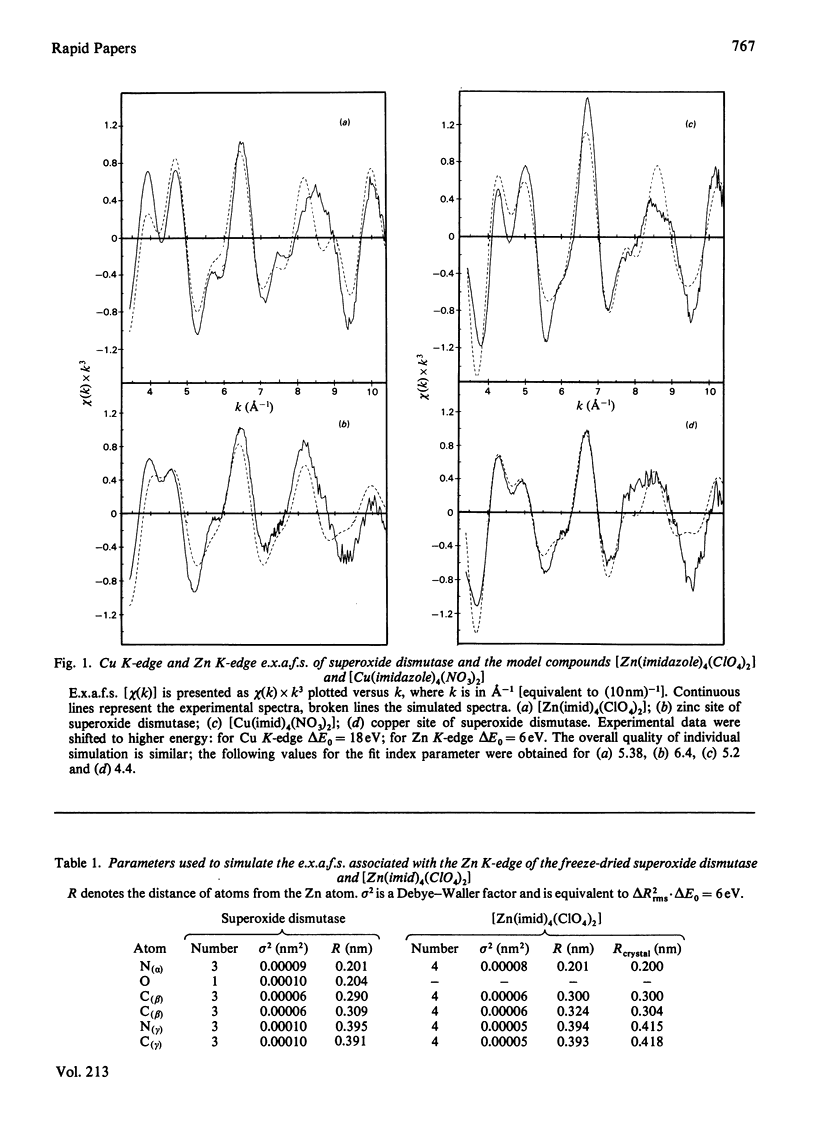
Abstract
Copper and zinc K-edge-extended X-ray-absorption fine structures were measured for the metal sites of freeze-dried bovine superoxide dismutase and the model compounds tetrakis(imidazole)cupric nitrate and tetrakis(imidazole)zinc perchlorate. Detailed simulation of the spectra indicates that the copper site of the enzyme is best fit by co-ordination of four imidazole groups with Cu-N(alpha) distances of 0.198 nm (1.98 A). The zinc site is best fit by three imidazole groups at 0.201 nm (2.01 A) and an oxygen (from aspartate) at 0.203 nm (2.03 A).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beem K. M., Rich W. E., Rajagopalan K. V. Total reconstitution of copper-zinc superoxide dismutase. J Biol Chem. 1974 Nov 25;249(22):7298–7305. [PubMed] [Google Scholar]
- Beem K. M., Richardson D. C., Rajagopalan K. V. Metal sites of copper-zinc superoxide dismutase. Biochemistry. 1977 May 3;16(9):1930–1936. doi: 10.1021/bi00628a027. [DOI] [PubMed] [Google Scholar]
- Lieberman R. A., Sands R. H., Fee J. A. A study of the electron paramagnetic resonance properties of single monoclinic crystals of bovine superoxide dismutase. J Biol Chem. 1982 Jan 10;257(1):336–344. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Perutz M. F., Hasnain S. S., Duke P. J., Sessler J. L., Hahn J. E. Stereochemistry of iron in deoxyhaemoglobin. Nature. 1982 Feb 11;295(5849):535–538. doi: 10.1038/295535a0. [DOI] [PubMed] [Google Scholar]
- Richardson J., Thomas K. A., Rubin B. H., Richardson D. C. Crystal structure of bovine Cu,Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1349–1353. doi: 10.1073/pnas.72.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotilio G., Morpurgo L., Giovagnoli C., Calabrese L., Mondovì B. Studies of the metal sites of copper proteins. Symmetry of copper in bovine superoxide dismutase and its functional significance. Biochemistry. 1972 May 23;11(11):2187–2192. doi: 10.1021/bi00761a028. [DOI] [PubMed] [Google Scholar]
- Whitney P. L. Affinity chromatography of carbonic anhydrase. Anal Biochem. 1974 Feb;57(2):467–476. doi: 10.1016/0003-2697(74)90102-x. [DOI] [PubMed] [Google Scholar]


