Abstract
High-grade prostatic intraepithelial neoplasia (HGPIN) is a well-characterised precursor lesion in prostate cancer. The term atypical intraductal proliferations (AIP) describes lesions with features that are far too atypical to be considered HGPIN, yet insufficient to be diagnosed as intraductal carcinoma of the prostate (IDCP). Here, a panel of biomarkers was assessed to provide insights into the biological relationship between IDCP, HGPIN, and AIP and their relevance to current clinicopathological recommendations. Tissue samples from 86 patients with prostate cancer were assessed by routine haematoxylin and eosin staining and immunohistochemistry (IHC) with a biomarker panel (Appl1/Sortilin/Syndecan-1) and a PIN4 cocktail (34βE12+P63/P504S). Appl1 strongly labelled atypical secretory cells, effectively visualising intraductal lesions. Sortilin labelling was moderate-to-strong in > 70% of cases, while Syndecan-1 was moderate-to-strong in micropapillary HGPIN/AIP lesions (83% cases) versus flat/tufting HGPIN (≤ 20% cases). Distinct biomarker labelling patterns for atypical intraductal lesions of the prostate were observed, including early atypical changes (flat/tufting HGPIN) and more advanced atypical changes (micropapillary HGPIN/AIP). Furthermore, the biomarker panel may be used as a tool to overcome the diagnostic uncertainty surrounding AIP by supporting a definitive diagnosis of IDCP for such lesions displaying the same biomarker pattern as cribriform IDCP.
Keywords: High-grade prostatic intraepithelial neoplasia, Atypical intraductal proliferation, Immunohistochemistry, Biomarkers, Diagnosis, Prostatic adenocarcinoma
Background
High-grade prostatic intraepithelial neoplasia (HGPIN) comprises three morphological patterns: flat, tufting, and micropapillary (MP) [1]. Atypical intraductal cribriform proliferations with cytological features of malignancy are no longer considered a HGPIN pattern, but now classified as IDCP (loose/dense), with patients displaying similar clinical behaviour to other IDCP patterns [2, 3]. In instances where atypical intraductal proliferative lesions are architecturally and/or cytologically more complex than HGPIN, but fall short of meeting the criteria of IDCP, the term atypical intraductal proliferations (AIP) is assigned to communicate diagnostic uncertainty. Currently, observations of HGPIN require conservative monitoring regimens without the justification of repeat biopsies [4], while AIP lesions warrant immediate follow-up, as such lesions may be indicative of unsampled IDCP and/or invasive prostate cancer [5].
The endosome lysosome biogenesis is integrally involved in all hallmarks of cancer, displaying altered biogenesis and function in prostate cancer biology [6–8]. A biomarker panel comprising Appl1, Sortilin, and Syndecan-1 has recently been used to define the complex biological changes contributing to prostate cancer pathogenesis and IDCP [9–11]. This study aimed to extend this research by evaluating the biomarker panel on prostatic precursor intraductal proliferative lesions to ascertain their suitability for inclusion within the current clinicopathological recommendations for guiding patient management.
Methods
Patient tissue was sourced from the Kathleen Cuningham Foundation Consortium for research into Familial Breast cancer (kConFab). Serial, formalin-fixed paraffin-embedded, radical prostatectomy sections from 87 patients with prostatic adenocarcinoma (Table 1) were stained with haematoxylin and eosin (H&E) and labelled by IHC (the biomarker panel and PIN4 cocktail). Tissue sections were stained with routine Ehrlich’s H & E (Australian Biostain Pty Ltd., VIC, Australia), using the Leica ST5010 Autostainer XL (Leica Biosystems, VIC, Australia) automated staining platform. IHC labelling was performed on a Ventana BenchMark Ultra platform (Roche Diagnostics Pty Ltd., NSW, Australia). Briefly, epitope retrieval was performed in CC1 buffer (at 95 °C for 32 min for the biomarker panel, and 64 min for the 34βE12+P63/P504S; Roche, Australia). Tissue sections were then incubated with monoclonal antibodies to Appl1, Sortilin, or Syndecan-1, for 1 h at 36 °C, or with 34βE12+P63 for 32 min and P504S 16 min at 36 °C. Detection and visualisation were performed using the OptiView DAB Detection Kit (Roche, Australia). Tissue sections were counterstained with Gill’s haematoxylin (Roche, Australia) and mounted with D.P.X. mounting medium (Thermo Fisher Scientific, VIC, Australia). All tissue slides were scanned using a Zeiss Axio Scan.Z1 in brightfield mode, with a Plan-Achromat 20× objective (Zeiss, Germany). In total, tissue from 87 patients was stained by H&E and labelled by IHC, but one patient was excluded due to technical issues during immunolabelling. Intraductal lesions that were located within 2 mm of the adenocarcinoma were assessed.
Table 1.
Patient characteristics
| Patient characteristics | ||||||
| Patients | 86 | |||||
| Patients with HGPIN or AIP | 64 | |||||
| Mean age (years) | 61.8 | |||||
| RP Grade Group | Total cases | No. patients with HGPIN or AIP | % Flat HGPIN (n) | % Tufting HGPIN (n) | % MP HGPIN (n) | % AIP (n) |
| 1 | 13 | 9 | 56 (5) | 78 (7) | 67 (6) | 33 (3) |
| 2 | 36 | 26 | 58 (15) | 81 (21) | 73 (19) | 23 (6) |
| 3 | 18 | 17 | 59 (10) | 65 (11) | 41 (7) | 71 (12) |
| 4 | 2 | 1 | 100 (1) | 100 (1) | 100 (1) | 100 (1) |
| 5 | 11 | 9 | 67 (6) | 56 (5) | 78 (7) | 67 (6) |
| NA | 6 | 2 | 50 (1) | 50 (1) | 50 (1) | 100 (2) |
| Total | 86 | 64 | 59 (38) | 72 (46) | 64 (41) | 47 (30) |
AIP atypical intraepithelial neoplasia, HGPIN high-grade prostatic intraepithelial neoplasia, MP micropapillary, RP radical prostatectomy, NA RP Grade Group not available
Results
Intraductal non-IDCP lesions in prostate carcinoma
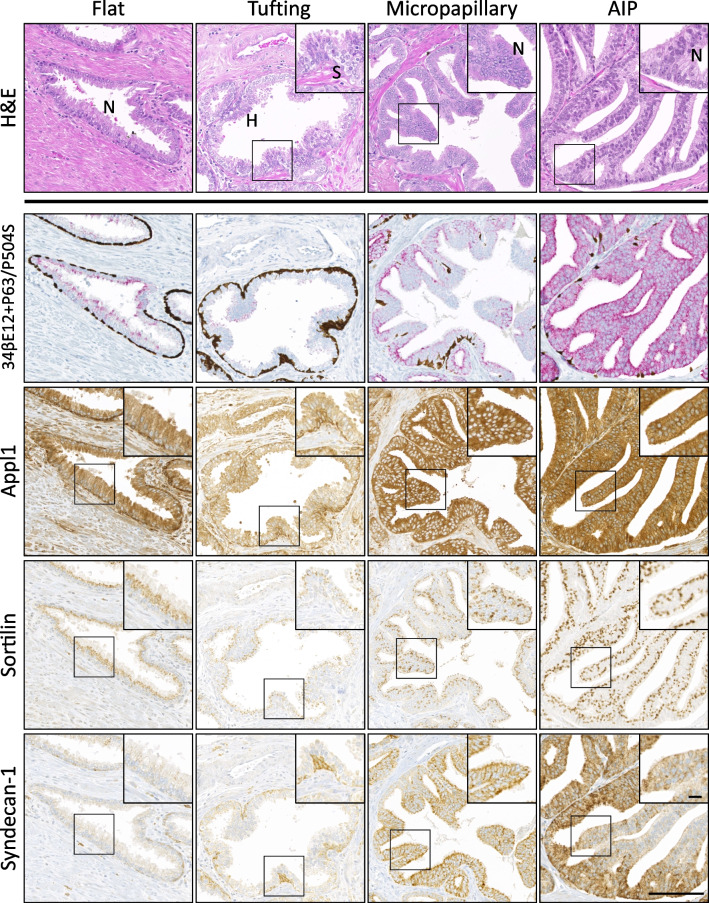
Histological and IHC assessment of radical prostatectomy tissue samples enabled the identification of four non-IDCP intraductal lesions, which were found in 76.7% of cases. These were classified according to morphological features: nuclear enlargement/stratification/crowding and prominent nucleoli with or without nuclear hyperchromasia (Fig. 1). PIN4 immunolabelling often revealed an incomplete basal cell layer (34βE12+P63) and demonstrated variable P504S labelling of atypical secretory epithelial cells in HGPIN (flat, tufting, and MP) and AIP lesions (Fig. 1). The most common patterns of HGPIN were tufting and MP (72% and 64%, respectively), followed by flat (59%), and lesions assigned as AIP (47%) (Table 1). Furthermore, less than 23% of patients (n = 15) had one lesion alone, wherein flat, tufting, and MP HGPIN was found equally (6% patients). A total of 33% patients had two lesions occurring together, with the most common being tufting and MP HGPIN (11% patients). A total of 22% of patients displayed three lesions, the most common combination being flat, tufting, and MP HGPIN (9% patients), while another 22% of the patients had four lesions occurring together.
Fig. 1.
Biomarker panel expression in precursor lesions of prostate cancer. Representative regions of precursor HGPIN (flat, tufting, MP) and AIP lesions were stained with routine H&E (top row) or immunohistochemically labelled with antibodies against basal cell/AMACR cocktail (34βE12+P63/P504S; second row), Appl1 (third row), Sortilin (fourth row), and Syndecan-1 (fifth row). H, nuclear hyperchromasia; N, prominent nucleoli; S, stratification and crowding. Scale bar in image represents 100 μm (20 μm in inset)
Discrete biomarker expression pattern in prostate cancer precursor lesions and AIP
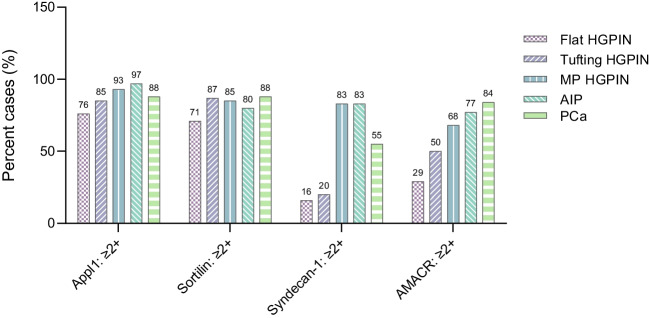
Appl1 labelled basal cells with high intensity, while atypical secretory epithelial cells in glands with HGPIN (flat, tufting, and MP) and AIP displayed moderate-to-high labelling intensity (≥ 76% cases, Fig. 2). Sortilin labelling was only detected in secretory cells, in a supranuclear location that was often diffuse in flat HGPIN (Fig. 1). Although Sortilin labelling was detected at a moderate-to-high intensity in all assessed patterns (≥ 71% cases), tufting HGPIN displayed a polarised distribution towards the periphery of the luminal proliferations. While Sortilin appeared disorganised in tufting HGPIN due to the pseudostratification of the cells, MP HGPIN and AIP displayed a highly organised distribution of Sortilin (Fig. 1). Syndecan-1 labelling intensity was moderate-to-strong in patterns with large epithelial proliferations (MP HGPIN and AIP; 83% cases), when compared to flat HGPIN or small luminal protrusions (tufting; ≤ 20% cases) (Fig. 2). The pattern of expression observed in MP HGPIN/AIP (Appl1 labelling with positive Sortilin and Syndecan-1) is similar to that in cribriform IDCP patterns [10]. The majority of the intraductal lesions were in the immediate vicinity of the invasive component (69%), while 31% were distant to the cancer, but within 2 mm distance. When the lesions were compared to the invasive component, the pattern of expression was similar, displaying moderate-to-high intensity of Appl1 and Sortilin in 88% of cases and moderate-to-high intensity of Syndecan-1 in 55% of cases. Notably, the majority of MP and AIP lesions (83%) had comparable or higher expression of Syndecan-1 than that expressed in the cancer component, versus levels observed in flat or tufting HGPIN lesions (20%) (Fig. 2).
Fig. 2.
Expression pattern in atypical intraductal lesions of the prostate. Appl1/Sortilin/Syndecan-1 and AMACR expression in HGPIN, AIP, and prostatic adenocarcinoma. Intensity was scored 0–3+, and moderate-to-high (≥ 2) expression is shown
Discussion
The markers Appl1, Sortilin, and Syndecan-1, which are at critical control points in the endosome-lysosome system, form a biomarker panel that can accurately map the pathogenesis in prostate cancer and assist Gleason grading to enable more accurate prediction of biochemical and clinical recurrence in patients [9, 11]. More recently, this biomarker panel has shed light on the retrograde spread theory of IDCP, wherein cribriform patterns displayed strong Appl1, Sortilin, and Syndecan-1 labelling patterns, while solid IDCP architecture had high intensity Appl1 and Syndecan-1 labelling, but minimal Sortilin labelling [10].
Here, the expression pattern of the biomarker panel was assessed in atypical intraductal proliferative lesions in prostate cancer, revealing distinct patterns of expression with Appl1, Sortilin, and Syndecan-1, including (1) labelling of Appl1/Sortilin in flat/tufting HGPIN and (2) labelling of Appl1, Sortilin, and Syndecan-1 in MP HGPIN and AIP. The observation that the MP HGPIN and AIP have a similar pattern of expression is not surprising, since morphologically both patterns comprise large epithelial proliferations, suggesting that MP is an architecturally more advanced lesion than flat/tufting HGPIN. In accordance, the tufting/flat HGPIN pattern is associated with a significantly lower risk of cancer upon follow-up, when compared to MP HGPIN and cribriform type HGPIN (a lesion now classified as either AIP or diagnosed as IDCP if there is sufficient atypia) [12].
While lesions assigned the term AIP share similar ERG+/PTEN status with IDCP, this expression profile is not observed in HGPIN lesions [13, 14], but these studies did not segregate HGPIN patterns. In this study, the segregation of patterns revealed that MP HGPIN lesions share a similar Appl1/Sortilin/Syndecan-1 profile to AIP/IDCP lesions [10], raising two important considerations. Firstly, according to current clinicopathological recommendations, AIP requires immediate repeat biopsy as this may be indicative of unsampled IDCP, but a similar consideration may now be warranted for MP HGPIN lesions displaying this labelling pattern. Secondly, some cases of AIP cannot be definitively diagnosed as IDCP for a variety of reasons, including incomplete sampling by needle biopsy. However, this study shows that some AIP lesions display the same Appl1/Sortilin/Syndecan-1 labelling pattern that is observed in IDCP (loose/dense cribriform) [10]. This suggests that the panel of biomarkers has utility in identifying IDCP in lesions previous termed AIP.
Management of patients with precursor lesions of prostate cancer is dependent on the accurate distinction between HGPIN patterns and those assigned AIP. The Appl1, Sortilin, and Syndecan-1 biomarker panel can depict either early atypical changes (flat/tufting HGPIN) or more advanced atypical changes (MP HGPIN/AIP). Furthermore, the biomarker panel may be used as a tool to overcome the diagnostic uncertainty surrounding AIP by supporting a definitive diagnosis of IDCP for such lesions displaying the same labelling pattern as cribriform IDCP. The results from this study shed light on precursor lesions of prostate cancer and present a novel panel of biomarkers that can be used to guide patient management.
Acknowledgements
We wish to thank Heather Thorne, Eveline Niedermayr, Sharon Guo, all the kConFab research nurses and staff, the heads and staff of the Family Cancer Clinics, and the Clinical Follow-Up Study (which has received funding from the NHMRC, the National Breast Cancer Foundation, Cancer Australia, and the National Institute of Health (USA)) for their contributions to this resource, and the many families who contribute to kConFab. kConFab is supported by a grant from the National Breast Cancer Foundation and previously by the National Health and Medical Research Council (NHMRC), the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia, and the Cancer Foundation of Western Australia. We also wish to thank Niamh Kernan, Jacqui B Crowley, and Prerna Tewari for assisting with development of the Ventana BenchMark Ultra protocol.
Author contribution
C.M, J.M.L, A.S, L.K, H.S, B.D, and D.A.B: intellectual input and experiment design. C.M, J.M.L, A.S, M.C.C, S.K, S.P, J.J.O’L, H.S, B.D, and D.A.B: concept development. C.M, J.M.L, B.S-Y.U, L.K, H.S, B.D, and D.A.B: interpretation and figure preparation. All authors were involved in manuscript drafting, revising the paper and gave final approval.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The research in this study was funded by the Envision Sciences Pty Ltd, the University of South Australia, a Cancer Council grant, a MTP connect/MRFF grant, and a NHMRC development grant.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethics approval for sample access and use of de-identified human prostate tissues from the kConFab was obtained through the institutional review board, Human Research Ethics Committee of the University of South Australia (Application IDs: 201907 and 0000036070). The study was performed in accordance with the Declaration of Helsinki.
Competing interests
D.A.B and J.J.O’L are shareholders for Envision Sciences Pty Ltd and benefit from this company’s research funding. D.A.B and I.R.D.J have a patent WO2014197937A1 that has been licensed by UniSA Ventures to Envision Sciences Pty Ltd for commercialisation. DAB is named as inventor on an additional patent, PCT/AU2020/050925, involving the invention used in this manuscript, which is owned by Envision Sciences Pty Ltd. C.M, J.M.L, A.S, S.P, B.S-Y.U, and I.R.D.J are employed by the University of South Australia using funding from Envision Sciences Pty Ltd.
Footnotes
The original online version of this article was revised: In this article the authors listed under kConFab Consortium should have been part of the main authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/29/2023
A Correction to this paper has been published: 10.1007/s00428-023-03666-8
References
- 1.Bostwick DG, Liu L, Brawer MK, Qian J (2004) High-grade prostatic intraepithelial neoplasia. Rev Urol 6:171–179. 10.1038/modpathol.3800053 [PMC free article] [PubMed] [Google Scholar]
- 2.Netto GJ, Amin MB, Berney DM et al (2022) The 2022 World Health Organization classification of tumors of the urinary system and male genital organs-part B: prostate and urinary tract tumors. Eur Urol 82:469–482. 10.1016/j.eururo.2022.07.002 [DOI] [PubMed] [Google Scholar]
- 3.Hickman RA, Yu H, Li J et al (2017) Atypical intraductal cribriform proliferations of the prostate exhibit similar molecular and clinicopathologic characteristics as intraductal carcinoma of the prostate. Am J Surg Pathol 41:550–556. 10.1097/PAS.0000000000000794 [DOI] [PubMed] [Google Scholar]
- 4.Morote J, Schwartzmann I, Celma A et al (2022) The current recommendation for the management of isolated high-grade prostatic intraepithelial neoplasia. BJU Int 129:627–633. 10.1111/bju.15568 [DOI] [PubMed] [Google Scholar]
- 5.Shah RB, Nguyen JK, Przybycin CG et al (2019) Atypical intraductal proliferation detected in prostate needle biopsy is a marker of unsampled intraductal carcinoma and other adverse pathological features: a prospective clinicopathological study of 62 cases with emphasis on pathological outcomes. Histopathology 75:346–353. 10.1111/his.13878 [DOI] [PubMed] [Google Scholar]
- 6.Johnson IR, Parkinson-Lawrence EJ, Shandala T et al (2014) Altered endosome biogenesis in prostate cancer has biomarker potential. Mol Cancer Res 12:1851–1862. 10.1158/1541-7786.MCR-14-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson IR, Parkinson-Lawrence EJ, Butler LM, Brooks DA (2014) Prostate cell lines as models for biomarker discovery: performance of current markers and the search for new biomarkers. Prostate 74:547–560. 10.1002/pros.22777 [DOI] [PubMed] [Google Scholar]
- 8.Johnson IR, Parkinson-Lawrence EJ, Keegan H et al (2015) Endosomal gene expression: a new indicator for prostate cancer patient prognosis? Oncotarget 6:37919–37929. 10.18632/oncotarget.6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martini C, Logan JM, Sorvina A et al (2023) Aberrant protein expression of Appl1, Sortilin and Syndecan-1 during the biological progression of prostate cancer. Pathology 55:40–51. 10.1016/j.pathol.2022.08.001 [DOI] [PubMed] [Google Scholar]
- 10.Sorvina A, Martini C, Prabhakaran S et al (2023) Appl1, Sortilin and Syndecan-1 immunohistochemistry on intraductal carcinoma of the prostate provides evidence of retrograde spread. Pathology. 10.1016/j.pathol.2023.05.004 [DOI] [PubMed]
- 11.Logan JM, Hopkins AM, Martini C et al (2023) Prediction of prostate cancer biochemical and clinical recurrence is improved by IHC-assisted grading using Appl1, Sortilin and Syndecan-1. Cancers (Basel) 15:12. 10.3390/cancers15123215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kronz JD, Allan CH, Shaikh AA, Epstein JI (2001) Predicting cancer following a diagnosis of high-grade prostatic intraepithelial neoplasia on needle biopsy: data on men with more than one follow-up biopsy. Am J Surg Pathol 25:1079–1085. 10.1097/00000478-200108000-00014 [DOI] [PubMed] [Google Scholar]
- 13.Lotan TL, Gumuskaya B, Rahimi H et al (2013) Cytoplasmic PTEN protein loss distinguishes intraductal carcinoma of the prostate from high-grade prostatic intraepithelial neoplasia. Mod Pathol 26:587–603. 10.1038/modpathol.2012.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah RB, Yoon J, Liu G, Tian W (2017) Atypical intraductal proliferation and intraductal carcinoma of the prostate on core needle biopsy: a comparative clinicopathological and molecular study with a proposal to expand the morphological spectrum of intraductal carcinoma. Histopathology 71:693–702. 10.1111/his.13273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.