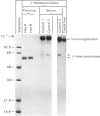
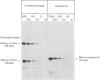
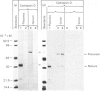
Abstract
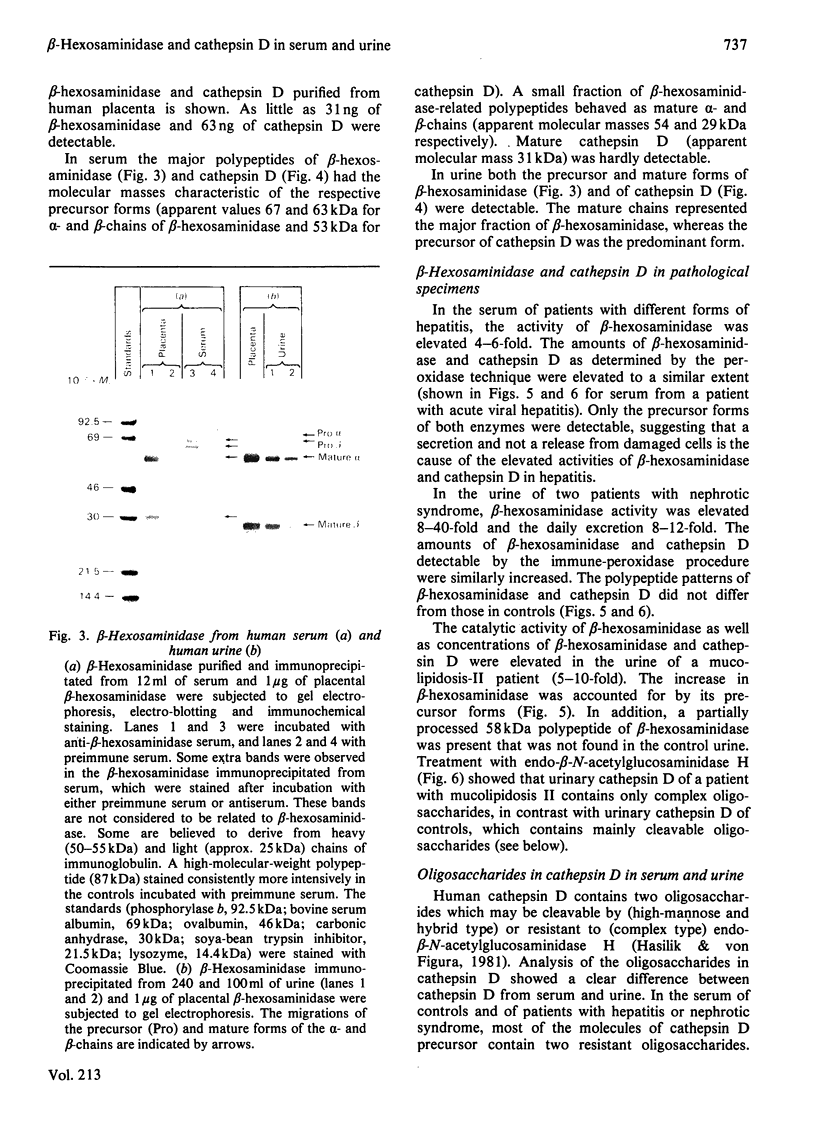
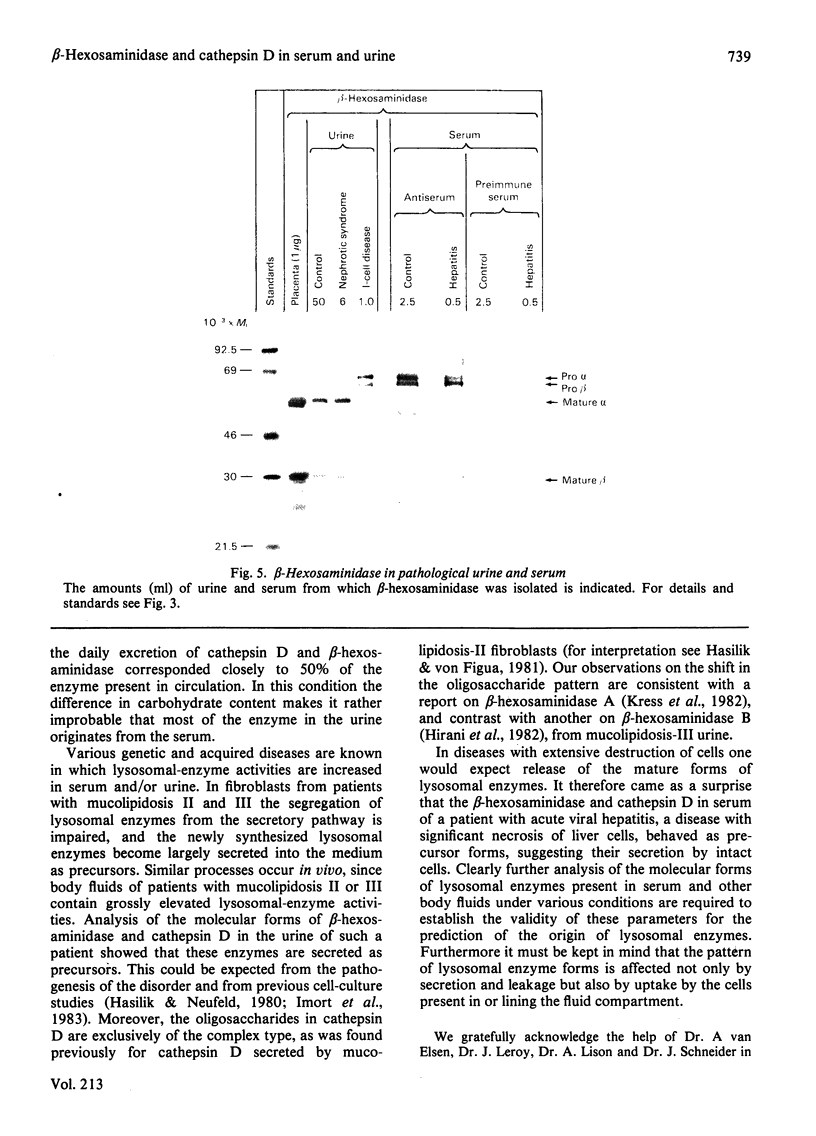
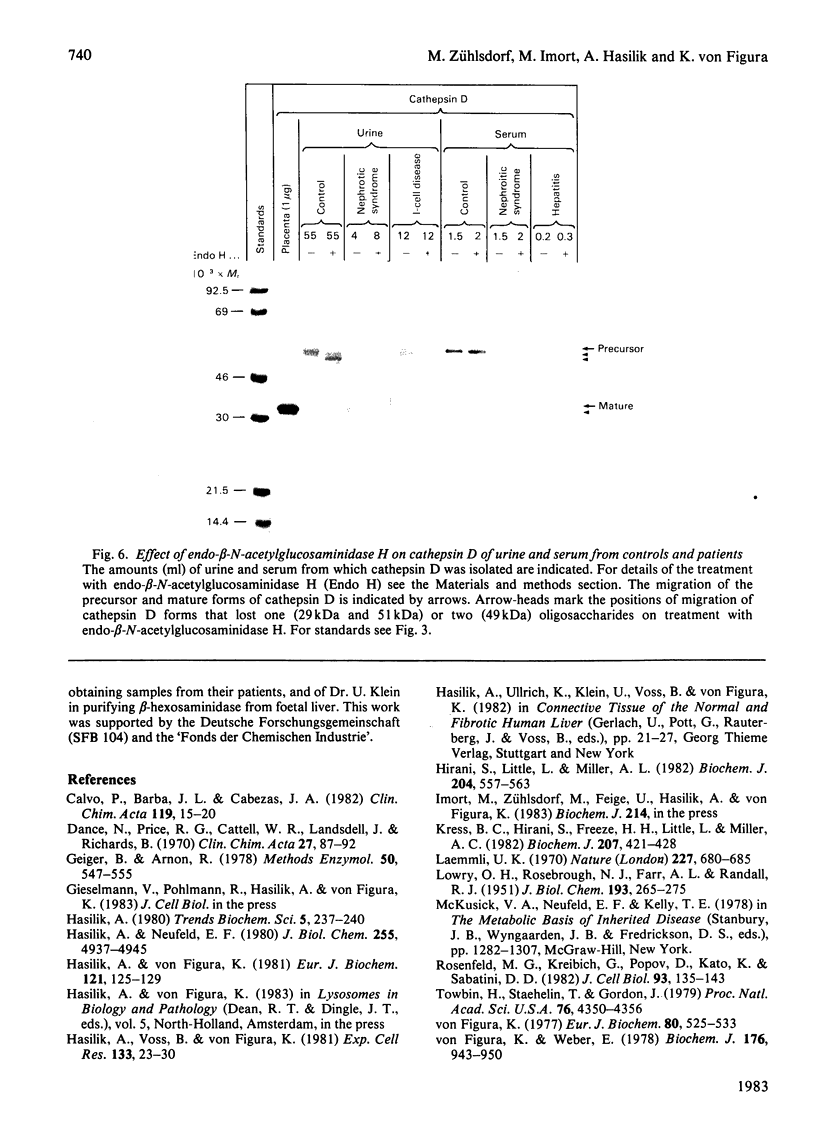
A procedure is described that allows the characterization of the molecular forms of beta-hexosaminidase and cathepsin D in controls and pathological specimens of human serum and human urine. The following observations were made. (1) In human serum, beta-hexosaminidase (alpha- and beta-chain) and cathepsin D are present predominantly in their high-molecular-weight precursor forms. In human urine, these enzymes exist as both precursor and mature forms. (2) Cathepsin D precursor from serum and urine differs in the number of oligosaccharides that are sensitive to endo-beta-N-acetylglucosaminidase H. Therefore the urine enzyme is not likely to originate from the serum. (3) The presence exclusively of precursors of beta-hexosaminidase and of cathepsin D in the sera of patients with hepatitis suggests that in hepatitis secretion of lysosomal enzymes is elevated, rather than the enzymes leaking from damaged cells. (4) In the urine of patients with nephrotic syndrome, beta-hexosaminidase and cathepsin D are present in grossly elevated amounts, but do not differ in the polypeptide patterns from controls. (5) In urine from a patient with mucolipidosis II, the elevated activity of beta-hexosaminidase is accounted for mainly by the precursor forms. Mature beta-chain of beta-hexosaminidase is lacking, and incompletely processed beta-hexosaminidase polypeptides are present. Both the precursor and the mature forms of cathepsin D are increased. They contain only complex oligosaccharides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvo P., Barba J. L., Cabezas J. A. Serum beta-N-acetylglucosaminidase, beta-D-glucosidase, alpha-D-glucosidase, beta-D-fucosidase, alpha-L-fucosidase and beta-D-galactosidase levels in acute viral hepatitis, pancreatitis, myocardial infarction and breast cancer. Clin Chim Acta. 1982 Feb 26;119(1-2):15–19. doi: 10.1016/0009-8981(82)90400-4. [DOI] [PubMed] [Google Scholar]
- Dance N., Price R. G., Cattell W. R., Lansdell J., Richards B. The excretion of N-acetyl-beta-glucosaminidase and beta-galactosidase by patients with renal disease. Clin Chim Acta. 1970 Jan;27(1):87–92. doi: 10.1016/0009-8981(70)90378-5. [DOI] [PubMed] [Google Scholar]
- Geiger B., Arnon R. Hexosaminidases A and B from human placenta. Methods Enzymol. 1978;50:547–555. doi: 10.1016/0076-6879(78)50061-x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hasilik A., Von Figura K. Oligosaccharides in lysosomal enzymes. Distribution of high-mannose and complex oligosaccharides in cathepsin D and beta-hexosaminidase. Eur J Biochem. 1981 Dec;121(1):125–129. doi: 10.1111/j.1432-1033.1981.tb06440.x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Voss B., Von Figura K. Transport and processing of lysosomal enzymes by smooth muscle cells and endothelial cells. Exp Cell Res. 1981 May;133(1):23–30. doi: 10.1016/0014-4827(81)90352-9. [DOI] [PubMed] [Google Scholar]
- Hirani S., Little L., Miller A. L. A study of highly purified mucolipidosis III urinary N-acetyl-beta-D-hexosaminidase B. Biochem J. 1982 May 15;204(2):557–563. doi: 10.1042/bj2040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress B. C., Hirani S., Freeze H. H., Little L., Miller A. L. Mucolipidosis III beta-N-acetyl-D-hexosaminidase A. Purification and properties. Biochem J. 1982 Dec 1;207(3):421–428. doi: 10.1042/bj2070421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Kreibich G., Popov D., Kato K., Sabatini D. D. Biosynthesis of lysosomal hydrolases: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution. J Cell Biol. 1982 Apr;93(1):135–143. doi: 10.1083/jcb.93.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K. Human alpha-N-acetylglucosaminidase. 1. Purification and properties. Eur J Biochem. 1977 Nov 1;80(2):523–533. [PubMed] [Google Scholar]
- von Figura K., Weber E. An alternative hypothesis of cellular transport of lysosomal enzymes in fibroblasts. Effect of inhibitors of lysosomal enzyme endocytosis on intra- and extra-cellular lysosomal enzyme activities. Biochem J. 1978 Dec 15;176(3):943–950. doi: 10.1042/bj1760943. [DOI] [PMC free article] [PubMed] [Google Scholar]