Abstract
Rhabdomyosarcoma (RMS) of the urinary bladder in adults and elderly is an exceptionally rare neoplasm that displays poorly differentiated solid (alveolar-like) small cell pattern, frequently indistinguishable from small cell neuroendocrine carcinoma (SCNEC). However, the histogenesis of RMS and SCNEC and their inter-relationship have not been well studied and remained controversial. We herein analyzed 23 SCNEC and 3 small round cell RMS of the bladder for neuroendocrine (synaptophysin + chromogranin A) and myogenic (desmin + myogenin) marker expression and for TERT promoter mutations. In addition, the RMS cohort and one SCNEC that was revised to RMS were tested for gene fusions using targeted RNA sequencing (TruSight Illumina Panel which includes FOXO1 and most of RMS-related other genes). Overall, significant expression of myogenin and desmin was observed in one of 23 original SCNEC justifying a revised diagnosis to RMS. On the other hand, diffuse expression of synaptophysin was noted in 2 of the 4 RMS, but chromogranin A was not expressed in 3 RMS tested. TERT promoter mutations were detected in 15 of 22 (68%) SCNEC and in two of three (67%) assessable RMS cases, respectively. None of the four RMS cases had gene fusions. Our data highlights phenotypic and genetic overlap between SCNEC and RMS of the urinary bladder. High frequency of TERT promoter mutations in SCNEC is in line with their presumable urothelial origin. In addition, the presence of TERT promoter mutation in 2 of 3 RMS and lack of FOXO1 and other gene fusions in all 4 RMSs suggest a mucosal (urothelial) origin, probably representing extensive monomorphic rhabdomyoblastic transdifferentiation in SCNEC.
Keywords: Small cell neuroendocrine carcinoma, Rhabdomyosarcoma, Urinary bladder, TERT promoter mutations, FOXO1 gene fusions
Introduction
Urothelial bladder cancer is the fifth most common cancer in men worldwide and one of the most cost consuming malignancies [1]. Divergent histomorphological differentiation accompanied by distinct clinical outcomes is a main characteristic of bladder cancer. Some urothelial carcinoma subtypes are associated with poor clinical outcome, but histology-tailored therapeutic recommendations are not available yet [2]. Among the reported bladder carcinoma subtypes, neuroendocrine cancer is a rare and clinically aggressive subtype accounting for < 1% of bladder tumors [3]. Small cell neuroendocrine carcinoma (SCNEC) of the urinary bladder is the major representative in the spectrum of neuroendocrine bladder cancer, but mixed types do occur [4]. From a clinical point of view, SCNEC is characterized by highly aggressive course heralded by early metastasis and worse prognosis with 80% of patients dying within 5 years after diagnosis [5].
Skeletal muscle differentiation occurs rarely across a variety of human malignancies. However, it has a greater tendency to occur among neural/neuroendocrine neoplasms [6]. Moreover, rhabdomyosarcoma (RMS) of the bladder in adults and elderly frequently shows solid small round cell pattern recapitulating poorly differentiated solid alveolar RMS of other sites. This pattern closely resembles SCNEC [7]. Nevertheless, clinical prognosis as well as therapeutic implications as for example choice of chemotherapy differs significantly for the two entities [3, 8].
The aim of this study was to compare SCNEC and adult-onset RMS of the bladder for skeletal muscle differentiation and neuroendocrine marker expression, respectively. In addition, we tested both cohorts for TERT promoter gene mutations (as surrogate for urothelial origin) and the RMS cohort for FOXO1 fusions (as marker for majority of alveolar RMS cases) using a large, targeted RNA fusion detection panel to assess the hypothesis, if both entities represent two phenotypic patterns of same histogenetic disease.
Materials and methods
Study cohort
Twenty-three cases of SCNEC and three RMS cases were retrieved from routine and consultation files of our departments. Histological reassessment was done in keeping with the currently valid version of the World Health Organization (WHO) 2022 classification for bladder tumors [9]. For each tumor, transurethral resection specimens were used for analysis.
Immunohistochemical analysis
Immunohistochemical analysis (IHC) was performed using a Ventana BenchMark Ultra autostainer (Ventana Medical Systems Inc, 1910 Innovation Park Drive, Tucson, Arizona, USA) according to the manufacturer’s instructions. Whole tissue consecutive, 3-µm cuts were made from embedded tissues. The following antibodies were used for analysis: desmin (D33, mouse monoclonal, Dako, dilution 1:50), myogenin (F5D, monoclonal mouse, Dako, dilution 1:50), synaptophysin (rabbit polyclonal, ThermoScientific, dilution 1:350), and chromogranin A (DAK-A3, monoclonal mouse, Dako, 1:400). Expression of these four markers was scored as strong, moderate or weak and diffuse or focal. Diverse other markers were used in a case-to-case basis according to the most pertinent differential diagnostic considerations at time of initial biopsy assessment.
DNA isolation
The manual microdissection of tumor tissue was performed carefully after previous annotation of the SCNEC as well as RMS area on a hematoxylin and eosin (H&E)-stained slide. DNA isolation was performed using the Maxwell16® LEV Blood DNA Kit (Promega, Mannheim, Germany) according to the manufacturer’s instructions.
TERT promoter analysis
The mutation analysis of the TERT promoter was performed as described elsewhere using SNaPshot analysis of the TERT core promoter with an ABI Prism 3500 Genetic Analyzer and the SNaPshot-Multiplex-Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions [10]. SNaPshot assays were designed to detect the three-hotspot mutations at positions − 146, − 124, and − 57 bp of the TERT promoter.
RNA isolation
For all RMS samples and one SCNEC with diffuse rhabdomyogenic features, RNA was isolated from formalin-fixed paraffin embedded (FFPE) tissue sections using RNeasy FFPE Kit (Qiagen, Hilden, Germany) and quantified spectrophotometrically using NanoDrop-1000 (Thermo Fisher Scientific, Waltham, MA, USA).
RNA fusion analysis
For all RMS samples and one SCNEC with myogenic features, molecular analysis was performed using the TruSight RNA Fusion panel (Illumina, Inc., San Diego, CA, USA) with 500 ng RNA as input according to the manufacturer’s protocol. Libraries were sequenced on a MiSeq system system (Illumina, Inc., San Diego, CA, USA) with > 3 million reads per case, and sequences were analyzed using the RNA-Seq Alignment workflow, version 2.0.1 (Illumina, Inc., San Diego, CA, USA). The Integrative Genomics Viewer (IGV), version 2.2.13 13 (Broad Institute, University of California, CA, USA) was used for data visualization [11].
FOXO1 FISH translocation analysis
In two RMS cases (revised SCNEC case 14 and RMS case 1) the ZytoLight®SPEC FOXO1 Dual Color Break Apart Probe (ZytoVision GmbH, Bremerhaven, Germany) was used to detect translocations involving the chromosomal region 13q14.11 harboring the FOXO1 gene and fluorescence in situ hybridization (FISH) study was performed following the manufacturer’s recommendations.
Results
Clinical characteristics and histological review of the SCNEC cohort
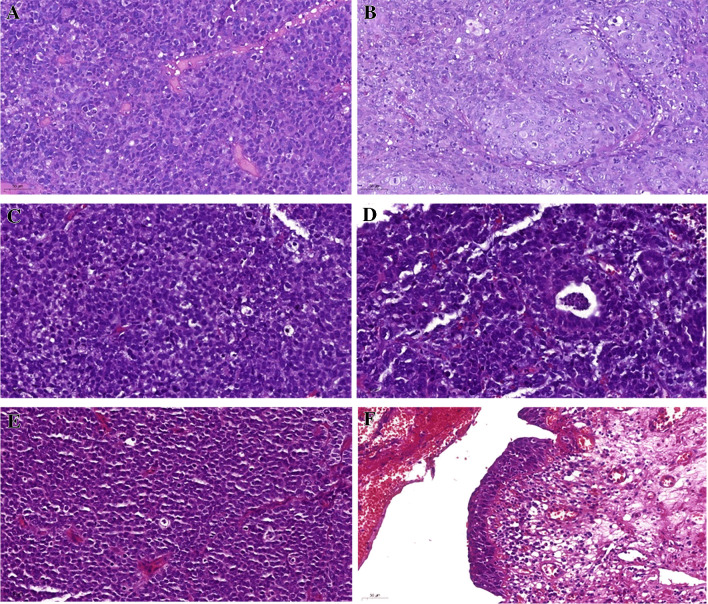
Table 1 summarizes the clinicopathological characteristics of the cohort. Ten (43.5%) out of 23 SCNEC cases affected females. The median age at diagnosis was 67 years (range 42 to 87 years). SCNEC histologically presented with small blue round cells with high-grade morphology disposed into solid sheets and nests, diffusely invading the bladder wall layers. Mitotic activity was brisk. A urothelial component was detected in 10 (43.5%) out of 23 cases, represented by carcinoma in situ (n = 1), conventional invasive urothelial carcinoma component (n = 6) and one case each with glandular, squamous, or sarcomatoid differentiation. Representative images of the morphological appearance of SCNEC and the associated urothelial components are illustrated in Fig. 1.
Table 1.
Study charactersitics of the analyzed SCNEC cohort
| Cases | Sex | Age | Stage | Grade-2016 | Grade-1973 | Urothelial Component |
|---|---|---|---|---|---|---|
| Small cell neuroendocrine carcinoma of the urinary bladder (Case 14 was revised to RMS due to uniform expression of desmin+myogenin) | ||||||
| 1 | Male | 66 | pT1 | High-grade | G3 | Conventional urothelial carcinoma |
| 2 | Male | 68 | pT1 | High-grade | G3 | Conventional urothelial carcinoma |
| 3 | Female | 65 | at least pT1 | High-grade | G3 | Conventional urothelial carcinoma |
| 4 | Female | 76 | pT1 | High-grade | G3 | Carcinoma in situ |
| 5 | Female | 61 | at least pT1 | High-grade | G3 | Not present |
| 6 | Male | 61 | at least pT1 | High-grade | G3 | Not present |
| 7 | Male | 67 | at least pT2 | High-grade | G3 | Not present |
| 8 | Female | 68 | pTa | High-grade | G3 | Glandular |
| 9 | Female | 66 | at least pT1 | High-grade | G3 | Conventional urothelial carcinoma |
| 10 | Male | 65 | at least pT1 | High-grade | G3 | Not present |
| 11 | Female | 79 | pT1 | High-grade | G3 | Not present |
| 12 | Male | 72 | pT1 | High-grade | G3 | Not present |
| 13 | Female | 55 | at least pT1 | High-grade | G3 | Not present |
| 14 | Male | 60 | at least pT1 | High-grade | G3 | Not present (diagnosis revised to RMS due to uniform expression of desmin+myogenin) |
| 15 | Male | 78 | pT1 | High-grade | G3 | Not present |
| 16 | Female | 65 | at least pT1 | High-grade | G3 | Not present |
| 17 | Male | 70 | at least pT1 | High-grade | G3 | Not present |
| 18 | Female | 42 | at least pT1 | High-grade | G3 | Not present |
| 19 | Male | 80 | at least pT1 | High-grade | G3 | Conventional urothelial carcinoma |
| 20 | Male | 82 | High-grade | G3 | Not present | |
| 21 | Female | 87 | at least pT1 | High-grade | G3 | Squamous |
| 22 | Male | 79 | at least pT1 | High-grade | G3 | Sarcomatoid |
| 23 | Male | 83 | at least pT1 | High-grade | G3 | Conventional urothelial carcinoma |
| Rhabdomyosarcoma cases of the urinary bladder | ||||||
| 1 | Male | 69 | ||||
| 2 | Male | 72 | ||||
| 3 | Female | 68 | ||||
Fig. 1.
Representative images of small cell neuroendocrine carcinoma (SCNEC) of the urinary bladder: SCNEC Case 23 presenting with small cell neuroendocrine (A) and urothelial (B) components. SCNEC Case 8 (C) was combined with focal glandular differentiation (D). SCNEC (E) component as well as carcinoma in situ (F) are shown for SCNEC Case 4. All images 200 × magnification
Immunohistochemical analysis of neuroendocrine and rhabdomyoblastic features in SCNEC
All SCNEC cases strongly expressed at least one neuroendocrine marker (mostly synaptophysin; 20/21). Variable chromogranin A expression was noted in 7 of 19 cases (moderate/strong in 6 and weak in one case). Diffuse (100%) expression of desmin and myogenin was detected in one case (Case 14), consistent with a revised diagnosis to solid RMS (Table 2). All other cases have not demonstrated any rhabdomyogenic marker expression.
Table 2.
Results of immunohistochemical markers and expression values of both cohorts
| Case | Neuroendocrine markers | Myogenic markers | ||||
|---|---|---|---|---|---|---|
| Synaptophysin | Chromogranin A | Myogenin | Desmin | |||
| Intensity | Intensity | Intensity | Percentage | Intensity | Percentage | |
| Small cell neuroendocrine carcinoma of the bladder | ||||||
| 1 | Not available | Not available | No expression | No expression | ||
| 2 | Not available | Not available | No expression | No expression | ||
| 3 | Strong | No expression | No expression | No expression | ||
| 4 | Strong | Moderate | No expression | No expression | ||
| 5 | Strong | No expression | No expression | No expression | ||
| 6 | Strong | No expression | No expression | No expression | ||
| 7 | Strong | No expression | No expression | No expression | ||
| 8 | Strong | No expression | No expression | No expression | ||
| 9 | Strong | No expression | No expression | No expression | ||
| 10 | Strong | Strong | No expression | No expression | ||
| 11 | Strong | Weak | No expression | No expression | ||
| 12 | Strong | No expression | No expression | No expression | ||
| 13 | Strong | Moderate | No expression | No expression | ||
| 14 | Strong | No expression | Strong | 100 | Strong | 100 |
| 15 | No expression | No expression | No expression | No expression | ||
| 16 | Strong | Moderate | No expression | No expression | ||
| 17 | Strong | No expression | No expression | No expression | ||
| 18 | Strong | Strong | No expression | No expression | ||
| 19 | Strong | No expression | No expression | No expression | ||
| 20 | Weak | No expression | No expression | No expression | ||
| 21 | Strong | Not available | No expression | No expression | ||
| 22 | Strong | Not available | No expression | No expression | ||
| 23 | Strong | Moderate | No expression | No expression | ||
| Rhabdomyosarcomas of the bladder | ||||||
| 1 | Strong | Strong | 70 | Strong | 80 | |
| 2 | No expression | No expression | Strong | 100 | Strong | 100 |
| 3 | No expression | No expression | Strong | 100 | Strong | 100 |
TERT promoter gene analysis of the SCNEC cohort
Excluding the one case that was revised to RMS, 16 of the 22 genuine SCNEC cases (68%) harbored a hotspot mutation of the TERT promoter gene (Table 3). Almost all mutations except one occurred 124 base pairs away from the TERT gene. Figure 3 shows representative images of the sequence results.
Table 3.
Results of TERT promoter mutation analysis of both cohorts
| Case | TERT promoter mutation analysis | |||
|---|---|---|---|---|
| − 57 | − 124 | − 146 | Mutational status | |
| Small cell neuroendocrine carcinoma of the bladder | ||||
| 1 | Wild type | Wild type | Wild type | Wild type |
| 2 | Not available | G > A | Wild type | Mutated |
| 3 | Wild type | G > A | Wild type | Mutated |
| 4 | Wild type | G > A | Wild type | Mutated |
| 5 | Wild type | G > A | Wild type | Mutated |
| 6 | Wild type | G > A | Wild type | Mutated |
| 7 | Not available | G > A | Wild type | Mutated |
| 8 | Wild type | G > A | Wild type | Mutated |
| 9 | Not available | Not available | Not available | Not available |
| 10 | Wild type | Wild type | Wild type | Wild type |
| 11 | Not available | Wild type | G > A | Mutated |
| 12 | Not available | G > A | Wild type | Mutated |
| 13 | Wild type | G > A | Wild type | Mutated |
| 14 | Wild type | G > A | Wild type | Mutated |
| 15 | Wild type | G > A | Wild type | Mutated |
| 16 | Wild type | G > A | Wild type | Mutated |
| 17 | Wild type | G > A | Wild type | Mutated |
| 18 | Wild type | Wild type | Wild type | Wild type |
| 19 | Wild type | Wild type | Wild type | Wild type |
| 20 | Wild type | G > A | Wild type | Mutated |
| 21 | Wild type | Wild type | Wild type | Wild type |
| 22 | Wild type | G > A | Wild type | Mutated |
| 23 | Wild type | Wild type | Wild type | Wild type |
| Rhabdomyosarcomas of the bladder | ||||
| 1 | Not available | Not available | Not available | Not available |
| 2 | Wild type | G > A | Wild type | Mutated |
| 3 | Wild type | Wild type | Wild type | Wild type |
Fig. 3.
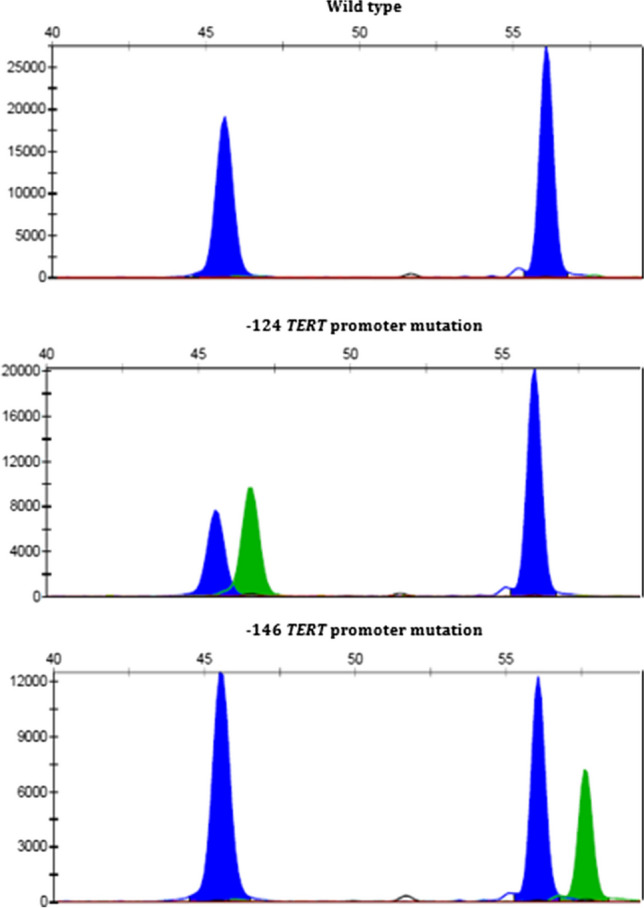
A Representative examples of the SNaPshot analysis of the TERT promoter hot spot mutations. Shown are Case 18 (upper), Case 12 (middle), and Case 11 (lower). The X-axis represents base pair length of the DNA fragment, and the Y-axis shows the intensity of fluorescence signal of the labeled nucleotide. The blue peak for each promoter mutations corresponds to a guanine and the green peak an adenine
Clinicopathological characteristics and histological review of the RMS cases
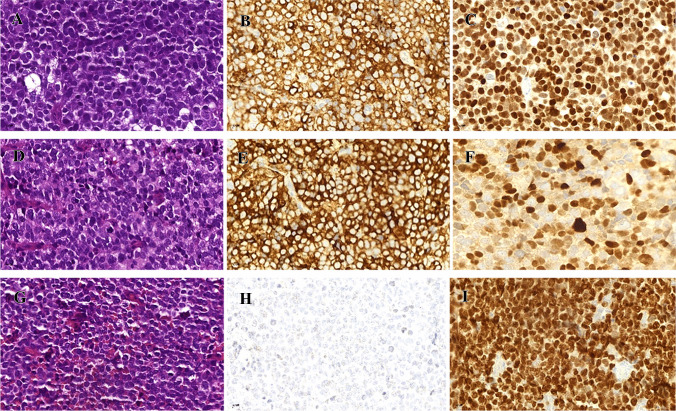
Table 1 summarizes the clinicopathological characteristics of the cases (including the revised SCNEC Case 14). The RMS affected three males and one female aged 60 to 72 years (median, 68). All tumors showed diffuse solid sheets of monotonous small round cells with variable dyscohesive pseudoalveolar arrangements and brisk mitotic and apoptotic activity, closely recapitulating solid-alveolar RMS of other sites. No true rhabdomyoblastic cells were seen on H&E-stained slides (Fig. 1B). A urothelial carcinoma component was lacking in all cases. Immunohistochemistry showed homogeneous expression of desmin and myogenin in 70% to 100% of cells; two cases demonstrated a strong synaptophysin expression. Representative images of the hsitology of RMS, and the different expression levels of the used immunohistochemical markers are shown in Fig. 2.
Fig. 2.
Representative images of the histology (A, D, and G) and immunohistochemical expression of synaptophysin (B, E, and H) and myogenin (C, F, and I) in RMS cases (upper row: SCNEC 14 that was revised to RMS; middle row: RMS Case 1; lower row: RMS Case 2); All images 400 × magnification
RNA fusion and TERT promoter findings
As a surrogate marker of solid alveolar RMS, we tested the three RMS cases and the 4th case that was revised from SCNEC to RMS for gene fusions known to be frequently encountered in RMS including FOXO1 and other fusions. None of the four cases showed a FOXO1 fusion or fusions involving any of the 507 gene included in the TruSight RNA Fusion Panel used. Notably, this panel includes also FUS, EWSR1, FOXO4, NCOA1, NCOA2, PAX3, VGLL3, and FGFR1 and is hence able to detect nearly all fusions known to be involved in adult RMS of different types. RMS Case 2 showed a LOC493754-AUTS2 fusion of unknown significance. Altogether, two of three (67%) assessable RMS cases showed a − 124 hot spot TERT promoter mutation (Fig. 3).
Discussion
Significant morphological and occasionally immunophenotypic overlap is observed between SCNEC and poorly differentiated RMS originating in different organs. However, in routine practice, this overlap has no significant diagnostic impact given that the anatomic sites affected by these two entities differ significantly in most cases. Additionally, most of alveolar RMS affect children and adolescents, whereas neuroendocrine carcinomas are unexpected or do not occur among this age group, limiting the differential diagnosis. However, topographic overlap between SCNEC and poorly differentiated RMS presenting in adults is noted in a few organs, in particular, in epithelial-lined visceral organs including the sinonasal cavities and the urinary bladder. Several studies have pointed out the frequent expression of pankeratin and neuroendocrine markers in alveolar RMS [12–14]. To date, the exact cell of origin of RMS originating within these epithelial-lined organs remained elusive. Likewise, the histogenetic relationship, if any, between these poorly differentiated RMS and SCNEC has not been sufficiently explored.
After the lung, the urinary bladder is the second most common site of origin of SCNEC. In concordance with the literature, SCNEC predominately occurs in older patients with a median age at diagnosis of 67.5 years in this study. However, in contrast to its pulmonary counterpart, SCNEC of the bladder is associated with other histologic subtype/s in around 40–50% of the cases [15]. In our current series, we observed a comparable frequency of urothelial carcinoma and variants as a component in 43.5% of SCNEC.
RMS of the urinary bladder is rare, primarily occurring in children and adolescents. There are few adult cases reported in the literature [16]. While most pediatric cases correspond to the embryonal subtype of RMS, bladder RMS in adults and elderly is predominantly poorly differentiated with small cell pattern, recapitulating solid alveolar RMS [7]. Their morphology overlaps significantly with SCNEC, frequently representing pitfalls or diagnostic challenge. The issue is further complicated by frequent variable immunoreactivity of RMS cells with low molecular weight keratins and neuroendocrine markers including CD56, synaptophysin, and, less frequently, chromogranin A. In many cases, the exact diagnosis of SCNEC versus solid small cell RMS of the urinary bladder might be arbitrary and only inclusion of desmin and myogenin and the presence or absence of a urothelial component might facilitate distinction.
Rhabdomyoblastic differentiation of variable extent has been reported in high-grade NEC of different organs [6]. Although considered rare, this phenomenon might be under-diagnosed given that rhabdomyogenic markers are only applied to cases with ambiguous diagnosis or uncertain differentiation and not in clear-cut SCNEC cases. Indeed, in this study, we observed diffuse expression of desmin and myogenin in one case originally reported as SCNEC which justified revising diagnosis to RMS. On the other hand, one of our original RMS cases also presented a high expression of neuroendocrine markers. These findings highlight close phenotypic overlap between SCNEC and RMS in the bladder.
A main characteristic of urinary bladder cancer are mutations in the TERT promoter gene. Hotspot mutations of this promoter were detected in around 70% of bladder cancer cases and were not associated with clinical or pathological parameters [17]. In a recent publication on 132 SCNEC of the bladder, TERT promoter mutations were detected in 68% of cases [18]. We herein report similar frequency in our cohort of 22 SCNEC (68%). In the context of a bladder neoplasm, detection of TERT promoter mutation has emerged as a surrogate marker for urothelial origin. As reported by Priemer et al., the TERT promoter mutational status can differentiate SCNEC of the bladder from those of prostatic origin [19].
In our current study, we found significant morphological and immunophenotypic overlap between SCNEC and RMS of the bladder. The only distinguishing diagnostic differences of RMS are the lack of a urothelial component and the presence of homogeneous rhabdomyoblastic immunophenotype. Our findings suggest a continuum of rhabdomyoblastic differentiation in both entities ranging from virtually absent in classical SCNEC on one end of the spectrum to being the sole pattern in tumors classified as RMS on the opposite end of the spectrum with possible intermediate variants in between, although we have not observed cases with focal or partial rhabdomyoblastic differentiation, but this might be the result of sampling errors. Consistent with this view, none of the four RMS cases tested with targeted RNA sequencing showed any of the gene fusions expected in genuine alveolar-type RMS. The common origin of SCNEC and RMS in the bladder was further strengthened by the high comparable frequency of TERT promoter mutations identified in both entities in our study (68% of SCNEC and 67% of RMS cases, respectively). According to the current literature, TERT promoter mutations are very rare in RMS and were detected in approximately 1.4% of RMS cases not stratified by the organ of origin [20]. On the other hand, > 80% of all alveolar RMS harbor a distinct balanced FOXO1 translocation, which is a characteristic and disease-defining genetic marker [8].
Thompson et al. [14] analyzed 52 alveolar RMS of the sinonasal tract in adults aged ≥ 18 years (mean age, 43) and detected low-molecular weight keratins overall in 54% of cases (CAM5.2 in 50% and AE1/AE3 in 36%). Moreover, the neuroendocrine markers CD56 (100%), synaptophysin (35%), and chromogranin (13%) were frequently expressed. However, despite this significant phenotypic overlap with sinonasal SCNEC, the histogenesis and molecular pathogenesis of adult RMS in these epithelial-lined organs seem quite distinct and site-specific. Notably, FOXO1 rearrangements have been detected by PCR studies in 81% of sinonasal RMS cases with PAX3 as fusion partner in 72.7% of cases [14]. On the contrary, none of our 4 RMS cases and none of four unclassified bladder RMSs in adults studied by Gupta et al. revealed a FOXO1 or other RMS-associated fusion [21]. A fusion involving PPP1R12A (fused to LIN7A or PTPRQ) was detected by Gupta et al. in two RMS cases (one reported as sarcomatoid!). However, these gene fusions have not been reported before, and it is not clear if they drive oncogenesis or represent passenger events. Admittedly, the fusion partners detected by Gupta et al. in two tumors are not included in the TruSight Illumina Panel we used in this study, so the relevance of this fusion needs to be verified in larger future studies.
Treatment of poorly differentiated RMS (including solid alveolar subtypes), in adults typically involves a combination of agents such as cyclophosphamide, vincristine, and doxorubicin [22]. In contrast, standard chemotherapy for SCNEC of the urinary bladder often utilizes platinum-based chemotherapy and etoposide [23]. Accordingly, separating these two disease entities has clear therapeutic relevance. However, distinguishing these two aggressive phenotypic diseases in routine practice is challenging and is significantly influenced by extent of sampling. Thorough sampling and careful histological evaluation to detect minor microscopic urothelial foci (in situ or invasive) is the most reliable clue for verifying a urothelial origin. However, in limited biopsies and in cases with uniform rhabdomyoblastic differentiation, only inclusion of desmin and myogenin in the immunohistochemical panel of any potential SCNEC would facilitate this distinction. Our study indicates that TERT promoter mutations are identified in 67% of assessable RMS cases, representing a potential surrogate for a urothelial origin. Enhanced recognition of RMS cases should help to confirm our hypothesis, and if confirmed, then to address the central question, whether the approach to treat adult RMS of the urinary bladder needs to be revised. Moreover, targeting the urothelial origin through additional modified therapeutic approaches could potentially lead to improved response rates.
In summary, our study confirms the reported significant morphological and immunophenotypic overlap between SCNEC and poorly differentiated RMS of the urinary bladder. We herein add molecular overlap with similar frequency of TERT promotor mutations in SCNEC (68%) and RMS (67%) in our study. The presence of TERT promoter mutations and lack of FOXO1 and other RMS gene fusions in all tested RMS cases are in line with a urothelial origin of most if not all RMS of the bladder in adults. Urinary bladder small round cell RMS in adults probably originates via monomorphic rhabdomyoblastic transdifferentiation in SCNEC and are likely distinct from genuine alveolar RMS in other organs/ soft tissue and bone sites. Our study is however limited by the low number of RMS cases due to rarity of this disease. Accordingly, extended analysis on more RMS cases is needed to further consolidate this notion.
Acknowledgements
We thank Natascha Leicht, Susanne Blank, and Christa Winkelmann und Verena Popp for excellent technical support.
Author contribution
AA and VB: conception and design of the work, acquisition, analysis and interpretation of the data, and drafting of the MS and revising it critically for important intellectual content and scientific integrity. VB, RS, AH, OH, and AA: acquisition, analysis and interpretation of the data, reading and revising the MS critically for important intellectual content and scientific integrity. All the authors have read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Declarations
This study was conducted in accordance with the Declaration of Helsinki and the protocol approved by the Ethics Committee of the Friedrich-Alexander University Erlangen-Nürnberg (No. 329_16B).
Conflict of interest
The senior author of this study (AA) is the Editor-in-Chief of Virchows Archiv. The authors declare no other competing interests.
Footnotes
This study was performed with active contribution of the late professor Ondrej Hes.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Malats N, Real FX (2015) Epidemiology of bladder cancer. Hematol Oncol Clin North Am 29(2):177–89 (vii) [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Beltran A, Cheng L (2006) Histologic variants of urothelial carcinoma: differential diagnosis and clinical implications. Hum Pathol 37(11):1371–1388 [DOI] [PubMed] [Google Scholar]
- 3.Kouba EJ, Cheng L (2017) Understanding the genetic landscape of small cell carcinoma of the urinary bladder and implications for diagnosis, prognosis, and treatment: a review. JAMA Oncol 3(11):1570–1578 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y et al (2019) Small cell carcinoma of the bladder: the characteristics of molecular alterations, treatment, and follow-up. Med Oncol 36(12):98 [DOI] [PubMed] [Google Scholar]
- 5.Ismaili N (2011) A rare bladder cancer–small cell carcinoma: review and update. Orphanet J Rare Dis 6:75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eusebi V et al (2000) Small cell neuroendocrine carcinoma with skeletal muscle differentiation: report of three cases. Am J Surg Pathol 24(2):223–230 [DOI] [PubMed] [Google Scholar]
- 7.Paner GP et al (2008) Rhabdomyosarcoma of the urinary bladder in adults: predilection for alveolar morphology with anaplasia and significant morphologic overlap with small cell carcinoma. Am J Surg Pathol 32(7):1022–1028 [DOI] [PubMed] [Google Scholar]
- 8.Kieran K, Shnorhavorian M (2016) Current standards of care in bladder and prostate rhabdomyosarcoma. Urol Oncol 34(2):93–102 [DOI] [PubMed] [Google Scholar]
- 9.Netto GJ, Amin MB, Berney DM et al (2022) The 2022 World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs-part B: prostate and urinary tract tumors. Eur Urol 82(5):469–482. 10.1016/j.eururo.2022.07.002 [DOI] [PubMed] [Google Scholar]
- 10.Hurst CD, Platt FM, Knowles MA (2014) Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur Urol 65(2):367–369 [DOI] [PubMed] [Google Scholar]
- 11.Robinson JT et al (2011) Integrative genomics viewer. Nat Biotechnol 29(1):24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahrami A et al (2008) Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall. Mod Pathol 21(7):795–806 [DOI] [PubMed] [Google Scholar]
- 13.Erlenbach-Wunsch K et al (2014) Expression of the LIM homeobox domain transcription factor ISL1 (Islet-1) is frequent in rhabdomyosarcoma but very limited in other soft tissue sarcoma types. Pathology 46(4):289–295 [DOI] [PubMed] [Google Scholar]
- 14.Thompson LDR et al (2018) Sinonasal tract alveolar rhabdomyosarcoma in adults: a clinicopathologic and immunophenotypic study of fifty-two cases with emphasis on epithelial immunoreactivity. Head Neck Pathol 12(2):181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng L et al (2004) Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patients. Cancer 101(5):957–962 [DOI] [PubMed] [Google Scholar]
- 16.Ahsaini M et al (2018) A rare pure embryonal rhabdomyosarcoma of the urinary bladder in an adult successfully managed with neoadjuvant chemotherapy and surgery: a case report. J Med Case Rep 12(1):329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allory Y et al (2014) Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol 65(2):360–366 [DOI] [PubMed] [Google Scholar]
- 18.Hoffman-Censits J et al (2021) Urothelial Cancers with small cell variant histology have confirmed high tumor mutational burden, frequent TP53 and RB mutations, and a unique gene expression profile. Eur Urol Oncol 4:297–300 [DOI] [PubMed]
- 19.Priemer DS et al (2018) Small-cell carcinomas of the urinary bladder and prostate: TERT promoter mutation status differentiates sites of malignancy and provides evidence of common clonality between small-cell carcinoma of the urinary bladder and urothelial carcinoma. Eur Urol Focus 4(6):880–888 [DOI] [PubMed] [Google Scholar]
- 20.Siraj AK et al (2020) Telomerase reverse transcriptase promoter mutations in cancers derived from multiple organ sites among middle eastern population. Genomics 112(2):1746–1753 [DOI] [PubMed] [Google Scholar]
- 21.Gupta S et al (2019) A comparison of adult rhabdomyosarcoma and high-grade neuroendocrine carcinoma of the urinary bladder reveals novel PPP1R12A fusions in rhabdomyosarcoma. Hum Pathol 88:48–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarrabi A, Perrin D, Kavoosi M et al (2023) Rhabdomyosarcoma: current therapy, challenges, and future approaches to treatment strategies. Cancers (Basel) 15(21):5269. 10.3390/cancers15215269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siefker-Radtke AO, Kamat AM, Grossman HB et al (2009) Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J Clin Oncol 27(16):2592–2597. 10.1200/JCO.2008.19.0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.