Abstract
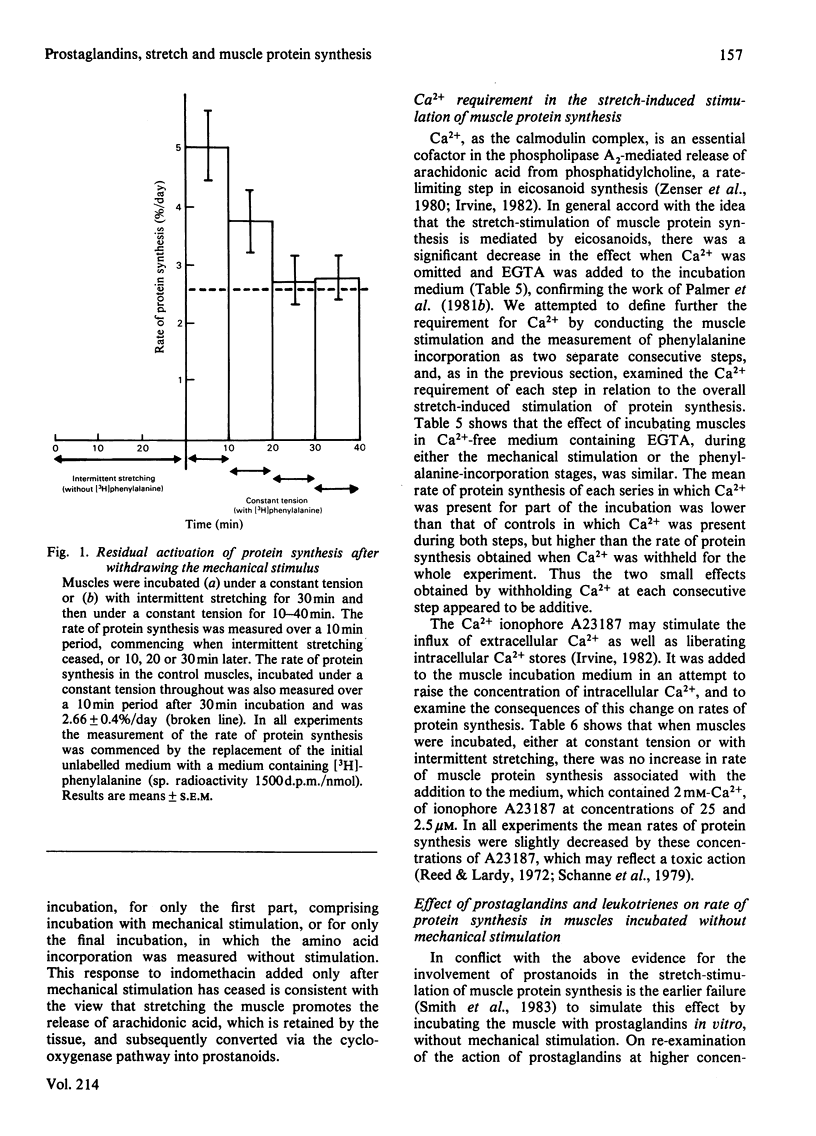
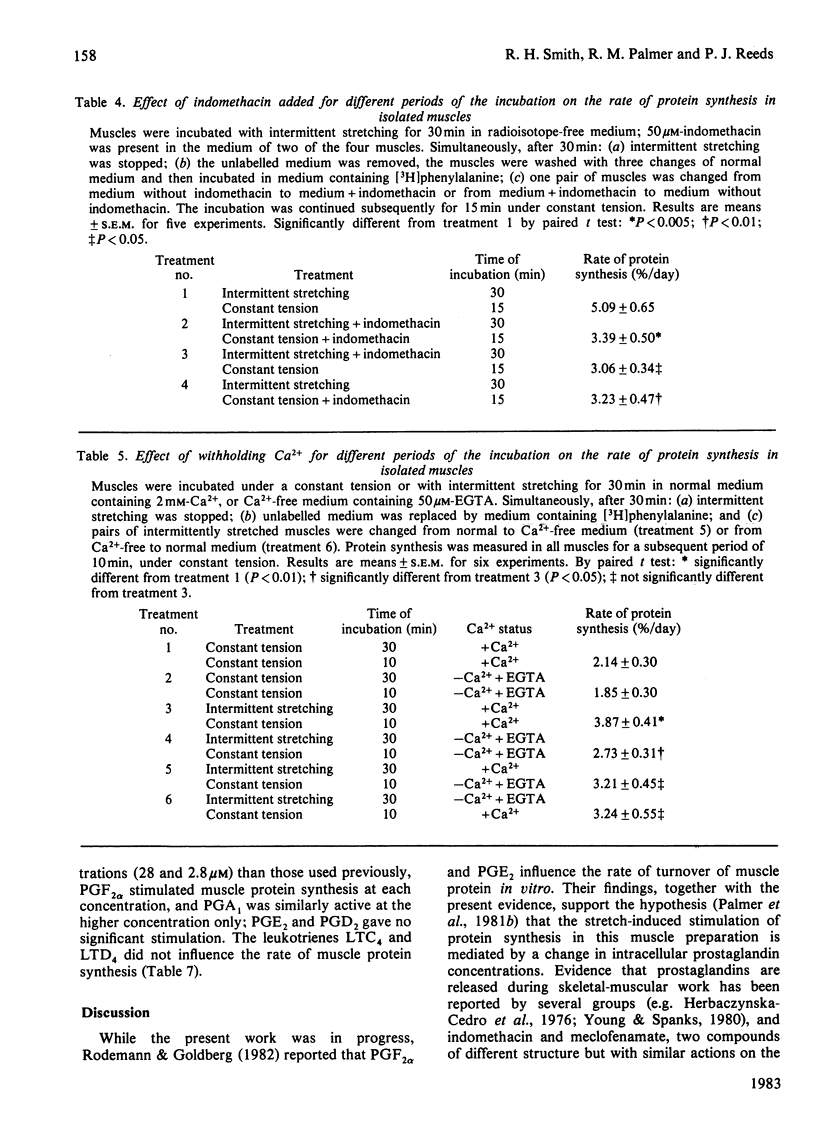
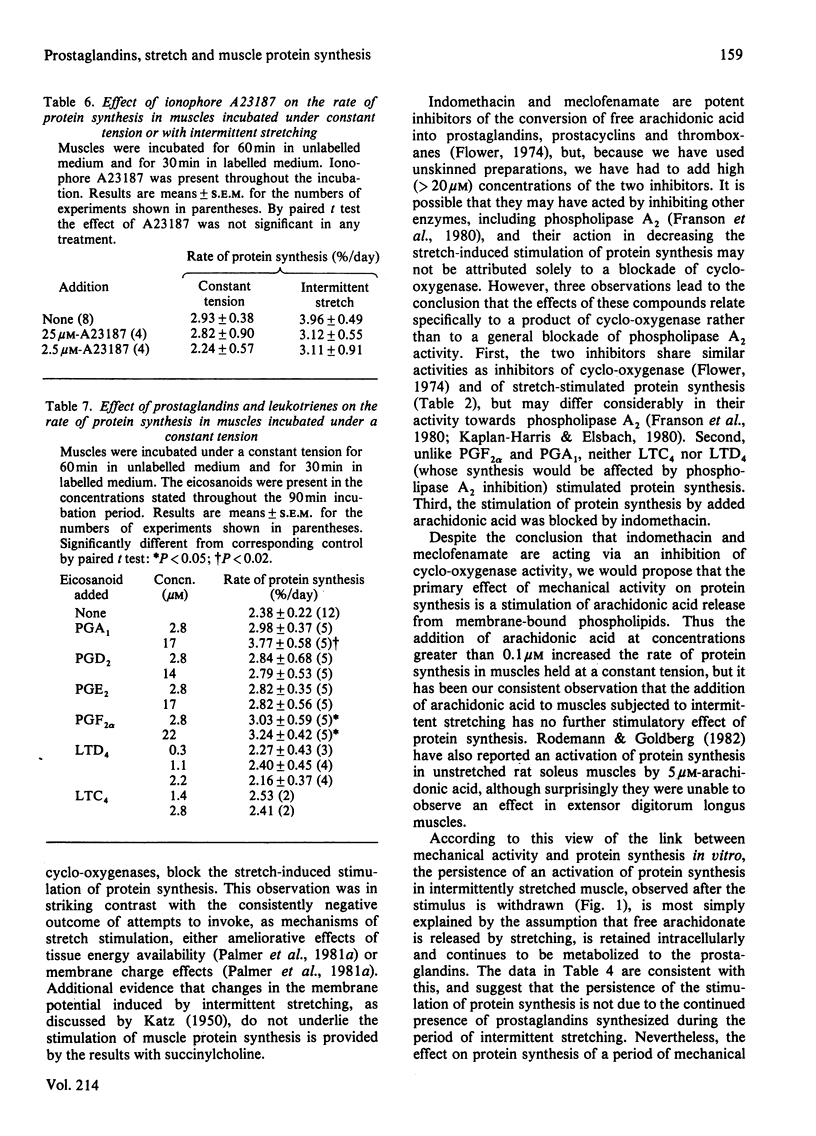
Protein synthesis was measured in isolated intact rabbit muscles by the incorporation of [3H]phenylalanine added at a high concentration (2.5 mM) to the incubation medium. Intermittent mechanical stretching substantially increased the rate of protein synthesis relative to that in control muscles incubated under a constant tension. Indomethacin and meclofenamic acid, inhibitors of the enzyme cyclo-oxygenase, which converts free arachidonic acid into the prostaglandins, prostacyclins and thromboxanes, decreased the rate of protein synthesis in intermittently stretched muscles, but had no effect on synthesis rates in the unstimulated controls. Arachidonic acid at concentrations of 0.2 and 1.0 microM gave a highly significant increase in the rate of protein synthesis in muscles incubated under a constant tension. The ability of arachidonic acid to increase protein-synthesis rates was abolished by the addition of indomethacin. Activation of protein synthesis by intermittent stretching persisted for 10-20 min after the stretch stimulation had ceased. Indomethacin, added either during the initial incubation with intermittent stretching or during the subsequent period when protein synthesis was measured after stimulation had ceased, decreased protein-synthesis rates. This decrease was similar whether indomethacin was present during the initial, final or entire incubation period. In experiments analogous with those in (4) above, when Ca2+ was withheld and EGTA added for the entire incubation, rates of protein synthesis were again decreased. The rates of protein synthesis observed when Ca2+ was present during either an initial stimulation phase or a final, unstimulated, measurement phase were similar, and were intermediate between control rates and those in muscles incubated without Ca2+ for the whole experiment. Two prostaglandins, F2 alpha (2.8 microM) and A1 (28 microM), increased rates of protein synthesis in unstimulated muscles, but prostaglandins E2 and D2 and the leukotrienes C4 and D4 failed to do so. It is concluded that the stretch-stimulated increase in protein synthesis may be caused by activation of membrane phospholipases, release of arachidonic acid and a consequent increase in prostaglandin synthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B. Indirekter Nachweis einer dehnungsinduzierten Ca++-Freisetzung aus dem sarkoplasmatischen Retikulum glyzerinisierter Skelett- und Herzmuskelpräparate. Basic Res Cardiol. 1979 Mar-Apr;74(2):177–202. doi: 10.1007/BF01907820. [DOI] [PubMed] [Google Scholar]
- Chapman D., Gómez-Fernández J. C., Goñi F. M. Intrinsic protein--lipid interactions. Physical and biochemical evidence. FEBS Lett. 1979 Feb 15;98(2):211–223. doi: 10.1016/0014-5793(79)80186-6. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Franson R. C., Eisen D., Jesse R., Lanni C. Inhibition of highly purified mammalian phospholipases A2 by non-steroidal anti-inflammatory agents. Modulation by calcium ions. Biochem J. 1980 Feb 15;186(2):633–636. doi: 10.1042/bj1860633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. The influence of passive stretch on the growth and protein turnover of the denervated extensor digitorum longus muscle. Biochem J. 1978 Aug 15;174(2):595–602. doi: 10.1042/bj1740595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbaczynska-Cedro K., Staszewska-Barczak J., Janczewska H. Muscular work and the release of prostaglandin-like substances. Cardiovasc Res. 1976 Jul;10(4):413–420. doi: 10.1093/cvr/10.4.413. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B. Depolarization of sensory terminals and the initiation of impulses in the muscle spindle. J Physiol. 1950 Oct 16;111(3-4):261–282. doi: 10.1113/jphysiol.1950.sp004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama T., Etlinger J. D. Calcium-dependent regulation of protein synthesis and degradation in muscle. Nature. 1979 May 24;279(5711):344–346. doi: 10.1038/279344a0. [DOI] [PubMed] [Google Scholar]
- Kaplan-Harris L., Elsbach P. The antiinflammatory activity of analogs of indomethacin correlates with their inhibitory effects on phospholipase A2 of rabbit polymorphonuclear leukocytes. Biochim Biophys Acta. 1980 May 28;618(2):318–326. doi: 10.1016/0005-2760(80)90038-7. [DOI] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Millward D. J. Turnover of muscle protein in the fowl. Changes in rates of protein synthesis and breakdown during hypertrophy of the anterior and posterior latissimus dorsi muscles. Biochem J. 1978 Nov 15;176(2):407–417. doi: 10.1042/bj1760407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Reeds P. J., Lobley G. E., Smith R. H. The effect of intermittent changes in tension on protein and collagen synthesis in isolated rabbit muscles. Biochem J. 1981 Sep 15;198(3):491–498. doi: 10.1042/bj1980491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett W. C., Jesse R. L., Cohen P. Trypsin-induced phospholipase activity in human platelets. Biochem J. 1976 Nov 15;160(2):405–408. doi: 10.1042/bj1600405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C. V. Cationic dependency of high affinity prostaglandin F2 alpha receptors in bovine corpus luteum cell membranes. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1242–1250. doi: 10.1016/0006-291x(75)90806-2. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Reeds P. J., Palmer R. M., Smith R. H. Protein and collagen synthesis in rat diaphragm muscle incubated in vitro: the effect of alterations in tension produced by electrical or mechanical means. Int J Biochem. 1980;11(1):7–14. doi: 10.1016/0020-711x(80)90274-8. [DOI] [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Rodemann H. P., Waxman L., Goldberg A. L. The stimulation of protein degradation in muscle by Ca2+ is mediated by prostaglandin E2 and does not require the calcium-activated protease. J Biol Chem. 1982 Aug 10;257(15):8716–8723. [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Shier W. T., DuBourdieu D. J. Role of phospholipid hydrolysis in the mechanism of toxic cell death by calcium and ionophore A23187. Biochem Biophys Res Commun. 1982 Nov 16;109(1):106–112. doi: 10.1016/0006-291x(82)91572-8. [DOI] [PubMed] [Google Scholar]
- TROWELL O. A. The culture of mature organs in a synthetic medium. Exp Cell Res. 1959 Jan;16(1):118–147. doi: 10.1016/0014-4827(59)90201-0. [DOI] [PubMed] [Google Scholar]
- Vogt W. Role of phospholipase A2 in prostaglandin formation. Adv Prostaglandin Thromboxane Res. 1978;3:89–95. [PubMed] [Google Scholar]
- Young E. W., Sparks H. V. Prostaglandins and exercise hyperemia of dog skeletal muscle. Am J Physiol. 1980 Feb;238(2):H191–H195. doi: 10.1152/ajpheart.1980.238.2.H191. [DOI] [PubMed] [Google Scholar]
- Zenser T. V., Herman C. A., Davis B. B. Effects of calcium and A23187 on renal inner medullary prostaglandin E2 synthesis. Am J Physiol. 1980 Apr;238(4):E371–E376. doi: 10.1152/ajpendo.1980.238.4.E371. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]


