Abstract
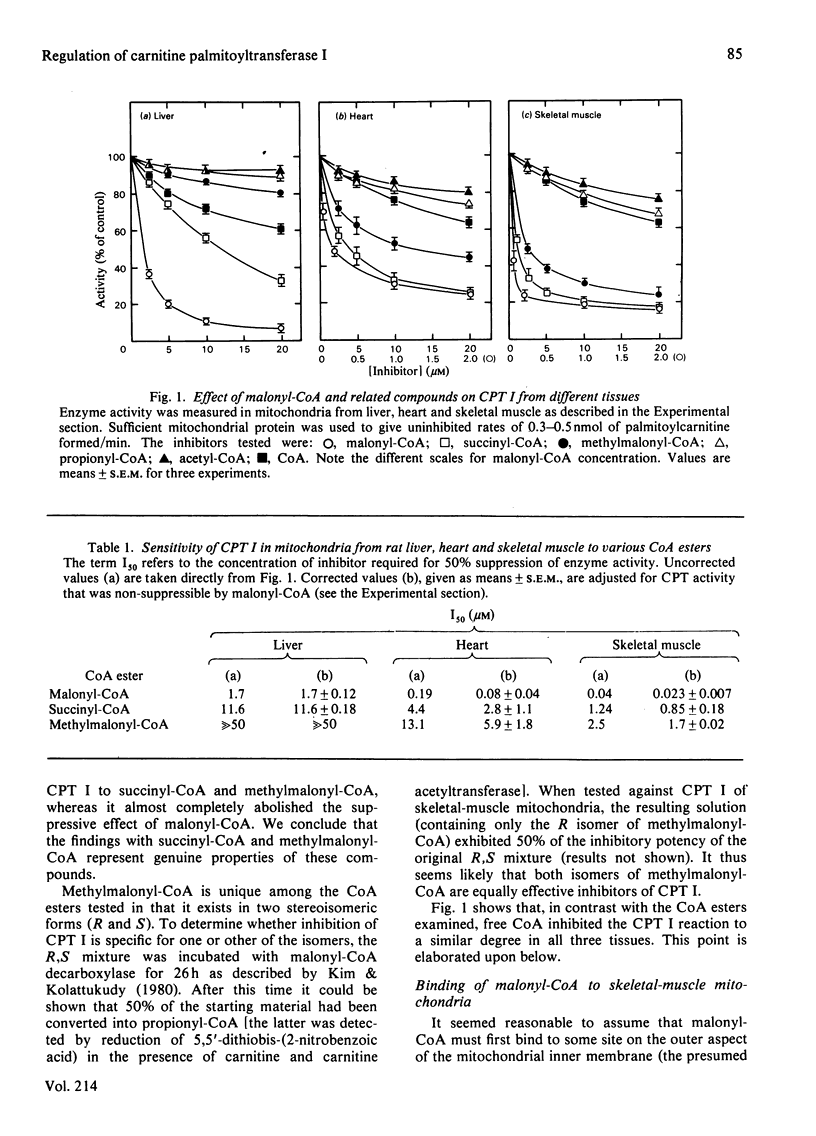
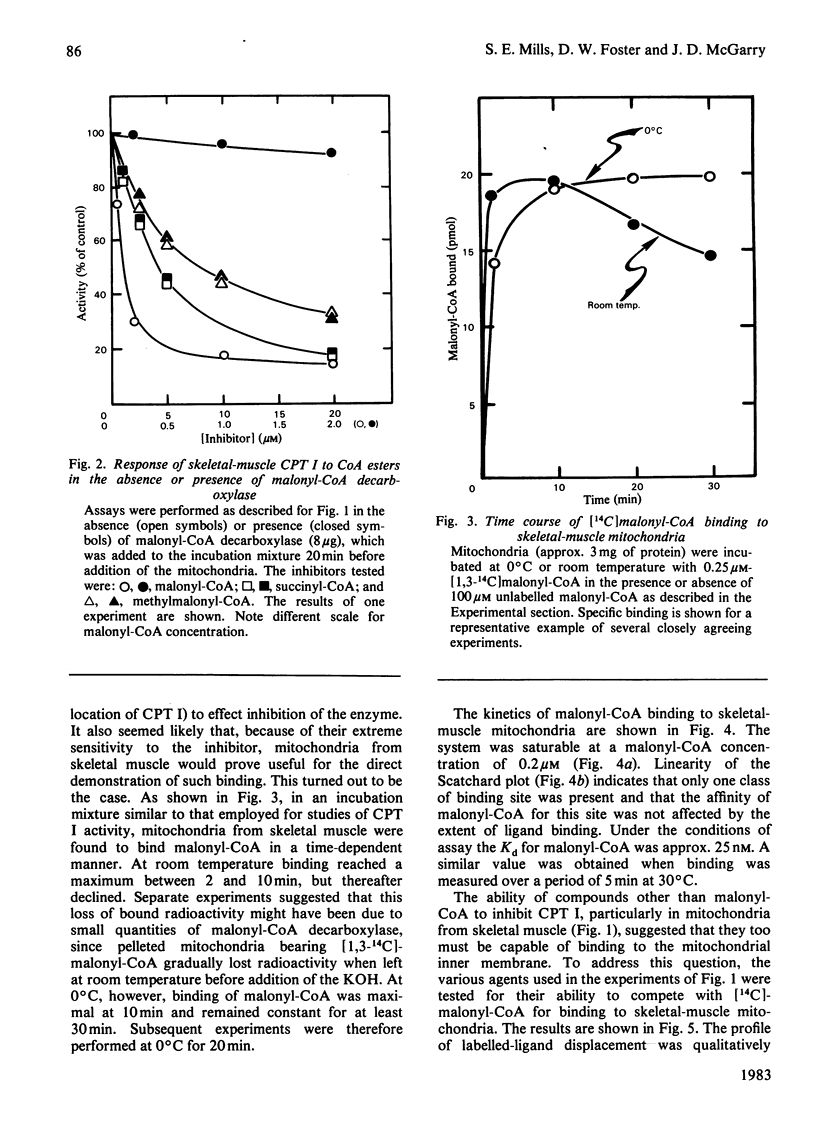
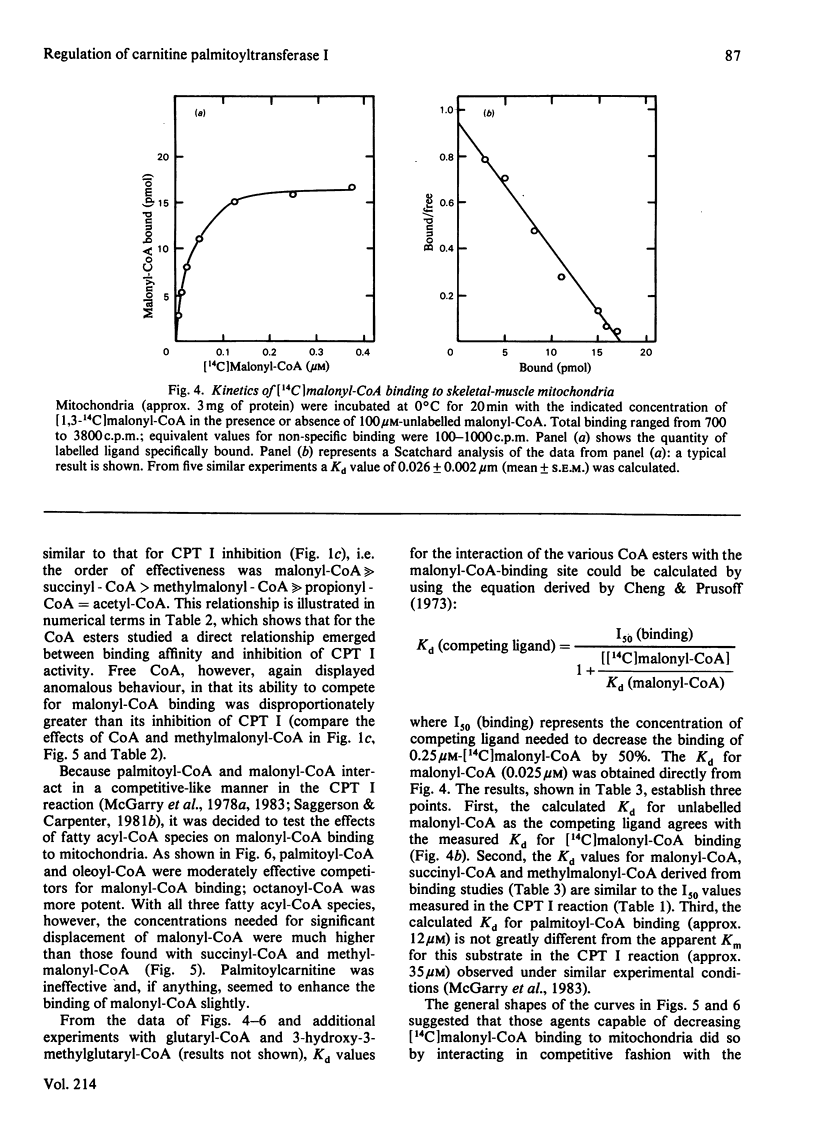
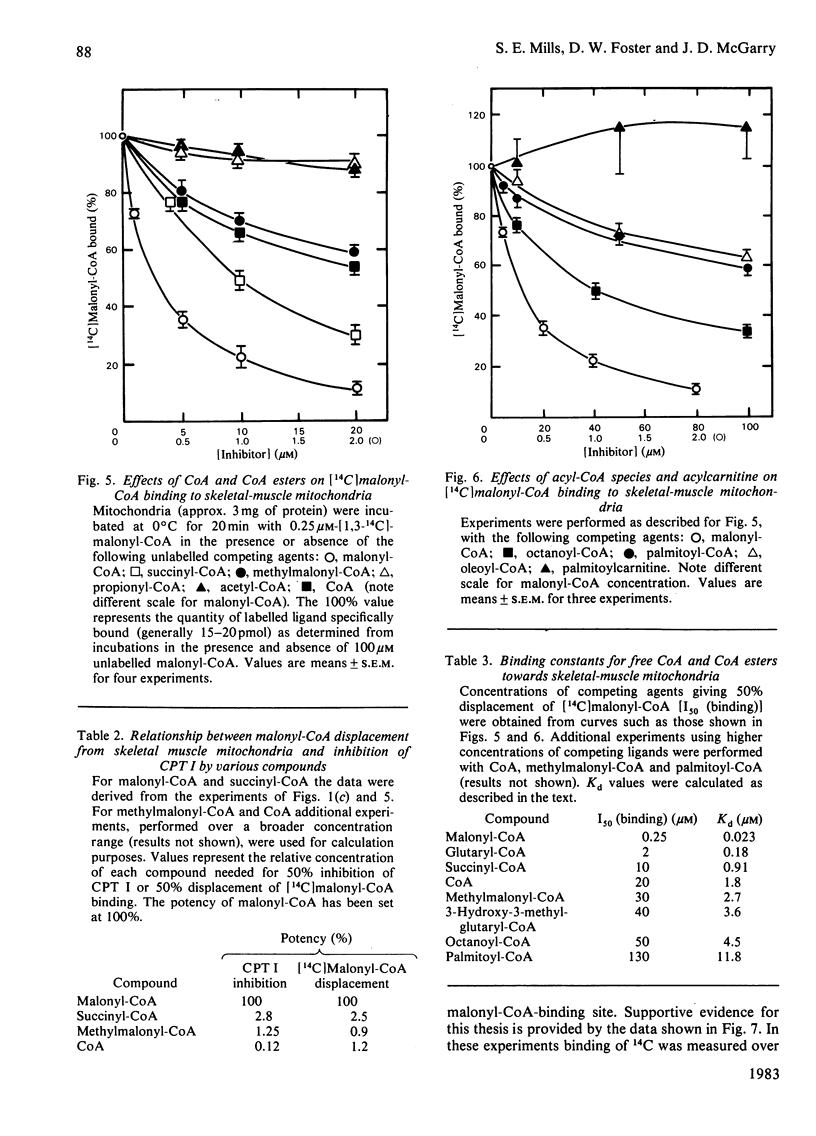
The sensitivity of carnitine palmitoyltransferase I (CPT I; EC 2.3.1.21) to inhibition by malonyl-CoA and related compounds was examined in isolated mitochondria from liver, heart and skeletal muscle of the rat. In all three tissues the same order of inhibitory potency emerged: malonyl-CoA much greater than succinyl-CoA greater than methylmalonyl-CoA much greater than propionyl-CoA greater than acetyl-CoA. For any given agent, suppression of CPT I activity was much greater in skeletal muscle than in liver, with the heart enzyme having intermediate sensitivity. With skeletal-muscle mitochondria a high-affinity binding site for [14C]malonyl-CoA was readily demonstrable (Kd approx. 25 nM). The ability of other CoA esters to compete with [14C]malonyl-CoA for binding to the membrane paralleled their capacity to inhibit CPT I. Palmitoyl-CoA also competitively inhibited [14C]malonyl-CoA binding, in keeping with its known ability to overcome malonyl-CoA suppression of CPT I. For reasons not yet clear, free CoA displayed anomalous behaviour in that its competition for [14C]malonyl-CoA binding was disproportionately greater than its inhibition of CPT I. Three major conclusions are drawn. First, malonyl-CoA is not the only physiological compound capable of suppressing CPT I, since chemically related compounds, known to exist in cells, also share this property, particularly in tissues where the enzyme shows the greatest sensitivity to malonyl-CoA. Second, malonyl-CoA and its analogues appear to interact with the same site on the mitochondrial membrane, as may palmitoyl-CoA. Third, the degree of site occupancy by inhibitors governs the activity of CPT I.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim Biophys Acta. 1981 Sep 24;665(3):628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Kolattukudy P. E. Stereospecificity of malonyl-CoA decarboxylase, acetyl-CoA carboxylase, and fatty acid synthetase from the uropygial gland of goose. J Biol Chem. 1980 Jan 25;255(2):686–689. [PubMed] [Google Scholar]
- Long C. S., Haller R. G., Foster D. W., McGarry J. D. Kinetics of carnitine-dependent fatty acid oxidation: implications for human carnitine deficiency. Neurology. 1982 Jun;32(6):663–666. doi: 10.1212/wnl.32.6.663. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Stark M. J., Foster D. W. Hepatic malonyl-CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem. 1978 Nov 25;253(22):8291–8293. [PubMed] [Google Scholar]
- NORUM K. R. PALMITYL-COA:CARNITINE PALMITYLTRANSFERASE. PURIFICATION FROM CALF-LIVER MITOCHONDRIA AND SOME PROPERTIES OF THE ENZYME. Biochim Biophys Acta. 1964 Jul 8;89:95–108. [PubMed] [Google Scholar]
- Saggerson E. D. Carnitine acyltransferase activities in rat liver and heart measured with palmitoyl-CoA and octanoyl-CoA. Latency, effects of K+, bivalent metal ions and malonyl-CoA. Biochem J. 1982 Feb 15;202(2):397–405. doi: 10.1042/bj2020397. [DOI] [PMC free article] [PubMed] [Google Scholar]


