Abstract
Purpose
Exercise offers various clinical benefits to older breast cancer survivors. However, studies report that healthcare providers may not regularly discuss exercise with their patients. We evaluated clinical and sociodemographic determinants of receiving advice about exercise from healthcare providers among older breast cancer survivors (aged ≥65 years).
Methods
We used data from the Surveillance, Epidemiology, and End Results cancer registries linked to the Medicare Health Outcomes Survey (MHOS) from 2008 to 2015. We included female breast cancer survivors, aged ≥65 years, who completed the MHOS survey ≥2 years after a breast cancer diagnosis in a modified Poisson regression to identify clinical and sociodemographic determinants of reportedly receiving advice about exercise from healthcare providers.
Results
The sample included 1,836 breast cancer survivors. The median age of the sample was 76 years (range: 72–81). Overall, 10.7% of the survivors were non-Hispanic Black, 10.1% were Hispanic, and 69.3% were non-Hispanic White. Only 52.3% reported receiving advice about exercise from a healthcare provider. Higher body mass index (BMI) and comorbid medical history that included diabetes, cardiovascular, or musculoskeletal disease were each associated with a higher likelihood of receiving exercise advice. Lower education levels, lower BMI, and never having been married were each associated with a lower likelihood of receiving exercise advice.
Conclusions
Nearly half of breast cancer survivors aged ≥65 years did not report receiving exercise advice from a healthcare provider, suggesting interventions are needed to improve exercise counseling between providers and survivors, especially with women with lower educational attainment who have never been married.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-024-07460-1.
Keywords: Breast cancer survivors, Exercise advice, Healthcare providers, Survivorship care
Introduction
Due to improved treatment regimens [1], breast cancer mortality rates in the U.S. have declined annually by 1.3% in the past decade [2]. Currently, there are close to four million female breast cancer survivors in the U.S., the majority (67%) of whom are over the age of 65 years [3, 4]. The most recent exercise guidelines for cancer survivors recommend individualized exercise prescriptions in order to improve cancer-related health outcomes, including anxiety, depressive symptoms, physical functioning, and health-related quality of life [5]. The American Cancer Society, the American College of Sports Medicine, and the American Institute for Cancer Research recommend the equivalent of ≥150–300 min/week of moderate intensity aerobic activity (e.g., walking, swimming, cycling), along with ≥2 days/week of muscle-strengthening exercise (e.g., lifting weights) [5–10]. Despite this, a recent analysis using the National Health Interview Survey indicated that less than half of breast cancer survivors in the U.S. (36%) currently meet aerobic exercise guidelines [11]. This proportion decreases with rising age due to corresponding increases in comorbidities, intimidation of starting exercise, and lack of transportation, parks and recreation space, and guidance on exercise [12]. Other factors relevant to the general population, such as body mass index (BMI), comorbidities, or marital status, also influence survivors’ participation in physical activity [13, 14].
The American Society of Clinical Oncology recommends that all cancer survivors receive exercise counseling [15]. Previous studies have shown that healthcare provider discussions about exercise with their patients can help increase exercise participation [16]. Most cancer survivors also report wanting to discuss exercise and cardiovascular health with their clinicians during survivorship care planning [17–21]. However, despite guidelines and evidence supporting the benefits of exercise, older cancer survivors continue to report limited to no discussions about exercise from their healthcare providers in clinical settings [22–25].
There is limited data to date on individual clinical and sociodemographic characteristics of breast cancer survivors specifically that may influence how or why healthcare providers engage in conversations about exercise in clinical settings. This information could potentially help healthcare systems and providers better consistently identify, engage, and support women who may be less likely to receive advice about exercise during survivorship care planning, while also reducing potential physician-related biases. In this population-based study, we aimed to identify clinical and sociodemographic characteristics associated with the likelihood of receiving advice about exercise from healthcare providers among older breast cancer survivors. Additionally, we evaluated the comorbid health conditions associated with reported receipt of exercise advice.
Methods
Data source and study population
We used the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) cancer registry data and the Centers for Medicare & Medicaid Services’ Medicare Health Outcomes Survey (MHOS), which is available within one data resource (SEER-MHOS). SEER is a national cancer surveillance network of 16 regional cancer registries covering approximately 28% of the U.S. population that collects information on cancer diagnosis, site, and histology, initial cancer therapies, and demographic characteristics [26]. We included all SEER participating sites in these analyses, which included Connecticut, Georgia, Hawaii, Idaho, Iowa, Kentucky, Louisiana, Massachusetts, New Jersey, New Mexico, New York, Utah, Greater California/Los Angeles/Greater Bay Area/San Francisco/Oakland/San Jose/Monterey, Atlanta/Greater Georgia/Rural Georgia, Seattle/Puget Sound, and Detroit [26]. MHOS is a patient-reported survey of a random sample of enrollees in Medicare Advantage that assesses health-related quality of life and evaluates performance of the plans [27].
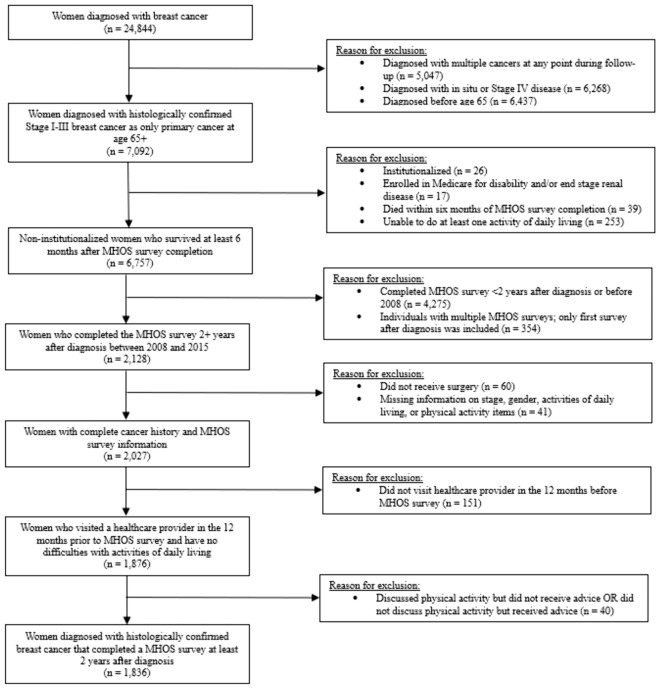
Consistent with previous literature, we included women diagnosed with histologically confirmed invasive breast cancer aged ≥65 years at diagnosis from SEER, and breast cancer survivors who had completed a MHOS survey at least two years after diagnosis [28–30]. We excluded participants who had missing data for reported levels of exercise and whose breast cancer diagnosis occurred at autopsy. In 2008, the U.S. national physical activity guidelines highlighted that healthcare providers should assess, counsel, and advise patients on exercise and how to do it safely [31]. Exercise guidelines for cancer survivors were introduced in 2010, which recommended two sessions per week of strength training and ≥150 min/week of aerobic activity [32]. Since physical activity guidelines for healthcare providers caring for this patient population were first published in 2008, we restricted our sample to include surveys completed from 2008 to 2015. The full list of inclusion and exclusion criteria can be found in Fig. 1. The final sample consisted of 1836 breast cancer survivors.
Fig. 1.
Inclusion and exclusion criteria
Exercise advice from healthcare providers
The outcome of interest in this study was reporting receiving exercise advice from a healthcare provider. The MHOS exercise advice item stated, “In the past 12 months, did a doctor or other health provider advise you to start, increase, or maintain your level of exercise or physical activity?” Respondents were asked to either answer ‘yes’ or ‘no’ [33].
Demographic characteristics
Based on previous studies [29, 34], we considered the following important covariates for reporting receipt of exercise counseling, including self-reported race and ethnicity (Hispanic, non-Hispanic Asian or Pacific Islander/American Indian/Alaska Native, non-Hispanic Black, and non-Hispanic White), marital status (married, divorced/separated/widowed, never married), household income (less than $20,000, $20,000–$39,999, $40,000–$79,999, $80,000 +), education level (less than high school, high school graduate or general education development (GED), some college/college graduate or more), and continuous age at survey in the analyses.
Breast cancer history
SEER summary stage (localized, regional), receipt of radiation therapy (yes, no), receipt of surgery (partial mastectomy, lumpectomy, excision of biopsy, modified radical mastectomy), tumor grade (I, II, III, IV), estrogen receptor status, progesterone receptor status, human epidermal growth factor receptor 2 (HER2) status, and age at diagnosis were included in the analyses.
Clinical characteristics
Based on expert opinion and a previous analysis of SEER-MHOS data [29], we also included information on other self-reported clinical characteristics, such as a woman’s history of falls in the last 12 months (yes, no), difficulty performing activities of daily living (yes, no) (includes bathing, dressing, eating, getting in/out of a chair, walking, using the toilet), BMI category (underweight: BMI <20 kg/m2; normal: BMI ≥20–24.9 kg/m2; overweight: BMI ≥25–29.9 kg/m2; obese/morbidly obese: BMI ≥30 kg/m2), general health status (excellent, very good, good, fair, poor), and comorbidity status (yes, no), including hypertension, cardiovascular disease (coronary artery disease, congestive heart failure, myocardial infarction, other heart conditions), pulmonary disease (emphysema, asthma, chronic obstructive pulmonary disease), diabetes, and musculoskeletal disease (arthritis of hip/knee or hand/wrist, osteoporosis, sciatica).
Study design and data analysis
Descriptive statistics were used to summarize sociodemographic characteristics, cancer history, and other clinical variables. We used a modified Poisson regression model with a least absolute shrinkage and selection operator (LASSO) to identify variables associated with the likelihood of receiving advice on exercise. LASSO was used to select variables to include in the final model, prevent overfitting of models, and improve model accuracy [35]. The overall model included difficulty performing activities of daily living, race/ethnicity, marital status, education, BMI category, fall history, receipt of radiation therapy, age at diagnosis, comorbidities (yes/no), tumor grade, and time between survey and diagnosis. We also ran five separate models for each comorbidity type (hypertension, cardiovascular disease, diabetes, pulmonary disease, or musculoskeletal disease) to reduce collinearity. We tested interactions between race/ethnicity, BMI, and education. We also ran additional exploratory subgroup analyses stratified by race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic). Statistical significance was set at p < 0.05. All analyses were conducted in RStudio, version 2023.06.1, and Stata, version 18.0 [36, 37]. This study was approved by the National Institutes of Health Institutional Review Board and was considered as exempt research based on use of de-identified pre-existing data.
Results
The sample included 1,836 women diagnosed with breast cancer. The median age of the sample at the time of the survey was 76 years (range: 72–81); 69.3% were non-Hispanic White, 43.4% had a college degree or higher, 38.8% were currently married, and 32.9% had a household income of less than $20,000 per year. Most women had at least one comorbidity (93.5%), with the three most commonly reported comorbidities being musculoskeletal disease (73.7%), hypertension (69.3%), and cardiovascular disease (28.3%); 37.3% reported difficulty performing an activity of daily living. The majority of women were diagnosed with breast cancer between ages 65–74 years (68.9%), and most women were diagnosed with localized invasive breast cancer (78.4%) and had received radiation (56.6%) (Table 1).
Table 1.
Characteristics of older breast cancer survivors in the overall sample and subgroups stratified by the receipt of exercise advice from a healthcare provider
| Characteristics | Overall Sample | Has your doctor given you exercise advice? | ||||
|---|---|---|---|---|---|---|
| N | % | Yes (n = 960) | No (n = 876) | |||
| n | Col%1 | n | Col%1 | |||
| Clinical characteristics | ||||||
| Age at diagnosis (Years) | ||||||
| 65–69 | 777 | 42.3 | 429 | 44.7 | 348 | 39.7 |
| 70–74 | 488 | 26.6 | 262 | 27.3 | 226 | 25.8 |
| 75–79 | 335 | 18.2 | 161 | 16.8 | 174 | 19.9 |
| ≥80 | 236 | 12.9 | 108 | 11.2 | 128 | 14.6 |
| Difficulty performing ≥1 activity of daily living | ||||||
| Yes | 684 | 37.3 | 382 | 39.8 | 302 | 34.5 |
| No | 1152 | 62.7 | 578 | 60.2 | 574 | 65.5 |
| Difficulty bathing | ||||||
| Yes | 234 | 12.7 | 131 | 13.6 | 103 | 11.8 |
| No | 1602 | 87.3 | 829 | 86.4 | 773 | 88.2 |
| Difficulty dressing | ||||||
| Yes | 147 | 8.0 | 88 | 9.2 | 59 | 6.7 |
| No | 1689 | 92.0 | 872 | 90.8 | 817 | 93.3 |
| Difficulty eating | ||||||
| Yes | 59 | 3.2 | 36 | 3.8 | 23 | 2.6 |
| No | 1777 | 96.8 | 924 | 96.2 | 853 | 97.4 |
| Difficulty getting in/out of a chair | ||||||
| Yes | 405 | 22.1 | 232 | 24.2 | 173 | 19.7 |
| No | 1431 | 77.9 | 728 | 75.8 | 703 | 80.3 |
| Difficulty walking | ||||||
| Yes | 592 | 32.2 | 326 | 34.0 | 266 | 30.4 |
| No | 1244 | 67.8 | 634 | 66.0 | 610 | 69.6 |
| Difficulty using the toilet | ||||||
| Yes | 104 | 5.7 | 61 | 6.4 | 43 | 4.9 |
| No | 1732 | 94.3 | 899 | 93.6 | 833 | 95.1 |
| Fall history in past 12 months | ||||||
| Yes | 452 | 24.6 | 240 | 25.0 | 212 | 24.2 |
| No/Missing | 1384 | 75.3 | 720 | 75.0 | 664 | 75.8 |
| BMI category | ||||||
| Underweight (<18.5 kg/m2) | 91 | 5.0 | 29 | 3.1 | 62 | 7.5 |
| Normal (≥18.5- <25 kg/m2) | 571 | 31.1 | 268 | 29.1 | 303 | 36.5 |
| Overweight (≥25- < 30kg/m2) | 558 | 30.4 | 309 | 33.6 | 249 | 30.0 |
| Obese/morbidly obese (≥30 kg/m2) | 531 | 28.9 | 315 | 34.2 | 216 | 26.0 |
| General health status | ||||||
| Excellent | 88 | 4.8 | 43 | 4.6 | 45 | 5.2 |
| Very good | 436 | 23.7 | 222 | 23.6 | 214 | 24.7 |
| Good | 774 | 42.2 | 410 | 43.6 | 364 | 42.1 |
| Fair | 436 | 23.7 | 229 | 24.3 | 207 | 23.9 |
| Poor | 72 | 3.9 | 37 | 3.9 | 35 | 4.0 |
| Comorbidities | ||||||
| ≥1 Comorbidity | 1717 | 93.5 | 912 | 95.0 | 805 | 91.9 |
| None | 119 | 6.5 | 48 | 5.0 | 71 | 8.1 |
| Hypertension | ||||||
| Yes | 1273 | 69.3 | 692 | 72.1 | 581 | 66.3 |
| No/Missing | 560 | 30.7 | 268 | 27.9 | 295 | 33.7 |
| Cardiovascular disease | ||||||
| Yes | 519 | 28.3 | 301 | 32.1 | 218 | 25.8 |
| No | 1264 | 68.8 | 638 | 67.9 | 626 | 74.2 |
| Pulmonary disease | ||||||
| Yes | 311 | 16.9 | 170 | 17.9 | 141 | 16.4 |
| No | 1499 | 81.6 | 780 | 82.1 | 719 | 83.6 |
| Diabetes | ||||||
| Yes | 502 | 27.3 | 305 | 32.2 | 197 | 22.8 |
| No/Missing | 1311 | 71.4 | 643 | 67.8 | 668 | 77.2 |
| Musculoskeletal disease | ||||||
| Yes | 1354 | 73.7 | 749 | 78.0 | 605 | 69.1 |
| No/Missing | 482 | 26.3 | 211 | 22.0 | 271 | 30.9 |
| Tumor grade | ||||||
| Grade I | 526 | 30.1 | 284 | 30.8 | 242 | 29.3 |
| Grade II | 806 | 46.2 | 429 | 46.6 | 377 | 45.7 |
| Grade III/IV | 414 | 22.5 | 208 | 21.7 | 206 | 23.5 |
| Missing | 90 | 4.9 | 39 | 4.1 | 51 | 5.8 |
| SEER summary stage | ||||||
| Localized | 1440 | 78.4 | 752 | 78.3 | 688 | 78.5 |
| Regional | 396 | 21.6 | 208 | 21.7 | 188 | 45.3 |
| Radiation | ||||||
| Yes | 1039 | 56.6 | 560 | 58.3 | 479 | 54.7 |
| No/Missing | 797 | 43.4 | 400 | 41.7 | 397 | 45.3 |
| Surgery | ||||||
| Partial mastectomy | 213 | 11.6 | 111 | 11.6 | 102 | 11.6 |
| Lumpectomy | 760 | 41.4 | 403 | 42.0 | 357 | 40.8 |
| Excision of biopsy | 265 | 14.4 | 141 | 14.7 | 124 | 14.2 |
| Total mastectomy | 259 | 14.1 | 131 | 13.6 | 128 | 14.6 |
| Modified radical mastectomy | 339 | 18.5 | 174 | 18.1 | 165 | 18.8 |
| Estrogen receptor status | ||||||
| Positive | 1516 | 86.7 | 805 | 88.0 | 711 | 85.4 |
| Negative | 232 | 13.3 | 110 | 12.0 | 122 | 14.6 |
| Progesterone receptor status | ||||||
| Positive | 1288 | 74.5 | 681 | 75.0 | 607 | 74.0 |
| Negative | 440 | 25.5 | 227 | 25.0 | 213 | 26.0 |
| HER2 receptor status | ||||||
| Positive | 43 | 7.9 | 19 | 6.7 | 24 | 9.1 |
| Negative | 504 | 92.1 | 263 | 93.3 | 241 | 90.9 |
| Age at survey (Median [IQR]) | 76 [72, 81] | 76 [72, 81] | 77 [73, 82] | |||
| Time between survey and diagnosis (median [IQR]) | 4.6 [3.1, 6.5] | 4.4 [3.1, 6.4] | 4.7 [3.0, 6.6] | |||
| Sociodemographic characteristics | ||||||
| Race/ethnicity | ||||||
| Hispanic | 185 | 10.1 | 110 | 11.5 | 75 | 8.6 |
| Non-Hispanic American Indian/Alaska Native/Asian or Pacific Islander | 175 | 10.0 | 98 | 10.2 | 77 | 8.8 |
| Non-Hispanic Black | 197 | 10.7 | 100 | 10.4 | 97 | 11.1 |
| Non-Hispanic White | 1272 | 69.3 | 650 | 67.8 | 622 | 71.4 |
| Marital status | ||||||
| Married | 713 | 38.8 | 391 | 41.6 | 322 | 37.7 |
| Divorced/separated/widowed | 992 | 54.0 | 513 | 54.5 | 479 | 56.2 |
| Never married | 89 | 4.8 | 37 | 3.9 | 52 | 6.1 |
| Education level | ||||||
| Less than high school | 370 | 20.2 | 176 | 18.9 | 194 | 22.9 |
| High school graduate or GED | 616 | 33.6 | 309 | 33.1 | 307 | 36.2 |
| Some college/college degree | 796 | 43.4 | 448 | 48.0 | 348 | 41.0 |
| Household income | ||||||
| < $20,000 | 604 | 32.9 | 317 | 40.7 | 287 | 43.4 |
| $20,000–$39,999 | 448 | 24.4 | 241 | 30.9 | 207 | 31.3 |
| $40,000–$79,999 | 283 | 15.4 | 158 | 20.3 | 125 | 18.9 |
| ≥$80,000 | 106 | 5.8 | 63 | 8.1 | 43 | 6.5 |
BMI Body mass index, HER2 human epidermal growth factor 2, GED general education development, IQR interquartile range
1Column percentage calculated (cell number/column total) × 100
E.g., Col. % for no difficulties in performing activities of daily living among those who received advice about exercise from a doctor (578/960) × 100 = 60.2%
The multivariable modified Poisson regression analysis showed that, from a sociodemographic standpoint, the strongest associated variables were education and marital status. Compared to breast cancer survivors with a college degree or more, those with less than a high school education or those with a high school degree or a GED had a 23% (Relative Risk (RR): 0.77; 95% confidence interval (CI): 0.67–0.88), and 12% (RR: 0.88; 95% CI: 0.80–0.98) lower likelihood of receiving exercise advice from a healthcare provider, respectively. Breast cancer survivors who were never married had a 21% lower likelihood of receiving exercise advice than those who were married (RR: 0.79; 95% CI: 0.61–1.03), and these results were trending towards significance. From a clinical standpoint, the strongest associated variables were BMI and age. With every one-year increase in age, survivors had a 1% lower likelihood of receiving exercise advice (RR: 0.99; 95% CI: 0.98–1.00); these results were trending towards significance. Compared to those with normal BMI (≥18.5–<25 kg/m2), breast cancer survivors who were underweight (<18.5 kg/m2) reported a 26% lower likelihood (RR: 0.74; 95% CI: 0.54–1.03) of receiving advice, while breast cancer survivors who were overweight (≥25–<30 kg/m2) and obese (≥30 kg/m2) reported a 21% (RR: 1.21; 95% CI: 1.08–1.36) and 26% (RR: 1.26; 95% CI: 1.11–1.42) higher likelihood of receiving advice, respectively. Survivors with at least one comorbidity had a 29% higher likelihood of receiving advice compared to those without any comorbidities (RR: 1.29; 95% CI: 1.03–1.62). Compared to non-Hispanic White breast cancer survivors, non-Hispanic Asian or Pacific Islander/American Indian/Alaska Native survivors had a 17% higher likelihood of receiving exercise advice (RR: 1.17; 95% CI 1.01–1.36) (Table 2). Difficulty performing ≥ 1 activity of daily living, fall history, receipt of radiation, and tumor grade were not associated with receipt of exercise advice from a healthcare provider. The interactions between race/ethnicity, BMI, and education were trending towards significance. However, there was inadequate sample size to conduct subgroup analyses.
Table 2.
Factors associated with the receipt of exercise advice from healthcare providers in a multivariable modified poisson regression analysis*
| Variable | Category | Relative risk | 95% Confidence interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| Clinical characteristics | ||||
| BMI | Normal (≥18.5–<25 kg/m2) | Reference | ||
| Underweight (<18.5 kg/m2) | 0.74 | 0.54 | 1.03 | |
| Overweight (≥25–<30 kg/m2) | 1.21 | 1.08 | 1.36 | |
| Obese/Morbidly Obese (≥30 kg/m2) | 1.26 | 1.11 | 1.42 | |
| Comorbidities | ≥1 Comorbidity | 1.29 | 1.03 | 1.62 |
| None | Reference | |||
| Difficulty performing ≥ 1 activity of daily living | Yes | 1.09 | 0.99 | 1.21 |
| No | Reference | |||
| Fall history in past 12 months | Yes | 1.04 | 0.94 | 1.16 |
| No | Reference | |||
| Radiation | Yes | 1.07 | 0.98 | 1.18 |
| No | Reference | |||
| Grade | Grade I | Reference | ||
| Grade II | 0.97 | 0.88 | 1.08 | |
| Grade III | 0.92 | 0.81 | 1.05 | |
| Grade IV | 0.46 | 0.08 | 2.54 | |
| Age at diagnosis | Continuous | 0.99 | 0.98 | 1.00 |
| Time from survey to diagnosis | Continuous | 0.99 | 0.98 | 1.01 |
| Sociodemographic characteristics | ||||
| Race/ethnicity | Non-Hispanic White | Reference | ||
| Non-Hispanic American Indian/Alaska Native/Asian or Pacific Islander | 1.17 | 1.01 | 1.36 | |
| Non-Hispanic Black | 0.98 | 0.83 | 1.14 | |
| Hispanic | 1.14 | 0.99 | 1.32 | |
| Marital status | Married | Reference | ||
| Divorced/separated/widowed | 1.02 | 0.93 | 1.13 | |
| Never married | 0.79 | 0.61 | 1.03 | |
| Education | College degree or more | Reference | ||
| High school graduate or GED | 0.88 | 0.80 | 0.98 | |
| Less than high school | 0.77 | 0.67 | 0.88 | |
*Represents a fully adjusted model; all variables in table were included in the model
BMI Body mass index, GED general education development
The results for exploratory subgroup analyses stratified by race and ethnicity are provided in Supplemental Table 1. Breast cancer survivors with less than a high school education were less likely to receive exercise advice from a healthcare provider across non-Hispanic White (RR: 0.71; 95% CI: 0.58–0.86) and Hispanic (RR: 0.61; 95% CI: 0.45–0.83) groups.
The comorbidity-specific models showed that breast cancer survivors with musculoskeletal disease (RR: 1.22; 95% CI: 1.08–1.37), diabetes (RR: 1.15; 95% CI: 1.04–1.27) and cardiovascular disease (RR: 1.11; 95% CI: 1.01–1.22) showed a higher likelihood of receiving exercise advice. Hypertension and pulmonary disease were not associated with reports of receiving exercise advice (Table 3; Supplemental Tables 2, 3, 4, 5, 6).
Table 3.
Individual comorbidities associated with the receipt of exercise advice from healthcare providers in multivariable modified poisson regression analyses
| Comorbidities | Relative risk | 95% Confidence interval | |
|---|---|---|---|
| Lower | Upper | ||
| Hypertension | 1.07 | 0.96 | 1.20 |
| Cardiovascular disease | 1.10 | 1.01 | 1.22 |
| Pulmonary disease | 1.01 | 0.90 | 1.14 |
| Diabetes | 1.15 | 1.04 | 1.27 |
| Musculoskeletal disease | 1.22 | 1.08 | 1.37 |
Each row represents a separate model; adjusted for difficulty performing activities of daily living, race/ethnicity, marital status, education, body mass index, fall history, radiation, tumor grade, age at diagnosis, time from survey to diagnosis, and any other comorbidities
Discussion
To our knowledge, this is the first study that assesses sociodemographic and contextual determinants of receiving exercise advice from healthcare providers solely among breast cancer survivors. Breast cancer survivors have unique needs relating to exercise; for instance, due to adverse effects of treatment, preexisting conditions, and lower exercise participation following diagnosis, women with breast cancer may have a higher risk of cardiovascular disease-related mortality than the general population [38, 39]. Additionally, breast cancer survivors may face reduced muscular strength and mobility due to treatment-related side effects [40, 41]. Therefore, it is important that clinicians discuss the benefits and potential risks of exercise with these women.
Our results show that only about half of the older breast cancer survivors (52.3%) had received exercise advice from their healthcare providers. This is consistent with a previous study [29] using the SEER-MHOS dataset (2008–2014), which found that only about half (53.4%) of older breast, colorectal, and prostate cancer survivors reported discussing exercise with their healthcare providers. Previous studies not specific to the cancer survivor population have also shown that, among adult patients, only about half (50.2%) of healthcare providers counsel their patients on either diet, exercise, or weight control [34, 42–44]. Healthcare providers cite numerous reasons for limited engagement in discussions about exercise in clinical practice, including the lack of time and clinical tools to support personalized discussions [22–24, 45]. Providers, especially those in primary care, report feeling time pressure during their visits with patients [46], and often there is not enough time for them to discuss exercise. Providers also report feeling like they have a lack of expertise and knowledge about exercise [47, 48]. Survey studies have shown that clinicians may not be aware of the current exercise guidelines, as it remains absent from the formal education of many healthcare professions [47, 48]. Additionally, providers may not be aware of the different types, frequency, and duration of exercise that a patient may need based on the patient’s risk of cardiovascular disease and other comorbidities [49, 50]. Healthcare providers who offer advice to cancer survivors may focus on discussing the benefits of exercise for physical and functional health gains rather than discussing the full range of benefits that exercise may offer breast cancer survivors, including improvements in quality of life during treatment [19]. Thus, it is important that clinicians are provided training and education about exercise, information on referral programs, and training on how to discuss exercise effectively in different patient populations.
We found that sociodemographic and contextual factors in this population are associated with the likelihood of receiving advice about exercise from a healthcare provider. Overall, lower levels of education were associated with a lower likelihood of receiving advice about exercise from a healthcare provider. A similar relationship was observed in non-Hispanic White and Hispanic women; however, these subgroup analyses were considered exploratory due to limited sample size. While we observed a strong relationship between lower levels of education and lower likelihood of being advised about exercise, potential reasons for this are unclear. Educational attainment has been shown to be a significant predictor of pre-existing exercise levels, with lower levels of education associated with less exercise [51]. This relationship could be driven by lower exercise self-efficacy, low income stability, and limited social support associated with lower levels of education [52]. It is possible that patients may have prompted this discussion among providers. It may be important to develop targeted interventions to increase discussions and exercise participation among these individuals since lower levels of education are associated with other concurrent, intersecting challenges to survivorship, including obesity, morbidity, and mortality [51]. Additionally, providers should consider a patients’ level of health literacy when delivering exercise advice; information should be given to patients at a level that they can understand in order to increase their self-efficacy, enabling them to feel confident about exercising [53, 54].
We also observed that survivors who had never been married were significantly less likely to report receiving exercise advice than those who had been married. However, studies show that married women report higher levels of exercise and leisure time activity compared to their single counterparts [13]. This may be explained by the marriage protection theory, which suggests that marriage provides health benefits due to increased social and financial support [55]. It is essential for providers to consider encouraging survivors who have never been married to also pursue exercise, especially among those who may not have access to other avenues of social support, given the potential benefits of marriage.
Among clinical factors, BMI had the strongest association with receiving exercise advice among breast cancer survivors. Older breast cancer survivors who had overweight or obesity showed a higher likelihood of receiving exercise advice. While there may certainly be additional benefits to exercise relevant to these populations, this can also potentially feed into pre-existing biases by providers that alienate patients of greater weight and worsen health outcomes if advice is not delivered effectively, while not adequately serving patients of normal weight who may benefit from greater muscle mass/functional status. Additionally, compared to normal weight, underweight survivors had a lower likelihood of receiving exercise advice. Underweight older survivors may be nearing death, so it might be inappropriate for clinicians to recommend exercise to these individuals [56].
With advancing age, breast cancer survivors were less likely to receive exercise advice from their healthcare providers, even when considering comorbidities. This is particularly concerning given that older women participate in the least exercise [57]. There could be inadvertent bias against older patients, where providers may have negative ageist stereotypes that may lead to poorer quality of care [58]. Providers need to encourage older breast cancer survivors to exercise, and future interventions are needed to ensure that providers deliver advice effectively to this patient population. Providers may consider offering exercise advice while considering different modalities, such as home-based exercise, that may increase exercise among this patient population [59].
Diabetes, cardiovascular, and musculoskeletal diseases were also associated with a higher likelihood of receiving exercise advice from a healthcare provider. However, in our study, pulmonary disease and hypertension were not associated with the receipt of exercise advice. Interestingly, prior studies have shown that exercise significantly reduces morbidity and mortality related to all of these conditions [60–64], encouraging the possibility of providers to expand the patients with comorbidities whom they encourage to exercise.
The intersection of cultural factors with the factors that we measured may influence breast cancer survivors’ ability to exercise, and these factors should be considered when providers engage in discussions about exercise with their patients. For example, in our study, we found that non-Hispanic American Indian/Alaska Native/Asian or Pacific Islander breast cancer survivors had a greater likelihood of receiving exercise advice. These findings are encouraging considering the lower levels of exercise [65–68] and higher rates of obesity and comorbidities reported in these groups [69]. Previous studies have shown that American Indian and Alaska Native women were more likely to exercise if they had higher levels of social support [70, 71]. However, most existing exercise interventions have failed to incorporate community-based strategies to help these women rely on their social environments [71]. Similarly, Asian American women report cultural reasons, lack of time due to keeping their traditions, and feeling like exercise was not appropriate for women as reasons for not exercising [72]. Community-based and culturally relevant exercise interventions that incorporate social and cultural norms, values, and beliefs may help engage these women in discussions about exercise. Interventions that are designed with community members, aligned with their values, delivered at a relevant health literacy level, and address specific barriers relevant to the community are more effective and can help increase the efficacy of exercise programs [73–75].
Our study presents several limitations. Due to the small sample size, while we could analyze single factors, we were unable to run subgroup analyses stratified by multiple factors (e.g., race and education; BMI and comorbidities). Additionally, the sample sizes for non-Hispanic Asian, Pacific Islander, American Indian, and Alaska Native breast cancer survivors were small. As a result, we were unable to conduct separate analyses for these women. There is a critical need to increase the inclusion of these underrepresented groups in registry data. As we focused on women aged over 65 years who may be clinically underserved with regard to exercise advice, our findings may not be generalizable to younger women. Our sample also only included women with Medicare Advantage plans, and patients who are on other types of health insurance may demonstrate different rates of receiving exercise advice from providers based on alternative factors (such as income). For instance, we were not able to evaluate the impact of the potential differences in physician services and payment structures in Medicare Advantage and fee-for-service Medicare plans that may influence exercise discussions [76, 77]. The SEER-MHOS dataset does not collect follow-up data on the uptake of exercise; therefore, we were unable to assess whether exercise discussions/advice with a healthcare provider resulted in a behavioral change or maintenance. Additionally, the SEER-MHOS dataset provided a single binary variable for receiving exercise advice from a clinician. Future research may include variables that describe the characteristics of exercise advice including ‘what’, ‘when’, ‘how’, and ‘why’ exercise advice was provided to the patient. Additionally, we were not able to include chemotherapy in our analyses due to the lack of completeness of the chemotherapy variable reported in SEER [78]. Also, we only used data from 2008 to 2015; as a result we were unable to evaluate the impact of the COVID-19 pandemic on the receipt of exercise advice from healthcare providers. As the data becomes available, future research may consider evaluating the impact of the COVID-19 pandemic on the receipt of exercise advice among older adults. Finally, given the nature of the SEER-MHOS questionnaire, our results may have been influenced by recall bias, where women who were already engaging in exercise were more likely to report that their clinicians recommended exercise. However, the intersection of older age and exercise patterns among breast cancer survivors remains a space with limited data.
In summary, our study provides important data to help healthcare providers identify, engage, and provide advice about exercise to older breast cancer survivors. Our findings also indicate that exercise prescriptions may require consideration of both individual clinical and sociodemographic factors. A clinical decision tool or a conversation aid specific to breast cancer survivors, extending the capabilities and level of individualization offered by existing tools such as ‘The Exercise and Screening for You’ tool [79], could potentially help address barriers to communicating the benefits of exercise in diverse breast cancer settings and could potentially help improve the overall quality of breast cancer survivorship care.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Disclaimer
Opinions and comments expressed in this paper belong to the authors and do not necessarily reflect those of the U.S. Government, Department of Health and Human Services, National Institutes of Health, or the National Institute on Minority Health and Health Disparities. The study funders had no role in the design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Author contributions
KW, OW, MS, and JJ contributed to the conception and design of this study and the acquisition, analysis, and interpretation of data. All authors contributed to drafting the work or revising it critically for important intellectual content and final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Open access funding provided by the National Institutes of Health. This study was supported by the Division of Intramural Research at the National Institute on Minority Health and Health Disparities of the National Institutes of Health (MD000022, Jayasekera) and the National Institutes of Health Distinguished Scholars program. This study used data from the SEER-MHOS linked data resource. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Centers for Medicare & Medicaid Services; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-MHOS database.
Data availability
The SEER-MHOS datasets analyzed during the current study are not publicly available due to the risk of re-identification. The data are available to investigators for research purposes, but approval is required to obtain the data.
Declarations
Conflict of interest
All authors declare no financial or non-financial competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kaitlyn M. Wojcik and Oliver W. A. Wilson are dual first authors.
References
- 1.Xu Y, Gong M, Wang Y, Yang Y, Liu S, Zeng Q (2023) Global trends and forecasts of breast cancer incidence and deaths. Sci Data 10(1):334. 10.1038/s41597-023-02253-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giaquinto AN et al (2022) Breast cancer statistics, 2022. CA Cancer J Clin 72(6):524–541. 10.3322/caac.21754 [DOI] [PubMed] [Google Scholar]
- 3.Bluethmann SM, Mariotto AB, Rowland JH (2016) Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev 25(7):1029–1036. 10.1158/1055-9965.EPI-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Giaquinto AN, Jemal A (2024) Cancer statistics, 2024. CA Cancer J Clin 74(1):12–49. 10.3322/caac.21820 [DOI] [PubMed] [Google Scholar]
- 5.Campbell KL et al (2019) Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51(11):2375–2390. 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rock CL et al (2022) American cancer society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin 72(3):230–262. 10.3322/caac.21719 [DOI] [PubMed] [Google Scholar]
- 7.Bull FC et al (2020) World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 54(24):1451–1462. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Physical activity guidelines for Americans. Medicine ACoS. https://www.acsm.org/education-resources/trending-topics-resources/physical-activity-guidelines/lists/guidelines-resources/physical-activity-guidelines-for-americans-2nd-edition. Accessed 8 Feb 2024
- 9.Physical activity and the risk of cancer, American Institute for Cancer Research, 2018. [Online]. https://www.wcrf.org/wp-content/uploads/2021/02/Physical-activity.pdf. Accessed 30 May 2024
- 10.Physical activity guidelines for Americans, 2nd Edition, Department of Health and Human Services, Washington, DC, 2018. [Online]. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed 30 May 2024
- 11.Cao C, Patel AV, Liu R, Cao Y, Friedenreich CM, Yang L (2023) Trends and cancer-specific patterns of physical activity, sleep duration, and daily sitting time among US cancer survivors, 1997–2018. J Natl Cancer Inst 115(12):1563–1575. 10.1093/jnci/djad146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bluethmann SM, Sciamanna CN, Winkels RM, Sturgeon KM, Schmitz KH (2018) Healthy living after cancer treatment: considerations for clinical and community practice. Am J Lifestyle Med 12(3):215–219. 10.1177/1559827618755681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettee KK et al (2006) Influence of marital status on physical activity levels among older adults. Med Sci Sports Exerc 38(3):541–546. 10.1249/01.mss.0000191346.95244.f7 [DOI] [PubMed] [Google Scholar]
- 14.Bensimhon DR, Kraus WE, Donahue MP (2006) Obesity and physical activity: a review. Am Heart J 151(3):598–603. 10.1016/j.ahj.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 15.Ligibel JA et al (2022) Exercise, diet, and weight management during cancer treatment: ASCO guideline. J Clin Oncol 40(22):2491–2507. 10.1200/JCO.22.00687 [DOI] [PubMed] [Google Scholar]
- 16.Orrow G, Kinmonth AL, Sanderson S, Sutton S (2013) Republished research: effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 47(1):27. 10.1136/bjsports-2012-e1389rep [DOI] [PubMed] [Google Scholar]
- 17.Alderman G, Semple S, Cesnik R, Toohey K (2020) Health care professionals’ knowledge and attitudes toward physical activity in cancer patients: a systematic review. Semin Oncol Nurs 36(5):151070. 10.1016/j.soncn.2020.151070 [DOI] [PubMed] [Google Scholar]
- 18.Brick R, Natori A, Moreno PI, Molinares D, Koru-Sengul T, Penedo FJ (2023) Predictors of cancer rehabilitation medicine referral and utilization based on the moving through cancer physical activity screening assessment. Support Care Cancer 31(4):216. 10.1007/s00520-023-07679-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daley AJ, Bowden SJ, Rea DW, Billingham L, Carmicheal AR (2008) What advice are oncologists and surgeons in the United Kingdom giving to breast cancer patients about physical activity? Int J Behav Nutr Phys Act 5(1):46. 10.1186/1479-5868-5-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López ME et al (2013) Ductal carcinoma in situ (DCIS): posttreatment follow-up care among Latina and non-Latina White women. J Cancer Surviv 7(2):219–226. 10.1007/s11764-012-0262-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arem H, Duan X, Ehlers DK, Lyon ME, Rowland JH, Mama SK (2021) Provider discussion about lifestyle by cancer history: a nationally representative survey. Cancer Epidemiol Biomark Prev 30(2):278–285. 10.1158/1055-9965.Epi-20-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong AJ, Faulkner G, Jones JM, Sabiston CM (2018) A qualitative analysis of oncology clinicians’ perceptions and barriers for physical activity counseling in breast cancer survivors. Support Care Cancer 26(9):3117–3126. 10.1007/s00520-018-4163-8 [DOI] [PubMed] [Google Scholar]
- 23.Glowacki K, Weatherson K, Faulkner G (2019) Barriers and facilitators to health care providers’ promotion of physical activity for individuals with mental illness: a scoping review. Ment Health Phys Act 16:152–168. 10.1016/j.mhpa.2018.10.006 [Google Scholar]
- 24.Hébert ET, Caughy MO, Shuval K (2012) Primary care providers’ perceptions of physical activity counselling in a clinical setting: a systematic review. Br J Sports Med 46(9):625. 10.1136/bjsports-2011-090734 [DOI] [PubMed] [Google Scholar]
- 25.Christian AH, O’Malley D, Barac A, Miller SM, Hudson SV (2017) Cardiovascular risk and communication among early stage breast cancer survivors. Patient Educ Couns 100(7):1360–1366. 10.1016/j.pec.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26."Surveillance, Epidemiology, and End Results Program." National Cancer Institute. https://seer.cancer.gov/. Accessed 21 Aug 2023
- 27.Medicare health outcomes survey. Health Services Advisory Group. https://www.hosonline.org/. Accessed 8 Feb 2024
- 28.Byrne M et al (2021) Trajectories of fatigue in a population-based sample of older adult breast, prostate, and colorectal cancer survivors: an analysis using the SEER-MHOS data resource. Support Care Cancer 29(12):7393–7402. 10.1007/s00520-021-06267-w [DOI] [PubMed] [Google Scholar]
- 29.Siembida EJ, Kent EE, Bellizzi KM, Smith AW (2020) Healthcare providers’ discussions of physical activity with older survivors of cancer: potential missed opportunities for health promotion. J Geriatr Oncol 11(3):437–443. 10.1016/j.jgo.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 30.Huang MH, Blackwood J, Godoshian M, Pfalzer L (2019) Predictors of falls in older survivors of breast and prostate cancer: a retrospective cohort study of surveillance, epidemiology and end results-Medicare health outcomes survey linkage. J Geriatr Oncol 10(1):89–97. 10.1016/j.jgo.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 31.2008 Physical activity guidelines for Americans. Department of Health and Human Services. https://health.gov/sites/default/files/2019-09/paguide.pdf. Accessed 20 March 2024
- 32.Schmitz KH et al (2010) American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42(7):1409–1426. 10.1249/MSS.0b013e3181e0c112 [DOI] [PubMed] [Google Scholar]
- 33.Physical activity measure. National Committee for Quality Assurance. https://www.ncqa.org/hedis/measures/physical-activity-in-older-adults/. Accessed 8 Feb 2024
- 34.Schonberg MA, Marcantonio ER, Wee CC (2006) Receipt of exercise counseling by older women. J Am Geriatr Soc 54(4):619–626. 10.1111/j.1532-5415.2006.00679.x [DOI] [PubMed] [Google Scholar]
- 35.Least absolute shrinkage and selection operator (LASSO). Columbia Mailman School of Public Health. https://www.publichealth.columbia.edu/research/population-health-methods/least-absolute-shrinkage-and-selection-operator-lasso. Accessed 18 March 2024
- 36.RStudio: Integrated Development for R. (2023). RStudio PBC, Boston.
- 37.Stata Statistical Software: Release 18. (2023). StataCorp LLC, College Station, TX.
- 38.Gernaat SAM et al (2017) Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat 164(3):537–555. 10.1007/s10549-017-4282-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galimzhanov A et al (2023) Cardiovascular outcomes in breast cancer survivors: a systematic review and meta-analysis. Eur J Prev Cardiol 30(18):2018–2031. 10.1093/eurjpc/zwad243 [DOI] [PubMed] [Google Scholar]
- 40.Owusu C et al (2018) Perspective of older African–American and non-hispanic white breast cancer survivors from diverse socioeconomic backgrounds toward physical activity: a qualitative study. J Geriatr Oncol 9(3):235–242. 10.1016/j.jgo.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J, Choi WJ, Jeong SH (2013) The effects of physical activity on breast cancer survivors after diagnosis. J Cancer Prev 18(3):193–200. 10.15430/jcp.2013.18.3.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith AW et al (2011) U.S. primary care physicians’ diet-, physical activity-, and weight-related care of adult patients. Am J Prev Med 41(1):33–42. 10.1016/j.amepre.2011.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed NU, Delgado M, Saxena A (2017) Trends and disparities in the prevalence of physicians’ counseling on exercise among the U.S. adult population, 2000–2010. Prev Med 99:1–6. 10.1016/j.ypmed.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 44.Wattanapisit A, Lapmanee S, Chaovalit S, Lektip C, Chotsiri P (2023) Prevalence of physical activity counseling in primary care: a systematic review and meta-analysis. Health Promot Perspect 13(4):254–266. 10.34172/hpp.2023.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pang A et al (2018) Physician practice patterns and barriers to counselling on physical activity in solid organ transplant recipients, (in eng). Ann Transplant 23:345–359. 10.12659/aot.908629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prasad K et al (2020) Time pressure during primary care office visits: a prospective evaluation of data from the healthy work place study. J Gen Intern Med 35(2):465–472. 10.1007/s11606-019-05343-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cantwell M et al (2018) Healthcare professionals’ knowledge and practice of physical activity promotion in cancer care: challenges and solutions. Eur J Cancer Care. 10.1111/ecc.12795 [DOI] [PubMed] [Google Scholar]
- 48.Nadler M, Bainbridge D, Tomasone J, Cheifetz O, Juergens RA, Sussman J (2017) Oncology care provider perspectives on exercise promotion in people with cancer: an examination of knowledge, practices, barriers, and facilitators. Support Care Cancer 25(7):2297–2304. 10.1007/s00520-017-3640-9 [DOI] [PubMed] [Google Scholar]
- 49.Riddell MC, Burr J (2011) Evidence-based risk assessment and recommendations for physical activity clearance: diabetes mellitus and related comorbidities. Appl Physiol Nutr Metab 36:S154–S189. 10.1139/h11-063 [DOI] [PubMed] [Google Scholar]
- 50.Perez-Terzic CM (2012) Exercise in cardiovascular diseases. PM R 4(11):867–873. 10.1016/j.pmrj.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 51.Becker S, Zimmermann-Stenzel M (2009) Physical activity, obesity, and educational attainment in 50- to 70-year-old adults, (in English). J Public Health-Heid 17(2):145–153. 10.1007/s10389-008-0222-9 [Google Scholar]
- 52.Cerin E, Leslie E (2008) How socio-economic status contributes to participation in leisure-time physical activity. Soc Sci Med 66(12):2596–2609. 10.1016/j.socscimed.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 53.Buja A et al (2020) Health literacy and physical activity: a systematic review. J Phys Act Health 17(12):1259–1274. 10.1123/jpah.2020-0161 [DOI] [PubMed] [Google Scholar]
- 54.Sheridan SL, Halpern DJ, Viera AJ, Berkman ND, Donahue KE, Crotty K (2011) Interventions for individuals with low health literacy: a systematic review. J Health Commun 16:30–54. 10.1080/10810730.2011.604391 [DOI] [PubMed] [Google Scholar]
- 55.August KJ, Sorkin DH (2010) Marital status and gender differences in managing a chronic illness: the function of health-related social control. Soc Sci Med 71(10):1831–1838. 10.1016/j.socscimed.2010.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roh L et al (2014) Mortality risk associated with underweight: a census-linked cohort of 31,578 individuals with up to 32 years of follow-up. BMC Public Health 14:371. 10.1186/1471-2458-14-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee C (1993) Factors related to the adoption of exercise among older women, (in english). J Behav Med 16(3):323–334. 10.1007/Bf00844763 [DOI] [PubMed] [Google Scholar]
- 58.Eymard AS, Douglas DH (2012) Ageism among health care providers and interventions to improve their attitudes toward older adults: an integrative review. J Gerontol Nurs 38(5):26–35. 10.3928/00989134-20120307-09 [DOI] [PubMed] [Google Scholar]
- 59.Physical activity: home-based exercise interventions for adults aged 65 years and older. Guide to Community Preventive Services. https://www.thecommunityguide.org/findings/physical-activity-home-based-exercise-interventions-adults-65-years-older.html. Accessed 14 June 2024
- 60.Kraus WE et al (2019) Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Med Sci Sports Exerc 51(6):1270–1281. 10.1249/MSS.0000000000001939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colberg SR et al (2016) Physical activity/exercise and diabetes: a position statement of the American diabetes association. Diabetes Care 39(11):2065–2079. 10.2337/dc16-1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodrigues EV, Gomes AR, Tanhoffer AI, Leite N (2014) Effects of exercise on pain of musculoskeletal disorders: a systematic review. Acta Ortop Bras 22(6):334–338. 10.1590/1413-78522014220601004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Semlitsch T et al (2013) Increasing physical activity for the treatment of hypertension: a systematic review and meta-analysis. Sports Med 43(10):1009–1023. 10.1007/s40279-013-0065-6 [DOI] [PubMed] [Google Scholar]
- 64.Spruit MA, Pitta F, McAuley E, ZuWallack RL, Nici L (2015) Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 192(8):924–933. 10.1164/rccm.201505-0929CI [DOI] [PubMed] [Google Scholar]
- 65.Folsom AR, Cook TC, Sprafka JM, Burke GL, Norsted SW, Jacobs DR (1991) Differences in leisure-time physical-activity levels between blacks and whites in population-based samples—the Minnesota heart survey, (in english). J Behav Med 14(1):1–9. 10.1007/Bf00844764 [DOI] [PubMed] [Google Scholar]
- 66.Bild DE, Jacobs DR Jr, Sidney S, Haskell WL, Anderssen N, Oberman A (1993) Physical activity in young black and white women. The CARDIA Study. Ann Epidemiol 3(6):636–644. 10.1016/1047-2797(93)90087-k [DOI] [PubMed] [Google Scholar]
- 67.Wing RR et al (1989) Obesity, obesity-related behaviors and coronary heart disease risk factors in black and white premenopausal women. Int J Obes 13(4):511–519 [PubMed] [Google Scholar]
- 68.Washburn RA, Kline G, Lackland DT, Wheeler FC (1992) Leisure time physical activity: are there black/white differences? Prev Med 21(1):127–135. 10.1016/0091-7435(92)90012-7 [DOI] [PubMed] [Google Scholar]
- 69.Paxton RJ et al (2012) Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer 118(16):4024–4031. 10.1002/cncr.27389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson JL, Wolfe VK, Wilson N, Pardilla MN, Perez G (2003) Personal, social, and environmental correlates of physical activity in native American women. Am J Prev Med 25:53–60. 10.1016/s0749-3797(03)00165-x [DOI] [PubMed] [Google Scholar]
- 71.Pedersen M, Harris KJ, Brown B, Anderson K, Lewis JP (2022) A systematic review of interventions to increase physical activity among American Indian and Alaska native older adults. Gerontologist 62(6):e328–e339. 10.1093/geront/gnab020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Im EO et al (2012) Asian American midlife women’s attitudes toward physical activity. J Obstet Gynecol Neonatal Nurs 41(5):650–658. 10.1111/j.1552-6909.2012.01392.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joseph RP, Keller C, Affuso O, Ainsworth BE (2017) Designing culturally relevant physical activity programs for African–American Women: a framework for intervention development. J Racial Ethn Health Disparities 4(3):397–409. 10.1007/s40615-016-0240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keller CS, Coe K, Moore N (2014) Addressing the demand for cultural relevance in intervention design. Health Promot Pract 15(5):654–663. 10.1177/1524839914526204 [DOI] [PubMed] [Google Scholar]
- 75.Conn VS, Chan K, Banks J, Ruppar TM, Scharff J (2013) Cultural relevance of physical activity intervention research with underrepresented populations. Int Q Commun Health Educ 34(4):391–414. 10.2190/IQ.34.4.g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baker LC, Bundorf MK, Devlin AM, Kessler DP (2016) Medicare advantage plans pay hospitals less than traditional medicare pays. Health Aff (Millwood) 35(8):1444–1451. 10.1377/hlthaff.2015.1553 [DOI] [PubMed] [Google Scholar]
- 77.Hung A, Stuart B, Harris I (2016) The effect of medicare advantage enrollment on mammographic screening. Am J Manag Care 22(2):e53–e59 [PubMed] [Google Scholar]
- 78.Noone AM et al (2016) Comparison of SEER treatment data with medicare claims. Med Care 54(9):e55–e64. 10.1097/MLR.0000000000000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.The EASY tool. Texas A&M Health. https://www.easyforyou.info/. Accessed 18 March 2024
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The SEER-MHOS datasets analyzed during the current study are not publicly available due to the risk of re-identification. The data are available to investigators for research purposes, but approval is required to obtain the data.