and treating cancer. Among the available therapeutic methods are chemotherapy (Dickens & Ahmed, 2018), molecularly targeted therapy (Piawah & Venook, 2019), gene therapy (Carrillo et al., 2018), radiation (Dobosz & Dzieciątkowski, 2019), immunotherapy (Chang et al., 2019), and phototherapy (Marabini et al., 2020). Indirectly, medicinal plant therapeutic compounds also help improve overall health by enhancing cellular signaling processes and activating endogenous defense mechanisms (Gopal et al., 2011). There are 240 species in the ubiquitous tropical genus Diospyros. Of those species, 59 are found in India, Japan, Thailand, South Africa, the Philippines, and Nigeria (Alex et al., 2012). The medium‐sized Gürke, the Gaub persimmon, is an endemic perennial to India. An ethnomedicinal plant called Diospyros peregrina (Gaertn.) Gürke's alcoholic fruit extract has hypoglycemic, diuretic, and anticancer properties (Philander, 2011). Several parts of the plant have a variety of medicinal uses outside its traditional usage for dysentery and menstrual problems. The fruit of D. peregrina contains soluble tannins, peregrinol, flavonoids, hexacosanol, hexacosane, betulinic acid, β‐sitosterol, and lupeol. D. peregrina was previously discovered to have anticancer properties by experimenting on mice with Ehrlich ascites carcinoma (Alex et al., 2012; Philander, 2011).
Herbalists in South Africa's Western Cape, which has a lot of different plants, said they used Dioscorea villosa root to treat diarrhea, intestinal worms, and too much flatulence (De Wet & Ngubane, 2014). Moreover, the root of the D. villosa plant was utilized to alleviate pain and dysmenorrhea in a rural area of northern Maputaland (Rajesh et al., 2017). Nanoparticles (NPs) isolated from the leaves of another plant, Diospyros ferrea (Wild.), also exhibited anticancer activity against MCF‐7 (Michigan Cancer Foundation‐7) cancer cell lines (Park et al., 2017). Investigations into the pharmacology of the calyx of Diospyros kaki Thunb. (DKC) have not been entirely explained, despite reports that it has significant polyphenol levels. DKC's anticancer efficacy and putative molecular mechanism against human colorectal cancer cells were analyzed using the MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide) test. Extracts of the tree Diospyros kaki calyx were tested for their ability to inhibit cell proliferation in 70% ethanol (DKC‐E70). Western blotting and reverse transcription polymerase chain reaction (RT‐PCR) were used to examine the effect of DKC‐E70 on cyclin D1 messenger RNA (mRNA) and protein expression. Results revealed that DKC‐E70 inhibited LoVo (epithelial cell line), SW480 (human colon adenocarcinoma cell line), and HT‐29 (human colorectal adenocarcinoma cell line). Although DKC‐E70 decreased cyclin D1 expression at both the protein and mRNA levels, the downregulation of cyclin D1 protein by DKC‐E70 may have been caused by the induction of cyclin D's degradation and transcriptional inhibition.
Furthermore, it was observed that the effect of DKC‐E70 on cyclin D1 degradation was diminished in the presence of MG132 (proteasome inhibitor). Moreover, DKC‐E70 phosphorylated cyclin D1's threonine‐286 (T286) and T286 mutated to alanine (T286A) reversed DKC‐downregulation E70's of cyclin D1. The phosphorylation of T286 by DKC‐E70 and consequent degradation of cyclin D1 were also seen to be inhibited when ERK1/2 (extracellular signal‐related kinases 1 and 2), p38, or GSK3 (glycogen synthase kinase 3) inhibitors were present. DKC‐E70 suppressed the expression of β‐catenin and TCF4 (transcription factor 4) and β‐catenin/TCF‐dependent luciferase activity in cyclin D1 transcriptional inhibition. Thus, this study implies cyclin D1 as one of the potential anticancer targets that can be downregulated via DKC‐E70 through cyclin D1 breakdown. Furthermore, downregulation can be carried out by T286 phosphorylation dependent on ERK1/2, p38, or GSK3 and cyclin D1 transcriptional suppression via Wnt signaling. Hence, based on these findings, DKC‐E70 may be possible for developing chemoprevention or therapeutic medicines for human colorectal cancer.
2. GENUS DIOSPYROS: AN OVERVIEW
The broad pantropical genus Diospyros comprises several hundred species, mostly evergreen trees and shrubs. Though a few species are deciduous, inhabiting the temperate regions, its best‐known features are the genus’ delicious fruits and dark wood. It derives its name from the Greek term “Diospyros,” which means “divine wheat” or “fruit of the gods.” Despite their fibrous texture, the edible fruits of the Diospyros species are rich in vitamins and minerals. Increased tannin concentration makes them extremely astringent before they are fully mature. Diospyros lotus, a plant native to south‐eastern Europe and south‐west Asia, produces little, yellow, or purplish‐black fruits called date‐plum that soften up and become edible when ripe. Likewise, D. kaki (Oriental persimmon) is a long‐standing, widespread cultivated plant primarily found in China and Japan (Sarkhosh et al., 2024). This species has distinct male and female parts. The calyx lobes are frequently star‐shaped that become larger as the fruits develop into enormous, spherical berries. A persimmon cultivar grown in Israel's Plain of Sharon is known as the Sharon fruit and is sold in supermarkets. The south‐eastern American plant Diospyros virginiana produces the fruits of American persimmon. However, several portions of the African Diospyros mespiliformis are also traditionally utilized for medicinal and practical reasons. For instance, edible jackal berry fruits are produced by this species. It is utilized to create a glaze that is used on ceramics. Elephants, raccoons, gorillas, and deer eat jackal berry fruits in the wild, serving as crucial seed dispersers. Since ancient times, ebony wood has been traded. This slow‐growing Diospyros ebenum species is currently endangered, and its legal timber export is restricted, provided the source for this highly valued ebony. Ebony wood is incredibly tough, primarily black, solid, durable, and quite glossy when polished. Apart from furniture, it is historically used for the black pieces in chess sets and, along with ivory, as piano keys and fretboards on stringed instruments like guitars and violins. The primary source of ebony now comes from woodlands in Central Africa and is called Diospyros crassiflora (Sodhi et al., 2023).
Over the range of the genus Diospyros, numerous uses catering to needs of human beings have also been documented. Unripe fruits of the Central American and Mexican Diospyros digyna are used as a fish poison, while unripe fruits of the Asiatic Diospyros oleifera produce persimmon oil for waterproofing (Nafiu et al., 2013). Diospyros abyssinica, often referred to as kôforonto and baforonto, is a species found in Mali and other parts of southern Africa that belongs to the Ebenaceae family. Moreover, it is also found in Angola, Guinea, Eritrea, and Ethiopia. Ayurvedic, African, and Chinese traditional medicine are just a few conventional medical systems using trees. Almost every component of these plants has been employed in medicine in some capacity, including as an astringent and a treatment for troubled digestion. In Zimbabwe, root juice from Albizia lebbeck is mixed with the bark and leaves of D. abyssinica to treat snakebites. The triterpenoids, such as betulin, betulinic acid, and lupeol, are the chief ingredients isolated from D. abyssinica. These are all well‐known anti‐inflammatory substances. The usage of this species in traditional medicine demonstrates that it has excellent therapeutic significance. The antioxidant activity of D. abyssinica root bark has been investigated. It extracted dichloromethane, petroleum ether, 80% aqueous ethanol, chloroform, and water (at 50°C and 100°C). It was discovered that the root bark of D. abyssinica is the most abundant source of extracted chemicals, with antioxidants making up 36.7% of the weight of the plant material. The 80% ethanol and methanol extracts showed the highest level of radical scavenging action for D. abyssinica. Thus, this plant appears to be an incredible source of antioxidants (Rauf et al., 2017).
Although Diospyros plants are used for edible produce, valuable wood, and decorative purposes, many folk medicinal techniques have also used the plant parts of many different species to treat bleeding, incontinence, sleeplessness, hiccoughs, diarrhea, etc. Several species of this genus have been used to isolate phytochemical components, such as polyphenols, terpenoids, lupanes, hydrocarbons, ursanes, tannins, and lipids, as well as benzopyrones, oleananes, naphthoquinones, and taraxerones. In vitro, in vivo, and clinical studies have demonstrated the biological efficacy of these plants as antimicrobial, antioxidant, analgesic, inflammatory, antidiabetic, thermogenic, anthelmintic, and enzyme‐inhibiting agents. This genus is a rich source of pharmacologically relevant components and expedites drug discovery. The edible fruit‐yielding varieties are D. kaki (Oriental persimmon), D. virginiana (North American persimmon), D. digyna (black sapote), D. lotus (date‐plum), and Diospyros rhombifolia (princess persimmon). The copper, calcium, iron, potassium, sodium, magnesium, and zinc elements are all present in D. kaki fruit mineral profiles (Park et al., 2017). Black ebony (D. ebenum and Diospyros melanoxylon) and striped ebony are the species that produce lumber (Diospyrus celebica and Diospyrus muns). Certain species, including D. crassiflora, are carved into wood for ornamental purposes.
The Diospyros species are widely used in traditional medicine in the tropical areas. As a tonic, powder, and poultice, fruits, barks, leaves, hardwoods, and roots have been used to treat a variety of ailments, including asthma, dermatitis, hypertension, atherosclerosis, lumbago, biliousness, sleeplessness, and bleeding, among others. This substance has several common uses, including as astringent, as laxative for constipation, carminative, sedative, febrifuge, antihypertensive, vermifuge, and antidiuretic (Carrillo et al., 2018). For instance, the traditional Chinese medicine (TCM) drug NaoXinQing, a standardized extract of D. kaki leaves, treats neurological disorders (Dobosz & Dzieciątkowski, 2019). Similarly, Diospyros lycioides is among the several medicinal plants utilized in Botswana to treat HIV/AIDS (human immunodeficiency virus/acquired immunodeficiency syndrome) (Marabini et al., 2020); in Nigeria, D. mespiliformis roots have been used as antimalarial agents (Chang et al., 2019). These plants are mentioned as traditional remedies in pharmacopoeia and medical texts. The identification of numerous bioactive components from this genus, however, has been made possible by bioassay‐guided fractionation using a variety of chromatographic methods, including gas chromatography (GC), high‐performance liquid chromatography (HPLC), and capillary electrophoresis (CE), as well as metabolomics methods like mass spectrometry (MS) and nuclear magnetic resonance (NMR) (Alex et al., 2012; Gopal et al., 2011). Such potent therapeutics include naphthoquinones (diospyrin, plumbagin, 8‐hydroxyisodiospyrin, and ebenone) (De Wet & Ngubane, 2014; Philander, 2011), anthraquinones, terpenoids (lupane, taraxerol, ursane, oleanane, and lupeol), steroids, lignans, flavonoids (myricetin), naphthalene (diospyrol), phenolic acids (gallic acid, diospyric acid, ellagic acid, ursolic acid, betulinic acid, and maslinic acid), 7‐methyljuglone, and amyrin (Rajesh et al., 2017). Moreover, research using binding energy and protein–ligand docking has improved the screening of therapeutic candidates and provided insight into the biological mechanisms.
3. PHYTOCHEMISTRY OF GENUS DIOSPYROS
Secondary metabolites, anthraquinones, terpenoids, steroids, and tannins, were identified in phytochemical analyses of extracts from the roots, leaves, bark, and hardwood fractions of the Diospyros lotus. Due to numerous bioactive chemical components, the plant's medicinal value can be correlated. The plant's crude methanolic extract contained both polar and nonpolar phytoconstituents. The plant is used as a folk remedy due to bioactive phytoconstituents that require an additional study. In the present investigation, the crude methanolic extract of D. lotus roots contains all the polar and nonpolar constituents found in the roots. The roots have no record of the presence of anthraquinones, terpenoids, or steroids. According to the literature, the genus Diospyros shows pesticidal and biological activities (Chopra & Nayar, 1956; Ghias et al., 2011; Kirtikar & Basu, 1918; Pérez‐Valera et al., 2024). The principal components extracted from Diospyros species are triterpenes and related steroid derivatives. Chopra and Nayar (1956) identified the triterpenes betulinic acid, betulin, and ursolic acid isolated from a dichloromethane extract of Diospyros leucomelas Poir. leaves using 1H‐ and 13C‐NMR spectroscopy. Yoshihira et al. (1970) isolated four naphthoquinones (7‐Methyljugulone, isodiospyrin, mamegakinone, and 7‐methyljugulone tetramer) and three triterpenoids (betulinic acid, taraxerol, and oxallobetulin) from a chloroform extract of D. lotus (L.) root (Kirtikar & Basu, 1918).
Further research is required to ascertain whether the unique activity of Diospyros is due to the presence of anthraquinones, terpenoids, or steroids. Traditional medicine uses anthraquinones, terpenoids, and steroids in the root extract. The quantity of extract extracted from the leaves was negligible compared to the roots. Terpenoids and tannins were identified in crude leaf extracts prepared with methanol, ethyl acetate, and chloroform, whereas bark extracts contained terpenoids, steroids, and anthraquinones. Although no research has been documented, the presence of these compounds in the bark may have anti‐inflammatory benefits. In addition to anthraquinones, terpenoids, and steroids, hardwood extracts contained anthraquinones (Jeffreys et al., 1983). Based on spectral analysis, a new triterpene was isolated from the fruit of D. peregrina and identified as lup 20(29)‐en‐3a, 27 diol (Maridass, 2008). Maridass (2008) investigated the chemical composition of Diospyros malabarica Desr. fruit oil using capillary GC and GC/MS experiments. More than 35 isolated components were effectively named beta‐bisabolene (β‐bisabolene) (25.86%) and trans‐methyl isoeugenol (31.86%) (del Carmen Recio et al., 1995). Several anti‐inflammatory compounds, including ursolic acid, betulin, and betulinic acid, are extracted from Diospyros leucomelas (Xiu‐Zhen et al., 1989). Isodiospyrin, beta‐amyrin (β‐amyrin), olean‐12‐en‐3‐one, and Bi‐naphthoquinone (Alake, 1994) are responsible for cytotoxicity in Diospyros morrisiana (Alake, 1994). D. tricolor was used to isolate diosquinone, an antibacterial compound (Loder et al., 1957). Due to phenolic compounds, Diospyros mollis is an effective anthelmintic (Chen et al., 2024; Marston et al., 1984). 7‐Methyljuglone, mamegakinone, and isodiospyrin are antifungal and molluscicidal compounds found in Diospyros usambarensis (Bouzayani et al., 2022). For phytochemical analysis of the plant's roots, stems, bark, leaves, and unripe fruit, D. malabarica extracts were prepared using water, methanol, ethanol, ethyl acetate, dichloromethane, and petroleum ether. All plant extracts were analyzed qualitatively for phytochemical constituents, such as flavonoids, tannins, terpenoids, and saponins, and quantitatively for total flavonoid content (TFC) and total phenol content (TPC). Table 1 shows phytochemical and chemical structures present in different species of Diospyros.
TABLE 1.
Phytochemicals and their chemical structures.
| Species | Class of phytochemical | Phytochemicals | Chemical structures |
|---|---|---|---|
| D. lotus | Pentacyclic triterpene | 1. Betulinic acid |
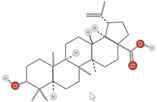
|
| 2. Taraxerol |

|
||
| D. leucomelas | Pentacyclic triterpene | 1. Betulinic acid |
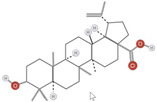
|
| 2. Betulin |
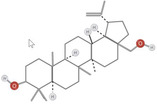
|
||
| 3. Ursolic acid |
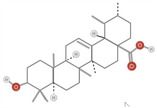
|
||
| D. peregrina | Pentacyclic triterpene | 1. Lup 20(29)‐ene‐3beta, 27 diol |

|
| D. malabarica | Sesquiterpenoids | 1. β‐Bisabolene |
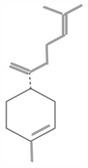
|
| Dimethoxybenzenes | 2. Trans‐methyl isoeugenol |
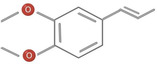
|
|
| D. morrisiana | Naphthoquinone | 1. Isodiospyrin |
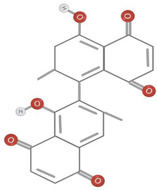
|
| Pentacyclic triterpene | 2. β‐Amyrin |
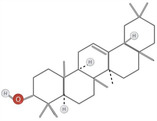
|
|
| 3. Olean‐12‐en‐3‐one |
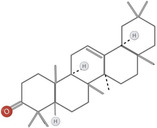
|
||
| Naphthoquinone | 4. Bi‐naphthoquinone |
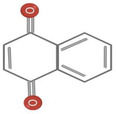
|
|
| D. tricolor | Naphthoquinone epoxide | 1. Diosquinone |
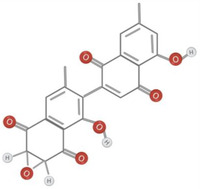
|
| D. usambarensis | Naphthoquinones | 1. Methyljuglone |
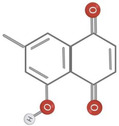
|
| 2. Mamegakinone |
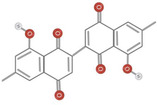
|
||
| 3. Isodiospyrin |
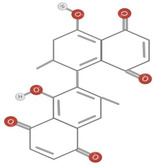
|
The presence of terpenoids, tannins, saponins, and flavonoids in D. malabarica was confirmed by qualitative phytochemical analysis employing a variety of solvents. In the phytochemical analysis, the blue–black color indicated the presence of tannins, while the frothy and yellow color indicated the occurrence of flavonoids and saponins, respectively. The eventual development of a reddish‐brown hue on the inner aspect of the leaf stated that the plant contained terpenoids. This phytochemical analysis confirmed that the various D. malabarica plant parts examined had a high concentration of flavonoids, crucial to the plant's antioxidant capacity (Alao et al., 2022). Similar phytochemicals, including terpenoids, saponins, and tannins, demonstrate that plants can inhibit the proliferation of bacteria and fungi. Likewise, saponins possess an anti‐obesity healing perspective (Sharifi‐Rad, 2016). Plants contain phytochemical substances that have been isolated and used to treat various health conditions and in the commercial production of dietary supplements and other nutrients. There were individual biological responses exhibited by each phytochemical, which could increase the likelihood of discovering new antibacterial components (Hassan & Ullah, 2019). Generally, phytochemicals positively affect human health, such as protection against disease, detoxification, infection, inflammation, and oxidation (Abdulhafiz et al., 2022). The significant in vitro antioxidant capacity of the ethanol extract of D. malabarica bark has been attributed to phytochemicals such as terpenoids and flavonoids. Priority research is being conducted on phytochemicals for dietary supplements and natural medications (Riaz et al., 2016). TPC and TFC values were also highest in methanol, suggesting that D. malabarica contains more polar phenolic compounds.
However, compared to other examined D. malabarica extracts, the bark extract contained the most significant concentrations of TFC and TPC. The phytochemical content (polyphenol, tannins, and flavonoids) is used to measure antioxidant capacity, and the results indicated that methanol bark extract, among other extracts, may be more effective in fighting free radicals (Chhetry et al., 2022; Othman et al., 2019). Polyphenols have been identified as the most potent antiviral, anti‐inflammatory, antioxidant, and antimicrobial agents among all phytochemicals (Ghosh et al., 2022). Antimicrobial and anti‐inflammatory properties and the ability to suppress tumor cell proliferation have been attributed to TFC and TPC. Previous research has also shown that phenolic chemicals play a significant role in D. malabarica's high antioxidant activity (Ali et al., 2022). Flavonoids are essential polyphenols for antibiotic activity because only flavonoids form complexes with microbial proteins, cell walls, and numerous other biologically active components (Zreen et al., 2022).
4. ETHNOPHARMACOLOGICAL ASPECTS OF GENUS DIOSPYROS
Ethnopharmacology is the key to discovering new primitive medications from medicinal plants. Abd El Halim reports that the preponderance of Diospyros species is found in tropical regions (Abd El Halim et al., 2014). All plant portions, including leaves, twigs, fruits, hardwood, barks, and roots, have been used to treat abdominal pain, asthma, dysentery, whooping cough, leprosy, menstrual problems, and dermatitis (Rauf et al., 2017).
Organs from various Diospyros species have been used in alternative medicine for decades. Ancient Chinese medical texts describe using persimmon leaves as a remedy, a nutritious drink, and a cosmetic ingredient, among other applications. There have been reports of the treatment of bleeding, burns, cardiovascular disease, chronic leg ulcers, internal bleeding, hematemesis, increased salivation, frostbite, lung distension, and snakebites (Xie et al., 2015; Zhang et al., 2017). The NaoXinQing tablet (a persimmon leaf extract tablet) has been registered as a trademark and included in the Chinese Pharmacopoeia for treating cerebral arteriosclerosis (Xie et al., 2015). In addition, the frosted leaves were described as an antihypertensive agent in traditional Japanese medicine, and the tea was widely consumed due to its antiaging properties, which are directly related to its high vitamin C content (Xie et al., 2015).
The bark of the well‐known species D. discolor was traditionally used to treat congestion, dysentery, diarrhea, and fever (Akter & Sarker, 2015). Other species, such asD. peregrina, were utilized to develop remedies for cholera, dysentery, diarrhea, diabetes, oral ulcers, and wounds (Saini et al., 2015). The unripe fruit juice of Diospyros blancoi is used as a natural remedy for diarrhea and as a first aid treatment for ulcers, and the bark, leaves, and roots are used to treat respiratory disorders and skin conditions, such as dermatitis (Khalipha et al., 2012; Morton, 1987). The stem and bark of D. blancoi were utilized as antioxidants, free radical scavengers, and anticancer agents (Khan et al., 2016). In Nigeria, it was reported that the roots of D. mespiliformis were used to treat malaria, and the bactericidal activity of its leaves and bark was observed. Diospyros lycioides has been used in Botswana for HIV/AIDS treatment (Rauf et al., 2017).
5. ANTICANCER POTENTIAL OF GENUS DIOSPYROS
Cancer has numerous causes and is exceedingly difficult to treat. Although toxicity to healthy cells should be avoided, it is essential for eliminating malignant cells. Diospyros species may prove beneficial in this context. Diospyrin and 8‐hydroxyisodiospyrin from D. lotus demonstrated remarkable anticancer activity. However, cancer is a heterogeneous, multifactorial complex disease, which the Diospyros compounds might not control (Rauf et al., 2017).
Han et al. reported that persimmon leaves inhibit nitrosamine‐induced squamous epithelial hyperplasia and malignancy in rats (Han et al., 1983). Park discovered an antitumor activity of persimmon leaves (Park et al., 1996). In A549 adenocarcinoma cells, persimmon leaf extract (PLE) and its galloylated homologs, such as PLEg (2"‐galloyl moiety), significantly increased the cytotoxicity of doxorubicin (DOX) by decreasing phosphorylation of the G2/M checkpoint (Kawakami et al., 2011). Different cancer cell lines were used to assess the anticancer potentials of an n‐hexane extract of Diospyros maritima Blume stems and were found to be very effective against all studied cell lines (Kuo et al., 1997). The diterpenoid diosmarioside D, isolated from methanol extracts of D. maritima leaves, exhibited potent cytotoxic activity against the lung adenocarcinoma (A549) cell line (Kawakami et al., 2018). Using an established NIH (National Institutes of Health) method, the cytotoxic potential of diosquinone, a naphthoquinone epoxide previously isolated from the bark of D. mespiliformis and D. tricolor, was assessed against 10 cancer cell lines. Diosquinone was found to be highly effective against a variety of cancer cell lines (Adeniyi et al., 2003). The IC50 (half‐maximal inhibitory concentration) values for the leaves, stem, and biosynthesized nanoparticles of D. villosa demonstrated their high cytotoxicity, revealing a potent inhibitory effect on the development of MCF‐7 and A549 cells (Table 2) (Adu et al., 2023).
TABLE 2.
Anticancer potential of Genus Diospyros along with their proposed modes of action.
| Species | Type of cancer | Cell lines | Part of plant | ED50 | Mode of action | References |
|---|---|---|---|---|---|---|
| D. lotus | Human breast cancer | MCF‐7 | Roots | 2.5–20 μM | Exhibits oxidative stress‐dependent apoptosis; The increment in cytosolic calcium [Ca(2+)](c) which leads to the apoptotic cell death triggered by diospyrin diethylether | (Rauf et al., 2017) |
| D. kaki | Squamous epithelial hyperplasia | A549 | Leaves | 30 μg/mL | Reduces the phosphorylation of checkpoint proteins, such as structural maintenance of chromosome 1, checkpoint kinase 1, and p53 in DOX‐treated ataxia telangiectasia mutated in a dose‐dependent manner | (Han et al., 1983) |
| D. maritima | Hepatoma | HEPA‐3B | Stems | 1.72 μg/mL | Cell growth inhibition | (Kawakami et al., 2018) |
| Cervix carcinoma | HELA | 1.92 μg/mL | ||||
| Colon carcinoma | COLO‐205 | 2.24 μg/mL | ||||
| Nasopharyngeal carcinoma | KB | 1.85 μg/mL | ||||
| Lung carcinoma | A549 | Leaves | 0.5 mg/mL | |||
| D. mespiliformis and D. tricolor | Human breast cancer | BC‐1 | Root bark | 0.2 μg/mL | Inhibitory action against human cancer cell lines through induction of apoptosis and cell cycle arrest | (Adeniyi et al., 2003) |
| Human fibrosarcoma | HT | 0.2 μg/mL | ||||
| Human lung cancer | LU‐1 | 0.2 μg/mL | ||||
| Human colon cancer | COL‐2 | 3.1 μg/mL | ||||
| Human nasopharyngeal carcinoma | KB | 0.2 μg/mL | ||||
| Viblastin non‐resistance human nasopharyngeal carcinoma | KB‐V(V + VLB) | 1 μg/mL | ||||
| Multiple drug resistance KB or viblastin‐resistant KB | KB‐V(V‐VLB) | 1.7 μg/mL | ||||
| Hormone‐dependent human prostrate cancer | LNCaP | 4.5 μg/mL | ||||
| Human glioblastoma | U373 | 0.18 μg/mL | ||||
| Human neuroblastoma | SKNSH | 0.2 μg/mL | ||||
| D. villosa | Human breast cancer | MCF‐7 | Leaves | 0.17 μg/mL | Induces apoptosis in cancer cells | (Adu et al., 2023) |
| Stem | 0.16 μg/mL | |||||
| Nanoparticles | 2.03 μg/mL | |||||
| Human alveolar basal epithelial cancer | A549 | Leaves | 7.76 μg/mL | |||
| Stem | 10.67 μg/mL | |||||
| Nanoparticles | 7.13 μg/mL | |||||
| Human embryonic kidney | HEK293 | Leaves | 158.5 μg/mL | |||
| Stem | 45.1 μg/mL | |||||
| Nanoparticles | 4.77 μg/mL |
5.1. In vitro studies of genus Diospyros
The organic plant extract of Diospyros chamaethamnus has a potent antiproliferative effect on cancer cell lines, according to in vitro testing. With respective IC50 values of 29.12, 16.08, and 24.67 μg/mL against UACC62 (melanoma cell line), TK10 (renal cell cancer), and MCF‐7 cells, D. chamaethamnus was the most potent (Dushimemaria et al., 2017). Diospyros quercina can be widely utilized in traditional Malagasy cancer treatment. D. quercina crude extract is an effective cytotoxic agent against P388 lymphocytic leukemia cell lines (Ruphin et al., 2014). The antiproliferative activity of D. lotus extract and isolated compounds against the inhibition of cell proliferation in nine human cancer cell lines, namely A375, ACHN (renal cell adenocarcinoma cell line), A549, CaCo‐2 (human colorectal adenocarcinoma cells), COR‐L23 (lung large cell carcinoma), MCF‐7, Huh‐7D12 (human hepatoma cell lines), and LNCaP (Lymph Node Carcinoma of the Prostate), was compared to the antiproliferative activity of one regular cell line, 142BR (Table 3). There was a dose–response relationship for each of the examined samples. With an IC50 of 12.20 μg/mL, D. lotus extract displayed the most significant inhibitory efficacy against COR‐L23 (Loizzo et al., 2009).
TABLE 3.
Summary of various in vitro, in vivo, and in silico studies related to Genus Diospyros.
| Species | Cell line | IC50 (μg/mL) | Potentials | References |
|---|---|---|---|---|
| In vitro studies | ||||
| D. chamaethamnus (organic extract) | UACC62 | 29.12 | Antiproliferative effect | (Dushimemaria et al., 2017) |
| TK10 | 16.08 | |||
| MCF‐7 | 24.67 | |||
| D. quercina (crude extract) | P388 | 0.851 ± 0.050 | Cytotoxic effect | (Ruphin et al., 2014) |
| D. lotus (methanolic extract) | A375 | 61.1 ± 2.5 | Antiproliferative effect | (Loizzo et al., 2009) |
| ACHN | >100 | |||
| A549 | 48.6 ± 2.2 | |||
| CaCo‐2 | 47.1 ± 2.8 | |||
| COR‐L23 | 12.2 ± 1.1 | |||
| MCF‐7 | >100 | |||
| LNCaP | >100 | |||
| 142BR | >100 | |||
| D. fleuryana (ethanolic extract) | KB | 15.8 | Cytotoxic effect | (Alex et al., 2012) |
| Hep | 29.75 | |||
| Lu | 53.33 | |||
| MCF‐7 | 60.23 | |||
| D. peregrina (methanolic extract) | MCF‐7 | 53.32 | Cytotoxic effect | (Ha et al., 2020) |
| Hep G2 | 79.95 | |||
| D. kaki (crude extract) | HCT116 | 27.22 | Cytotoxic effect | (Chen et al., 2020) |
| A549 | 56.85 | |||
| Hep G2 | 12.78 | |||
| BT20 | 26.66 | |||
| U2OS | 48.72 | |||
| MDB‐MA‐321 | 19.33 | |||
| D. montana (methanolic extract) | Hep G2 | 22 | Anticancer effect | (Sujatha et al., 2023) |
| In vivo studies | ||||
| D. kaki | Human hepatocellular carcinoma | Flavonoids | Inhibits their proliferation via the PDGFR–Rac–JNK pathway | (Kim et al., 2020) |
| D. kaki | Mouse skin carcinogenesis | Naphthoquinone derivatives | Inhibitory effect on DMBA–TPA | (Kapadia et al., 1997) |
| D. kaki | Human lung non‐small carcinoma cells | Kaempferol | Apoptosis of NCI‐H460 | (Leung et al., 2007) |
| D. blancoi | Mice Ehrlich ascites carcinoma cells | Saponins, triterpenes, tannins | Inhibition of EAC cells‐induced tumor‐bearing mice | (Howlader et al., 2012) |
| Species | Phytochemical | Docking score kcal/mol | Target protein | Protein residues | |
|---|---|---|---|---|---|
| In silico studies | |||||
| D. kaki | Acarbose | −7.4615 | 6CCY | GLU234, GLU278, and ASP292, ASN79; PHE161 and HIS194 LYS276 and LYS189 | (Muhammad et al., 2022) |
| D. kaki | Quercetin 3‐O‐glucoside | −6.86404133 | 6CCY | GLU234; ASP291. LYS158 and GLU278 | (Muhammad et al., 2022) |
Rauf and colleagues discovered that the traditional medicinal plant D. lotus possesses significant cytotoxic activity and can be an effective anticancer medication (Rauf et al., 2021). The phytochemical analysis of the ethyl acetate (EtOAc) extract of Diospyros fleuryana leaves resulted in the isolation of 8′‐hydroxyisodiospyrin, a compound with anticancer potential (Alex et al., 2012). In both the Hep G2 (hepatocellular carcinoma) and MCF‐7 cell lines, the dichloromethane fraction of D. peregrina fruit extract exhibited the highest cytotoxicity (Ha et al., 2020). D. kaki inhibited the proliferation of human breast, colorectal, and hepatic cancer cells in vitro by inducing apoptosis and oxidative stress (Chen et al., 2020). Increased DNA damage, autophagy, and decreased mitochondrial membrane potential all contribute to the potent anticancer effect of silver oxide nanoparticles (Ag2ONPs) synthesized from the methanolic bark extract of Diospyros montana against hepatocellular carcinoma (Hep G2) cells (Sujatha et al., 2023).
5.2. In vivo studies of genus Diospyros
Hazara and coworkers examined the anticancer effects of diospyrin and its derivatives on 13 cancer cell lines. It was discovered that the acetylamine derivative increased cytotoxicity in HT‐29 colon cancer cells in particular. The findings suggest that cell death may involve mitochondrial pathways (Akter & Sarker, 2015). Park and colleagues' in vivo experiments on Sarcoma‐180 cells revealed the anticancer activity of D. kaki leaves (Park et al., 1996). D. kaki induces the apoptosis of cancer cells and inhibits their proliferation via the PDGFR–Rac–JNK (platelet‐derived growth factor receptor–Rac–c‐Jun N‐terminal kinase) pathway (Table 3) (Kim et al., 2020). In a two‐stage in vivo mouse skin carcinogenesis experiment, naphthoquinone derivatives, such as naphthazarin and juglone, exhibited potent inhibitory effects on promoting DMBA–TPA (7,12‐dimethylbenz(a)anthracene–TPA) tumors (Kapadia et al., 1997). The antioxidant system is activated by kaempferol, a pharmacologically active component of D. kaki, leading to apoptosis in human lung non‐small carcinoma cells (Leung et al., 2007; Nuzzo et al., 2022). Howlader and associates discovered cytotoxic effects in a D. blancoi ethanol extract in 2012 (Howlader et al., 2012). Using Ehrlich ascites carcinoma cells, Khan and coworkers evaluated the in vivo anticancer activity of D. blancoi and found that D. blancoi extracts showed substantial cytotoxic activity (Khan et al., 2016).
5.3. In silico studies of genus Diospyros
In silico docking is a useful method in computational drug discovery. It involves modeling the interactions between smaller molecules (drugs) and target protein receptors or enzymes to predict their binding affinity (Raza, Ahmad, et al., 2017; Raza, Jiang, et al., 2017; Raza, Khan, et al., 2017; Raza, Wei, et al., 2017). The extent of these interactions can affect the biosafety, pharmacological response, delivery rate, therapeutic efficiency, and the design of new drugs (Raza, Ahmad, et al., 2017; Raza, Jiang, et al., 2017; Raza, Khan, et al., 2017; Raza, Wei, et al., 2017). In silico investigations on the target enzymes validated the results of in vitro experiments, studies of binding orientation, and ligand–enzyme interactions. Muhammad and colleagues tested the effectiveness of D. kaki polyphenols in inhibiting AKT1 (AKT Serine/Threonine Kinase 1) (6CCY)‐driven cancer growth. The bioactive chemicals from D. kaki were found to inhibit AKT1 in an in silico analysis, suggesting they may have anticancer potential (Muhammad et al., 2022). Muhammad and colleagues examined the efficacy of D. kaki polyphenols in combating the cancer‐causing protein AKT1 (6CCY). In an in silico study, bioactive compounds derived from D. kaki were shown to inhibit AKT1, suggesting they have anticancer potential (Rauf et al., 2016). Novel anticancer dimeric naphthoquinones derived from D. lotus exhibit significant anticarcinogenic activity due to their superior docking statistics compared to the norm (Rauf et al., 2021).
6. CLINICAL STUDIES
Human body extensively uses ligands such as natural compounds from plants which have brilliant affinities toward transporter proteins like human serum albumin, lipoprotein, and glycoprotein (Raza, Ahmad, et al., 2017; Raza, Jiang, et al., 2017; Raza, Khan, et al., 2017; Raza, Wei, et al., 2017). Drug resistance has become a critical global issue. Consequently, the discovery of latest, less lethal drugs provides a basic and urgent implication in the field of drug discovery (Raza, Ahmad, et al., 2017; Raza, Jiang, et al., 2017; Raza, Khan, et al., 2017; Raza, Wei, et al., 2017). In their study, Kaushik and colleagues found that by inhibiting the proliferation of regulatory T (Treg) cells in the microenvironment of breast and lung cancer tumors, D. peregrina significantly boosts the immune system's ability to protect against these diseases (Kaushik et al., 2022). Roy et al. demonstrated that D. peregrina‐mediated immunomodulation of lymphocytes isolated from the blood of breast cancer patients promotes lymphocytic proliferation, induces type 1 cytokines (IL‐12 (interleukin‐12), IFN‐γ (interferon gamma)), and produces the tumor‐killing agent nitric oxide (NO) (Roy et al., 2021). Harun Al Rashid and his colleagues investigated the anticancer properties of Diospyros melanoxylon Roxb. They discovered that ursolic acid isolated from D. melanoxylon inhibits cancer development, progression, and metastasis in multiple cancer types, making it an effective cancer prevention and treatment agent (Al Rashid et al., 2017). D. malabarica fruit preparation (DFP) upregulates the expression of type 1 specific cytokines and enhances tumor suppression by modulating several epigenetic markers to elicit a protective immune response against tumors (Bhootra et al., 2023).
7. MOLECULAR MECHANISM
Gewirtz hypothesized in 1999 that the primary lethal mechanism of the quinonoid class of synthetic substances is bioreduction, followed by interaction with molecular oxygen and the formation of reactive oxygen species (ROS) (Gewirtz, 1999). Kayashima and colleagues first proposed apoptosis as a form of cell death caused by reactive oxygen species (ROS) in 2009 (Kayashima et al., 2009). It is becoming increasingly apparent that reactive oxygen species (ROS) play a crucial role in mediating the apoptotic process, which quinonoid compounds employ as an anticancer strategy (Lee et al., 2012). Kumar et al. demonstrated that the bisnaphthoquinonoid derivative diospyrin diethylether induces apoptosis in response to oxidative stress in various human cancer cell lines and tumor models. It was discovered that diospyrin diethylether induces apoptosis in MCF‐7 human breast cancer cells; consequently, the researchers examined the effect of an increase in cytosolic calcium [Ca(2+)] (c) on this process. In addition, they have cast light on the redox signaling induced by diospyrin diethylether, which integrates calcium‐dependent calpain/caspase 12 activation and mitochondrial changes to highlight the induction of apoptotic cell death (Kumar et al., 2012). According to studies, plumbagin inhibits angiogenesis and tumor formation in human umbilical vein endothelial cells (HUVECs) in vitro and mouse models of human colon carcinoma and prostate cancer. Following vascular endothelial growth factor receptor‐2 (VEGFR2) activation, the rat sacroma (Ras) signaling pathway was the intervening mechanism (Lai et al., 2012). According to Nguyen and colleagues’ findings, kaempferol could induce apoptosis in the lung cancer cell line (A459) and inhibit the mitogen‐activated protein kinase (MAPK) phosphorylation pathway and c‐Fos and nuclear factor of activated T cells (NFATc) in bone marrow cells (Nguyen et al., 2003). In U‐2OS human osteosarcoma cells, kaempferol could significantly inhibit the mRNA expression levels of extracellular signal‐regulated kinase (ERK), JNK, and p38 proteins. Phosphatidylinositol‐3‐kinase (PI3K) direct binding efficacy of kaempferol in inhibiting PI3K/Akt (protein kinase B) pathway, thereby inhibiting nuclear factor kappa B (NF‐κB) and activator protein 1 (AP‐1) activities, has been confirmed, suggesting its role in cellular functions, including angiogenesis and apoptosis (Choudhary et al., 2023).
8. CONCLUSION
The Diospyros genus is considered a promising source of potent anticancer constituents comprising diverse phytochemicals with inherent therapeutic properties. In this review, the detailed investigation underlines the multidimensional nature of this genus, covering not only its rich phytochemistry but also highlighting its significance against various cancers. The comprehensive evidence derived from multiple in vitro, in vivo, and in silico studies explained in this article reinforces the fact that Diospyros species possess several bioactive compounds, which can be further explored in different clinical trials to validate their use as cancer therapeutics. As the scientific community strives to bridge the gap between traditional knowledge and modern research, the Genus Diospyros may provide a new paradigm in nature's pharmacopoeia, justifying sustained exploration and scrutiny for its invaluable contributions to anticancer drug discovery.
AUTHOR CONTRIBUTIONS
Abdur Rauf: Conceptualization (lead); data curation (lead); investigation (lead); methodology (lead); validation (equal); visualization (equal); writing – original draft (lead); writing – review and editing (equal). Zuneera Akram: Conceptualization (equal); investigation (supporting); methodology (supporting); validation (supporting); visualization (equal); writing – review and editing (supporting). Nabia Hafeez: Investigation (supporting); methodology (supporting); validation (equal); visualization (equal); writing – review and editing (supporting). Anees Ahmed Khalil: Conceptualization (supporting); data curation (supporting); investigation (equal); methodology (equal); validation (equal); visualization (supporting); writing – original draft (equal); writing – review and editing (equal). Ahood Khalid: Investigation (supporting); methodology (supporting); validation (supporting); visualization (equal); writing – review and editing (supporting). Zoya Abid: Methodology (supporting); validation (supporting); visualization (supporting); writing – review and editing (supporting). Hassan A. Hemeg: Data curation (supporting); investigation (supporting); methodology (supporting); validation (equal); visualization (equal); writing – review and editing (supporting). Abdullah S. M. Aljohani: Investigation (supporting); methodology (supporting); validation (equal); visualization (equal); writing – review and editing (supporting). Waleed Al Abdulmonem: Investigation (supporting); methodology (supporting); validation (equal); visualization (equal); writing – review and editing (supporting). Mohammed Mansour Quradha: Conceptualization (equal); investigation (supporting); methodology (supporting); validation (equal); visualization (equal); writing – original draft (supporting); writing – review and editing (supporting).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
Authors would like to thank the University of Lahore and University of Swabi for their support and help during the drafting and finalizing of this manuscript.
Rauf, A. , Akram, Z. , Hafeez, N. , Khalil, A. A. , Khalid, A. , Abid, Z. , Hemeg, H. A. , Aljohani, A. S. M. , Al Abdulmonem, W. , & Quradha, M. M. (2024). Anticancer therapeutic potential of genus Diospyros: From phytochemistry to clinical applications—A review. Food Science & Nutrition, 12, 7033–7047. 10.1002/fsn3.4375
Contributor Information
Abdur Rauf, Email: mashaljcs@yahoo.com.
Anees Ahmed Khalil, Email: aneesahmedkhalil@gmail.com.
Mohammed Mansour Quradha, Email: mm.quradh@seiyunu.edu.ye.
DATA AVAILABILITY STATEMENT
The dataset supporting the conclusions of this article is included within the report.
REFERENCES
- Abd El Halim, A. M. , Hafeez, R. H. , & Safwat, A. A. (2014). Taxonomic revision of Ebenaceae in Egypt. Current Science International, 3(4), 414–425. [Google Scholar]
- Abdulhafiz, F. , Reduan, M. F. H. , Hamzah, Z. , Kari, Z. A. , Dawood, M. A. , & Mohammed, A. (2022). Acute oral toxicity assessment and anti‐hyperuricemic activity of Alocasia longiloba extracts on Sprague‐Dawley rats. Saudi Journal of Biological Sciences, 29(5), 3184–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeniyi, B. A. , Robert, M. F. , Chai, H. , & Fong, H. H. S. (2003). In vitro cytotoxicity activity of diosquinone, a naphthoquinone epoxide. Phytotherapy Research, 17(3), 282–284. [DOI] [PubMed] [Google Scholar]
- Adu, O. T. , Naidoo, Y. , Lin, J. , Dwarka, D. , Mellem, J. , Murthy, H. N. , & Dewir, Y. H. (2023). Cytotoxic potential of Diospyros villosa leaves and stem bark extracts and their silver nanoparticles. Plants, 12(4), 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter, S. , & Sarker, A. (2015). Antimicrobial activities of seeds of Diospyros blancoi and Baccuarea ramiflora . International Journal of Advances in Pharmacy, Biology and Chemistry, 4(1), 789–793. [Google Scholar]
- Al Rashid, M. H. , Mandal, V. , Mandal, S. C. , & Amirthalingam, R. (2017). Diospyros Melanoxylon Roxb in cancer prevention: Pharmacological screening, pharmacokinetics and clinical studies. International Journal of Pharmacognosy, 4(7), 217–223. [Google Scholar]
- Alake, L. B. (1994). Antibacterial activity of diosquinone isolated from Diospyros tricolor . Planta Medica, 60(5), 477. [DOI] [PubMed] [Google Scholar]
- Alao, I. I. , Oyekunle, I. P. , Iwuozor, K. O. , & Emenike, E. C. (2022). Green synthesis of copper nanoparticles and investigation of its anti‐microbial properties. Advanced Journal of Chemistry‐Section B, 4(1), 39–52. [Google Scholar]
- Alex, A. T. , Nawagamuwa, N. H. , Joseph, A. , Rao, J. V. , Mathew, J. A. , & Udupa, N. (2012). In vitro anti‐cancer and anti‐oxidant activity of different fractions of Diospyros peregrina unripe fruit extract. Free Radicals and Antioxidants, 2(4), 45–49. [Google Scholar]
- Ali, A. , Parisi, A. , & Normanno, G. (2022). Polyphenols as emerging antimicrobial agents. In Emerging modalities in mitigation of antimicrobial resistance (pp. 219–259). Springer International Publishing. [Google Scholar]
- Anusewicz, D. , Orzechowska, M. , & Bednarek, A. K. (2020). Lung squamous cell carcinoma and lung adenocarcinoma differential gene expression regulation through pathways of notch, hedgehog, Wnt, and ErbB signalling. Scientific Reports, 10(1), 21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhootra, S. , Jill, N. , Rajak, R. , Shanmugam, G. , Rakshit, S. , Kannanthodi, S. , Thakkar, V. , George, M. , & Sarkar, K. (2023). Diospyros malabarica fruit preparation mediates immunotherapeutic modulation and epigenetic regulation to evoke protection against non–small cell lung cancer (NSCLC). Journal of Ethnopharmacology, 314, 116525. [DOI] [PubMed] [Google Scholar]
- Bouzayani, B. , Koubaa, I. , Frikha, D. , Samet, S. , Ben Younes, A. , Chawech, R. , Maalej, S. , Allouche, N. , & Mezghani Jarraya, R. (2022). Spectrometric analysis, phytoconstituents isolation and evaluation of in vitro antioxidant and antimicrobial activities of Tunisian Cistanche violacea (Desf). Chemical Papers, 76(5), 3031–3050. [Google Scholar]
- Bray, F. , Ferlay, J. , Soerjomataram, I. , Siegel, R. L. , Torre, L. A. , & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. [DOI] [PubMed] [Google Scholar]
- Carrillo, M. A. , Zhen, A. , & Kitchen, S. G. (2018). The use of the humanized mouse model in gene therapy and immunotherapy for HIV and cancer. Frontiers in Immunology, 9, 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, M. , Wang, M. , Wang, M. , Shu, M. , Ding, B. , Li, C. , Pang, M. , Cui, S. , Hou, Z. , & Lin, J. (2019). A multifunctional cascade bioreactor based on hollow‐structured Cu2MoS4 for synergetic cancer chemo‐dynamic therapy/starvation therapy/phototherapy/immunotherapy with remarkably enhanced efficacy. Advanced Materials, 31(51), 1905271. [DOI] [PubMed] [Google Scholar]
- Chen, L. I. , Guo, Y. , Alsaif, G. , & Gao, Y. (2020). Total flavonoids isolated from Diospyros kaki L. f. leaves induced apoptosis and oxidative stress in human cancer cells. Anticancer Research, 40(9), 5201–5210. [DOI] [PubMed] [Google Scholar]
- Chen, T. V. , Nghia, N. T. , Nhat Truong, L. V. , & Khanh Linh, N. H. (2024). Ethnomedicinal, phytochemical, and pharmacological properties of Diospyros mollis Griff.: A review. Natural Product Communications, 19(2), 1934578X241233459. [Google Scholar]
- Chhetry, A. K. , Dhakal, S. , Chaudhary, L. , Karki, K. , Khadka, R. B. , Chaudhary, G. P. , Bastola, T. , Poudel, A. , Aryal, P. , & Pandey, J. (2022). Study of antibacterial activity of root bark, leaves, and pericarp extracts of Diploknema butyracea and evaluation of prospective antioxidant activity. Journal of Tropical Medicine, 2022, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra, R. N. , & Nayar, S. L. (1956). Glossary of Indian medicinal plants. Council of Scientific and Industrial Research. [Google Scholar]
- Choudhary, R. , Singh, A. , Upadhyay, A. , Singh, R. , Thangalakshmi, S. , Dar, A. H. , Bajpai, V. K. , & Shukla, S. (2023). Exotic god fruit, persimmon (Diospyros kaki): Pharmacological importance and human health aspects. eFood, 4(1), e52. [Google Scholar]
- De Wet, H. , & Ngubane, S. C. (2014). Traditional herbal remedies used by women in a rural community in northern Maputaland (South Africa) for the treatment of gynaecology and obstetric complaints. South African Journal of Botany, 94, 129–139. [Google Scholar]
- del Carmen Recio, M. , Giner, R. M. , Manez, S. , Gueho, J. , Julien, H. R. , Hostettmann, K. , & Rios, J. L. (1995). Investigations on the steroidal anti‐inflammatory activity of triterpenoids from Diospyros leucomelas . Planta Medica, 61(1), 9–12. [DOI] [PubMed] [Google Scholar]
- Dickens, E. , & Ahmed, S. (2018). Principles of cancer treatment by chemotherapy. Surgery (Oxford), 36(3), 134–138. [Google Scholar]
- Dobosz, P. , & Dzieciątkowski, T. (2019). The intriguing history of cancer immunotherapy. Frontiers in Immunology, 10, 2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushimemaria, F. , Du Preez, C. I. , & Mumbengegwi, D. R. (2017). Randomized anticancer and cytotoxicity activities of Guibourtia coleosperma and Diospyros chamaethamnus . African Journal of Traditional, Complementary, and Alternative Medicines, 14(4), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz, D. (1999). A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochemical Pharmacology, 57(7), 727–741. [DOI] [PubMed] [Google Scholar]
- Ghias, U. , Abdur, R. , Bina, S. S. , & Syed, Q. S. (2011). Preliminary comparative phytochemical screening of Diospyros lotus Stewart. Middle‐East Journal of Scientific Research, 10(1), 78–81. [Google Scholar]
- Ghosh, A. , Sarmah, P. , Patel, H. , Mukerjee, N. , Mishra, R. , Alkahtani, S. , Varma, R. S. , & Baishya, D. (2022). Nonlinear molecular dynamics of quercetin in Gynocardia odorata and Diospyros malabarica fruits: Its mechanistic role in hepatoprotection. PLoS One, 17(3), e0263917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal, Y. V. , Ravindranath, A. , Kalpana, G. , Raju, A. B. , & Redddy, V. P. (2011). Antitumor activity of Diospyros peregrina on Ehrlich ascites carcinoma in mice. Journal of Scientific Research, 3(2), e95–e102. [Google Scholar]
- Ha, N. T. T. , Van Cuong, P. , Tra, N. T. , Van Tuyen, N. , Cham, B. T. , & Tung, H. H. (2020). Cytotoxic naphthoquinones from diospyros fleuryana leaves. Discovery Phytomedicine, 7(1), 42–46. [Google Scholar]
- Han, K. H. , Han, D. C. , & Zhang, Y. M. (1983). Pharmacological effect and clinical application of Diospyros kaki leaves. Research on Zhong Cheng Yao, 7, 27–28. [Google Scholar]
- Hassan, A. , & Ullah, H. (2019). Antibacterial and antifungal activities of the medicinal plant veronica biloba. Journal of Chemistry, 2019, 1–7. [Google Scholar]
- Howlader, M. S. I. , Sayeed, M. S. B. , Ahmed, M. U. , Mohiuddin, A. K. , Labu, Z. K. , Bellah, S. F. , & Islam, M. S. (2012). Characterization of chemical groups and study of antioxidant, antidiarrhoeal, antimicrobial and cytotoxic activities of ethanolic extract of Diospyros blancoi (family: Ebenaceae) leaves. Journal of Pharmacy Research, 5(6), 3050–3052. [Google Scholar]
- Jeffreys, J. A. D. , Bin Zakaria, M. , Waterman, P. G. , & Zhong, S. (1983). A new class of natural product: Homologues of juglone bearing 4‐hydroxy‐5‐methyl‐coumarin‐3‐yl units from diospyros species. Tetrahedron Letters, 24(10), 1085–1088. [Google Scholar]
- Kapadia, G. J. , Balasubramanian, V. , Tokuda, H. , Konoshima, T. , Takasaki, M. , Koyama, J. , Tagahaya, K. , & Nishino, H. (1997). Anti‐tumor promoting effects of naphthoquinone derivatives on short term Epstein‐Barr early antigen activation assay and in mouse skin carcinogenesis. Cancer Letters, 113(1–2), 47–53. [DOI] [PubMed] [Google Scholar]
- Kaushik, R. R. , Koranne, M. , Rao, M. S. , Rakshit, S. , Shanmugam, G. , George, M. , & Sarkar, K. (2022). Role of Diospyros peregrina fruit preparation in suppressing regulatory T (Treg) cells in the tumor microenvironment of breast and lung cancer. Phytomedicine Plus, 2(4), 100353. [Google Scholar]
- Kawakami, K. , Nishida, H. , Tatewaki, N. , Nakajima, Y. , Konishi, T. , & Hirayama, M. (2011). Persimmon leaf extract inhibits the ATM activity during DNA damage response induced by doxorubicin in A549 lung adenocarcinoma cells. Bioscience, Biotechnology, and Biochemistry, 75(4), 650–655. [DOI] [PubMed] [Google Scholar]
- Kawakami, S. , Nishida, S. , Nobe, A. , Inagaki, M. , Nishimura, M. , Matsunami, K. , Otsuka, H. , Aramoto, M. , Hyodo, T. , & Yamaguchi, K. (2018). Eight ent‐Kaurane Diterpenoid Glycosides named Diosmariosides A–H from the leaves of Diospyros maritima and their cytotoxic activity. Chemical and Pharmaceutical Bulletin, 66(11), 1057–1064. [DOI] [PubMed] [Google Scholar]
- Kayashima, T. , Mori, M. , Yoshida, H. , Mizushina, Y. , & Matsubara, K. (2009). 1, 4‐naphthoquinone is a potent inhibitor of human cancer cell growth and angiogenesis. Cancer Letters, 278(1), 34–40. [DOI] [PubMed] [Google Scholar]
- Khalipha, A. B. R. , Ahmed, F. , & Rahman, M. M. (2012). Antioxidant and antidiarrhoeal potentiality of Diospyros blancoi. International Journal of Pharmacology, 8(5), 403–409. [Google Scholar]
- Khan, M. A. , Rahman, M. M. , Sardar, M. N. , Arman, M. S. I. , Islam, M. B. , Khandakar, M. J. A. , Rashid, M. , Sadik, G. , & Alam, A. K. (2016). Comparative investigation of the free radical scavenging potential and anticancer property of Diospyros blancoi (Ebenaceae). Asian Pacific Journal of Tropical Biomedicine, 6(5), 410–417. [Google Scholar]
- Kim, H. S. , Suh, J. S. , Jang, Y. K. , Ahn, S. H. , Raja, G. , Kim, J. C. , Jung, Y. , Jung, S. H. , & Kim, T. J. (2020). Anti‐cancer potential of persimmon (Diospyros kaki) leaves via the PDGFR‐Rac‐JNK pathway. Scientific Reports, 10(1), 18119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtikar, K. R. , & Basu, B. D. (1918). Indian medicinal plants (Vol. 2). publisher not identified Basu, Bhuwaneśwari Âśrama.
- Kumar, B. , Kumar, A. , Ghosh, S. , Pandey, B. N. , Mishra, K. P. , & Hazra, B. (2012). Diospyrin derivative, an anticancer quinonoid, regulates apoptosis at endoplasmic reticulum as well as mitochondria by modulating cytosolic calcium in human breast carcinoma cells. Biochemical and Biophysical Research Communications, 417(2), 903–909. [DOI] [PubMed] [Google Scholar]
- Kuo, Y. H. , Chang, C. I. , Li, S. Y. , Chou, C. J. , Chen, C. F. , Kuo, Y. H. , & Lee, K. H. (1997). Cytotoxic constituents from the stems of Diospyros maritima . Planta Medica, 63(4), 363–365. [DOI] [PubMed] [Google Scholar]
- Lai, L. , Liu, J. , Zhai, D. , Lin, Q. , He, L. , Dong, Y. , Zhang, J. , Lu, B. , Chen, Y. , Yi, Z. , & Liu, M. (2012). Plumbagin inhibits tumour angiogenesis and tumour growth through the Ras signalling pathway following activation of the VEGF receptor‐2. British Journal of Pharmacology, 165(4b), 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Yeon, J. H. , Kim, H. , Roh, W. , Chae, J. , Park, H. O. , & Kim, D. M. (2012). The natural anticancer agent plumbagin induces potent cytotoxicity in MCF‐7 human breast cancer cells by inhibiting a PI‐5 kinase for ROS generation. PLoS One, 7, e45023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, H. W. C. , Lin, C. J. , Hour, M. J. , Yang, W. H. , Wang, M. Y. , & Lee, H. Z. (2007). Kaempferol induces apoptosis in human lung non‐small carcinoma cells accompanied by an induction of antioxidant enzymes. Food and Chemical Toxicology, 45(10), 2005–2013. [DOI] [PubMed] [Google Scholar]
- Loder, J. W. , Mongolsuk, S. , Robertson, A. , & Whalley, W. B. (1957). Diospyrol, a constituent of Diospyros mollis. Journal of the Chemical Society (Resumed), 430, 2233–2237. 10.1039/JR9570002233 [DOI] [Google Scholar]
- Loizzo, M. R. , Said, A. , Tundis, R. , Hawas, U. W. , Rashed, K. , Menichini, F. , Frega, N. G. , & Menichini, F. (2009). Antioxidant and antiproliferative activity of Diospyros lotus L. extract and isolated compounds. Plant Foods for Human Nutrition, 64, 264–270. [DOI] [PubMed] [Google Scholar]
- Marabini, L. , Melzi, G. , Lolli, F. , Dell'Agli, M. , Piazza, S. , Sangiovanni, E. , & Marinovich, M. (2020). Effects of Vitis vinifera L. leaves extract on UV radiation damage in human keratinocytes (HaCaT). Journal of Photochemistry and Photobiology B: Biology, 204, 111810. [DOI] [PubMed] [Google Scholar]
- Maridass, M. (2008). Phytochemicals from genus diospyros (L.) and their biological activities. Ethnobotanical Leaflets, 2008(1), 28. [Google Scholar]
- Marston, A. , Msonthi, J. D. , & Hostettmann, K. (1984). Naphthoquinones of Diospyros usambarensis; their molluscicidal and fungicidal activities. Planta Medica, 50(3), 279–280. [DOI] [PubMed] [Google Scholar]
- Morton, J. F. (1987). Fruits of warm climates. Creative Resource Systems, Inc. [Google Scholar]
- Muhammad, I. , Nayab, G. E. , Rahman, N. , Niaz, S. , Ali, A. , & Khan, H. (2022). Anticancer potential of Diospyros kaki (persimmon) polyphenols against AKT1 (6CCY): Docking based in silico study. PHYTONutrients, 1, 57–68. [Google Scholar]
- Nafiu, M. O. , Salawu, M. O. , & Kazeem, M. I. (2013). Antioxidant activity of African medicinal plants. In Medicinal plant research in Africa (pp. 787–803). Elsevier. [Google Scholar]
- Nguyen, T. T. T. , Tran, E. , Ong, C. K. , Lee, S. K. , Do, P. T. , Huynh, T. T. , Nguyen, T. H. , Lee, J. J. , Tan, Y. , Ong, C. S. , & Huynh, H. (2003). Kaempferol‐induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK‐MAPK. Journal of Cellular Physiology, 197(1), 110–121. [DOI] [PubMed] [Google Scholar]
- Nuzzo, G. , Senese, G. , Gallo, C. , Albiani, F. , Romano, L. , d'Ippolito, G. , Manzo, E. , & Fontana, A. (2022). Antitumor potential of immunomodulatory natural products. Marine Drugs, 20(6), 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman, L. , Sleiman, A. , & Abdel‐Massih, R. M. (2019). Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Frontiers in Microbiology, 10, 911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, K. Y. , Moon, S. H. , & Kim, K. H. (1996). Antitumor effect of persimmon leaves in vivo using sarcoma‐180 cells. Journal of the Korean Society of Food Science and Nutrition (Korea Republic), 25, 865–870. [Google Scholar]
- Park, S. B. , Park, G. H. , Song, H. M. , Son, H. J. , Um, Y. , Kim, H. S. , & Jeong, J. B. (2017). Anticancer activity of calyx of Diospyros kaki Thunb. Through downregulation of cyclin D1 via inducing proteasomal degradation and transcriptional inhibition in human colorectal cancer cells. BMC Complementary and Alternative Medicine, 17(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Valera, O. , Torres‐Martínez, R. , Nieto‐Camacho, A. , Valencia, I. , Javier Espinosa‐García, F. , & Delgado, G. (2024). Larvicidal activity against Spodoptera frugiperda of some constituents from two diospyros species. In silico pesticide‐likeness properties, acetylcholinesterase activity and molecular docking. Chemistry & Biodiversity, 21(2), e202301871. [DOI] [PubMed] [Google Scholar]
- Philander, L. A. (2011). An ethnobotany of Western cape Rasta bush medicine. Journal of Ethnopharmacology, 138(2), 578–594. [DOI] [PubMed] [Google Scholar]
- Piawah, S. , & Venook, A. P. (2019). Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer, 125(23), 4139–4147. [DOI] [PubMed] [Google Scholar]
- Rajesh, V. , Sophiya, J. , Jacob, S. , Arumugam, P. , & Jayaraman, P. (2017). Biosynthesis of silver nanoparticles using Diospyros ferrea (Willd.) bakh. Leaves and evaluation of its antioxidant, anti‐inflammatory, antimicrobial and anticancer activity. Journal of Bionanoscience, 11(1), 24–33. [Google Scholar]
- Rauf, A. , Khan, A. , Abu‐Izneid, T. , Alhumaydhi, F. A. , Bawazeer, S. , Raza, M. , Khan, H. , Patel, S. , & Al‐Harrasi, A. (2021). Novel anticancer dimeric naphthoquinones from Diospyros lotus having anti‐tumor, anti‐inflammatory and multidrug resistance reversal potential: In vitro, in vivo and in silico evidence. Anti‐Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry‐Anti‐Cancer Agents), 21(15), 2089–2097. [DOI] [PubMed] [Google Scholar]
- Rauf, A. , Uddin, G. , Khan, H. , Raza, M. , Zafar, M. , & Tokuda, H. (2016). Anti‐tumour‐promoting and thermal‐induced protein denaturation inhibitory activities of β‐sitosterol and lupeol isolated from Diospyros lotus L. Natural Product Research, 30(10), 1205–1207. [DOI] [PubMed] [Google Scholar]
- Rauf, A. , Uddin, G. , Patel, S. , Khan, A. , Halim, S. A. , Bawazeer, S. , Ahmad, K. , Muhammad, N. , & Mubarak, M. S. (2017). Diospyros, an under‐utilized, multi‐purpose plant genus: A review. Biomedicine & Pharmacotherapy, 91, 714–730. [DOI] [PubMed] [Google Scholar]
- Raza, M. , Ahmad, A. , Yue, F. , Khan, Z. , Jiang, Y. , Wei, Y. , Raza, S. , He, W. W. , Khan, F. U. , & Qipeng, Y. (2017). Biophysical and molecular docking approaches for the investigation of biomolecular interactions between amphotericin B and bovine serum albumin. Journal of Photochemistry and Photobiology B: Biology, 170, 6–15. [DOI] [PubMed] [Google Scholar]
- Raza, M. , Jiang, Y. , Wei, Y. , Ahmad, A. , Khan, A. , & Qipeng, Y. (2017). Insights from spectroscopic and in‐silico techniques for the exploitation of biomolecular interactions between human serum albumin and paromomycin. Colloids and Surfaces B: Biointerfaces, 157, 242–253. [DOI] [PubMed] [Google Scholar]
- Raza, M. , Khan, Z. , Ahmad, A. , Raza, S. , Khan, A. , Mohammadzai, I. U. , & Zada, S. (2017). In silico 3‐D structure prediction and molecular docking studies of inosine monophosphate dehydrogenase from plasmodium falciparum. Computational Biology and Chemistry, 71, 10–19. [DOI] [PubMed] [Google Scholar]
- Raza, M. , Wei, Y. , Jiang, Y. , Ahmad, A. , Raza, S. , Ullah, S. , Han, Y. , Khan, Q. U. , & Yuan, Q. (2017). Molecular mechanism of tobramycin with human serum albumin for probing binding interactions: Multi‐spectroscopic and computational approaches. New Journal of Chemistry, 41(16), 8203–8213. [Google Scholar]
- Riaz, H. , Nosheen, S. , Kiran, S. , Jahan, N. , Abrar, S. , & Riaz, S. (2016). Investigation of free radical scavenging and immunomodulatory activity of Anethum graveolens (Sowa). Oxidation Communications, 39, 3012–3026. [Google Scholar]
- Roy, A. , Ghosh, A. , Sinha, K. , Mitra, B. , Rakshit, S. , George, M. , & Sarkar, K. (2021). Diospyros peregrina fruit preparation mediated immunomodulation of lymphocytes isolated from the blood of breast cancer patients. Iranian Journal of Immunology, 18(2), 111–118. [DOI] [PubMed] [Google Scholar]
- Ruphin, F. P. , Baholy, R. , Emmanuel, R. , Amelie, R. , Martin, M. T. , & Koto–te‐Nyiwa, N. (2014). Isolation and structural elucidation of cytotoxic compounds from the root bark of Diospyros quercina (Baill.) endemic to Madagascar. Asian Pacific Journal of Tropical Biomedicine, 4(3), 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini, P. , Mukherjee, N. , Mukherjee, S. , Roy, P. , Gayen, P. , Kumar, D. , Pal, B. C. , & Sinha Babu, S. P. (2015). Diospyros perigrena bark extract induced apoptosis in filarial parasite Setaria cervi through generation of reactive oxygen species. Pharmaceutical Biology, 53(6), 813–823. [DOI] [PubMed] [Google Scholar]
- Sarkhosh, A. , Habibi, F. , Shahid, M. A. , Sargent, S. A. , & Brecht, J. K. (2024). Dried persimmon fruit: A year‐round available product: HS1479, 4/2024. EDIS, 2024(2), 1–7. [Google Scholar]
- Sharifi‐Rad, J. (2016). Herbal antibiotics: Moving back into the mainstream as an alternative for “superbugs”. Cellular and Molecular Biology, 62(9), 1–2. [PubMed] [Google Scholar]
- Sodhi, K. K. , Shree, P. , Mishra, L. C. , Mishra, G. , Kumar, M. , & Singh, D. K. (2023). Promising compounds of plant origin and their synthetic analogs against trypanosomes. In Natural product based drug discovery against human parasites: Opportunities and challenges (pp. 411–429). Singapore. [Google Scholar]
- Sujatha, V. , Kaviyasri, G. , Venkatesan, A. , Thirunavukkarasu, C. , Acharya, S. , Dayel, S. B. , Al‐Ghamdi, S. , Abdelzaher, M. H. , Shahid, M. , & Ramesh, T. (2023). Biomimetic formation of silver oxide nanoparticles through Diospyros montana bark extract: Its application in dye degradation, antibacterial and anticancer effect in human hepatocellular carcinoma cells. Journal of King Saud University, Science, 35(3), 102563. [Google Scholar]
- Sung, H. , Ferlay, J. , Siegel, R. L. , Laversanne, M. , Soerjomataram, I. , Jemal, A. , & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. [DOI] [PubMed] [Google Scholar]
- Xie, C. , Xie, Z. , Xu, X. , & Yang, D. (2015). Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. Journal of Ethnopharmacology, 163, 229–240. [DOI] [PubMed] [Google Scholar]
- Xiu‐Zhen, Y. , Yao‐Haur, K. , Tsong‐Jyh, L. , To‐Shii, S. , Chung‐Hsiung, C. , Mcphail, D. R. , & Mcphail, A. T. (1989). Cytotoxic components of diospyros morrisiana. Phytochemistry, 28(5), 1541–1543. [Google Scholar]
- Yoshihira, K. , Tezuka, M. , & Natori, S. (1970). Naphthoquinone derivatives from diospyros SPP.: Bisisodiospyrin, a tetrameric naphthoquinone. Tetrahedron Letters, 11(1), 7–10. [Google Scholar]
- Zhang, Y. , Zhu, W. , Deng, X. Y. , Peng, J. M. , & Li, C. M. (2017). Both non‐covalent and covalent interactions were involved in the mechanism of detoxifying effects of persimmon tannin on Chinese cobra PLA2. Fitoterapia, 120, 41–51. [DOI] [PubMed] [Google Scholar]
- Zreen, Z. , Hameed, A. , Kiran, S. , Farooq, T. , & Zaroog, M. S. (2022). A comparative study of Diospyros malabarica (Gaub) extracts in various polarity‐dependent solvents for evaluation of phytoconstituents and biological activities. BioMed Research International, 2022, 4746223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the report.


