Abstract
Pueraria lobata root and Glycine max (L.) Merrill are rich in phytoestrogens. However, these bioactive ingredients have limited bioavailability due to their high molecular weight. In this study, we extracted two natural products and fermented with Lactiplantibacillus plantarum before mixing the fermented extracts (FPE‐FGE). To understand whether FPE‐FGE could alleviate menopause with dyslipidemia, we examined their effects on ovariectomy (OVX)‐induced dyslipidemia in mice. Oral administration of the FPE‐FGE (1:9, 3:7, and 9:1) did not affect safety‐related biomarkers, such as uterus index (%), vagina index (%), aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, and creatinine. Furthermore, FPE‐FGE (1:9, 3:7, and 9:1) increased the levels of 17β‐estradiol (E2) and expression of uterus estrogen receptor β (ERβ); there was little effect on the expression of uterus estrogen receptor α (ERα), and reduced the levels of gonadotropins, such as luteinizing hormone (LH) and follicle‐stimulating hormone (FSH). However, only the FPE‐FGE (3:7) reduced the levels of blood lipids, including total cholesterol (TC) and LDL‐cholesterol (LDL‐C). Accordingly, FPE‐FGE (3:7) upregulated endothelial nitric oxide synthase (eNOS), nitric oxide (NO), cyclic guanosine monophosphate (cGMP), and protein kinase G (PKG). In conclusion, FPE‐FGE (3:7) attenuated the menopausal dyslipidemia by upregulating eNOS‐NO‐cGMP signaling pathway.
Keywords: aglycone, eNOS‐NO‐cGMP signaling pathway, fermentation, Lactiplantibacillus plantarum, menopause
FPE‐FGE (3:7), which includes a combination of various glycosides, can reduce the levels of blood lipids by upregulating the eNOS‐NO‐cGMP signaling pathway.
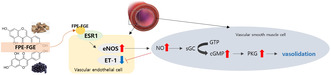
1. INTRODUCTION
Cardiovascular disease is the leading cause of mortality among women worldwide (Chang et al., 2011; Garcia et al., 2016). In particular, the cardiovascular disease risk significantly increases in women experiencing menopause due to dyslipidemia (Anagnostis et al., 2022; Wild et al., 2021). Menopause has been approximated in experimental female rodents by treating them with ovariectomy (OVX) surgery (Souza et al., 2019), which induces the reduction of 17β‐estradiol (E2) and stimulates luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) production (Kim et al., 2014; Luderer & Schwartz, 1994). However, increased LH suppresses the endothelial nitric oxide synthase (eNOS)‐nitric oxide (NO)‐cyclic guanosine monophosphate (cGMP) signaling pathway, resulting in lipid accumulation (Meng et al., 2019), and FSH induces dyslipidemia through inhibiting hepatic cholesterol metabolism (Song et al., 2016). In contrast, E2 induces eNOS and NO release, activating the eNOS‐NO‐cGMP signaling pathway (Vera‐Arzave et al., 2019). In addition, E2 regulates the secretion of LH and FSH through the negative feedback mechanism (Shaw et al., 2010).
Furthermore, NO is an essential vasodilator produced by the vascular endothelium via eNOS (Lamping et al., 2000; Manicam et al., 2017), and diffuses from the vascular endothelium to the smooth muscle cell, activating soluble guanylyl cyclase (sGC); this results in increased cGMP production. Subsequently, the cGMP activates protein kinase G (PKG), resulting in vasodilation (Bucci et al., 2012; Chen et al., 2023); this leading to vasodilation‐mediated anti‐atherosclerotic effects by improving vasomotor dysfunction (Raman et al., 2011; Tentolouris et al., 2000). Therefore, regulating the eNOS‐NO‐cGMP signaling pathway is a beneficial strategy for treating menopause‐induced dyslipidemia in women.
Pueraria lobata roots and Glycine max (L.) Merrill are rich in phytoestrogens, such as the isoflavone glycosides puerarin, daidzin, and genistin (Choi et al., 2020; Wagle et al., 2019). Isoflavone glycosides exert estrogenic effects in women experiencing menopause as their chemical structure is similar to estrogen, lowering blood cholesterol (Shidfar et al., 2009; Taku et al., 2007). However, these bioactive ingredients have a high molecular weight, so the absorption rate in the body is poor (Piskula et al., 1999; Zhang et al., 2011). In recent years, isoflavone glycosides have been fermented to reduce glucose, resulting in an increased absorption rate in the body (Nagino et al., 2016; Okabe et al., 2011).
Therefore, in a previous study, we extracted two natural products and fermented with Lactiplantibacillus plantarum before mixing the fermented extracts (FPE‐FGE) and checked the expression of estrogen receptors in cells treated with FPE‐FGE (1:9, 3:7, 5:5, and 9:1) to determine the best ratio. The FPE‐FGE (3:7) was the most effective because upon treatment with this mixture, the levels of ESR1 were lower and those of ERβ were higher than those in the E2 group (Ha et al., 2023). We also found that the FPE‐FGE (3:7) additively or synergistically ameliorates the depressive‐like behavior of ovariectomized mice by upregulating the hippocampal brain‐derived neurotrophic factor (Ha et al., 2023). However, the fermented extract mixture's anti‐dyslipidemic effects have not been studied in vivo.
In this study, to study the anti‐dyslipidemic effects of FPE‐FGE (3:7), we extracted Pueraria lobata roots and Glycine max (L.) Merrill and fermented with L. plantarum before being freeze‐dried and combined in differing ration to further investigated the dyslipidemia‐related biomarkers in OVX mice.
2. MATERIALS AND METHODS
2.1. Fermentation of Pueraria lobata root and Glycine max (L.) Merrill
Fifty‐six LAB strains were isolated from Korean traditional fermented food “kimchi” and identified, as previously reported (Ha et al., 2023). The isolated bacteria were cultured in the general lactic acid bacteria (LAB) De Man, Rogosa, and Sharpe broth (Becton, Dickinson and Company, Radnor, PA, USA) and used for the fermentation of Pueraria lobata root or Glycine max (L.) Merrill extracts. The fermented Pueraria lobata root and Glycine max (L.) Merrill extracts were freeze‐dried for future use.
2.2. High‐performance liquid chromatography (HPLC) analysis
The analysis was performed using an Agilent Technologies 1200 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA). A C18 column (Phenomonex Kinetex, CA, USA) was used to analyze all samples. The mobile phases were water (solvent A) and acetonitrile with 0.1% acetic acid (solvent B) in the gradient mode (Ha et al., 2023).
2.3. Animals
Female Institute of Cancer Research (ICR) mice (6 weeks old; 20 ± 2 g) were purchased from JA BIO, Inc. (Suwon, Korea). Mice were kept in wire cages under ventilated conditions (20–20°C, 50 ± 10% relative humidity, and 12 h light–dark cycle) and fed commercial standard laboratory mouse chow and water ad libitum.
All experiments were performed following the National Institute of Health (NIH) and CHA Bio Complex guidelines. All animal experiments were approved by the Institutional Animal Care and Use Committee of the CHA Bio Complex (IACUC No. 220163).
2.4. Preparation of mice with menopause and dyslipidemia
After acclimation, all the mice, except those in the sham operation group, were surgically ovariectomized. Specifically, after 1 week of acclimation, mice were subjected to a bilateral ovary removal or sham surgery by using sterilized surgical equipment under anesthesia. Menopausal dyslipidemia was induced in the female mice for 6 weeks. After induction of dyslipidemia, freeze‐dried FPE‐FGE (1:9, 3:7, and 9:1) was orally gavaged and administered once daily for 8 weeks. The 1× mouse dose (5.2 mg/kg) was used to evaluate the anti‐dyslipidemic effects, following the method of a previous study (Ha et al., 2023).
2.5. Blood chemistry tests
For the determination of the safety‐related biomarkers aspartate aminotransferase (AST), alanine aminotransferase (ALT), and blood urea nitrogen (BUN), and the blood lipids total cholesterol (TC), HDL‐cholesterol (HDL‐C), LDL‐cholesterol (LDL‐C), and triglyceride (TG), plasma samples were transferred to an automatic biochemical analyzer (Accute BA™, Toshiba Corp., Tokyo, Japan). Creatinine (CRE) was measured using a commercial kit (#80350, Crystal Chem, Inc., Elk Grove Village, IL, USA).
2.6. Immunoblotting
To determine estrogen receptor α (ERα) and estrogen receptor β (ERβ), uterus tissue was homogenized by Kimble® vortex mixer (DWK Life Sciences, Millville, NJ, USA), following the method of a previous study (Ha et al., 2023). Electrophoresed proteins were transferred to a membrane, blocked with 5% nonfat dried‐milk proteins, and probed with the corresponding antibodies against ERα [sc‐8002 (1:1000), Santa Cruz Biotechnology, Inc., Dallas, TX, USA], ERβ [sc‐390243 (1:1000), Santa Cruz Biotechnology, Inc.]. The membrane was incubated with horseradish peroxidase‐conjugated secondary antibodies. Proteins were visualized with an enhanced chemiluminescence system (ImageQuant™ LAS 500, Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.7. Enzyme‐linked immunosorbent assay (ELISA)
For the determination of E2, LH, and FSH, whole blood was drawn from the abdominal aorta and inferior vena cava, collected in anticoagulant tubes (Becton, Dickinson and Company [BD], Franklin Lakes, NJ, USA), centrifuged, and plasma samples collected, following the method of a previous study (Ha et al., 2023). In addition, for the determination of eNOS‐NO‐cGMP signaling‐pathway‐related biomarkers eNOS, cGMP, and PKG, the abdominal aorta tissue was lysed according to the enzyme‐linked immunosorbent (ELISA) kit supplier's protocol. The levels of biomarkers were assayed using commercial ELISA kits as follows: E2 (CSB‐E05109m; Cusabio Technology LLC, Houston, TX, USA), LH (CSB‐E12770m; Cusabio Technology LLC), FSH (CSB‐E06871m; Cusabio Technology LLC), eNOS (ab230938; Abcam, Cambridge, UK), cGMP (ab133052; Abcam), and PKG (LS‐F9645; LifeSpan BioSciences Inc. [LS Bio], Shirley, MA, USA).
2.8. Modified diazotization assay using the Griess method
To determine NO, plasma samples were transferred to a 96‐well plate, induced by adding sulfanilamide into the reaction buffer, and incubated for 10 min at room temperature. Then the final reaction was activated by adding naphthyl ethylenediamine to the stabilizer buffer and incubated for 10 min at room temperature. The concentration of NO was measured using a commercial kit (21023; iNtRON Biotechnology Inc., Seongnam, Korea).
2.9. Statistical analysis
All experimental values are reported as the mean ± SD using GraphPad Prism 9 (GraphPad Software, Inc., San Diego, CA, USA). Significant differences were analyzed using a one‐way analysis of variance (ANOVA) followed by Dunnett's post‐hoc tests (p < .05).
3. RESULTS AND DISCUSSION
3.1. Fermentation of Pueraria lobata root or Glycine max (L.) Merrill extracts
Food‐origin lactic acid bacteria are characterized by high intestinal reach and settlement due to their high acid resistance and bile resistance (Chang et al., 2010; Jung et al., 2019). Therefore, we isolated 56 LABs from fermented food (Ha et al., 2023) and inoculated them into Pueraria lobata root or Glycine max (L.) Merrill extracts. After fermentation, we evaluated the concentrations of glycosides or aglycones such as daidzein and genistein (data not shown).
Among these, L. plantarum CCHR1 (KCCM13337P) was selected as a suitable strain for Pueraria lobata root extract and L. plantarum CCHR2 (KCCM13338P) was found suitable for Glycine max (L.) Merrill extract. No special substances were detected in the FPE (Figure 1a,b), and the content of daidzein and genistein increased noticeably in the FGE (Figure 1c,d).
FIGURE 1.
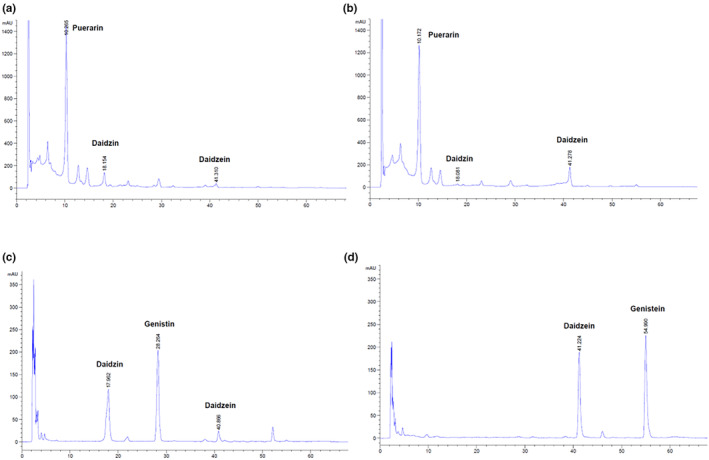
Analysis of changes in representative substances after fermentation. Pueraria lobata root extract (a); fermented P. lobata root extract (b); Glycine max (L.) Merrill extract (c); fermented Glycine max (L.) Merrill extract (d).
These results suggest that food‐origin lactic acid bacteria have relatively good growth potential in the external environment and thus good fermentation ability for each natural product.
3.2. Effects of administering FPE‐FGE on the safety in OVX mice
The Republic of Korea's Ministries of Food and Drug Safety (Korea MFDS) presents safety evaluation indicators through the guidelines for functional foods. On the basis of this, FPE‐FGE (3:7) did not affect the safety‐related biomarkers, such as uterus index (%), vagina index (%), AST, ALT, BUN, and CRE. Therefore, to further investigate the safety of FPE‐FGE (1:9, 3:7, and 9:1) in vivo by oral administration to OVX mice daily for 8 weeks, we evaluated the uterus index (%), vagina index (%), AST, ALT, BUN, and CRE (Figure 2a). The OVX surgery induced severe uterine and vaginal atrophy, resulting in the reduction of uterus index (%) and vagina index (%) to 88.4% and 79.6%. The intraperitoneal injection of E2 (10 μg/kg) inhibited OVX‐induced uterine and vaginal atrophy to 209.2% and 210.3%. However, orally administration of FPE‐FGE (1:9, 3:7, and 9:1) were not significant (Figure 2b,c).
FIGURE 2.
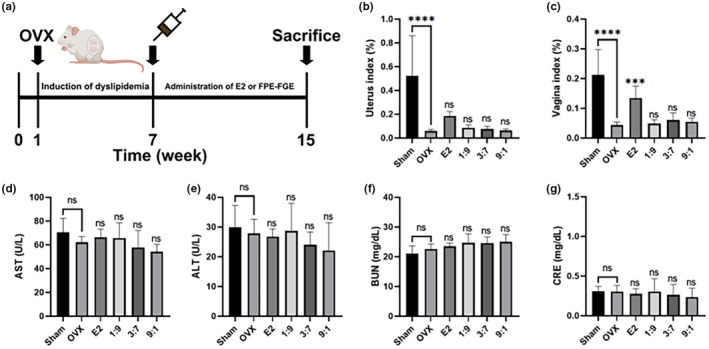
Safety of the administration of FPE‐FGE (1:9, 3:7, and 9:1) in OVX mice. Experimental design, (a); effects on the uterus index (%) (b); vagina index (%) (c); aspartate aminotransferase (AST) (d); alanine aminotransferase (ALT) (e); blood urea nitrogen (BUN) (f); and creatinine (CRE) (g). Values are indicated as mean ± SD (n = 7). *p < .05, **p < .01, ***p < .001, and ****p < .0001 compared with the OVX group. FPE‐FGE, mixture of fermented Pueraria lobata root and Glycine max (L.) Merrill extracts; OVX, ovariectomized.
Next, we measured the liver function, including the plasma levels of AST and ALT, and kidney function, including the plasma levels of BUN and CRE. No significant difference was found in E2 (10 μg/kg) or FPE‐FGE (1:9, 3:7, and 9:1) (Figure 2d–g). These results suggest that FPE‐FGE (1:9, 3:7, and 9:1) were safe functional food that does not affect liver and kidney function.
3.3. Phytoestrogenic effects of administering FPE‐FGE in OVX mice
To evaluate the phytoestrogenic effects of FPE‐FGE (1:9, 3:7, and 9:1) in OVX mice, we measured the plasma levels of E2 and the expression of uterus ERα and ERβ. The OVX surgery induced a reduction of E2 levels to 34.4%. Intraperitoneal injection of E2 (10 μg/kg) or oral administration of FPE‐FGE (1:9, 3:7, and 9:1) increased the OVX‐induced reduction of E2 to 39.4% or 46.7%, 40.6%, and 35.0% (Figure 3a).
FIGURE 3.
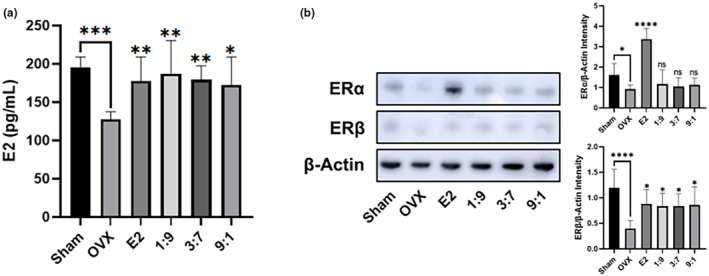
Phytoestrogenic effects of the oral administration of FPE‐FGE (1:9, 3:7, and 9:1) in OVX mice. Effects on 17β‐estradiol (E2) (a), expression of estrogen receptor α (ERα) and ERβ (b). Values are indicated as mean ± SD (n = 7). *p < .05, **p < .01, ***p < .001, and ****p < .0001 compared with the OVX group. FPE‐FGE, a mixture of fermented Pueraria lobata root and Glycine max (L.) Merrill extracts; OVX, ovariectomized.
Next, we measured the expression of uterus ERα and ERβ. The OVX surgery reduced ERα and ERβ expression to 43.0% and 66.7%. However, intraperitoneal injection of E2 (10 μg/kg) inhibited the OVX‐induced reduction of ERα and ERβ expression to 266.4% and 120.9%. However, oral administration of FPE‐FGE (1:9, 3:7, and 9:1) did not affect the expression of ERα. In contrast, oral administration of FPE‐FGE (1:9, 3:7, and 9:1) increased the expression of ERβ to 109.5%, 110.1%, and 115.3% (Figure 3b).
Women experiencing menopause may have various physiological responses, which can lead to dyslipidemia, hot flashes, sleep problems, depression, and osteoporosis. These symptoms begin with decreased E2 levels (Minkin, 2019). The transmission of E2 signals through estrogen receptors, such as ERα and ERβ, is essential to women's genital function (Pastore et al., 2012). However, ERα is frequently mutated in breast cancer and linked to ligand‐independent growth and metastasis (Li et al., 2022). In addition, breast cancer is known to be catalyzed by estrogen and is associated with the expression of ERα (Anbalagan & Rowan, 2015). In contrast, ERβ inhibits breast cancer cell migration and invasion (Song et al., 2019). In addition, ERβ inhibits the anti‐proliferative functions in ERα‐positive cells, such as MCF‐7 cells (Hapangama et al., 2015). Therefore, downregulating the expression of ERα and upregulating the expression of ERβ is a beneficial strategy for preventing breast cancer.
3.4. Effects of administering FPE‐FGE on OVX mice's gonadotropin levels
To investigate whether oral administration of FPE‐FGE (1:9, 3:7, and 9:1) could inhibit LH and FSH in OVX mice, we measured the plasma levels of LH and FSH. The OVX surgery induced an increase in LH and FSH to 14.2% and 86.4%. However, intraperitoneal injection of E2 (10 μg/kg) inhibited the increase in LH and FSH to 23.7% and 47.2%. In addition, oral administration of FPE‐FGE (1:9, 3:7, and 9:1) inhibited the increase of LH to 12.4%, 18.9%, and 16.9% and FSH to 44.1%, 49.2%, and 42.9% (Figure 4a,b).
FIGURE 4.
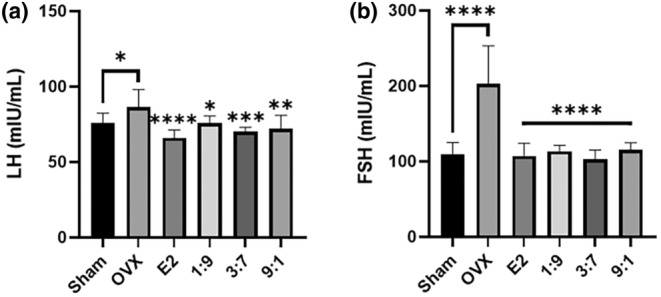
Effects of the oral administration of FPE‐FGE (1:9, 3:7, and 9:1) on gonadotropin levels in OVX mice. Effects on luteinizing hormone (LH) (a) and follicle‐stimulating hormone (FSH) (b). Values are indicated as mean ± SD (n = 7). *p < .05, **p < .01, ***p < .001, and ****p < .0001 compared with the OVX group. FPE‐FGE, mixture of fermented Pueraria lobata root and Glycine max (L.) Merrill extracts; OVX, ovariectomized.
Menopause in women induces a reduction of E2, resulting in increased gonadotropin levels, including LH and FSH (Kim et al., 2014; Luderer & Schwartz, 1994). Accordingly, elevated LH and FSH levels are anticipated in OVX mice (Luderer & Schwartz, 1994). Gonadotropins are essential to reproductive health; however, elevated LH and FSH levels contribute to the acceleration of dyslipidemia by inhibiting NO synthesis (Meng et al., 2019); NO inhibits atherosclerosis by improving vasomotor dysfunction (Raman et al., 2011; Tentolouris et al., 2000). Therefore, to understand the anti‐dyslipidemia effects of the oral administration of FPE‐FGE (1:9, 3:7, and 9:1) on OVX mice, the inhibitory effects of gonadotropins or inducible effects of the eNOS‐NO‐cGMP signaling pathway‐related biomarkers have been examined.
3.5. Effects of administering FPE‐FGE on OVX‐induced dyslipidemia in mice
Next, we measured the plasma levels of TC, HDL‐C, LDL‐C, and TG to evaluate the curative effects of FPE‐FGE (1:9, 3:7, and 9:1) on OVX‐induced dyslipidemia in mice. The OVX surgery increased the TC and LDL‐C to 25.5%, 94.9%. However, the intraperitoneal injection of E2 (10 μg/kg) or oral administration of FPE‐FGE (3:7) repressed an increase in TC and LDL‐C (Figure 5a,b). However, the HDL‐C and TG had no significant effect (data not shown).
FIGURE 5.
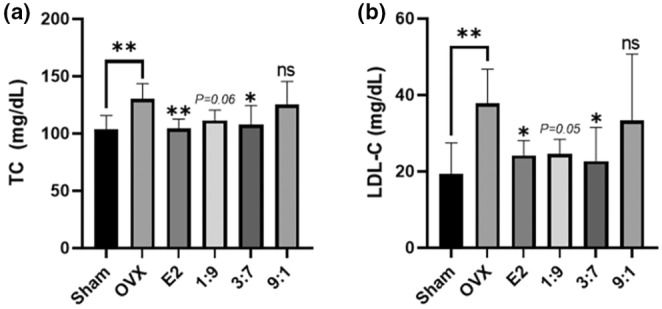
Anti‐dyslipidemic effects of the oral administration of FPE‐FGE (1:9, 3:7, and 9:1) in OVX mice. Effects on total cholesterol (TC) (a) and LDL‐cholesterol (LDL‐C) (b). Values are indicated as mean ± SD (n = 7). *p < .05, **p < .01, ***p < .001, and ****p < .0001 compared with the OVX group. FPE‐FGE, a mixture of fermented Pueraria lobata root and Glycine max (L.) Merrill extracts; OVX, ovariectomized.
3.6. Effects of administering FPE‐FGE on OVX mice's eNOS‐NO‐cGMP signaling pathway
Next, to investigate the mechanism of curative effects of FPE‐FGE (3:7), we measured the eNOS‐NO‐cGMP signaling pathway‐related biomarkers, including eNOS, NO, cGMP, and PKG. Increased expression of eNOS in endothelial cells also increases the occurrence of NO, which in turn increases the expression of cGMP in smooth muscle cells. This pathway eventually induces vasodilation and strengthens blood circulation (Cho et al., 2018).
The OVX surgery induced a reduction of the abdominal aortic eNOS, cGMP PKG to 38.7%, 57.4%, 53.9%, and plasma levels of NO to 66.6%. However, intraperitoneal injection of E2 (10 μg/kg) inhibited the reduction of eNOS, NO, cGMP, and PKG to 42.6%, 104.1%, 131.3%, and 61.3%. Interestingly, oral administration of FPE‐FGE (3:7) inhibited the reduction of eNOS, NO, cGMP, and PKG to 49.1%, 133.9%, 63.7%, and 37.9%. However, oral administration of FPE‐FGE (1:9 and 9:1) had no significant effect (Figure 6a–d). These results suggest that FPE‐FGE (3:7), which includes a combination of various glycosides, such as puerarin, daidzin, and genistin, and aglycones, such as daidzein and genistein, additively or synergistically, can reduce the levels of blood lipids by upregulating eNOS‐NO‐cGMP signaling pathway.
FIGURE 6.
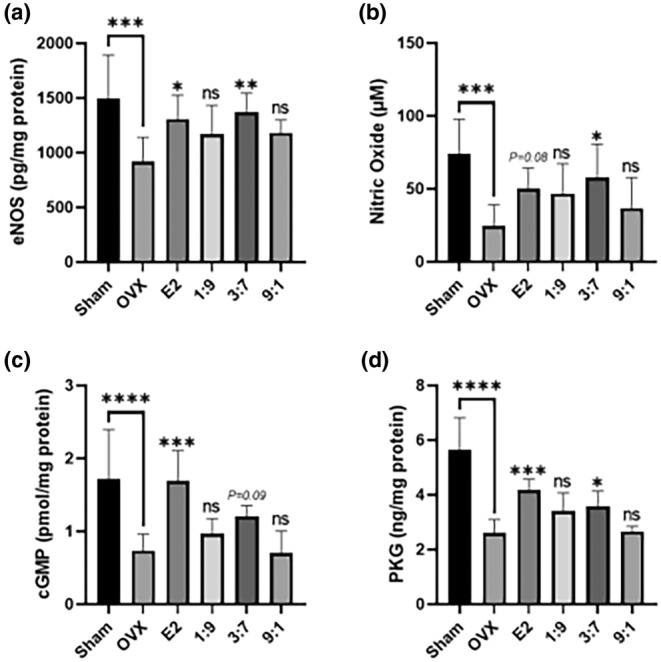
Effects of the oral administration of FPE‐FGE (1:9, 3:7, and 9:1) on nitric oxide synthase‐nitric oxide‐cyclic guanosine monophosphate (eNOS‐NO‐cGMP) signaling pathway in OVX mice. Effects on endothelial nitric oxide synthase (eNOS) (a), nitric oxide (NO) (b), cyclic guanosine monophosphate (cGMP) (c), and protein kinase G (PKG) (d). Values are indicated as mean ± SD (n = 7). *p < .05, **p < .01, ***p < .001, and ****p < .0001 compared with the OVX group. FPE‐FGE, a mixture of fermented Pueraria lobata root and Glycine max (L.) Merrill extracts; OVX, ovariectomized.
Flavonoids are known to enhance the production of NO, causing endothelium‐dependent vasorelaxation in the rat aorta (Benito et al., 2002). However, there were no studies that confirmed the change in the eNOS‐NO‐cGMP signaling pathway according to the ratio of glycosides and nonglycosides among isoflavones. This study is the first study to investigate the eNOS‐NO‐cGMP signaling pathway according to the ratio of glycosides and aglycones, and it has been proven that a mixture of glycosides and aglycones ratio (3:7) is the most effective in improving the cardiovascular system.
4. CONCLUSION
Oral administration of FPE‐FGE (1:9, 3:7, and 9:1) increased the levels of E2 and the expression of uterus ERβ but did not significantly affect the expression of uterus ERα. Moreover, FPE‐FGE (1:9, 3:7, and 9:1) reduced the levels of gonadotropins, such as LH and FSH. These results suggest that FPE‐FGE (1:9, 3:7, and 9:1) can exert phytoestrogenic effects by increasing the levels of E2 through the expression of uterus ERβ. Especially, FPE‐FGE (3:7), which includes a combination of various glycosides or aglycones, such as daidzein and genistein, additively or synergistically attenuated the menopausal dyslipidemia by upregulating eNOS‐NO‐cGMP signaling pathway. Although there is a limitation that there are no clinical trials, there are sufficient grounds to move on to clinical trials because significant results have been obtained in animal experiments.
In conclusion, combinations of various phytoestrogens can exert phytoestrogenic effects in women experiencing menopause with dyslipidemia through the OVX mice. If further clinical trials confirm the same symptom improvement, it could become potentially commercial functional foods for improving menopausal symptoms.
AUTHOR CONTRIBUTIONS
Insuk Choi: Conceptualization (lead); resources (lead). Hyo‐Min Jang: Methodology (equal); writing – original draft (lead). Jimyeong Ha: Methodology (equal); visualization (equal); writing – review and editing (lead).
FUNDING INFORMATION
This research was supported by the CMG Pharmaceutical Co., Ltd. and the CHA Advanced Research Institute (CARI‐RN‐84).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Jungeun Park for providing technical assistance in the laboratory.
Jang, H.‐M. , Ha, J. , & Choi, I. (2024). Anti‐dyslipidemic effects of Pueraria lobata root and Glycine max (L.) Merrill extracts fermented with Lactiplantibacillus plantarum in ovariectomized mice. Food Science & Nutrition, 12, 7544–7551. 10.1002/fsn3.4356
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Anagnostis, P. , Lambrinoudaki, I. , Stevenson, J. C. , & Goulis, D. G. (2022). Menopause‐associated risk of cardiovascular disease. Endocrine Connections, 11(4), e210537. 10.1530/EC-21-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbalagan, M. , & Rowan, B. G. (2015). Estrogen receptor alpha phosphorylation and its functional impact in human breast cancer. Molecular and Cellular Endocrinology, 418(3), 264–272. 10.1016/j.mce.2015.01.016 [DOI] [PubMed] [Google Scholar]
- Benito, S. , Lopez, D. , Saiz, M. P. , Buxaderas, S. , Sanchez, J. , Puig‐Parellada, P. , & Mitjavila, M. T. (2002). A flavonoid‐rich diet increases nitric oxide production in rat aorta. British Journal of Pharmacology, 135(4), 910–916. 10.1038/sj.bjp.0704534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci, M. , Papapetropoulos, A. , Vellecco, V. , Zhou, Z. , Zaid, A. , Giannogonas, P. , Cantalupo, A. , Dhayade, S. , Karalis, K. P. , Feil, R. , & Cirino, G. (2012). cGMP‐dependent protein kinase contributes to hydrogen sulfide‐stimulated vasorelaxation. PLoS ONE, 7(12), e53319. 10.1371/journal.pone.0053319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H. S. , Odongua, N. , Ohrr, H. , Sull, J. W. , & Nam, C. M. (2011). Reproductive risk factors for cardiovascular disease mortality among postmenopausal women in Korea: The Kangwha cohort study. Menopause, 18(11), 1205–1212. 10.1097/gme.0b013e31821adb43 [DOI] [PubMed] [Google Scholar]
- Chang, J. H. , Shim, Y. Y. , Cha, S. K. , & Chee, K. M. (2010). Probiotic characteristics of lactic acid bacteria isolated from Kimchi. Journal of Applied Microbiology, 109(1), 220–230. 10.1111/j.1365-2672.2009.04648.x [DOI] [PubMed] [Google Scholar]
- Chen, L. , Zhou, X. , Deng, Y. , Yang, Y. , Chen, X. , Chen, Q. , Liu, Y. , Fu, X. , Kwan, H. Y. , You, Y. , Jin, W. , & Zhao, X. (2023). Zhenwu decoction ameliorates cardiac hypertrophy through activating sGC (soluble guanylate cyclase) – cGMP (cyclic guanosine monophosphate) – PKG (protein kinase G) pathway. Journal of Ethnopharmacology, 300, 115705. 10.1016/j.jep.2022.115705 [DOI] [PubMed] [Google Scholar]
- Cho, I. H. , Kang, B. W. , Yun‐Jae, P. , Lee, H. J. , Park, S. , & Lee, N. (2018). Ginseng berry extract increases nitric oxide level in vascular endothelial cells and improves cGMP expression and blood circulation in muscle cells. Journal of Exercise Nutrition & Biochemistry, 22(3), 6–13. 10.20463/jenb.2018.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y. M. , Yoon, H. , Lee, S. , Ko, H. C. , Shin, M. J. , Lee, M. C. , Hur, O. S. , Ro, N. Y. , & Desta, K. T. (2020). Isoflavones, anthocyanins, phenolic content, and antioxidant activities of black soybeans (Glycine max (L.) Merrill) as affected by seed weight. Scientific Reports, 10(1), 19960. 10.1038/s41598-020-76985-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, M. , Mulvagh, S. L. , Bairey Merz, C. N. , Buring, J. E. , & Manson, J. E. (2016). Cardiovascular disease in women: Clinical perspectives. Circulation Research, 118(8), 1273–1293. 10.1161/CIRCRESAHA.116.307547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha, J. , Jang, H. M. , & Choi, I. (2023). Effects of Pueraria lobata root and Glycine max (L.) Merrill extracts fermented with Lactiplantibacillus plantarum on the depressive‐like behavior in ovariectomized mice via upregulation of hippocampal BDNF. Journal of Functional Foods, 108, 105776. 10.1016/j.jff.2023.105776 [DOI] [Google Scholar]
- Hapangama, D. K. , Kamal, A. M. , & Bulmer, J. N. (2015). Estrogen receptor β: The guardian of the endometrium. Human Reproduction Update, 21(2), 174–193. 10.1093/humupd/dmu053 [DOI] [PubMed] [Google Scholar]
- Jung, J. H. , Kim, S. J. , Lee, J. Y. , Yoon, S. R. , You, S. Y. , & Kim, S. H. (2019). Multifunctional properties of Lactobacillus plantarum strains WiKim83 and WiKim87 as a starter culture for fermented food. Food Science & Nutrition, 7(8), 2505–2516. 10.1002/fsn3.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. H. , Choi, Y. Y. , Han, J. M. , Lee, H. S. , Hong, S. B. , Lee, S. G. , & Yang, W. M. (2014). Ameliorative effects of Schizandra chinensis on osteoporosis via activation of estrogen receptor (ER)‐α/‐β. Food & Function, 5(7), 1594–1601. 10.1039/c4fo00133h [DOI] [PubMed] [Google Scholar]
- Lamping, K. G. , Nuno, D. W. , Shesely, E. G. , Maeda, N. , & Faraci, F. M. (2000). Vasodilator mechanisms in the coronary circulation of endothelial nitric oxide synthase‐deficient mice. American Journal of Physiology. Heart and Circulatory Physiology, 279(4), H1906–H1912. 10.1152/ajpheart.2000.279.4.H1906 [DOI] [PubMed] [Google Scholar]
- Li, Z. , McGinn, O. , Wu, Y. , Bahreini, A. , Priedigkeit, N. M. , Ding, K. , Onkar, S. , Lampenfeld, C. , Sartorius, C. A. , Miller, L. , Rosenzweig, M. , Cohen, O. , Wagle, N. , Richer, J. K. , Muller, W. J. , Buluwela, L. , Ali, S. , Bruno, T. C. , Vignali, D. A. A. , & Oesterreich, S. (2022). ESR1 mutant breast cancers show elevated basal cytokeratins and immune activation. Nature Communications, 13(1), 2011. 10.1038/s41467-022-29498-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luderer, U. , & Schwartz, N. B. (1994). Acute changes in pulsatile LH and FSH secretion after ovariectomy in rats: Treatment with oestradiol for 24 h suppresses LH, but not FSH, for at least 48 h. Reproduction, 100(2), 613–621. 10.1530/jrf.0.1000613 [DOI] [PubMed] [Google Scholar]
- Manicam, C. , Ginter, N. , Li, H. , Xia, N. , Goloborodko, E. , Zadeh, J. K. , Musayeva, A. , Pfeiffer, N. , & Gericke, A. (2017). Compensatory vasodilator mechanisms in the ophthalmic artery of endothelial nitric oxide synthase gene knockout mice. Scientific Reports, 7(1), 7111. 10.1038/s41598-017-07768-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, X. , Li, X. , Xu, X. , Li, P. , Chen, Y. , Fu, X. , & Xu, X. (2019). Elevated luteinizing hormone contributes to atherosclerosis formation by inhibiting nitric oxide synthesis via PI3K/Akt pathway. Vascular Pharmacology, 121, 106582. 10.1016/j.vph.2019.106582 [DOI] [PubMed] [Google Scholar]
- Minkin, M. J. (2019). Menopause: Hormones, lifestyle, and optimizing aging. Obstetrics and Gynecology Clinics, 46(3), 501–514. 10.1016/j.ogc.2019.04.008 [DOI] [PubMed] [Google Scholar]
- Nagino, T. , Kano, M. , Masuoka, N. , Kaga, C. , Anbe, M. , Miyazaki, K. , Kamachi, K. , Isozaki, M. , Suzuki, C. , Kasuga, C. , & Tanaka, A. (2016). Intake of a fermented soymilk beverage containing moderate levels of isoflavone aglycones enhances bioavailability of isoflavones in healthy premenopausal Japanese women: A double‐blind, placebo‐controlled, single‐dose, crossover trial. Bioscience of Microbiota, Food and Health, 35(1), 9–17. 10.12938/bmfh.2015-011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe, Y. , Shimazu, T. , & Tanimoto, H. (2011). Higher bioavailability of isoflavones after a single ingestion of aglycone‐rich fermented soybeans compared with glucoside‐rich non‐fermented soybeans in Japanese postmenopausal women. Journal of the Science of Food and Agriculture, 91(4), 658–663. 10.1002/jsfa.4228 [DOI] [PubMed] [Google Scholar]
- Pastore, M. B. , Jobe, S. O. , Ramadoss, J. , & Magness, R. R. (2012). Estrogen receptor‐α and estrogen receptor‐β in the uterine vascular endothelium during pregnancy: Functional implications for regulating uterine blood flow. Seminars in Reproductive Medicine, 30(1), 46–61. 10.1055/s-0031-1299597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskula, M. K. , Yamakoshi, J. , & Iwai, Y. (1999). Daidzein and genistein but not their glucosides are absorbed from the rat stomach. FEBS Letters, 447(2–3), 287–291. 10.1016/s0014-5793(99)00307-5 [DOI] [PubMed] [Google Scholar]
- Raman, K. G. , Gandley, R. E. , Rohland, J. , Zenati, M. S. , & Tzeng, E. (2011). Early hypercholesterolemia contributes to vasomotor dysfunction and injury associated atherogenesis that can be inhibited by nitric oxide. Journal of Vascular Surgery, 53(3), 754–763. 10.1016/j.jvs.2010.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, N. D. , Histed, S. N. , Srouji, S. S. , Yang, J. , Lee, H. , & Hall, J. E. (2010). Estrogen negative feedback on gonadotropin secretion: Evidence for a direct pituitary effect in women. The Journal of Clinical Endocrinology and Metabolism, 95(4), 1955–1961. 10.1210/jc.2009-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidfar, F. , Ehramphosh, E. , Heydari, I. , Haghighi, L. , Hosseini, S. , & Shidfar, S. (2009). Effects of soy bean on serum paraoxonase 1 activity and lipoproteins in hyperlipidemic postmenopausal women. International Journal of Food Sciences and Nutrition, 60(3), 195–205. [DOI] [PubMed] [Google Scholar]
- Song, P. , Li, Y. , Dong, Y. , Liang, Y. , Qu, H. , Qi, D. , Lu, Y. , Jin, X. , Guo, Y. , Jia, Y. , Wang, X. , Xu, W. , & Quan, C. (2019). Estrogen receptor β inhibits breast cancer cells migration and invasion through CLDN6‐mediated autophagy. Journal of Experimental & Clinical Cancer Reseach, 38(1), 354. 10.1186/s13046-019-1359-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y. , Wang, E. S. , Xing, L. L. , Shi, S. , Qu, F. , Zhang, D. , Li, J. Y. , Shu, J. , Meng, Y. , Sheng, J. Z. , Zhou, J. H. , & Huang, H. F. (2016). Follicle‐stimulating hormone induces postmenopausal dyslipidemia through inhibiting hepatic cholesterol metabolism. The Journal of Clinical Endocrinology & Metabolism, 101(1), 254–263. 10.1210/jc.2015-2724 [DOI] [PubMed] [Google Scholar]
- Souza, V. R. , Mendes, E. , Casaro, M. , Antiorio, A. T. F. , Oliveira, F. A. , & Ferreira, C. M. (2019). Description of ovariectomy protocol in mice. In Pre‐clinical models: Techniques and protocols (pp. 303–309). Springer Link. 10.1007/978-1-4939-8994-2_29 [DOI] [PubMed] [Google Scholar]
- Taku, K. , Umegaki, K. , Sato, Y. , Taki, Y. , Endoh, K. , & Watanabe, S. (2007). Soy isoflavones lower serum total and LDL cholesterol in humans: A meta‐analysis of 11 randomized controlled trials. The American Journal of Clinical Nutrition, 85(4), 1148–1156. 10.1093/ajcn/85.4.1148 [DOI] [PubMed] [Google Scholar]
- Tentolouris, C. , Tousoulis, D. , Davies, G. , Stefanadis, C. , Trikas, A. , Goumas, G. , & Toutouzas, P. (2000). The impact of risk factors for atherosclerosis on the vasomotor effects of inhibition of nitric oxide synthesis in patients with normal angiograms. Cardiology, 94(1), 26–30. 10.1159/000007042 [DOI] [PubMed] [Google Scholar]
- Vera‐Arzave, C. , Pacheco‐Yepez, J. , Mejía‐Barradas, C. M. , Cárdenas‐Jaramillo, L. M. , Campos‐Rodríguez, R. , & Abarca‐Rojano, E. (2019). 17β‐estradiol replacement therapy induces eNOS, nNOS and estrogen receptor β in hypophysectomized rats with inflamed footpads. Journal of Biological Regulators and Homeostatic Agents, 33(5), 1395–1403. 10.23812/19-64A [DOI] [PubMed] [Google Scholar]
- Wagle, A. , Seong, S. H. , Jung, H. A. , & Choi, J. S. (2019). Identifying an isoflavone from the root of Pueraria lobata as a potent tyrosinase inhibitor. Food Chemistry, 276, 383–389. 10.1016/j.foodchem.2018.10.008 [DOI] [PubMed] [Google Scholar]
- Wild, R. A. , Hovey, K. M. , Andrews, C. , Robinson, J. G. , Kaunitz, A. M. , Manson, J. E. , Crandall, C. J. , Paragallo, R. , Shufelt, C. , & Merz, C. N. (2021). Cardiovascular disease (CVD) risk scores, age, or years since menopause to predict cardiovascular disease in the Women's Health Initiative. Menopause, 28(6), 610–618. 10.1097/GME.0000000000001753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Pan Siu, A. K. , Lin, G. , & Zuo, Z. (2011). Intestinal absorbability of three Radix Puerariae isoflavones including daidzein, daidzin and puerarin. Chinese Medicine, 6, 1–8. 10.1186/1749-8546-6-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


