Abstract
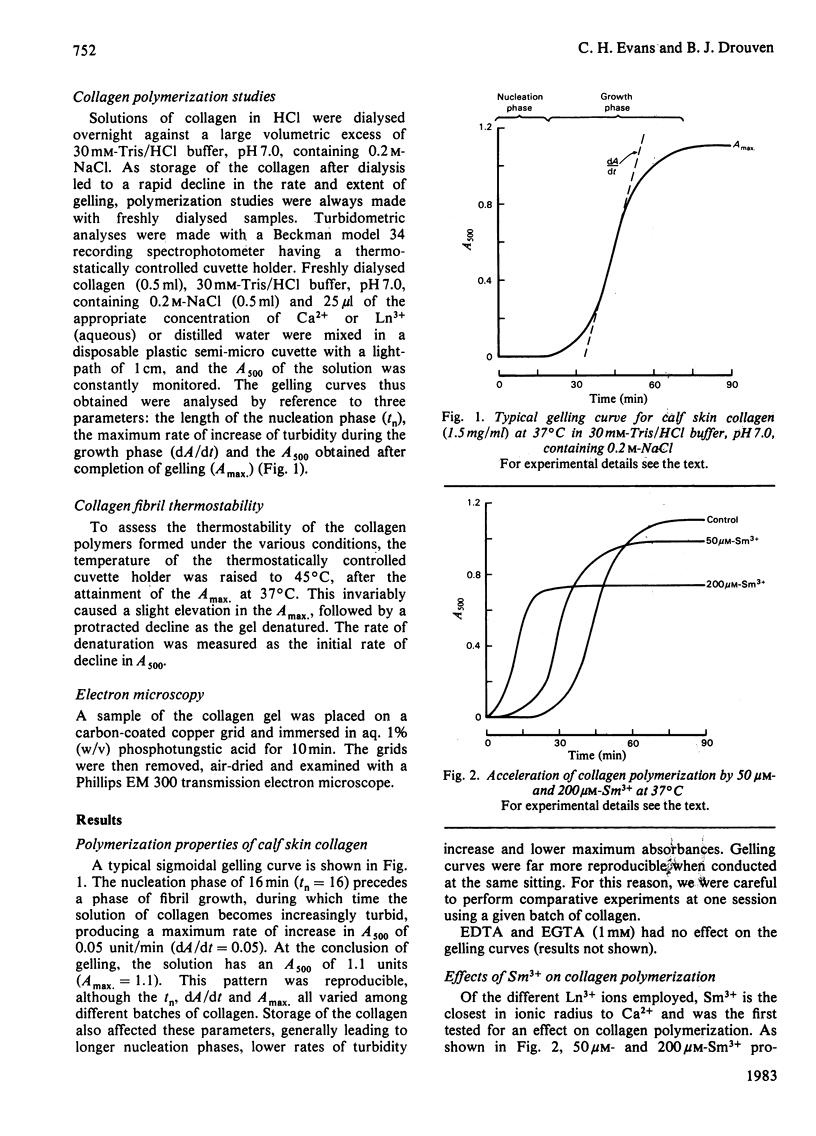
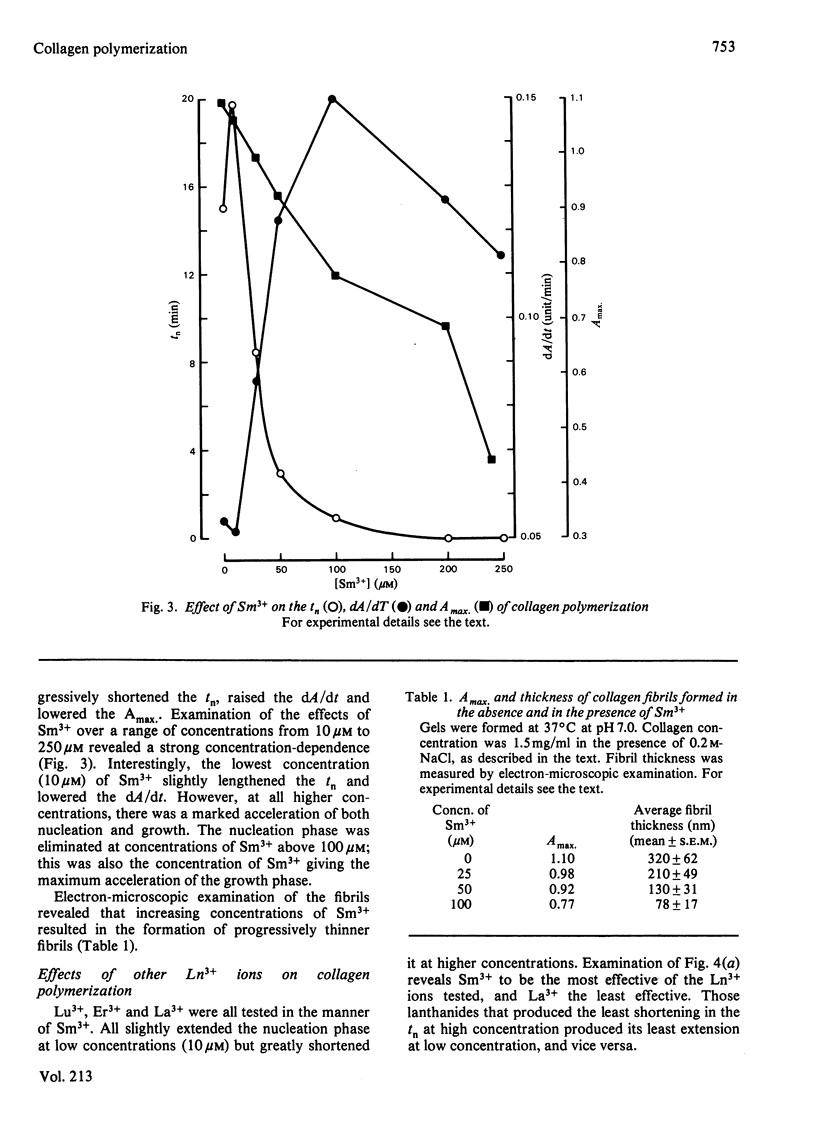
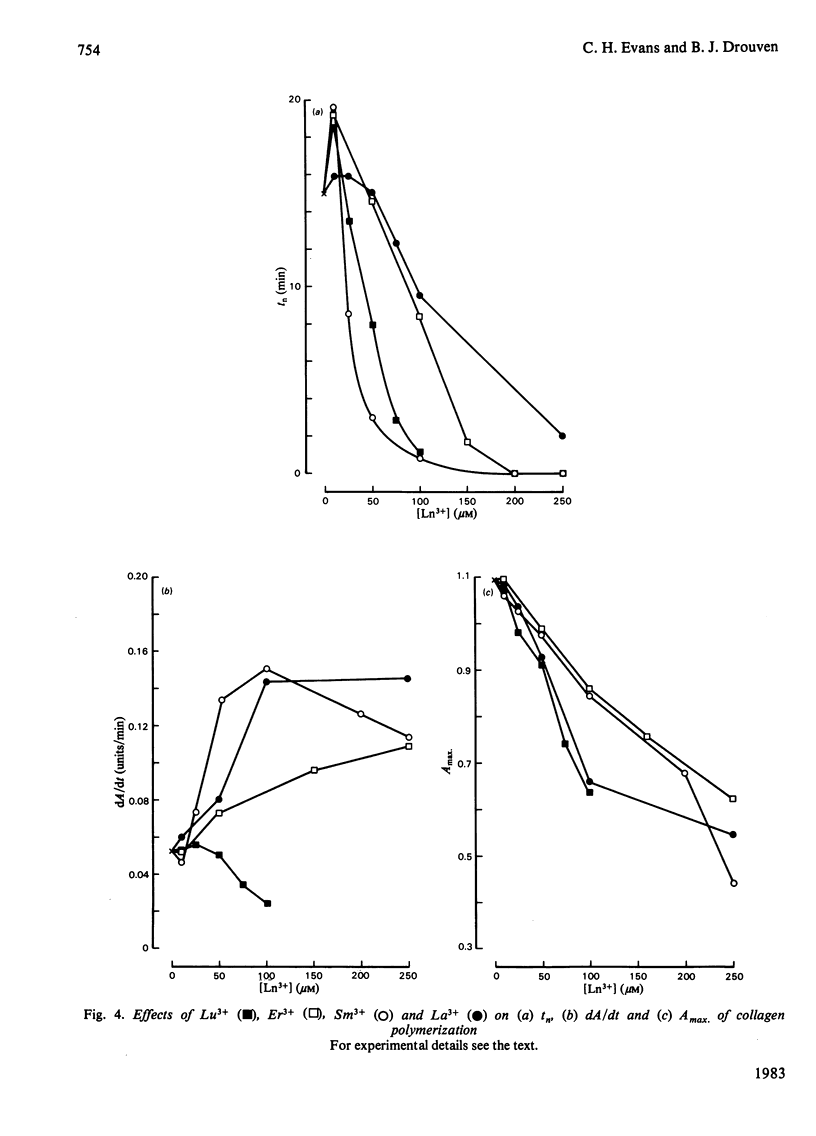
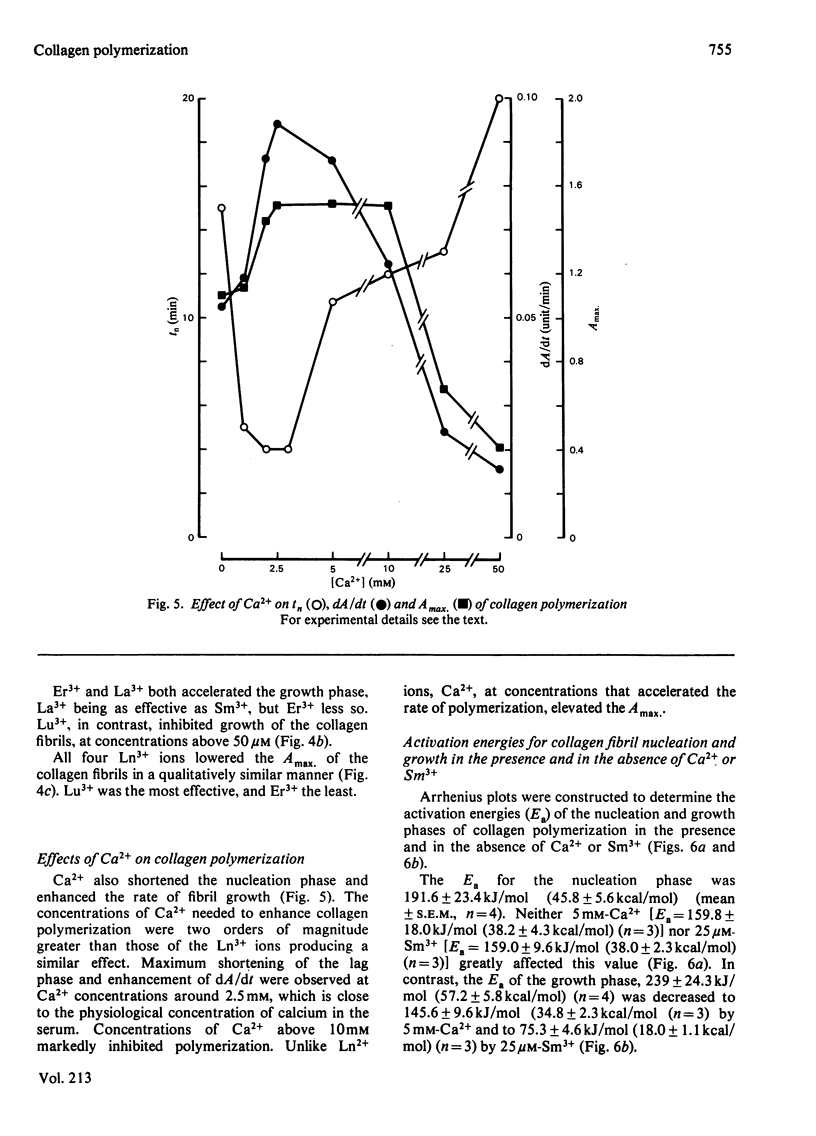
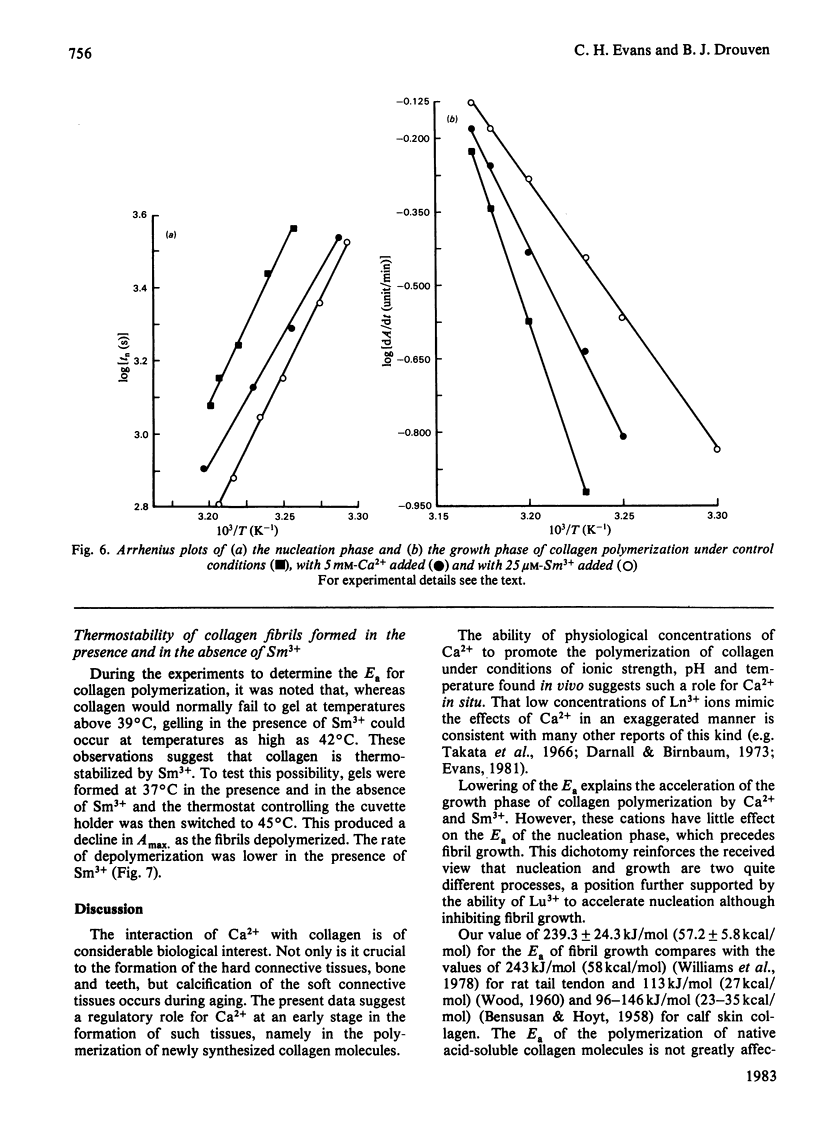
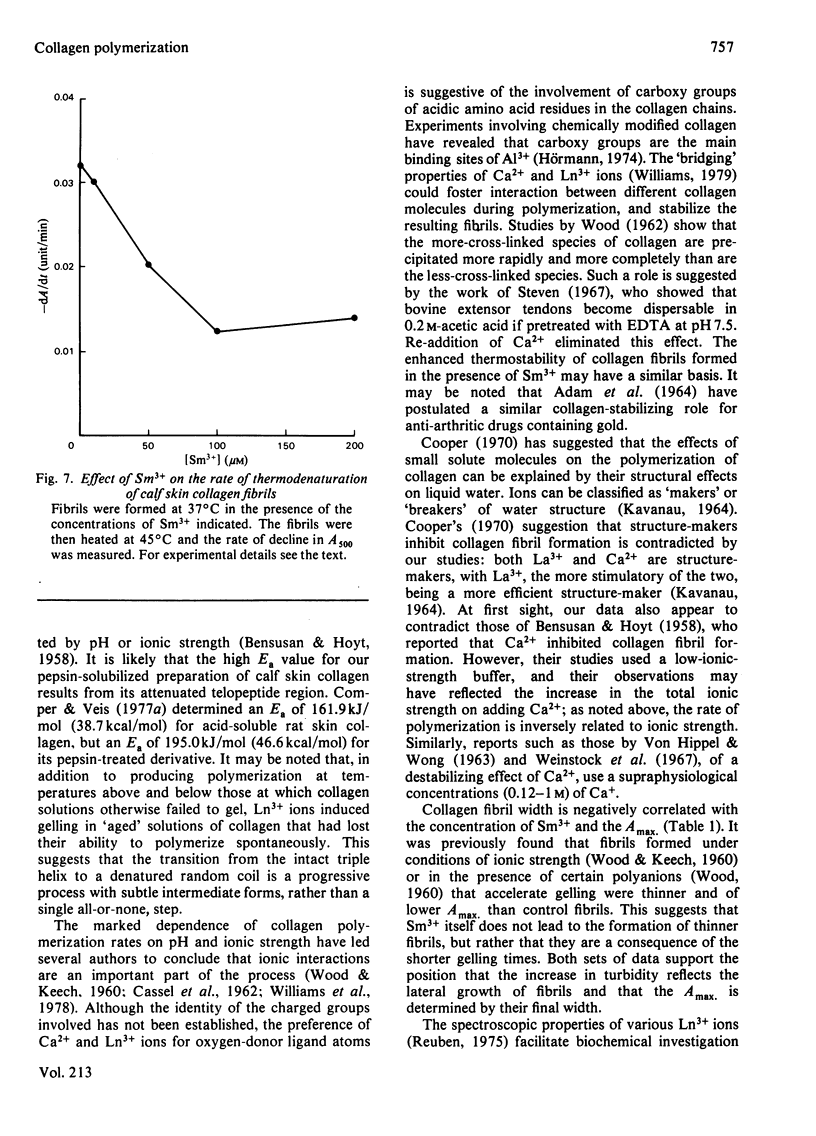
Ca2+ (1-5 mM) and lanthanide (20-250 microM) ions enhance the rate of polymerization of purified calf skin collagen (1.5 mg/ml) at pH 7.0 in the presence of 30mM-Tris/HCl and 0.2 M-NaCl. Both the nucleation phase and the growth phase of polymerization are accelerated. The activation energy of the growth phase, 239.3 +/- 24.3 kJ/mol (57.2 +/- 5.8 kcal/mol), is decreased to 145.6 +/- 9.6 kJ/mol (34.8 +/- 2.3 kcal/mol) by 5 mM-Ca2+ and to 75.3 +/- 4.6 kJ/mol (18.0 +/- 1.1 kcal/mol) by 25 microM-Sm3+. In contrast, the activation energy of the nucleation phase, 191.6 +/- 23.4 kJ/mol (45.8 +/- 5.6 kcal/mol), is only slightly decreased by Ca2+ or Sm3+. Collagen fibrils formed in the presence of Sm3+ are thinner than control fibrils, and more thermoresistant.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M., Bartl P., Deyl Z., Rosmus J. Reaction of gold with collagen in vivo. Experientia. 1964 Apr 15;20(4):203–204. doi: 10.1007/BF02135400. [DOI] [PubMed] [Google Scholar]
- Comper W. D., Veis A. Characterization of nuclei in in vitro collagen fibril formation. Biopolymers. 1977 Oct;16(10):2133–2142. doi: 10.1002/bip.1977.360161005. [DOI] [PubMed] [Google Scholar]
- Comper W. D., Veis A. The mechanism of nucleation for in vitro collagen fibril formation. Biopolymers. 1977 Oct;16(10):2113–2131. doi: 10.1002/bip.1977.360161004. [DOI] [PubMed] [Google Scholar]
- Cooper A. Thermodynamic studies of the assembly in vitro of native collagen fibrils. Biochem J. 1970 Jul;118(3):355–365. doi: 10.1042/bj1180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall D. W., Birnbaum E. R. Lanthanide ions activate alpha-amylase. Biochemistry. 1973 Aug 28;12(18):3489–3491. doi: 10.1021/bi00742a021. [DOI] [PubMed] [Google Scholar]
- Evans C. H., Mears D. C. Binding of the bone-seeking agent 99mTc-1-hydroxyethylidene-1,1-diphosphonic acid to cartilage and collagen in vitro and its stimulation by Er3+ and low pH. Calcif Tissue Int. 1980;32(2):91–94. doi: 10.1007/BF02408527. [DOI] [PubMed] [Google Scholar]
- Evans C. H., Tew W. P. Isolation of biological materials by use of erbium (III)--induced magnetic susceptibilities. Science. 1981 Aug 7;213(4508):653–654. doi: 10.1126/science.7256262. [DOI] [PubMed] [Google Scholar]
- Silver F. H., Trelstad R. L. Linear aggregation and the turbidimetric lag phase: type I collagen fibrillogenesis in vitro. J Theor Biol. 1979 Dec 7;81(3):515–526. doi: 10.1016/0022-5193(79)90049-3. [DOI] [PubMed] [Google Scholar]
- Steven F. S. The effect of chelating agents on collagen interfibrillar matrix interactions in connective tissue. Biochim Biophys Acta. 1967 Aug 15;140(3):522–528. doi: 10.1016/0005-2795(67)90526-0. [DOI] [PubMed] [Google Scholar]
- Takata M., Pickard W. F., Lettvin J. Y., Moore J. W. Ionic conductance changes in lobster axon membrane when lanthanum is substituted for calcium. J Gen Physiol. 1966 Nov;50(2):461–471. doi: 10.1085/jgp.50.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew W. P. Use of the coulombic interactions of the lanthanide series to identify two classes of Ca2+ binding sites in mitochondria. Biochem Biophys Res Commun. 1977 Sep 23;78(2):624–630. doi: 10.1016/0006-291x(77)90225-x. [DOI] [PubMed] [Google Scholar]
- VONHIPPEL P. H., WONG K. Y. THE COLLAGEN GELATIN PHASE TRANSITION. I. FURTHER STUDIES OF THE EFFECTS OF SOLVENT ENVIRONMENT AND POLYPEPTIDE CHAIN COMPOSITION. Biochemistry. 1963 Nov-Dec;2:1387–1398. doi: 10.1021/bi00906a035. [DOI] [PubMed] [Google Scholar]
- WOOD G. C., KEECH M. K. The formation of fibrils from collagen solutions. 1. The effect of experimental conditions: kinetic and electron-microscope studies. Biochem J. 1960 Jun;75:588–598. doi: 10.1042/bj0750588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD G. C. The formation of fibrils from collagen solutions. 2. A mechanism of collagen-fibril formation. Biochem J. 1960 Jun;75:598–605. doi: 10.1042/bj0750598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD G. C. The heterogeneity of collagen solutions and its effect on fibril formation. Biochem J. 1962 Aug;84:429–435. doi: 10.1042/bj0840429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock A., King P. C., Wuthier R. E. The ion-binding characteristics of reconstituted collagen. Biochem J. 1967 Mar;102(3):983–988. doi: 10.1042/bj1020983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. R., Gelman R. A., Poppke D. C., Piez K. A. Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J Biol Chem. 1978 Sep 25;253(18):6578–6585. [PubMed] [Google Scholar]
- Williams R. J. Irish area section guest lecture. Biochem Soc Trans. 1979 Jun;7(3):481–509. doi: 10.1042/bst0070481. [DOI] [PubMed] [Google Scholar]


