Abstract
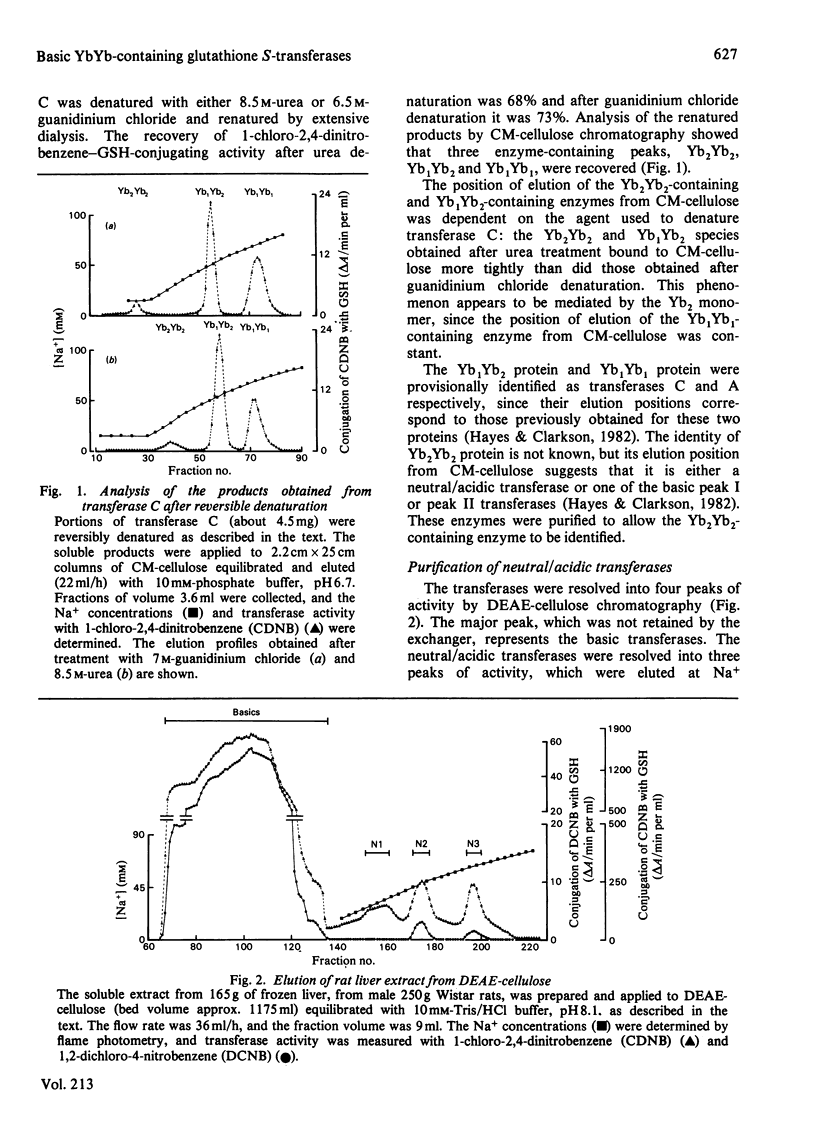
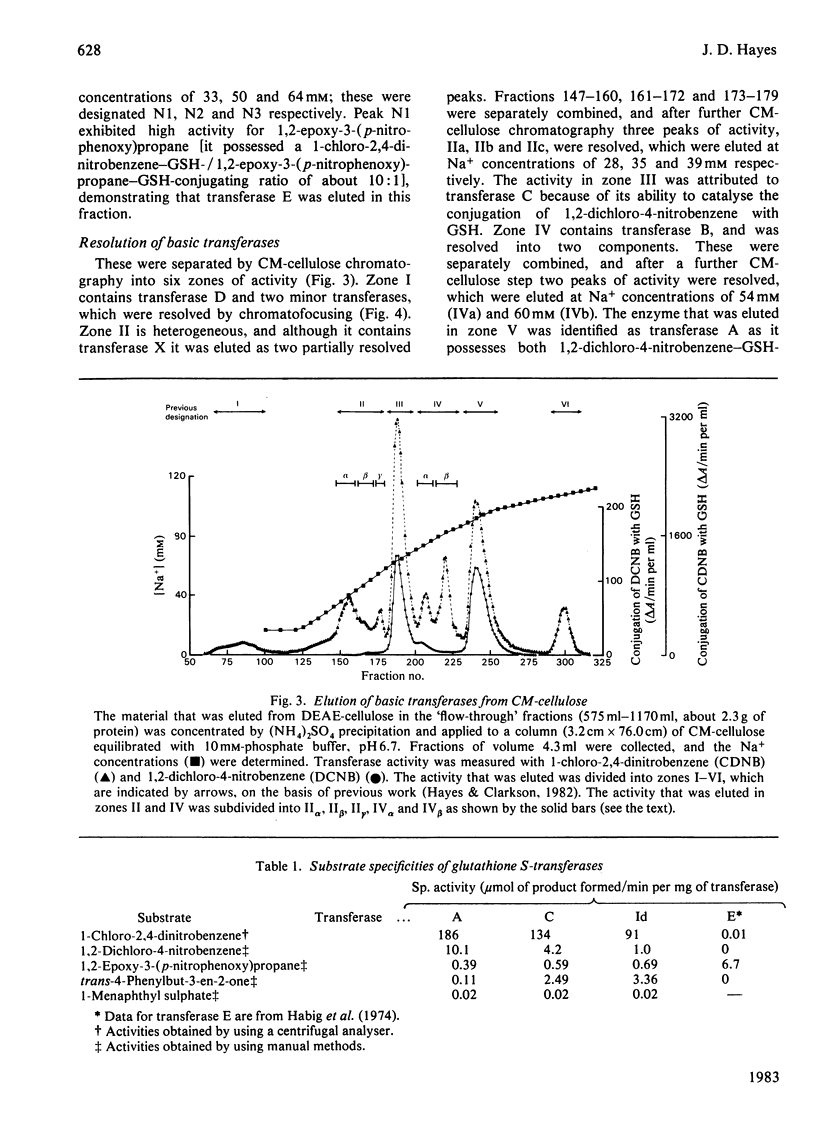
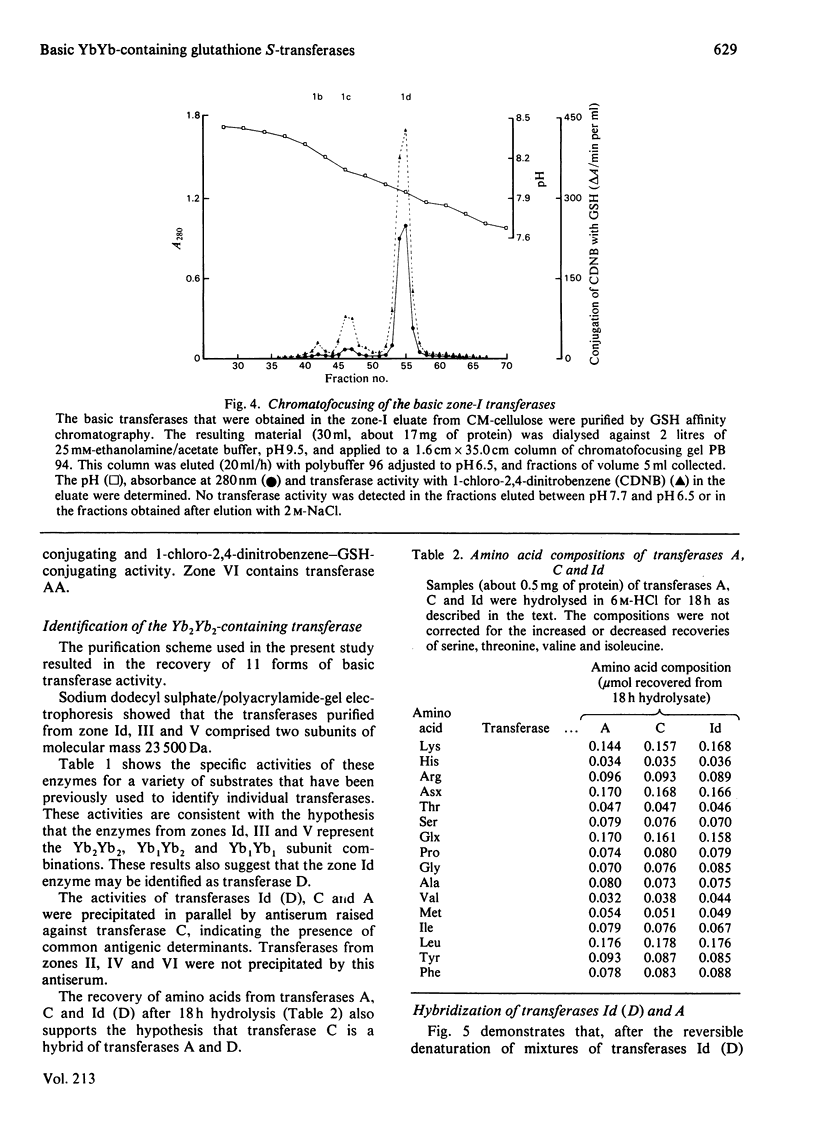
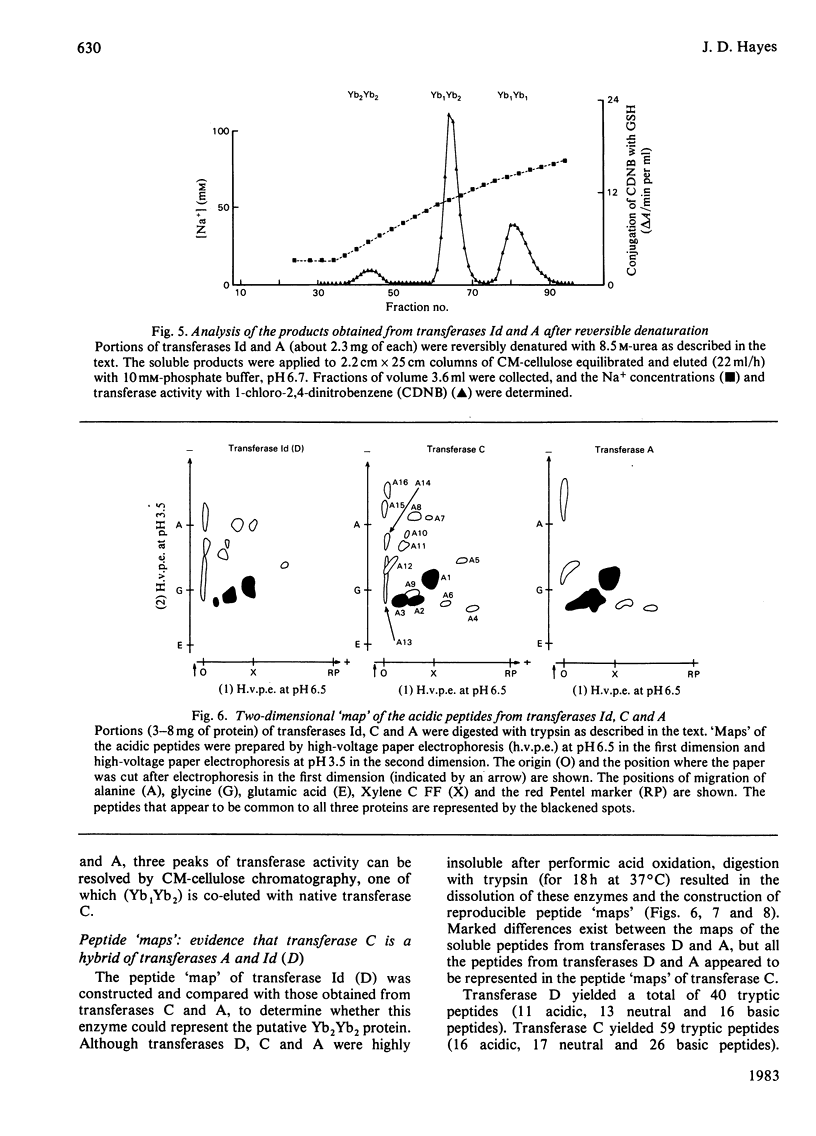
A purification scheme was devised that resulted in the resolution of a number of basic glutathione S-transferases from rat liver, three of which contained two subunits of molecular mass 23500 Da (i.e. Yb monomers). These were identified as transferases D, C and A by their elution positions from CM-cellulose and their specific activities towards a variety of substrates. Hybridization, immunotitration and peptide 'mapping' experiments demonstrated that transferases D, C and A comprise Yb2Yb2, Yb1Yb2 and Yb1Yb1 subunits.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Brown L. H. The amino acid sequence of Pseudomonas fluorescens azurin. Biochem J. 1967 Sep;104(3):784–825. doi: 10.1042/bj1040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass N. M., Kirsch R. E., Tuff S. A., Marks I., Saunders S. J. Ligandin heterogeneity : evidence that the two non-identical subunits are the monomers of two distinct proteins. Biochim Biophys Acta. 1977 May 27;492(1):163–175. doi: 10.1016/0005-2795(77)90223-9. [DOI] [PubMed] [Google Scholar]
- Beale D., Ketterer B., Carne T., Meyer D., Taylor J. B. Evidence that the Ya and Yc subunits of glutathione transferase B (ligandin) are the products of separate genes. Eur J Biochem. 1982 Sep 1;126(3):459–463. doi: 10.1111/j.1432-1033.1982.tb06802.x. [DOI] [PubMed] [Google Scholar]
- Fjellstedt T. A., Allen R. H., Duncan B. K., Jakoby W. B. Enzymatic conjugation of epoxides with glutathione. J Biol Chem. 1973 May 25;248(10):3702–3707. [PubMed] [Google Scholar]
- Gillham B. The reaction of aralkyl sulphate esters with glutathione catalysed by rat liver preparations. Biochem J. 1971 Feb;121(4):667–672. doi: 10.1042/bj1210667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferase AA from rat liver. Arch Biochem Biophys. 1976 Aug;175(2):710–716. doi: 10.1016/0003-9861(76)90563-4. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hayes J. D., Clarkson G. H. Purification and characterization of three forms of glutathione S-transferase A. A comparative study of the major YaYa-, YbYb- and YcYc-containing glutathione S-transferases. Biochem J. 1982 Dec 1;207(3):459–470. doi: 10.1042/bj2070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Strange R. C., Percy-Robb I. W. A study of the structures of the YaYa and YaYc glutathione S-transferases from rat liver cytosol. Evidence that the Ya monomer is responsible for lithocholate-binding activity. Biochem J. 1981 Aug 1;197(2):491–502. doi: 10.1042/bj1970491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Strange R. C., Percy-Robb I. W. Identification of two lithocholic acid-binding proteins. Separation of ligandin from glutathione S-transferase B. Biochem J. 1979 Sep 1;181(3):699–708. doi: 10.1042/bj1810699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketley J. N., Habig W. H., Jakoby W. B. Binding of nonsubstrate ligands to the glutathione S-transferases. J Biol Chem. 1975 Nov 25;250(22):8670–8673. [PubMed] [Google Scholar]
- Ketterer B., Beale D., Taylor J. B., Meyer D. J. The genetic relationships and inducibility of soluble glutathione transferases of the rat liver. Biochem Soc Trans. 1983 Aug;11(4):466–467. doi: 10.1042/bst0110466. [DOI] [PubMed] [Google Scholar]
- Kitahara A., Sato K. Immunological relationships among subunits of glutathione S-transferases A, AA, B and ligandin and hybrid formation between AA and ligandin by guanidine hydrochloride. Biochem Biophys Res Commun. 1981 Dec 15;103(3):943–950. doi: 10.1016/0006-291x(81)90901-3. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Jensson H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J Biol Chem. 1982 Sep 10;257(17):9909–9912. [PubMed] [Google Scholar]
- Pabst M. J., Habig W. H., Jakoby W. B. Mercapturic acid formation: the several glutathione transferases of rat liver. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1123–1128. doi: 10.1016/0006-291x(73)90616-5. [DOI] [PubMed] [Google Scholar]
- Pattinson N., Collins D., Campbell B. Covalent coupling of cholic acid to aminohexylamino-Sepharose 4B and its use in affinity chromatography of serum albumin. J Chromatogr. 1980 Jan 18;187(2):409–412. doi: 10.1016/s0021-9673(00)80473-2. [DOI] [PubMed] [Google Scholar]
- Pattinson N. Purification by affinity chromatography of glutathione S-transferases A and C from rat liver cytosol. Anal Biochem. 1981 Aug;115(2):424–427. doi: 10.1016/0003-2697(81)90028-2. [DOI] [PubMed] [Google Scholar]
- Scully N. C., Mantle T. J. Tissue distribution and subunit structures of the multiple forms of glutathione S-transferase in the rat. Biochem J. 1981 Jan 1;193(1):367–370. doi: 10.1042/bj1930367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977 Oct;82(2):334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Smith G. J., Ohl V. S., Litwack G. Ligandin, the glutathione S-transferases, and chemically induced hepatocarcinogenesis: a review. Cancer Res. 1977 Jan;37(1):8–14. [PubMed] [Google Scholar]
- Wolkoff A. W., Ketley J. N., Waggoner J. G., Berk P. D., Jakoby W. B. Hepatic accumulation and intracellular binding of conjugated bilirubin. J Clin Invest. 1978 Jan;61(1):142–149. doi: 10.1172/JCI108912. [DOI] [PMC free article] [PubMed] [Google Scholar]


